Beneficial Effects of Temperate Forage Legumes that Contain Condensed Tannins
Abstract
:1. Introduction: Structural Diversity and Function of Condensed Tannins:
2. Condensed Tannins in Temperate Forage Legumes
2.1. Grass and Legume Forages
2.2. The Beneficial Role of Some Condensed Tannins in Ruminant Digestion
3. Ruminant Production on CT Legumes in the Western U.S.
3.1. Conventional Beef Production
3.2. Beef Production on a CT-Containing Forage Legume
| Time in System (day) | ||||||
| CON | USU NAT | NAT | USU BFT | GFD | USU GFD | |
| Pre-weaned beef calf | 207 | 215 | 207 | 215 | 207 | 215 |
| Stocker | 123 | 216 | 159 | 216 | 159 | 216 |
| Yearling finishing | 110 | 111 | 110 | 111 | 313 | 111 |
| Total in days | 440 | 542 | 476 | 542 | 679 | 542 |
| Total in months | 14.6 | 18 | 15.8 | 18 | 22.6 | 18 |
| Weight (kg) | ||||||
| CON | USU NAT | NAT | USU BFT | GFD | USU GFD | |
| Pre-weaned beef calf | 245 | 289 | 245 | 289 | 226 | 289 |
| Stocker | 122 | 162 | 122 | 162 | 67 | 162 |
| Yearling finishing | 204 | 193 | 163 | 106 | 192 | 61 |
| Total weight | 571 | 644 | 530 | 557 | 486 | 512 |
| Dressing percentage (%) | 63.8 | 58.1 | 63.3 | 62.1 | 57.5 | 57.0 |
| Cattle required for 1 × 109 kg red meat | 2,745,005 | 2,672,625 | 2,980,715 | 2,891,034 | 3,585,836 | 3,426,535 |
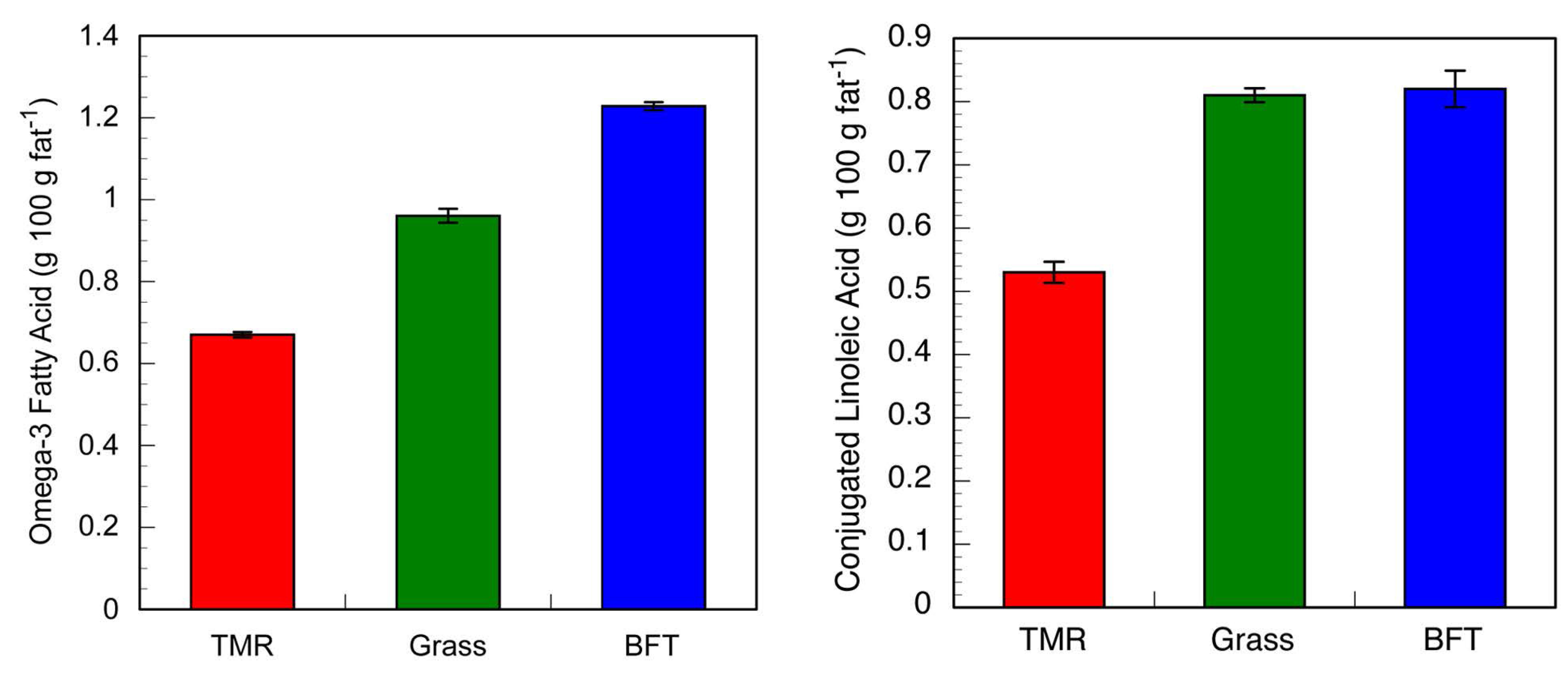
3.3. Dairy Production on a CT-Containing Forage Legume
| Year | BFT Intake | SEM | Grass Intake | SEM | BFT Milk Production | SEM | Grass Milk Production | SEM |
|---|---|---|---|---|---|---|---|---|
| kg/ha | kg/cow/day | |||||||
| 2012 | 1603 | 235 | 773 | 149 | 30 | 1.4 | 25 | 1.6 |
| 2013 | 2183 | 256 | 1301 | 181 | 35 | 0.7 | 30 | 2.1 |
4. Chemical Interactions of Condensed Tannins within a Dietary Context
4.1. Interaction with Proteins
4.2. Interactions with Carbohydrates
4.3. Interactions with Saponins
4.4. Interactions with Alkaloids
4.5. Interactions with Terpenes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mueller-Harvey, I.; Caygill, J.C. Tannins: Their nature and biological significance. In Secondary Plant Products: Antinutritional and Beneficial Actions in Animal Feeding; Nottingham University Press: Nottingham, UK, 1999. [Google Scholar]
- Jones, E.T.; Mangan, J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia Scop.) with fraction 1 leaf protein and with submixillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Robbins, C.T.; Weerasuriya, Y.; Wilson, T.C.; McArthur, C. Tannin chemistry in relation to digestion. J. Range Manag. 1992, 45, 57–62. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Zucker, W.V. Tannins: Does structure determine function? An ecological perspective. Am. Nat. 1983, 121, 335–365. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.; Sharma, S.B. Tansley review. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar]
- Xie, D.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Hanlin, R.L.; Hrmova, M.; Harbertson, J.F.; Downey, M.O. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 2010, 16, 173–188. [Google Scholar] [CrossRef]
- Feeny, P. Effect of oak leaf tannins on larval growth of the winter moth Operopthera brumata. J. Insect Physiol. 1968, 14, 805–817. [Google Scholar] [CrossRef]
- McKey, D. The distribution of secondary compounds within plants. In Herbivores, Their Interaction with Secondary Plant Metabolites; Rosenthal, G.W., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 55–133. [Google Scholar]
- Waghorn, G.C. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Ayres, M.P.; Clausen, T.P.; MacLean, S.F., Jr.; Redman, A.M.; Reichardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Madritch, M.D.; Bailey, J.K.; LeRoy, C.J.; Fischer, D.G.; Rehill, B.J.; Lindroth, E.L.; Hagerman, A.E.; Wooley, S.C.; Hart, S.C.; et al. From genes to ecosystems: The genetic basis of condensed tannins and their role in nutrient regulation in a Populus model system. Ecosystems 2008, 11, 1005–1020. [Google Scholar] [CrossRef]
- Priolo, A.; Bella, M.; Lanza, M.; Galofaro, V.; Biondi, L.; Barbagallo, D.; Ben Salem, H.; Pennisi, P. Carcass and meat quality of lambs fed fresh sulla (Hedysarum coronarium L.) with or without polyethylene glycol or concentrate. Small Rum. Res. 2005, 59, 281–288. [Google Scholar] [CrossRef]
- Priolo, A.; Vasta, V.; Fasone, V.; Lanza, C.M.; Scerra, M.; Biondi, L.; Bella, M.; Whittington, F.M. Meat odour and flavour and indoles concentration in ruminal fluid and adipose tissue of lambs fed green herbage or concentrates with or without tannins. Animal 2009, 3, 454–460. [Google Scholar] [CrossRef]
- Waghorn, G.C.; McNabb, W.C. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc. Nutr. Soc. 2003, 62, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.L.; Waghorn, G.C.; Watkins, K.A.; Bryant, M.A. Feeding birdsfoot trefoil (Lotus corniculatus) reduces the environmental impacts of dairy farming. Proc. N. Z. Soc. Anim. Prod. 2009, 69, 179–183. [Google Scholar]
- Barry, T.N.; McNeill, D.M.; McNabb, W.C. Plant secondary compounds: Their impact on nutritive value and upon animal production. In Proceedings of the XIX International Grassland Conference, Sao Paulo, Brazil, 11–21 February 2001; Wageningen Academic Publishers: Wageningen, The Netherlands, 2001; pp. 445–452. [Google Scholar]
- Min, B.R.; Hart, S.P. Tannins for suppression of internal parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar]
- Niezen, J.H.; Charleston, W.A.G.; Robertson, H.A.; Shelton, D.; Waghorn, G.C.; Green, R. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and development of immunity to gastrointestinal nematodes. Vet. Parasit. 2002, 105, 229–245. [Google Scholar] [CrossRef]
- Min, B.R.; Pomroy, W.E.; Hart, S.P.; Sahlu, T. The effect of short-term consumption of a forage containing condensed tannins on gastro-intestinal nematode parasite infections in grazing wether goats. Small Rum. Res. 2004, 51, 279–283. [Google Scholar] [CrossRef]
- Min, B.R.; Fernandez, J.M.; Barry, T.N.; McNabb, W.C.; Kemp, P.D. The effect of condensed tannins in Lotus corniculatus upon reproductive efficiency and wool production in ewes during autumn. Anim. Feed Sci. Technol. 2001, 92, 185–202. [Google Scholar] [CrossRef]
- Waghorn, G.C. Beneficial effects of low concentrations of condensed tannins in forages fed to ruminants. In Microbial and Plant Opportunities to Improve Lignocellulose Utilization by Ruminants; Akin, D.E., Ljungdahl, L.G., Wilson, J.R., Harris, P.J., Eds.; Elsevier Science Publisher: New York, NY, USA, 1990. [Google Scholar]
- Chung, Y.-H.; McGeough, E.J.; Acharya, S.; McAllister, T.A.; McGinn, S.M.; Harstad, O.M.; Beauchemin, K.A. Enteric methane emission, diet digestibility, and nitrogen excretion from beef heifers fed sainfoin or alfalfa. J. Anim. Sci. 2013, 91, 4861–4874. [Google Scholar] [CrossRef]
- Woodward, S.L.; Waghorn, G.C.; Laboyrie, P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 160–164. [Google Scholar]
- Paolini, V.; Dorchies, P.; Hoste, H. Effects of sainfoin hay on gastrointestinal nematode infections in goats. Vet. Rec. 2003, 152, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.J.; Miller, J.; Hall, J.O.; Clemensen, A.K.; Stott, R.; Snyder, D.; Provenza, F.D. Preference for tanniferous (Onobrychis viciifolia) and non-tanniferous (Astragalus cicer) forage plants by sheep in response to challenge infection with Haemonchus contortus. Small Rum. Res. 2013, 112, 199–207. [Google Scholar] [CrossRef]
- Schreurs, N.M.; Lane, G.A.; Tavendale, M.H.; Barry, T.N.; McNabb, W.C. Pastoral flavor in meat product from ruminant fed fresh forages and it amelioration by forage condensed tannins. Anim. Feed Sci. Technol. 2008, 146, 193–221. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed Sci. Technol. 2008, 147, 223–246. [Google Scholar] [CrossRef]
- Luciano, G.; Monahan, F.J.; Vasta, V.; Biondi, L.; Lanza, M.; Priolo, A. Dietary tannins improve lamb meat colour stability. Meat Sci. 2009, 81, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, V.T.; Kemp, P.D.; Thom, E.R.; Waghorn, G.C.; Woodfield, D.R.; Woodward, S.L. Agronomic evaluation of white clover selected for increased floral condensed tannin. Proc. N. Z. Grassl. Assoc. 2003, 65, 139–145. [Google Scholar]
- Hancock, K.R.; Collette, V.; Fraser, K.; Greig, M.; Xue, H.; Richardson, K.; Jones, C.; Rasmussen, S. Expression of the R2R3-MYB transcription factor TaMYB14 from Trifolium arvense activates proanthocyanidin biosynthesis in the legumes Trifolium repens and Medicago sativa. Plant Physiol. 2012, 159, 1204–1220. [Google Scholar] [CrossRef] [PubMed]
- McCaslin, M. The commercial potential for genetic engineering in alfalfa. In Proceedings of the 38th North American Alfalfa Improvement Conference, Sacramento, CA, USA, 27–31 July 2002; pp. 16–18. Available online: http://www.naaic.org/Meetings/National/2002meeting/2002Abstracts/McCaslinSymposium.pdf (accessed on 12 May 2015).
- University of Nebraska-Lincoln Ag BioSafety Education Center. Available online: http://agbiosafety.unl.edu/education/timeline.htm (accessed on 15 May 2015).
- Majak, W.; McAllister, T.A.; McCartney, D.; Stanford, K.; Cheng, K.J. Bloat in Cattle; Alberta Agriculture and Rural Development: Edmonton, AB, Canada, 2003.
- Barry, T.N.; McNabb, W.C. Review article: The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Symposium on factors influencing the voluntary intake of herbage by ruminants: Voluntary intake in relation to chemical composition and digestibility. J. Anim. Sci. 1965, 24, 834–843. [Google Scholar]
- Jung, H.G.; Allen, M.S. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 1995, 73, 2774–2790. [Google Scholar] [PubMed]
- Wen, L.; Kallenbach, R.L.; Williams, J.E.; Roberts, C.A.; Beuselinck, P.R.; McGraw, R.L.; Benedict, H.R. Performance of steers grazing rhizomatous and nonrhizomatous birdsfoot trefoil in pure stands and in tall fescue mixtures. J. Anim. Sci. 2002, 80, 1970–1976. [Google Scholar] [PubMed]
- Hoekstra, N.J.; Schulte, R.P.O.; Struik, P.C.; Lantinga, E.A. Pathways to improving the N efficiency of grazing bovines. Eur. J. Agron. 2007, 26, 363–374. [Google Scholar] [CrossRef]
- Satter, L.D.; Roffler, R.E. Nitrogen requirement and utilization in dairy cattle. J. Dairy Sci. 1975, 58, 1219–1237. [Google Scholar] [CrossRef]
- Barry, T.N.; Manley, T.R. Interrelationships between the concentrations of total condensed tannin, free condensed tannin and lignin in Lotus sp. and their possible consequences in ruminant nutrition. J. Sci. Food Agric. 1986, 37, 248–254. [Google Scholar] [CrossRef]
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [PubMed]
- Barry, T.N.; Duncan, S.J. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 1. Voluntary intake. Br. J. Nutr. 1984, 51, 493–504. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Barry, T.N. Alternative temperate forages containing secondary compounds for improving sustainable productivity in grazing ruminants. Anim. Feed Sci. Technol. 2005, 120, 179–201. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Barry, T.N.; López-Villalobos, N. Organic matter digestibility of condensed tannin-containing Lotus corniculatus and its prediction in vitro using cellulase/hemicellulase enzymes. Anim. Feed Sci. Technol. 2006, 125, 61–71. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Ulyatt, M.J.; John, A.; Fisher, M.T. The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus L. Br. J. Nutr. 1987, 57, 115–126. [Google Scholar] [CrossRef]
- Douglas, G.B.; Wang, Y.; Waghorn, G.C.; Barry, T.N.; Purchas, R.W.; Foote, A.G.; Wilson, G.F. Liveweight gain and wool production of sheep grazing Lotus corniculatus and lucerne (Medicago sativa). N. Z. J. Agric. Res. 1995, 38, 95–104. [Google Scholar] [CrossRef]
- Marten, G.C.; Jordan, R.M. Substitution value of birdsfoot trefoil for alfalfa-grass in pasture systems. Agron. J. 1979, 71, 55–59. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Ward, R.E.; Griggs, T.C.; Min, B.R.; Aiken, G.E. Average daily gain and blood fatty acid composition of cattle grazing the non-bloating legumes birdsfoot trefoil and cicer milkvetch in the Mountain West. Prof. Anim. Sci. 2011, 27, 574–583. [Google Scholar]
- Marten, G.C.; Ehle, F.R.; Ristau, E.A. Performance and photosensitization of cattle related to forage quality of four legumes. Crop Sci. 1987, 27, 138–145. [Google Scholar] [CrossRef]
- Smith, S.B.; Johnson, B.J. Marbling: Management of Cattle to Maximize the Deposition of intramuscular Adipose Tissue; Cattlemen’s Beef Board and National Cattlemen’s Beef Association: Centennial, CO, USA, 2014. [Google Scholar]
- American Grassfed Association. Grassfed and Grass Pastured Ruminant Standards; American Grassfed Association: Denver, CO, USA, 2014. [Google Scholar]
- Shattuck, K. Where corn is king, a new regard for grass-fed beef. Available online: http://healthimpactnews.com/2013/why-grass-fed-beef-is-good-for-your-health/ (accessed on 13 May 2015).
- Van Elswyk, M.E.; McNeill, S.H. Impact of grass/forage feeding versus grain finishing on beef nutrients and sensory quality: The U.S. experience. Meat Sci. 2014, 96, 535–540. [Google Scholar]
- Grabber, J.H.; Riday, H.; Cassida, K.A.; Griggs, T.C.; Min, D.H.; MacAdam, J.W. Yield, morphological characteristics, and chemical composition of European- and Mediterranean-derived birdsfoot trefoil cultivars grown in the colder continental United States. Crop Sci. 2014, 54, 1893–1901. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Griggs, T.C. Irrigated Birdsfoot Trefoil Variety Trial: Forage Yields; Utah State University Cooperative Extension Service: Logan, UT, USA, 2013. [Google Scholar]
- Hunt, S.R.; MacAdam, J.W.; Griggs, T.C. Seeding rate, oat companion crop and planting season effects on organic establishment of irrigated birdsfoot trefoil in the Mountain West USA. Crop Sci. 2015, in press. [Google Scholar]
- Pitcher, L.R. Beef Average Daily Gain and Enteric Methane Emissions on Birdsfoot Trefoil, Cicer Milkvetch and Meadow Brome Pastures. Master’s Thesis, Utah State University, Logan, UT, USA, December 2015. [Google Scholar]
- Capper, J.L. Is the grass always greener? Comparing the environmental impact of conventional, natural and grass-fed beef production systems. Animals 2012, 2, 127–143. [Google Scholar] [CrossRef]
- Chail, A.; Legako, J.F.; Martini, S.; MacAdam, J.W. Consumer sensory evaluation of forage and conventional feedlot finished beef ribeye steaks. Meat Sci. 2014, 101, 120–121. [Google Scholar] [CrossRef]
- Chail, A.; Legako, J.F.; Martini, S.; Ward, R.; MacAdam, J.W. Comparison of proximate composition, pH and fatty acids of beef ribeye steaks from forage and conventional feedlot finished cattle. In Proceedings of the Reciprocal Meat Conference, Lincoln, NB, USA, 12–17 June 2015; Available online: https://guidebook.com/guide/24176/poi/3363090/?pcat=132880 (acccessed on 15 May 2015).
- Maughan, B.; Provenza, F.D.; Tansawat, R.; Maughan, C.; Martini, S.; Ward, R.; Clemensen, A.; Song, X.; Cornforth, D.; Villalba, J.J. Importance of grass-legume choices on cattle grazing behavior, performance, and meat characteristics. J. Anim. Sci. 2014, 92, 2309–2324. [Google Scholar] [CrossRef] [PubMed]
- MacAdam, J.W.; Griggs, T.C. Irrigated Birdsfoot Trefoil Variety Trial: Forage Nutritive Value; Utah State University Cooperative Extension Service: Logan, UT, USA, 2013. [Google Scholar]
- Hunt, S.R.; MacAdam, J.W.; Reeve, J.R. Establishment of birdsfoot trefoil (Lotus corniculatus) pastures on organic dairy farms in the Mountain West USA. Org. Agric. 2015, 5, 63–77. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Karn, J.F.; Hendrickson, J.R. Dry matter yields of cool-season grass monocultures and grass-alfalfa binary mixtures. Agron. J. 2001, 93, 463–467. [Google Scholar] [CrossRef]
- Smith, L.W.; Goering, H.K.; Gordon, C.H. Relationships of forage compositions with rates of cell wall digestion and indigestibility of cell walls. J. Dairy Sci. 1972, 55, 1140–1147. [Google Scholar] [CrossRef]
- Crampton, E.W.; Donefer, E.; Lloyd, L.E. A nutritive value index for forages. J. Anim. Sci. 1960, 19, 538–544. [Google Scholar]
- Robbins, C.T.; Hanley, T.A.; Hagerman, A.E.; Hjeljord, O.; Baker, D.L.; Schwartz, C.C.; Mautz, W.W. Role of tannins in defending plants against ruminants: Reduction in protein availability. Ecology 1987, 68, 98–107. [Google Scholar] [CrossRef]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D.; Gibson, N.; López-Ortíz, S. Veterinary medicine: The value of plant secondary compounds and diversity in balancing consumer and ecological health. In Sustainable Food Production Includes Human and Environmental Health; Springer Netherlands: Dordrecht, The Netherlands, 2014; Volume 3, pp. 165–190. [Google Scholar]
- Rogosic, J.; Estell, R.E.; Skobic, D.; Martinovic, A.; Maric, S. Role of species diversity and secondary compound complementarity on diet selection of Mediterranean shrubs by goats. J. Chem. Ecol. 2006, 32, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Manzocco, L.; Nuñez, M.J.; Nicoli, M.C. Interaction among phenols in food fortification: Negative synergism on antioxidant capacity. J. Agric. Food Chem. 2004, 52, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of β-lactoglobulin with resveratrol and its biological implications. Biomacromolecules 2008, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.N.; Manley, T.R.; Duncan, S.J. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. Br. J. Nutr. 1986, 55, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.D.; McAllister, T.A.; Yanke, J.; Cheng, K.J.; Muir, A.D. Effects of condensed tannins on endoglucanase activity and filter paper digestion by Fibrobacter succinogenes S85. Appl. Environ. Microbiol. 1993, 59, 2132–2138. [Google Scholar] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Guçlu-Ustundag, O.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Hu, W.L.; Liu, J.X.; Ye, J.A.; Wu, Y.M.; Guo, Y.Q. Effect of tea saponin on rumen fermentation in vitro. Anim. Feed Sci. Technol. 2005, 120, 333–339. [Google Scholar] [CrossRef]
- Sinha Babu, S.P. Saponins and its possible role in the control of helminth parasites. In Recent Progress in Medicinal Plants; Sharma, S.K., Govil, J.N., Singh, V.K., Eds.; Studium Press: New Delhi, India, 2005; Volume 10, pp. 405–418. [Google Scholar]
- Lozano, G.A. Parasitic stress and self-medication in wild animals. Adv. Study Behav. 1998, 27, 291–317. [Google Scholar]
- Freeland, W.J.; Janzen, D.H. Strategies in herbivory by mammals: The role of plant secondary compounds. Am. Nat. 1974, 108, 269–286. [Google Scholar] [CrossRef]
- Freeland, W.J.; Calcott, P.H.; Anderson, L.R. Tannins and saponin: Interaction in herbivore diets. Biochem. Syst. Ecol. 1985, 13, 189–193. [Google Scholar] [CrossRef]
- Copani, G.; Hall, J.O.; Miller, J.; Priolo, A.; Villalba, J.J. Plant secondary compounds as complementary resources: Are they always complementary? Oecologia 2013, 172, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Mori, K.; Shiota, M. Effects of interaction of tannins and coexisting substances. III Formation and solubilization of precipitates with alkaloids. J. Pharm. Soc. Jpn. 1982, 102, 854–858. [Google Scholar]
- Charlton, A.J.; Davis, A.L.; Jones, D.P.; Lewis, J.R.; Davies, A.P.; Haslam, E.; Williamson, M.P. The self-association of the black tea polyphenol theaflavin and its complexation with caffeine. J. Chem. Soc. 2000, 2, 317–322. [Google Scholar] [CrossRef]
- Catanese, F.; Distel, R.A.; Villalba, J.J. Effects of supplementing endophyte-infected tall fescue with sainfoin and polyethylene glycol on the physiology and ingestive behavior of sheep. J. Anim. Sci. 2014, 92, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Provenza, F.D.; Wiedmeier, R.D.; Villalba, J.J. Influence of saponins and tannins on intake and nutrient digestion of alkaloid-containing foods. J. Sci. Food Agric. 2012, 92, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Lyman, T.D.; Provenza, F.D.; Villalba, J.J.; Wiedmeier, R.D. Cattle preferences differ when endophyte-infected tall fescue, birdsfoot trefoil, and alfalfa are grazed in different sequences. J. Anim. Sci. 2011, 89, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Lyman, T.D.; Provenza, F.D.; Villalba, J.J.; Wiedmeier, R.D. Phytochemical complementarities among endophyte-infected tall fescue, reed canarygrass, birdsfoot trefoil and alfalfa affect cattle foraging. Animal 2012, 6, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lyman, T.D.; Provenza, F.D.; Villalba, J.J. Sheep foraging behavior in response to interactions among alkaloids, tannins and saponins. J. Sci. Food Agric. 2008, 88, 824–831. [Google Scholar] [CrossRef]
- Owens, J.; Provenza, F.D.; Wiedmeier, R.D.; Villalba, J.J. Supplementing endophyte-infected tall fescue or reed canarygrass with alfalfa or birdsfoot trefoil increases forage intake and digestibility by sheep. J. Sci. Food Agric. 2012, 92, 987–992. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D.; Clemensen, A.K.; Larsen, R.; Juhnke, J. Preference for diverse pastures by sheep in response to intraruminal administrations of tannins, saponins and alkaloids. Grass Forage Sci. 2011, 66, 224–236. [Google Scholar] [CrossRef]
- Hagerman, A.E. Radial diffusion method for determining tannin in plant extracts. J. Chem. Ecol. 1987, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.J.; Spackman, C.; Goff, B.; Klotz, J.L.; MacAdam, J.W. Interaction between a tannin-containing legume and endophyte-infected tall fescue seed on lambs’ feeding behavior and physiology. In Proceedings of the American Society of Animal Science Annual Meeting, Orlando, FL, USA, 12–16 July 2015; Available online: http://m.jtmtg.org/abs/t/62691 (accessed on 15 February 2015).
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mote, T.; Villalba, J.J.; Provenza, F.D. Foraging sequence influences the ability of lambs to consume foods containing tannins and terpenes. Appl. Anim. Behav. Sci. 2008, 113, 57–68. [Google Scholar] [CrossRef]
- Seefeldt, S.S. Consequences of selecting Ramboulliet ewes for Mountain Big Sagebrush (Artemisia tridentata ssp. vaseyana) dietary preference. Rangeland Ecol. Manag. 2005, 58, 380–384. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D.; GouDong, H. Experience influences diet mixing by herbivores: Implications for plant biochemical diversity. Oikos 2004, 107, 100–109. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacAdam, J.W.; Villalba, J.J. Beneficial Effects of Temperate Forage Legumes that Contain Condensed Tannins. Agriculture 2015, 5, 475-491. https://doi.org/10.3390/agriculture5030475
MacAdam JW, Villalba JJ. Beneficial Effects of Temperate Forage Legumes that Contain Condensed Tannins. Agriculture. 2015; 5(3):475-491. https://doi.org/10.3390/agriculture5030475
Chicago/Turabian StyleMacAdam, Jennifer W., and Juan J. Villalba. 2015. "Beneficial Effects of Temperate Forage Legumes that Contain Condensed Tannins" Agriculture 5, no. 3: 475-491. https://doi.org/10.3390/agriculture5030475
APA StyleMacAdam, J. W., & Villalba, J. J. (2015). Beneficial Effects of Temperate Forage Legumes that Contain Condensed Tannins. Agriculture, 5(3), 475-491. https://doi.org/10.3390/agriculture5030475







