Abstract
Two hardwood species, namely red oak and yellow-poplar, were separated into their bark, sapwood and heartwood components. The samples were tested for calorific value, specific gravity, proximate analysis, mineral composition, chemical composition, ultimate analysis, and thermo-chemical decomposition behavior. In addition, the thermo-chemical decomposition behaviors of cellulose, xylan, and lignin polymers were also tested. Thermo-chemical decomposition behavior was assessed using a thermo-gravimetric (TGA) system by heating the sample from 50 °C to 700 °C at the heating rates of 10, 30 and 50 °C/min under nitrogen. The activation energy was calculated for various fractional conversion values using the isoconversion method. The results showed that char yields of lignin, cellulose and xylan were 41.43%, 4.45% and 1.89%, respectively, at the end of pyrolysis. Furthermore, cellulose, xylan and lignin decomposed dramatically in the temperature range of 320 °C to 360 °C, 150 °C to 230 °C and 100 °C to 410 °C, respectively, with decomposition peaks occurring at 340 °C, 200 °C and 340 °C, respectively. In addition, the maximum activation energy for cellulose was 381 kJ/mol at 360 °C and for xylan it was 348 kJ/mol at 210 °C.
1. Introduction
Renewable energy is currently attracting worldwide interest, as it is partly the solution to environmental concerns arising from the overuse of fossil fuels. Consumption of fossil fuels releases carbon stored beneath the earth’s crust for millennia into the atmosphere in the form of greenhouse gas (GHG) emission, gasses responsible for global warming. On the other hand, biomass when used as fuel releases carbon previously sequestered from the atmosphere, thereby, theoretically not increasing GHG levels. The GHG emissions during the combustion of biomass are assumed to be the same as that absorbed through the photosynthesis process during biomass growth [1], especially in the case of forest and agricultural biomass [2]. However, an overall life cycle analysis has shown an increase in GHG emissions for biomass utilization for biofuels [3]. The increased GHG emissions were the result of the use of fossil energy during various operations involved in supply-chain logistics, for example, harvesting, transport, preprocessing, and inefficient conversion technologies [4]. On the other hand Sharma et al. [5] reported that biomass collection from prolonged sustainably managed forests actually reduces net GHG emissions, thereby, making it carbon negative. In the United States, the total primary energy consumption from various biomass resources is currently 4%, and it is expected to increase to 10% by the year 2035 [6]. Most of the biomass utilization for bioenergy accounts for generation of electricity, heat and liquid transportation fuels. Among this biomass feedstock, roughly, more than 50% is in form of woody biomass [7]. The current contribution of woody biomass is 130 million dry tons, and it is expected reach to 210 and 225 million dry tons by 2022 and 2030, respectively [7]. Woody biomass is mainly used for electricity production, heating homes and providing process heat for industrial facilities through three primary thermal-conversion methods—combustion, gasification and pyrolysis [8,9,10,11]. The process air requirements vary for these three thermal-conversion methods. For combustion, gasification and pyrolysis the air consumption is excess, partial and nonexistent, respectively [12].
Pyrolysis is inherently present in combustion and gasification processes. It is an outstanding conversion method as it can directly generate solid, liquid and gaseous products under anoxic conditions [12]. The wood polymers (cellulose, hemicellulose and lignin) in any biomass and the pyrolytic conditions are the primary factors that control pyrolysis reactions and resulting products [13]. Any typical woody biomass contains 40%–50% cellulose, 25%–35% hemicellulose and 10%–40% lignin [14,15]. In addition, the pyrolytic conditions including pyrolysis temperature, pyrolysis pressure, vapor-phase residence time and heating rate affect the chemical reactions responsible for producing various chemical compounds present in bio-oils [16].
Wood polymers of woody biomass (cellulose, hemicellulose and lignin) interact during pyrolysis. In the temperature range of 150 °C to 180 °C, the stability of cellulose is reduced [17]. Furthermore, residual cellulose is protected by lignin after the temperature reaches 300 °C [17]. Previous studies used data on weight loss as a function of temperature generated from TGA (Thermogravimetric analysis) equipment to study pyrolysis kinetics of woody biomass [18]. Different experimental methods and mathematical models have been used to carry out the TGA experiments. For example, the isothermal method uses constant temperatures while non-isothermal heating ramps the temperature from ambient to the target temperature at a given heating rate. The data generated from TGA include temperature, time and weight changes during the pyrolysis process and these data can be used to calculate the activation energies of different pyrolysis phases [14] by means of a variety of mathematical models.
Activation energy, a quantified factor of pyrolysis kinetics, has been studied by several different mathematical methods [18]. Gaur and Reed [18] have documented the following methods for processing TGA data: Coat and Redfern method-1964; Gyulai and Greenhow method-1974; Doyle’s method-1961; Zsako method-1973; Satava and Skvara method-1969; Freeman and Carroll method-1958; Ingraham and Marrier method; Vachuska and Voboril method; Varhegyi’s Integral Solution-1978; and Gaur and Reed method-1994.
While determination of pyrolysis kinetics is critical to the understanding of the pyrolysis process for a given biomass, only a handful of studies have reported kinetics for hardwood [19,20]. However, no study has documented the effects of the physical components of hardwood (sapwood, heartwood and bark) during the pyrolysis process. The wood polymers in sapwood, heartwood and lignin are expected to be different and, in this case, may lead to different pyrolysis results.
This paper presents thermo-chemical decomposition behavior of hardwood’s physical components and model wood-polymers—cellulose, hemicellulose (xylan) and lignin polymers—using thermo-gravimetric analysis and the isoconversion method. The use of the isoconversion method has been successfully documented for pyrolysis kinetics determination [21,22]. It was hypothesized that pyrolysis kinetics of sapwood, heartwood, and bark will be different and that the isoconversion method will be valid for estimation of Arrhenius parameters for all fractional conversion values.
2. Experimental Procedures
Fresh samples of yellow-poplar and red oak were collected from the West Virginia University (WVU) Research Forest. The samples were separated into twelve sub-samples of bark, sapwood and heartwood (Figure 1). The physical components (bark, sapwood and heartwood) were ground to less than 1 mm particle size using a mill (Model: ED5, Thomas, Chillicothe, MO, USA), and subsequently oven dried for 24 h at 103 °C. In addition, pure cellulose (9004-34-6, Fisher, Hanover Park, IL, USA), xylan (58-86-6, TCI, Portland, OR, USA) and lignin (Indulin AT, Meadwestvaco, Charlston, SC, USA) were obtained from commercial venders as representative of wood-polymers. All samples were analyzed for the cell-wall specific gravity, calorific value, ultimate analysis (carbon, hydrogen, nitrogen, and sulfur content), proximate analysis (fixed carbon, volatile matter, and ash content) and thermo-chemical decomposition behavior. In addition, the wood samples were tested for mineral composition and chemical composition by the Agricultural and Environmental Sciences Laboratory, University of Georgia.
Specific gravity (true grind density) was measured according to ASAE Standard S269.4 [23] using a Multipycnometer (Model: Manual Multipycnometer, Quantachrome, FL, USA). Calorific value was measured using a bomb calorimeter (Model: 6300 Calorimeter, Parr Instrument Company, Moline, IL, USA) according to the ASTM D5865 standard [24] without accounting for heat of reactions for nitric and sulfuric acids. Ultimate analysis was performed using a CHNS analysis (Model: Series II CNHS/O Analyzer 2400, PerkinElmer, Waltham, MA, USA). Proximate analysis (moisture, volatile matter, fixed carbon and ash) was carried out using a proximate analyzer (Model: LECO 701, LECO Corporation, St. Joseph, MI, USA) following the ASTM D3174 standard [25]. Mineral composition, Acid Detergent Fiber (ADF), Neutral Detergent Fiber (NDF) and Acid Detergent Lignin (ADL) were measured at the University of Georgia Agricultural and Environmental Sciences Lab, Athens (UGA-AESL), GA, USA. Data on fiber analysis and ash content were employed to determine cellulose, hemicellulose, and lignin content according to following equations:
%Hemicellulose = %NDF − %ADF
%Cellulose = %ADF − (%Lignin + %Ash)
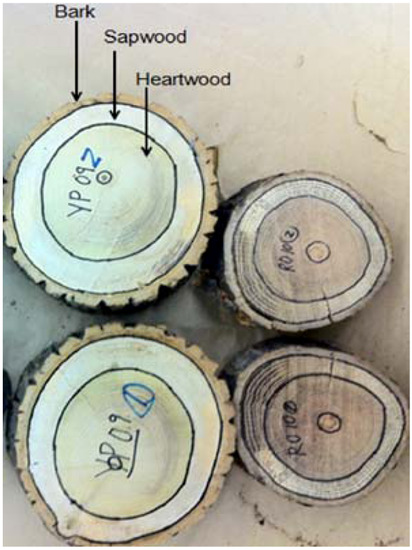
Figure 1.
Yellow-poplar and red oak samples showing bark, sapwood and heartwood.
Thermo-chemical decomposition behavior was assessed using a thermo-gravimetric analyzer (TGA) (Model: Q50, TA Instruments, Schaumburg, IL, USA). The TGA experiments were performed by heating a 8–12 mg sample from 50 °C to 700 °C at three heating rates of 10 °C, 30 °C, and 50 °C/min under a nitrogen flow of 50 cm3/min. Data obtained from thermo-gravimetric analysis were analyzed for any changes in thermo-chemical decomposition behavior. The TGA data were analyzed to determine the Arrhenius activation energy (E) and pre-exponential constant (A) using the isoconversion method described by Kim et al. [26]. In this method, the rate of fractional weight loss, dX/dt, is expressed as a function of conversion f(X) = (1 − X)n, where X is fractional conversion ( 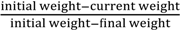 ) and “n” is the order of the reaction:
) and “n” is the order of the reaction:
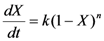 The reaction rate constant (k) was substituted by the following Arrhenius expression:
The reaction rate constant (k) was substituted by the following Arrhenius expression:
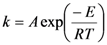 where R is universal gas constant and T is the absolute temperature in K. Upon substituting Equation 4 into Equation 3 and taking the natural logarithm, Equation 3 is transformed into the following equation:
where R is universal gas constant and T is the absolute temperature in K. Upon substituting Equation 4 into Equation 3 and taking the natural logarithm, Equation 3 is transformed into the following equation:
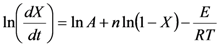
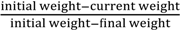 ) and “n” is the order of the reaction:
) and “n” is the order of the reaction:
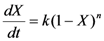
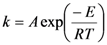
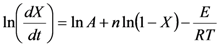
The activation energy (E) in Equation 5 was determined from the relationship between ln(dX/dt) and (1/T) for fractional weight loss (X). Consequently, a family of graphical curves was obtained for a series of X (0.01, 0.02,…, 0.9) values of fractional conversion. For example, the corresponding temperatures at 0.5 conversion of the cellulose samples were 608.15, 622.15, and 628.15 K, respectively, for the heating rates 10, 30, and 50 °C/min. The values of ln(dX/dt) were: −1.3451, −0.41228, and −0.09330, with corresponding 1/T values of 1.644331 × 10−3, 1.607329 × 10−3, 1.591976 × 10−3 (K−1). The intercept ln(A(1 − X)n was calculated from the linear relationship for the corresponding conversions assuming the order of reaction to be either 0 or 1.
3. Results and Discussion
3.1. Feedstock Characterization
Physical and chemical properties including calorific value, cell wall specific gravity, ultimate analysis, proximate analysis, mineral analysis of the physical components of woody biomass (bark, sapwood and heartwood) and wood polymers (cellulose, xylan and lignin) were determined as references for further analysis. Table 1 shows the results of cell-wall specific gravity and calorific values for all samples. As shown in Table 1, there are no significant differences among the cell-wall specific gravity of bark, sapwood and heartwood samples of red oak and yellow-poplar (p-value > 0.05). Similar results were observed for the calorific value data which were consistent with Corder’s findings [27]. Adebayo et al. [28] had measured specific gravity of wooden blocks of size 10 × 10 × 40 mm of sapwood and heartwood of red oak and yellow-poplar samples. They reported average overall specific gravity of sapwood and heartwood logs (not of cell-wall) to be 0.46 and 0.46 for red oak, and 0.58 and 0.61 for yellow-poplar, respectively [28]. The cell-wall specific gravity, reported in this paper, is always greater than that of wood because it does not account for pores present. The result was in agreement with a previous study [29]. The difference between the overall specific gravity and cell-wall specific gravity is related to the porosity of the wood matrix. Porosity directly affects heat transfer and mass transfer during biomass pyrolysis [30]. Miller and Bellan [30] have reported that high initial porosity reduced heat transfer rates during pyrolysis. However, given small sample size (10–12 mg) in the present thermogravimetric experiments, it is unlikely that heat and mass transfer limitation effects would be observed.

Table 1.
Calorific value and cell wall specific gravity measurements (mean ± standard deviation, 2 replications, 2 measurements on each replication) for samples of yellow-poplar and red oak’s components (bark, sapwood and heartwood) and wood-polymers.
| Property | Calorific value (MJ/kg) | Cell wall specific gravity | |
|---|---|---|---|
| Red oak | Bark | 18.86 ± 0.44 | 1.69 ± 0.07 |
| Sapwood | 18.78 ± 0.51 | 1.85 ± 0.18 | |
| Heartwood | 18.38 ± 0.36 | 1.79 ± 0.12 | |
| Yellow-poplar | Bark | 19.54 ± 1.02 | 1.74 ± 0.16 |
| Sapwood | 18.81 ± 0.88 | 1.85 ± 0.14 | |
| Heartwood | 18.65 ± 0.86 | 1.92 ± 0.25 | |
| Wood polymers | Cellulose | - * | 1.27 |
| Xylan | - * | 1.53 | |
| Lignin | 25.79 ± 0.09 | 1.25 | |
* Not determined by the equipment due to repeated failure in sample ignition.
Table 2 shows the results of the ultimate analysis. No substantial differences were observed for carbon, hydrogen, and sulfur contents among bark, sapwood and heartwood. The average carbon content was 46%–48%, hydrogen content was 6%, and sulfur content was 0.2%. The nitrogen content was the highest for bark followed by sapwood and then heartwood. In addition, the carbon and sulfur content in the lignin polymer was significantly higher than in the cellulose and xylan polymers (p < 0.05). The significantly higher sulfur content in the lignin polymer may be explained by the pulping and rough purifying process by which it was extracted. During one pulping process, superfluous sulfur compounds are used as chemical additives [31]. Additionally, when looking at the molar ratio for cellulose, it had 1.78 mols of hydrogen and 0.89 mols of oxygen for each mol of carbon. Therefore, its molar formula may be written as C1H1.78O0.89, after ignoring nitrogen and sulfur. Similarly, molar formulae for xylan and lignin may be written as C1H1.99O0.97 and C1H1.14O0.33, respectively. Therefore, lignin contains the least amount of hydrogen and oxygen per mol of carbon. Similarly, molar formulae calculated for yellow-poplar sapwood, heartwood, and bark were C1H1.52O0.73, C1H1.50O0.72, and C1H1.48O0.67, respectively. Therefore, bark should contain less oxygen and hydrogen than sapwood and heartwood and hence it should decompose at high temperatures producing more bio-char and phenolic rich bio-oil than the other two components.
Ultimate analysis is also directly related to the higher heating value of the material [32,33]. The Dulong formula (HHV = 0.338C + 1.428(H-O/8) + 0.095S) has been widely used for calculating higher heating values using C, H, and O content of biomass; however, the high ash content of biomass may limit the applicability of this formula.

Table 2.
Ultimate analysis (carbon, hydrogen, nitrogen and sulfur content) of bark, sapwood, and heartwood of red oak and yellow-poplar and wood-polymers (cellulose, xylan and lignin).
| Property | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Sulfur (%) | |
|---|---|---|---|---|---|
| Red oak | Bark | 47.52 ± 1.44 | 5.86 ± 0.25 | 0.56 ± 0.00 | 0.24 ± 0.02 |
| Sapwood | 46.62 ± 0.08 | 5.89 ± 0.01 | 0.26 ± 0.01 | 0.18 ± 0.04 | |
| Heartwood | 46.97 ± 0.21 | 5.87 ± 0.01 | 0.07 ± 0.01 | 0.16 ± 0.01 | |
| Yellow-poplar | Bark | 48.16 ± 0.50 | 5.93 ± 0.04 | 0.76 ± 0.02 | 0.18 ± 0.06 |
| Sapwood | 47.05 ± 0.06 | 5.96 ± 0.01 | 0.21 ± 0.03 | 0.21 ± 0.01 | |
| Heartwood | 47.53 ± 0.30 | 5.93 ± 0.06 | 0.13 ± 0.01 | 0.21 ± 0.01 | |
| Wood polymers | Cellulose | 42.00 ± 0.04 | 6.24 ± 0.06 | 0.99 ± 0.07 | 0.67 ± 0.04 |
| Xylan | 40.14 ± 0.42 | 6.65 ± 0.04 | 0.51 ± 0.09 | 0.64 ± 0.00 | |
| Lignin | 61.68 ± 0.01 | 5.85 ± 0.02 | 1.29 ± 0.10 | 2.06 ± 0.02 | |
Table 3 shows the results of the mineral composition of the physical components (sapwood, heartwood and bark) of red oak and yellow-poplar. The phosphorus, potassium, calcium, magnesium, manganese, aluminum, zinc concentrations in bark were higher than in sapwood and heartwood for both red oak and yellow-poplar. The mineral compositions in sapwood and heartwood are approximately same. Minerals present in biomass play a key role in catalyzing pyrolysis reactions [34,35]. Raveendran et al. [34] reported that cations, for example potassium and sodium, are responsible for accelerated catalytic decomposition of cellulose and hemicellulose.

Table 3.
Mineral concentration in parts per million (ppm) of the physical components (sapwood, heartwood, bark) of red oak and yellow-poplar.
| Sample | Red oak | Yellow-poplar | ||||
|---|---|---|---|---|---|---|
| Bark | Sapwood | Heartwood | Bark | Sapwood | Heartwood | |
| Phosphorus | 408 | 127 | <60 | 257 | <60 | <60 |
| Potassium | 3282 | 1875 | 684 | 1977 | 798 | 826 |
| Calcium | 11670 | 450 | 63 | 8530 | 482 | 1016 |
| Magnesium | 601 | 536 | 241 | 668 | 319 | 429 |
| Manganese | 1690 | 309 | 93 | 1415 | 272 | 364 |
| Iron | 109 | 107 | 54 | 105 | 56 | 51 |
| Aluminum | 78 | 14 | 5 | 248 | 22 | 19 |
| Copper | 2.9 | 2.6 | 1.8 | 5.6 | 1.7 | 1.5 |
| Zinc | 13.3 | 5.2 | 6.3 | 13.0 | 5.4 | 5.1 |
| Sodium | 40 | 18 | 58 | 23 | 34 | 19 |
Data presented in Table 3 show that a considerable amount of sodium and potassium is present in bark, sapwood, and heartwood for both red oak and yellow-poplar. Therefore, there may not be any observable differences in thermal decompositions of samples due to catalysis because all the samples would be catalyzed equally. Ren et al. [36] observed enhanced conversion of nitrogen present in biomass to NH3, HCN, NO, and HCNO at low temperatures when biomass was treated with KOH and CaO. Therefore, addition of minerals is desirable for fast thermal degradation of biomass but it is not recommended for nitrogen rich biomass to avoid unnecessary nitrogen loss.
In the proximate analysis, the average fixed carbon and volatile matter contents in bark, sapwood and heartwood of yellow-poplar and red oak were around 20% and 80%, respectively (Table 4). This result agrees with previous studies [37,38]. The ash content in bark (2% to 3%) was significant higher than in sapwood and heartwood (around 0.4%), (p < 0.01). Also, the ash and fixed carbon contents in lignin polymer were significantly higher than in cellulose and xylan polymers, and the volatile matter in lignin polymer was significantly lower than in the cellulose and xylan polymers (p < 0.01). The fixed carbon content of xylan (around 1.66%) was significantly lower than in cellulose (12.56%) and lignin (36.71%), (p < 0.01). The proximate analysis provides vital information about how biomass is expected to behave during pyrolysis. The volatile matter content is related to the amount of biomass, excluding water, which can be volatilized by the application of heat. During pyrolysis, these volatiles end-up in either gaseous products or bio-oils. However, proximate analysis provides no information as to where the volatile matter of biomass will end-up, i.e., in the bio-oil or gases. In addition, the fixed carbon represents the ash-free carbon residue left after pyrolysis. In the absence of mass transfer limitations in large batch pyrolysis experiments, quantities of bio-char produced from pyrolysis should be theoretically equal to the sum of fixed carbon content and ash content measured from proximate analysis. However, in practical situations, actual maximum bio-char yields on an ash-free basis were reported to be 57% (chestnut wood) and 80% (oak wood) of their respective fixed carbon contents [16].

Table 4.
Proximate analysis (moisture, ash, volatile and fixed carbon (% d.b.) of bark, sapwood, and heartwood of red oak and yellow-poplar and wood-polymers (cellulose, xylan and lignin).
| Property | Moisture (%) | Ash (%) | Volatile (%) | Fixed Carbon (%) | |
|---|---|---|---|---|---|
| Red oak | Bark | 6.94 ± 0.06 | 3.08 ± 0.52 | 77.50 ± 1.20 | 19.43 ± 1.73 |
| Sapwood | 0.42 ± 0.01 | 0.36 ± 0.00 | 81.58 ± 0.02 | 18.07 ± 0.02 | |
| Heartwood | 6.16 ± 0.04 | 0.13 ± 0.01 | 81.84 ± 0.08 | 18.05 ± 0.09 | |
| Yellow-poplar | Bark | 7.53 ± 0.04 | 2.24 ± 0.04 | 77.22 ± 0.17 | 20.55 ± 0.13 |
| Sapwood | 6.76 ± 0.00 | 0.48 ± 0.02 | 83.53 ± 0.20 | 16.00 ± 0.22 | |
| Heartwood | 6.75 ± 0.01 | 0.50 ± 0.02 | 83.60 ± 0.21 | 15.91 ± 0.19 | |
| Wood polymers | Cellulose | 5.29 ± 0.00 | 0.00 ± 0.05 | 87.47 ± 0.17 | 12.56 ± 0.12 |
| Xylan | 0.00 ± 0.01 | 0.00 ± 0.00 | 98.38 ± 0.12 | 1.66 ± 0.13 | |
| Lignin | 3.03 ± 0.01 | 2.19 ± 0.08 | 61.10 ± 0.28 | 36.71 ± 0.37 | |
The contents of cellulose, hemicellulose and lignin in red oak and yellow-poplar are presented in Table 5 and Figure 2. Lignin content in bark (around 18% to 20%) was relatively higher than in sapwood and heartwood (in the range of 10% to 15%). In contrast, cellulose and hemicellulose contents in red oak bark (around 30% and 20%, respectively) were relatively lower than in sapwood and heartwood (around 50% and 26%, respectively). Cellulose, hemicellulose and lignin contents in sapwood and heartwood were approximately similar. Similarly, Usia et al. [39] reported that the cellulose contents in sapwood, heartwood and bark of oak were 53.11%, 48.61% and 26.29%, respectively, and the lignin contents in three components were 28.71%, 24.14% and 33.14%, respectively. In Schowalter’s report [40], the concentration of cellulose in oak was 0.52 (g·g−1) in sapwood, 0.46 (g·g−1) in heartwood, 0.31 (g·g−1) in inner bark and 0.18 (g·g−1) in outer bark. Percent of biomass reported in the “others” category in Table 5 and Figure 2 are lumped quantities of fat, protein, extractives, pectin, etc., which were not individually measured.
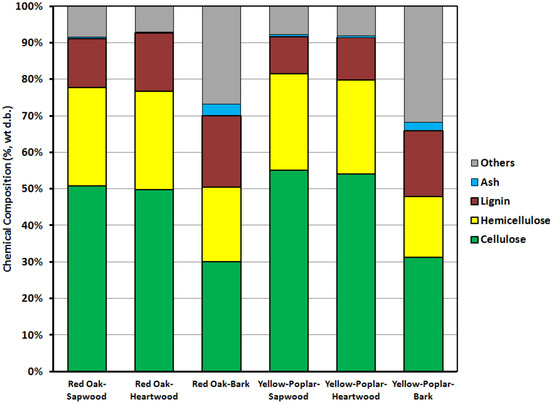
Figure 2.
Percent cellulose, hemicellulose and lignin contents (d.b.) in bark, sapwood and heartwood of red oak and yellow-poplar samples.

Table 5.
Percent cellulose, hemicellulose and lignin contents (d.b.) in bark, sapwood and heartwood of red oak and yellow-poplar samples.
| Property | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Others (%) | |
|---|---|---|---|---|---|
| Red oak | Bark | 30.02 | 20.45 | 19.58 | 26.87 |
| Sapwood | 50.87 | 26.86 | 13.38 | 8.52 | |
| Heartwood | 49.82 | 26.90 | 15.97 | 7.18 | |
| Yellow-poplar | Bark | 31.54 | 16.73 | 17.98 | 31.82 |
| Sapwood | 55.07 | 26.46 | 10.17 | 7.83 | |
| Heartwood | 54.08 | 25.74 | 11.61 | 8.07 | |
Cellulose, hemicellulose and lignin are the main organic polymers that decompose during pyrolysis [13,35,41,42,43,44]. Each polymer behaves distinctly during pyrolysis. Cellulose degrades at 240–350 °C producing anhydrocellulose and levoglucosan. The latter is produced when a glucosan radical forms, and it does not get a chance to bridge with oxygen present in the cellulose polymer. The in-depth chemistry of this reaction is presented by Mohan et al. [14]. Unlike cellulose, which has only glucose in its chain structure, hemicellulose contains heteropolysaccharide and thermally degrades in the temperature range of 130–194 °C [14]. The differences in thermal decomposition chemistry of hemicellulose and cellulose are not well-known. The thermal decomposition of lignin occurs at 280 to 500 °C yielding phenol via cleavage of ether and carbon-carbon linkages. According to Mohan et al. [14] lignin produces more residual char than cellulose or hemicellulose. The liquid component of lignin pyrolysis is composed of menthol, acetic acid, acetone, and water; and the gaseous component is mainly methane, ethane, and carbon monoxide. Qu et al. [43] reported that bio-oil yields were 65% from cellulose pyrolysis, 53% from xylan pyrolysis, and 40% from lignin pyrolysis. Both Raveendran et al. and Qu et al. [13,43] showed that the three biomass polymers decompose independently without interfering with each other.
3.2. Thermo-Chemical Decomposition Behavior of Cellulose, Xylan and Lignin
3.2.1. Thermogravimetric Analysis
Figure 3 shows the TGA and Differential Thermogravimeric (DTG) graphics of cellulose, xylan and lignin heated from 50 °C to 700 °C at the heating rate of 10 °C/min under an inert atmosphere. The DTG curves present the rate of fractional conversion and the TGA curves show the percentage weight remaining over the temperature range. As shown in the TGA graphic (Figure 3a), cellulose produced 4.45% char, xylan produced 1.89% char and lignin produced 41.43% char when heated to 700 °C. The char yields should match with the sum of fixed carbon and ash content measured from proximate analysis. Similar results of lignin and cellulose char yields were presented earlier [13,45]. The reported char yields from cellulose pyrolysis are 2.5% for Whatman cellulose, 14.0% for wood cellulose [13], and 7% for a fibrous powder form of cellulose [35]. Similarly, char yield reported for hemicellulose is 20% for xylan extracted from birchwood [35] and 30% for xylan [13].The reported char yield for lignin is 40.6% for alkali lignin, 47.1% for acid lignin [13] and 40% for alkali lignin in brown powder form [35]. Raveendran et al. [13] reported that cellulose present in different biomass differs due to its varying crystallinity, which not only affects the char yield but also the thermal degradation behavior. Char produced from xylan was lower than that reported in the literature. This difference is attributed to the dissimilar variety of xylan resulting in different structural and chemical properties.
When looking at the DTG graphics, cellulose decomposed dramatically in the temperature range of 300 °C to 400 °C with a large decomposition peak at 340 °C. Xylan decomposed in the temperature range of 150 °C to 400 °C with a large decomposition peak at 200 °C. Degradation of lignin occurred in the temperature range of 100 °C to 700 °C with a tiny degradation peak at 340 °C (Figure 3b). Similar results for lignin and cellulose degradation temperature range and peak location were presented in the literature [13,45]. However, the large peak for xylan degradation was reported at 260 °C [45] and 300 °C [13]. Furthermore, the reported decomposition temperature range for xylan was between 200 °C and 350 °C [13,45]. Different results may be caused by a dissimilar variety of xylan and TGA test conditions. Yang et al. [45] used xylan from birchwood (Sigma-Aldrich Ghemie GmbH, Munich, Germany). Raveendran et al. [13] heated xylan from room temperature to 1000 °C at the heating rate of 50 °C/min under an inert atmosphere.
Different thermal degradation behavior of cellulose, hemicellulose, and lignin is attributed to their individual chemical natures [35]. Among the three polymers, hemicellulose (xylan) has random amorphous structures with reactive acetyl groups that are easily broken down during acid hydrolysis. An average polymer of hemicellulose contains only 150 monomers of repeating saccharides. Unlike cellulose, which has only glucose in its chain structure, hemicellulose contains heteropolysaccharide and thermally degrades in the temperature range of 130–194 °C [14]. In contrast, cellulose is a linear polymer of glucose (5000–10,000 glucose units). The cellulose degrades at 240–350 °C producing anhydrocellulose and levoglucosan [14]. Cellulosic polymer contains some crystalline and some amorphous regions. The crystalline regions are resistant to acid hydrolysis or solvent penetration. Ligninic polymers are highly branched, substituted, mononuclear aromatic polymers forming a lignocellulosic complex in the biomass, and this amorphous structure of lignin accounts for 16% to 33% of the mass of woody biomass [14]. The thermal decomposition of lignin occurs at 280 to 500 °C yielding phenol via cleavage of ether and carbon-carbon linkages.
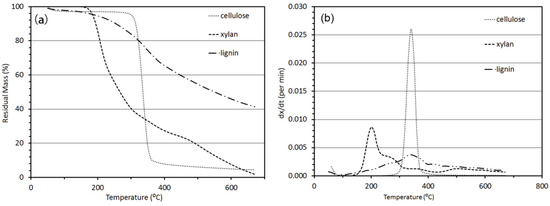
Figure 3.
Thermo-gravimetric analysis results showing (a) TGA-percentage weight remaining, and (b) DTG-rate of fractional changes when samples of cellulose, xylan and lignin are heated from 50 °C to 700 °C at the heating rate of 10 °C/min under an inert atmosphere.
3.2.2. Activation Energy Analysis
Figure 4 shows the activation energy and dX/dt curves of cellulose, xylan and lignin as a function of fractional conversion from 0.01 to 0.9. Valid activation energy values of cellulose, xylan and lignin were calculated in the fractional conversion range of 0.01 to 0.9, 0.01 to 0.4 and 0.01 to 0.5, respectively (Figure 4). The activation energy values in the fractional conversion range of 0.5 to 0.9 and 0.6 to 0.9 for xylan and lignin are not reported here due to their negative numerical values. The negative values could be the result of following three reasons: (1) the isoconversion model is not valid for certain fractional conversion values; (2) the Arrhenius law may not be applicable to certain fractional conversion values; (3) overlapping multiple reactions are taking place. Therefore, the authors state that the isoconversion model does not appear to be appropriate for every value of fractional conversion.
As shown in Figure 4a, the activation energy of cellulose ranged from 208 kJ/mol to 381 kJ/mol for the fractional conversion range of 0.2 to 0.9, which corresponds to the temperature range of 320 °C to 360 °C. A similar result for the cellulose activation energy (242 kJ/mol) was presented by Bradbury et al. [46] using an isothermal method. Chen and Kou [47] reported activation energy values of 124 kJ/mol for cellulose but in the temperature range of 200 °C to 300 °C under isothermal heating conditions. Also, cellulose activation energies of 194 kJ/mol and 195 kJ/mol in the temperature range of 300 °C to 400 °C were reported by Kissinger and Kamal’s method [48]. These different values of activation energies are the result of not only different calculational methods as documented by Gaur and Reed [18] and Chen and Kuo [47] but also the different crystallinity of the cellulose being used in these studies.
As shown in Figure 4b, the activation energy of xylan was in the range of 88 kJ/mol to 348 kJ/mol in the fractional conversion range of 0.1 to 0.4 with an additional high activation energy of 745 kJ/mol shown at the fractional conversion of 0.37 corresponding to a temperature of 225 °C. Otherwise for temperatures from 185 to 215 °C, the activation energy values fall between 150 and 250 kJ/mol. Similar, activation energy values have been reported in the literature, for example, 252 kJ/mol in the temperature range of 200 °C to 300 °C using isothermal heating [47]. Some other studies reported activation energy values of 109 kJ/mol in the temperature range of 225–265 °C and 105 kJ/mol in the temperature range of 270 to 320 °C [49]. Again, varying values of activation energy for xylan are directly related to the source of xylan and the method used in calculating the activation energy.
As shown in Figure 4c, the activation energy of lignin constantly increased as a response to rising temperature from 50 to 370 °C in the fractional conversion range of 0.01 to 0.5. A major increase in activation energy was calculated in the fractional conversion range of 0.4 to 0.5 corresponding to the temperature range of 340 to 370 °C. The maximum activation energy of lignin was 801 kJ/mol at a fractional conversion of 0.5. Lignin activation energy was reported to be 38 kJ/mol [47] under isothermal heating conditions, and 284 kJ/mol [50] in the temperature range of 200 °C to 300 °C Different activation energy values of lignin may be attributed to the calculation methods, source of lignin, and the method used in the extraction of the lignin. For example, Ramiah [49] reported that lignin extracted from spruce wood sawdust by periodate oxidation and hydrolysis had an activation energy value of 54.39 kJ/mol whereas lignin extracted from douglas fir by digesting in sulfuric acid and hydrolysis had an activation energy value of 79.50 kJ/mol. Also, lignin type changes with wood species. Softwood and hardwood have varying proportions of three monomers (p-coumaryl alcohol; coniferyl alcohol; and sinapyl alcohol), which are precursors to lignin polymers. Hardwood lignin contains mainly coniferyl and sinapyl-based alcohols whereas softwood lignin contains mainly coniferyl alcohol with little p-coumaryl alcohol.
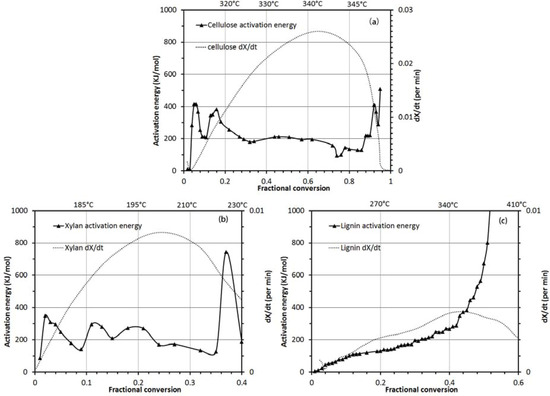
Figure 4.
Activation energy and dX/dt curves of (a) cellulose, (b) xylan and (c) kraft lignin as a function of fractional conversion from 0.01 to 0.9.
3.3. Thermo-Chemical Decomposition Behavior of Wood Components
3.3.1. Thermogravimetric Analysis
Figure 5 presents the DTG and TGA graphics for red oak and yellow-poplar’s components (bark, sapwood and heartwood) heated from 50 °C to 700 °C at the heating rate of 10 °C/min under an inert atmosphere. The TGA graphic for red oak shows char yields of 15.61% for sapwood, 15.60% for heartwood, and 23.45% for bark. Theoretically, these yields should have been equal to the sum of fixed carbon and ash content (as-received basis) measured from the proximate analysis. However, estimated char yields from proximate analysis were 16.97% for sapwood, 17.04% for heartwood, and 20.93% for bark. Similar observations were recorded for the yellow-poplar samples. Experimental char yields were 14.74% for sapwood, 14.26% for heartwood, and 22.69% for bark samples of yellow-poplar. An attempt was made to calculate the char yields for sapwood, heartwood, and bark using their chemical composition data presented in Table 5, TGA char-yield data for individual model wood polymers (cellulose, xylan, and lignin) presented in Section 3.2, and assumed 26.9% char yield from extractives [13]. The calculated and measured char yields (calculated, measured) were (10.55%, 15.61%) for sapwood, (11.22%, 15.60%) for heartwood, and (17.02%, 23.45%) for red oak samples. As can be seen, the char yields calculated from models of wood polymers and the chemical composition of wood are different from the experimentally measured char yields. These differences are directly related to the differences in the chemical nature of cellulose, xylan, and lignin used as model wood polymers and those actually present in the sapwood, heartwood, and bark samples. Raveendran et al. [13] reported that crystallinity of cellulose varies from 34.4 for Whatman cellulose to 91.5 for highly crystalline cellulose used in chromatographic instrument columns and it directly affects the thermal decomposition of wood. Wood cellulose crystallinity is somewhere around 68.9. Due to the difference in crystallinity, wood cellulose produced 14% char whereas Whatman cellulose produced 2.5% char. Therefore, low-crystallinity cellulose produces low quantities of char because it has more amorphous regions to react during pyrolysis than highly crystalline cellulose. Raveendran et al. [13] did not find such large differences between xylan and wood extracted hemicellulose char yields. Char yields for lignin polymers may vary from 47.1 for acid-extracted lignin to 40.6 for alkali-extracted lignin [13]. There are other methods for lignin extraction as discussed in Fengel and Wegeber [31] and each extraction method significantly alters the amorphous chemical structure of lignin, which might explain different char yields from different lignins obtained from TGA data.
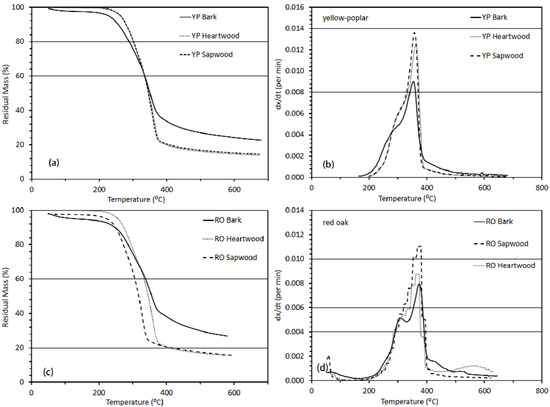
Figure 5.
Thermo-gravimetric analysis results showing percentage weight remaining when samples of (a) yellow-poplar and (c) red oak’s components (bark, sapwood and heartwood), and rate of fractional changes when samples (b) yellow-poplar and (d) red oak’s components (bark, sapwood and heartwood) heated from 50 °C to 700 °C at the heating rate of 10 °C/min under an inert atmosphere.
The TGA graphics were used to calculate the DTG graphics and plot the rate of fractional conversion as a function of temperature. The DTG graphics provide visual information on when pyrolysis reactions are taking place and the rates of fractional conversion. Within the DTG graphics, there are three aspects to notice: first, location of peaks, second, relative height of peaks, and third, broadness of the DTG graphic on the temperature scale. In the DTG graphic (Figure 5), two obvious peaks were observed for bark, sapwood and heartwood in the temperature ranges of 250 °C to 350 °C and 350 °C to 400 °C, respectively (Figure 5b,d). The first peak at 280 °C accounted for the degradation of hemicellulose and the later peak at 360 °C was mainly due to cellulose decomposition. Raveendran et al. [13] reported the following prominent events that can be observed in DTG: (1) mainly moisture evolution below 100 °C; (2) start of extractive decomposition between 100 and 250 °C, mainly hemicellulose decomposition between 250 and 350 °C, cellulose decomposition between 350 and 500 °C and lignin decomposition beyond 500 °C. Interestingly, cellulose and hemicellulose peaks are more separated for bark samples, whereas, the hemicellulose peak is visible only as a shoulder for the sapwood and heartwood samples. These separations may very well be explained by noting the cellulose-to-hemicellulose ratios calculated from chemical composition data presented in Table 5. The calculated cellulose-to-hemicellulose ratios were 1.9 for sapwood and heartwood and 1.5 for bark of the red oak samples. Similarly, the ratios were 2.1 for sapwood and heartwood and 1.9 for bark samples of the yellow-poplar. It is evident that a low cellulose-to-hemicellulose ratio produces a clear separation of the hemicellulose peak which is why the red oak bark showed more visible separation of the hemicellulose peak than yellow-poplar bark.
Another visible difference was the height of peaks which is related to the rate of decomposition. At both peaks, sapwood and heartwood showed higher decomposition rates than bark for both yellow-poplar and red oak. This behavior is caused by the amount of cellulose or hemicellulose present in the biomass. For example, sapwood had the highest cellulose content; therefore, it showed the largest peak height at 360 °C. In contrast, bark had the lowest cellulose content; therefore, it showed the lowest peak height at the same temperature. Finally, broadness of the DTG curve is associated with lignin and extractive decomposition because both lignin and extractives decompose in a very broad temperature range. The only difference between the two is that extractives decompose at higher rates than lignin but at lower temperatures [13]. Raveendran et al. [13] reported that the extractives derived from wood decomposed between 150 and 400 °C, whereas lignin decomposed between 250 and 450 °C. Therefore, a high lignin-to-extractive ratio should provide insight into the broadness of DTG curve. If the DTG curve is broadened towards the right side of the cellulose peak, the lignin-to-extractive ratio is low and vice versa. From the chemical composition data in Table 5, this ratio was 0.6 for yellow-poplar bark and 0.7 for red oak bark. Therefore, extra broadness is visible on the right side of the cellulose peak for red oak bark in the DTG graphics.
3.3.2. Activation Energy Analysis
Figure 6 shows the activation energy and dX/dt curves of (a) bark, (b) heartwood and (c) sapwood for yellow-poplar as a function of fractional conversion from 0.01 to 0.9. These activation energy values should be the result of the combined effects of the individual wood polymers. The key observations about the activation energy pattern for bark, heartwood and sapwood in Figure 6 are the following: (1) A “three-zone” degradation occurred for sapwood and heartwood (<0.2 fractional conversion; 0.2–0.9 fractional conversion; and >0.9 fractional conversion); (2) A “three-zone” degradation occurred for bark (<0.4 fractional conversion, 0.4 to 0.8 fractional conversion, and >0.8 fractional conversion); and (3) activation energy values for bark remained higher than for the other two wood components at all conversions.
The three-zone degradation for sapwood and heartwood (<0.2 fractional conversion; 0.2–0.9 fractional conversion; and >0.9 fractional conversion) may be explained by their respective zone-temperatures and the chemical compositions of sapwood and heartwood (moisture, extractive, cellulose, hemicellulose, and lignin contents). The activation energy below 0.2 fractional conversions ranged from 265 to 296 kJ/mol, which may very well be related to moisture evolution and extractive decompositions. Both sapwood and heartwood had a total of 14.3% of moisture and extractive content. Also, up to fractional conversion of 0.2, degradation took place below 295 °C, which is the temperature range for moisture evolution and extractive decomposition [13]. The activation energy for moisture evolution below 100 °C is reported to be in the range of 29.35 to 33.78 kJ/mol, which is basically related to the evaporation of free and some bound water [51]. Above 100 °C but below 295 °C, all bound water including water of constitution (water molecules connected to cellulose polymers by high-energy hydrogen bonds) is driven off. In the same temperature range, extractives decompose. In the second zone, mainly cellulose and hemicellulose decompose between 0.2 and 0.9 fractional conversion and temperatures between 295 °C to 365 °C. Both sapwood and heartwood had approximately a total of 76% cellulose and hemicellulose and cumulatively 90% of sapwood and heartwood was made-up of moisture, extractives, cellulose, and hemicellulose. The activation energy between 0.2 and 0.9 fractional conversions stayed around 240 to 260 kJ/mol. The reported activation energy values for 0.70 conversion are 209 kJ/mol [19] and 265 kJ/mol [52]. Finally, activation energy above fractional conversion of 0.9 fluctuated between 286 to 449 kJ/mol, mainly for the decomposition of the remaining 10% lignin. Beall [53] reported activation energy of wood lignin decomposition to be 135.98 kJ/mol in the temperature range of 398 °C to 439 °C, which was calculated by a differential method.
As mentioned before, decomposition of bark also took place in three fractional conversion zones (<0.4 fractional conversion, 0.4 to 0.8 fractional conversion, and >0.8 fractional conversion) but activation energy values for bark remained higher than the other two wood components for all conversions. The first decomposition zone for bark lasted until 0.4 fractional conversion below 315 °C, which reflected decomposition of its combined 37% moisture and extractives. The activation energy in this decomposition zone was between 260 and 296 kJ/mol. In the second fractional conversion zone (0.4 to 0.8), the combined 44% hemicellulose and cellulose degraded with an activation energy in the range of 256 to 309 kJ/mol until the temperature reached 375 °C. After the fractional conversion of 0.8 for bark, the activation energy reached as high as 943 kJ/mol due to lignin decomposition as temperatures soared above 600 °C. Additionally, overall activation energy values for bark decomposition were attributed to the high extractive and lignin content of the bark compared to the sapwood and heartwood.
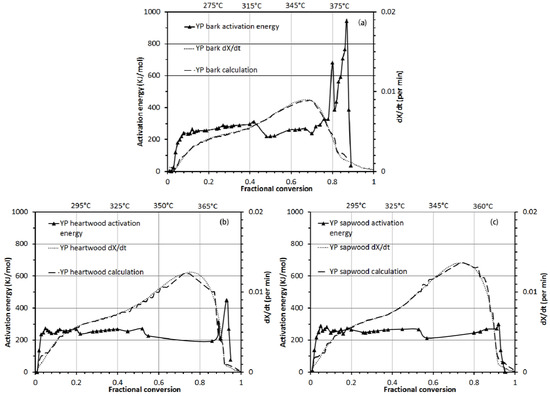
Figure 6.
Activation energy and dX/dt (experimental and calculated) curve of (a) bark, (b) heartwood and (c) sapwood of yellow-poplar as a function of fractional conversion from 0.01 to 1.0.
Figure 6 also shows plot for dX/dt calculated using activation energy values, pre-exponential factor values, and an assumed order of reaction equal to 1. The calculated dX/dt curve closely followed the experimental dX/dt curve, which suggested that the isoconversion method applied in this paper for the determination of the kinetics is valid for wood pyrolysis.
4. Conclusions
Biomass components (bark, sapwood and heartwood) of red oak and yellow-poplar and wood polymers (cellulose, xylan and lignin) were evaluated for calorific value, cell wall specific gravity, proximate analysis, mineral analysis and ultimate analysis. There is no significant difference for the results of the cell wall specific gravity, calorific value and ultimate analysis among bark, sapwood and heartwood for red oak and yellow-poplar. In addition, the thermo-chemical decomposition behavior of the individual wood polymers (cellulose, xylan and lignin) and red oak and yellow-poplar components (bark, sapwood and heartwood) was also examined. Cellulose showed activation energy values in the range of 208 to 381 kJ/mol during decomposition whereas xylan and lignin had maximum activation energy values of 348 kJ/mol and 801 kJ/mol at fractional conversions of 0.4 and 0.5, respectively. The activation energy requirement for wood components remained within the range of 233 kJ/mol to 388 kJ/mol until 365 °C and then peaked to roughly 943 kJ/mol, 449 kJ/mol and 298 kJ/mol for bark, sapwood and heartwood at 375 °C where major energy input for lignin decomposition is needed. Also, it was observed that the isoconversion methods may not work for all the fractional conversion values for individual wood polymers but the method worked well for the wood components.
Acknowledgments
This research was funded by the United States Department of Agriculture’s McStennis Grant.
References
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Lifecycle Greenhouse Gas Emissions Due to Increased Biofuel Production; ICF International: Fairfax, VA, USA, 2009. Available online: http://epa.gov/oms/renewablefuels/rfs2-peer-review-model.pdf (accessed on 9 January 2013).
- Nitschke, C.R.; Innes, J.L. Integrating climate change into forest management in south-central British Columbia: An assessment of landscape vulnerability and development of a climate-smart framework. For. Ecol. Manag. 2008, 256, 313–327. [Google Scholar] [CrossRef]
- Sharma, N.; Rowe, R. Managing the world’s forests. Financ. Dev. 1992, 29, 31. [Google Scholar]
- Annual Energy Outlook 2012; Technical Report DOE/EIA-0383(2012) for EIA: Washington, DC, USA, 2012.
- U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; Technical Report for U.S. Department of Energy: Oak Ridge, TN, USA, 2011.
- Balat, M. Biomass energy and biochemical conversion processing for fuels and chemicals. Energy Sources Part A Recovery Util. Environ. Eff. 2006, 28, 517–525. [Google Scholar]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour.Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kothari, R.; Tyagi, V.V. Thermo chemical conversion of biomass—Eco friendly energy routes. Renew. Sustain. Energy Rev. 2012, 16, 1801–1816. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Antal, M.J., Jr.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Beall, F.C.; Eickner, H.W. Thermal Degradation of Wood Components: A Review of the Literature; U.S. Forest Products Laboratory: Madison, WI, USA, 1970. [Google Scholar]
- Gaur, S.; Reed, T.B. Thermal Data for Natural and Synthetic Fuels; Marcel Decker, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Herrera, A.; Soria, S.; de Araya, C. A kinetic study on the thermal decomposition of six hardwood species. Eur. J. Wood Wood Prod. 1986, 44, 357–360. [Google Scholar] [CrossRef]
- Kim, S.-S.; Jeon, J.-K.; Park, Y.-K.; Kim, S. Thermal pyrolysis of fresh and waste fishing nets. Waste Manag. 2005, 25, 811–817. [Google Scholar]
- Kim, S.-S.; Kim, S. Pyrolysis characteristics of polystyrene and polypropylene in a stirred batch reactor. Chem. Eng. J. 2004, 98, 53–60. [Google Scholar] [CrossRef]
- ASAE Standards S269.4. In Cubes, Pellets, and Crumbles—Definitions and Methods for Determining Density, Durability, and Moisture Content; ASAE: St. Joseph, MI, USA, 1998.
- ASTM Committee on Standards. In Standard Test Method for Gross Calorific Value of Coal and Coke; Standard No. ASTM D5865-98a; The American Society for Tesing and Materials: West Conshohocken, PA, USA, 1998.
- ASTM Committee on Standards. In Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal; Standard No. ASTM D3174-12; The American Society for Tesing and Materials: West Conshohocken, PA, USA, 2011.
- Kim, S.-S.; Agblevor, F.A. Pyrolysis characteristics and kinetics of chicken litter. Waste Manag. 2007, 27, 135–140. [Google Scholar] [CrossRef]
- Corder, S.E. Properties and uses of bark as an energy source. In Proceedings of the XVI IUFRO World Congress, Oslo, Norway, 20 June–2 July 1976.
- Adebayo, A.; Wang, J.; Dawson-Andoh, B.; McNeel, J.; Armstrong, J. Assessment of Appalachian hardwood residue properties and potentials for bioenergy utilization. Wood Fiber Sci. 2009, 41, 74–83. [Google Scholar]
- Vincent, J.F. From cellulose to cell. J. Exp. Biol. 1999, 202, 3263–3268. [Google Scholar]
- Miller, R.S.; Bellan, J. Analysis of reaction products and convertion time in the pyrolysis of cellulose and wood particles. Combust. Sci. Technol. 1996, 119, 331–373. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Xu, C.; Lad, N. Production of heavy oils with high caloric values by direct liquefaction of woody biomass in sub/near-critical water. Energy Fuels 2007, 22, 635–642. [Google Scholar]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. Influence of mineral matter on pyrolysis of palm oil wastes. Combust. Flame 2006, 146, 605–611. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Wu, X.; Liang, C.; Chen, X.; Shen, J.; Tang, G.; Wang, Z. Effect of mineral matter on the formation of nox precursors during biomass pyrolysis. J. Anal. Appl. Pyrolysis 2009, 85, 447–453. [Google Scholar] [CrossRef]
- Demirbaş, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Adebayo, A.B. Pretreatments and Energy Potentials of Appalachian Hardwood Residues for Biofuel Production. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 2010. [Google Scholar]
- Usia, M.; Kara, S. The chemical composition of wood and bark of Cedrus libani A. Rich. Eur. J. Wood Wood Prod. 1997, 55, 268–268. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Zhang, Y.L.; Sabin, T.E. Decomposition and nutrient dynamics of oak Quercus spp. logs after five years of decomposition. Ecography 1998, 21, 3–10. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Wang, S.; Luo, Z. Interactions of biomass components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyrolysis 2011, 90, 213–218. [Google Scholar] [CrossRef]
- Qu, T.; Guo, W.; Shen, L.; Xiao, J.; Zhao, K. Experimental study of biomass pyrolysis based on three major components: Hemicellulose, cellulose, and lignin. Ind. Eng. Chem. Res. 2011, 50, 10424–10433. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Jeon, J.-K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic pyrolysis of biomass components over mesoporous catalysts using PY-GC/MS. Catal. Today 2012, in press. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2005, 20, 388–393. [Google Scholar]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy 2011, 36, 6451–6460. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Vázquez, A. Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites. Polym. Degrad. Stab. 2004, 84, 13–21. [Google Scholar] [CrossRef]
- Ramiah, M.V. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose, and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Murugan, P.; Mahinpey, N.; Johnson, K.E.; Wilson, M. Kinetics of the pyrolysis of lignin using thermogravimetric and differential scanning calorimetry methods. Energy Fuels 2008, 22, 2720–2724. [Google Scholar] [CrossRef]
- Mirzaee, E.; Rafiee, S.; Keyhani, A.; Emam-Djomeh, Z. Determining of moisture diffusivity and activation energy in drying of apricots. Res. Agric. Eng. 2009, 55, 114–120. [Google Scholar]
- Singh, K.; Zondlo, J.W.; Wang, J.; Sivanandan, L.; Brar, J.S. Influence of environmental decomposition of logging residues on fuel properties. Biol. Eng. Trans. 2012, 5, 163–176. [Google Scholar]
- Beall, F. Thermogravimetric analysis of wood lignin and hemicelluloses. Wood. Fiber Sci. 1969, 1, 215–226. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).