1. Introduction
Soil microbiomes are the primary drivers of nitrogen cycling in agricultural systems, mediating all major nitrogen transformations and controlling nutrient availability [
1]. In alkaline semi-arid soils, microbial communities face unique constraints from high pH, low moisture, and nutrient limitations that fundamentally alter their structure and function. Understanding how green manuring with legumes influences microbial community assembly and metabolic pathways in degraded carbonate chernozems is essential for developing effective restoration strategies.
Agricultural intensification leads to taxonomic homogenization of soil microbiomes [
2], where multiple taxa converge toward similar metabolic functions despite different phylogenetic origins [
3]. In grasslands receiving elevated nutrient inputs, microbial communities show consistent shifts toward copiotrophic taxa regardless of geographic location or specific management practice [
4]. This convergence may explain why different organic amendments sometimes produce similar outcomes despite distinct carbon and nitrogen inputs, raising questions about whether green manuring can overcome such convergence pressures in degraded soils.
Green manuring with leguminous crops represents a biological approach to soil restoration, yet its effectiveness in alkaline soils remains inconsistent. During decomposition of plant residues with high C:N ratios (>20:1), microbial communities initially immobilize nitrogen, with the duration depending on residue quality and environmental conditions [
5]. In soils with pH > 8.5, decomposition is further complicated by reduced enzyme activity and precipitation of organic substrates with calcium [
6]. These constraints are particularly relevant for the degraded steppe soils of Central Asia.
The Virgin Lands Campaign (1954–1962) converted 25 million hectares of Kazakhstani steppe to wheat production [
7], triggering organic matter losses of 30–50% within three decades [
8]. Current soil organic carbon levels in Northern Kazakhstan average 2–3%, compared to 4–6% in undisturbed steppes [
9]. This degradation reduces microbial habitat quality through aggregate breakdown and decreased pore connectivity. Reversing this degradation requires understanding how soil microbiomes respond to restoration practices.
Key microbial groups involved in nitrogen cycling may respond differently to green manuring in alkaline soils. Ammonia-oxidizing archaea (AOA) within 0 often outnumber ammonia-oxidizing bacteria in soils, with ratios ranging from equal amounts to over 3000-fold depending on soil conditions [
10]. These archaea use the most energy-efficient aerobic CO
2 fixation pathway (3-hydroxypropionate/4-hydroxybutyrate cycle) [
11], while their high affinity for ammonia allows them to thrive at low substrate concentrations [
12]. Nitrite-oxidizing bacteria (
Nitrospirae), including newly discovered complete ammonia oxidizers (comammox), play crucial roles in completing nitrification across diverse environments [
13].
Among leguminous green manures, sweet clover (
Melilotus officinalis (L.)) demonstrates exceptional adaptation to semi-arid conditions through deep taproots (2–3 m), salt tolerance, and biomass production of 8–12 t ha
−1 [
14]. This species promotes specific rhizobial populations through root exudation of flavonoids and phenolic compounds [
15,
16], while its coumarin content can reach 0.2–0.9% dry weight under stress conditions [
17]. These characteristics make sweet clover a promising candidate for soil restoration in degraded steppe ecosystems.
However, phosphorus availability often limits microbial activity in carbonate-rich soils despite adequate total P, as calcium-phosphate precipitation reduces bioavailable P by 10–100-fold compared to neutral soils [
18]. In agricultural soils, phosphorus distribution in particle size fractions controls microbial growth efficiency and carbon use [
19], with P-limitation affecting ATP-dependent processes and nucleic acid synthesis [
20]. Understanding these nutrient constraints is critical for predicting green manure effectiveness.
The aim of this study was to assess the influence of first-year sweet clover green manuring on the structure and functional characteristics of microbial communities in southern carbonate chernozem soils of Northern Kazakhstan. We hypothesized that: (1) first-year sweet clover green manuring would increase soil nitrate-N availability and shift the microbiome towards nitrogen-cycling taxa compared to clean fallow; and (2) sweet clover incorporation would result in more diverse functional profiles reflecting enhanced biological nitrogen fixation and organic matter inputs. This pilot study provides baseline data for developing long-term restoration strategies in alkaline steppe soils.
2. Materials and Methods
2.1. Study Site and Experimental Design
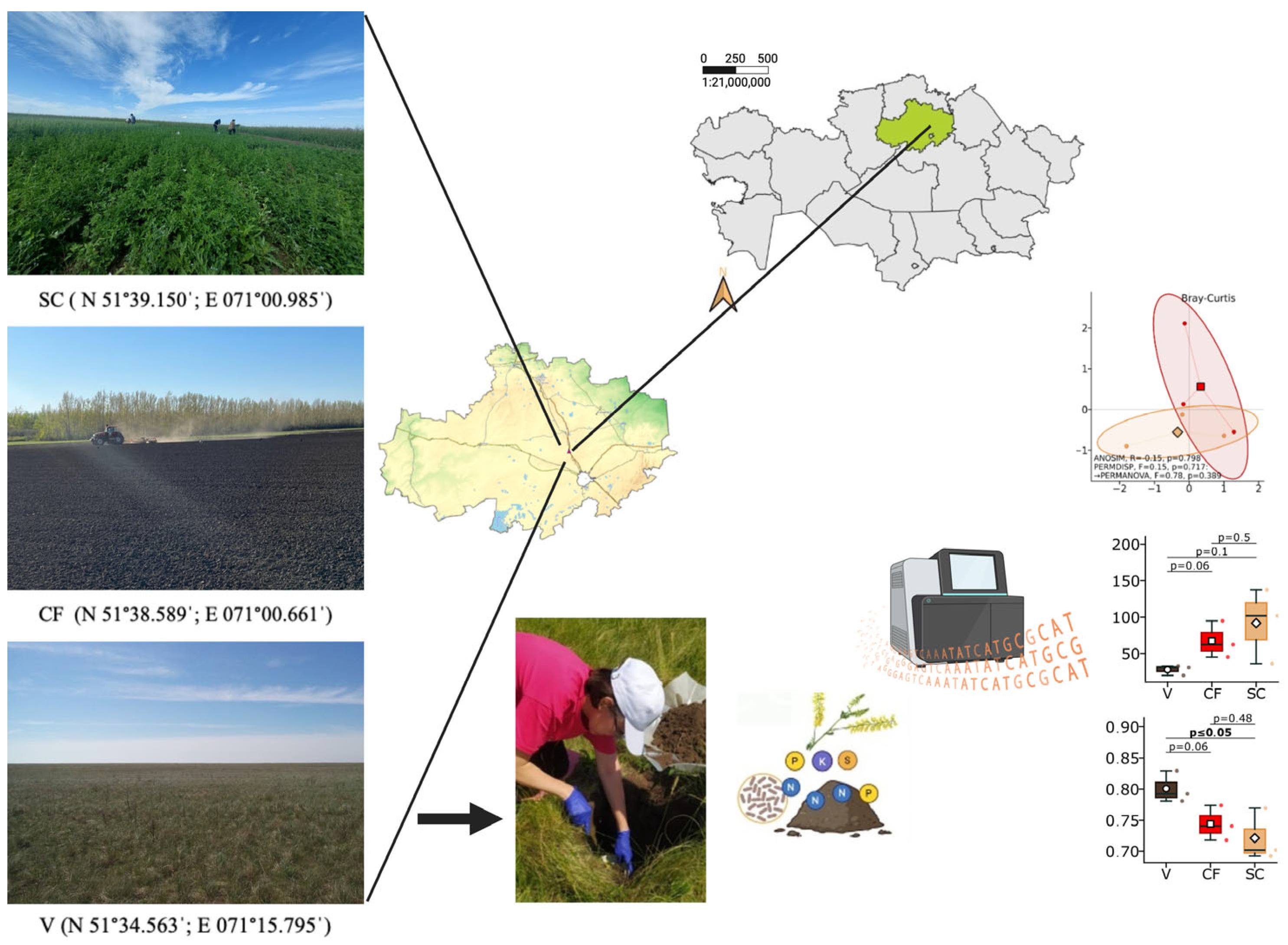
The research was carried out in field experiments established at the A.I. Barayev Research and Production Center for Grain Farming (51°39′ N, 71°01′ E), Shortandy District, Akmola Region, the steppe zone of Northern Kazakhstan (
Figure 1).
Climatic conditions in the research area are distinctly continental, dry. The annual precipitation level is 342.4 mm (35–40% falls into summer period, 15–20% autumn, 17–25% winter and 20–25% spring period). The soil freezes through to the depth of 1.2–2 m and thaws slowly in spring. The frost-free period lasts for 102–130 days, and in some years, up to 168 days. Spring is short, with a steep increase in air temperature and heavy winds. In the summer, the average air temperature is 20.0–23.3 °C, and maximal values can reach +42 …+44 °C, while temperature on the soil surface rises up to +50 …+60 °C. As a result, the climate of Northern Kazakhstan is characterized by irregularity year-wise. In 2024, meteorological conditions in general were favorable for sweet clover growth and development in spite of unstable temperature regime and uneven distribution of precipitation. Weather conditions during the period of sweet clover green biomass formation were similar to long-term annual averages, which ensured good plant development (July) before plowing under thereof. An excessive amount of precipitation in August (106.6 mm, the annual average norm being 66.8 mm) contributed to decomposition of sweet clover plant residues. The temperature background was around 17.4 °C.
The soil represents southern calcareous chernozem (WRB: Chernozem Calcic [
21]), which is characterized by high carbonate content (up to 5%). With regard to granulometric composition, the soils are heavy clay loams with a pulverous structure. The arable layer is 30 cm thick; the humus horizon is up to 50 cm. The supply rate of nitrogen total form is 0.3%, phosphorus 0.1%, while calcium (up to 80%) and magnesium (11%) prevail in the absorbed layer. Soil рН is around 7.6–7.9, which is slightly alkaline. The soils are characterized by a non-washing regime; the groundwater depth level is below 10 m. Soil moisture supply totally depends on precipitation level. Available water content by the sowing time usually does not exceed 110 mm, while by autumn the content thereof in the root zone decreases to the level of plant wilting. Substantial seasonal fluctuations of soil moisture and carbonate content cause degree of arable layer compaction.
2.1.1. Yellow Sweet Clover of the First Year of Existence (Green Manure) (SC)
The plot where the experiment with yellow sweet clover was established had been fallowed for three years; before that, wheatgrass perennial grass was grown on that plot. The year before sowing yellow sweet clover, the plot was cultivated as clean fallow, which included four shallow subsurface cultivations with a KPSh-3 sweep cultivator and one autumn deep cultivation with a deep tiller PG-3-5 down to 25–27 cm to accumulate soil moisture. This system of fallowing is traditional for the zone of investigation.
In the spring, the soil was harrowed, smoothed with BIG-3 soil spikers, cultivated with seedbed cultivators, and rolled before and after sweet clover planting. Sweet clover Altynbas cultivar was sown after fallow on 26 April 2014 (on the planting date recommended for the given zone) with the direct-connected grass planter, using scarified seeds (without seed inoculation with biopreparations), with the seeding rate of 3.5 million fertile seeds per 1 hectare (8 kg/ha) with row spacing of 15 cm. Repetition was quadruple. The area of the registration plot was 120 m
2. The yielding capacity of green (aboveground) mass of first-year yellow sweet clover was identified according to [
22], and yielding capacity of underground (root) mass according to [
23].
Sweet clover for green manure was plowed under at the full bud stage (mid July) in the first year of the plant life with a mounted plow (PN-3-35) down to the depth of the plowing horizon. After the plowing under, the sweet clover furrow slice was cultivated with a disk harrow (BDM 6 × 4P). Location of the variant (*SC) is 51°39.150′ N; 071°00.985′ E.
2.1.2. Clean Fallow (CF)
The control was clean fallow that had not been seeded with farm crops for three years. Prior to that, wheatgrass perennial grass was grown on that plot. During the growing period the clean fallow was cultivated four times with blade weeder KPSh-3 to control weeds; fertilizers were not applied. The location of the variant (CF) was 51°38.589′ N; 071°00.661′ E.
2.1.3. Virgin Steppe (V)
The virgin plot located in the Shortandy District, Amola Region, was selected as the reference standard. The soil of the virgin plot had never been in crop rotation and retained its natural vegetation, prevailing species being
Stipa lessingiana Trin. et Rupr.,
Stipa capillata L.,
Festuca valesiaca Gand. subsp.
sulcata.,
Artemisia austriaca Jacq. The virgin plot is a natural preserve in the steppe zone of North Kazakhstan. The variant location (V) was 51°34.563′ N; 071°15.795′ E. For metagenomic and agrochemical analyses, three independent composite soil samples per treatment were collected (
n = 3 for SC, CF, and V), corresponding to spatially separated field replicates. For the virgin steppe site, the three biological replicates represent spatially separated sampling points within the preserved area (minimum distance of 50 m between sampling points). It should be noted that agricultural treatments (SC, CF) and the virgin steppe reference are located at different coordinates, which introduces potential site effects when comparing these systems (see
Section 4.7).
2.2. Biochemical Analysis of Aboveground and Underground (Roots) Biomass of Yellow Sweet Clover
Nitrogen content in the dry matter of yellow sweet clover aboveground and underground (roots) biomass was identified per the Kjeldahl Method with the use of a combustion apparatus and water vapor distillation (UDK-139, Velp Scientifica, Usmate Velate, Italy) in compliance with ISO 5983-1:2005 [
24]. Phosphorus content was identified photometrically pursuant to GOST 26657-97 [
25]. Potassium content was identified by the flame photometry method as per GOST 30504-97 [
26].
2.3. Soil Sampling and Agrochemical Analysis
Complex soil samples (0–20 cm) were collected in early September with a sampler (hole borer) from each plot, using a systemic grid with 10 subsamples per plot. Soil samples were dried to air-dried basis, then they were crushed and sifted through a 2 mm sieve to identify agrochemical characteristics. Soil pH was measured with potentiometric method in soil: water suspension 1:5. Nitrate nitrogen was extracted from the soil with KCl solution, with subsequent reduction with hydrazine to nitrites and was identified with spectrophotometer Cary 50 (Varian Optical Spectroscopy Instruments, Melbourne, Australia) per GOST 26488-85 [
27]. Available phosphorus and potassium were extracted with ammonium carbonate solution (the Machigin method) and measured with spectrophotometry (P) and flame photometry (K) per GOST 26205-91 [
28]. The organic matter (humus) was analyzed by way of oxidation with potassium dichromate (the Tyurin method) per GOST 26213-2021 [
29]. Note on analytical standards: National standards (GOST) were employed for soil and plant analyses as these methods are specifically validated for steppe soil conditions. International equivalents are provided for reference: GOST 26488-85 [
27] for nitrate determination is comparable to ISO 14255:1998 (KCl extraction) [
30]. GOST 26205-91 (Machigin method) [
28] corresponds to a modified Olsen P method adapted for calcareous soils, and GOST 26213-2021 [
29] for organic matter follows the principles of the Walkley-Black chromic acid oxidation method [
31]. All analyses were performed in an accredited laboratory (ISO/IEC 17025:2017) [
32] ensuring international comparability of results.
2.4. DNA Extraction and Metagenomic Sequencing
DNA extraction from soil samples was performed using the ZymoBiomics DNA Microprep kit (Cat. No. D4301, Zymo Research, Irvine, CA, USA), with concentrations determined spectrophotometrically using a NanoDrop 2000/2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Sterile water served as the negative control. Whole-genome shotgun metagenomic sequencing was conducted according to standard Illumina protocols by Novogene Corporation (Beijing, China) on the NovaSeq 6000 (Illumina, San Diego, CA, USA) platform, generating an average of 6 Gb of data per sample.
2.5. Microbial Symbiont Identification
Identification of nitrogen-fixing symbionts was performed through taxonomic profiling of metagenomic reads against reference genomes of known rhizobial species. Successful establishment of symbiotic relationships was confirmed by detection of nif gene clusters and nodulation genes (nodABC) with coverage depth > 10× and identity > 97%. Symbiont abundance was calculated as genome equivalents per gram of soil based on single-copy core gene normalization.
2.6. Bioinformatics and Statistical Analysis
Raw sequencing data were processed using KneadData v0.12.0 for quality control [
33], including removal of low-quality reads (Q < 20), adapter sequences, and host contamination. Taxonomic profiling was performed using MetaPhlAn 4 (database mpa_vJan21_CHOCOPhlAnSGB_202103) [
34] and functional annotation with HUMAnN 3.0 [
35] against the UniRef90 database. The average sequencing depth was 23.9 million reads per sample, with minimum threshold set at 10 million reads for inclusion in analysis. Quality assessment performed with FastQC v0.11.9 [
36] showed consistently high per-base quality scores (median Phred > Q30). Levels of sequence duplication were low, and no overrepresented adapter sequences were detected.
Statistical analyses were performed in R v4.2.2 [
37] and Python v3.9.16 [
38]. Alpha diversity metrics (ACE, Shannon, Simpson, and Pielou’s evenness indices) were calculated using the vegan package. Beta diversity was assessed using Bray–Curtis dissimilarity and Jaccard indices (scikit-bio v0.6.2). Differential abundance analysis was conducted using MaAsLin2 v1.12.0 [
39] with treatment and block as fixed factors. Given the exploratory nature of this first metagenomic study in alkaline steppe soils, we used FDR-adjusted
p < 0.25 for initial screening of differential features, with
p < 0.05 considered significant for primary hypotheses.
PERMANOVA (adonis2 function, vegan package) assessed community structure differences with 999 permutations. ANOSIM and PERMDISP tests evaluated group separation and dispersion homogeneity. Correlation analysis examined correlations between microbial taxa, metabolic pathways, and soil properties using Spearman correlation (r > |0.7|,
p < 0.01). Principal component analysis (PCA) visualized community structure patterns. LEfSe [
40] identified biomarker taxa with LDA score > 2.0.
Marker taxa were defined as microbial species showing statistically significant differential abundance between groups, identified using Linear discriminant analysis Effect Size (LEfSe) with the following parameters: alpha value for factorial Kruskal–Wallis test = 0.05, threshold on the logarithmic LDA score for discriminative features = 2.0. Only taxa present in >50% of samples within at least one treatment group were considered for biomarker analysis.
3. Results
3.1. Plant Food Compound Content in Yellow Sweet Clover
Green mass yielding capacity of the plowed under yellow sweet clover of the first year was 11.6 t/ha. Before plowing under of yellow sweet clover, plant selection was carried out to identify plant food compounds in the dry matter of the aboveground and underground (roots) biomass. During the first year of growing, yellow sweet clover accumulated 3.24 t/ha of dry matter in the aboveground portion and 1.75 t/ha in the underground portion (roots) in the arable layer. In the aboveground biomass of yellow sweet clover, nitrogen content in the dry matter was 2.63%, phosphorus 0.30%, and potassium 2.01%. The yield from 1 ha of dry matter in the aboveground portion (3.24 t/ha) was: nitrogen 85.2 kg, phosphorus 9.7 kg, and potassium 65.1 kg. In the underground biomass of yellow sweet clover (roots), nitrogen content in the dry matter was 2.65%, phosphorus 0.28%, and potassium 1.60%. The yield from 1 ha of dry matter in the underground portion (1.75 t/ha) was: nitrogen 46.4 kg, phosphorus 4.9 kg, and potassium 28.0 kg. The total dry matter yield was 4.99 t/ha (3.24 t/ha aboveground + 1.75 t/ha underground). The total amount of nutrients (aboveground and underground mass combined) applied when plowed under in the variant of yellow sweet clover of the first year was as follows: nitrogen 131.6 kg/ha, phosphorus 14.6 kg/ha, and potassium 93.1 kg/ha.
3.2. Comparative Agrochemical Characteristics of Soil
The agrochemical analysis revealed significant alterations in soil properties following agricultural conversion from virgin steppe to cultivated systems (
Table 1). One-way ANOVA indicated significant treatment effects for most parameters except exchangeable potassium and sulfur.
Soil nitrate-nitrogen content showed unexpected patterns following sweet clover incorporation. Despite successful crop establishment, N- concentrations in sweet clover plots did not differ significantly from clean fallow plots. Both agricultural treatments showed substantial enrichment compared to the virgin steppe reference site (F2,6 = 42.7, p ≤ 0.001, η2 = 0.93). The numerically lower nitrate content in sweet clover plots suggests potential nitrogen immobilization during early decomposition processes at 50 days post-incorporation. Organic matter content showed severe depletion under cultivation. Sweet clover and clean fallow treatments showed no significant difference between each other, but both differed substantially from virgin steppe (5.5 ± 0.9%; F2,6 = 24.6, p ≤ 0.001, η2 = 0.89). This indicates rapid degradation following agricultural conversion. Soil pH increased significantly from virgin steppe (8.3 ± 0.2) to agricultural systems, reaching 9.0 ± 0.1 in both sweet clover and clean fallow plots (F2,6 = 24.2, p ≤ 0.001, η2 = 0.89). Available phosphorus (P2O5) demonstrated uniform enrichment under cultivation, with sweet clover and clean fallow showing nearly identical concentrations (p = 1.0). Both treatments exceeded virgin steppe levels by 3.9-fold (F2,6 = 34.9, p ≤ 0.001, η2 = 0.92), likely reflecting enhanced mineral weathering rather than management-specific effects.
Exchangeable potassium showed no significant treatment effects (F2,6 = 1.03, p = 0.41), though concentrations exceeded 1000 mg/kg in both sweet clover and clean fallow treatments, representing 8.4% and 10.4% increases over virgin steppe. These levels approach thresholds that can interfere with calcium-magnesium uptake in alkaline soils. Different superscripts within rows indicate significant differences at p < 0.05 (Tukey’s HSD test). The F-value shows the ratio of variance between groups to variance within groups; η2 (partial eta squared) shows the proportion of total variance explained by treatment effects.
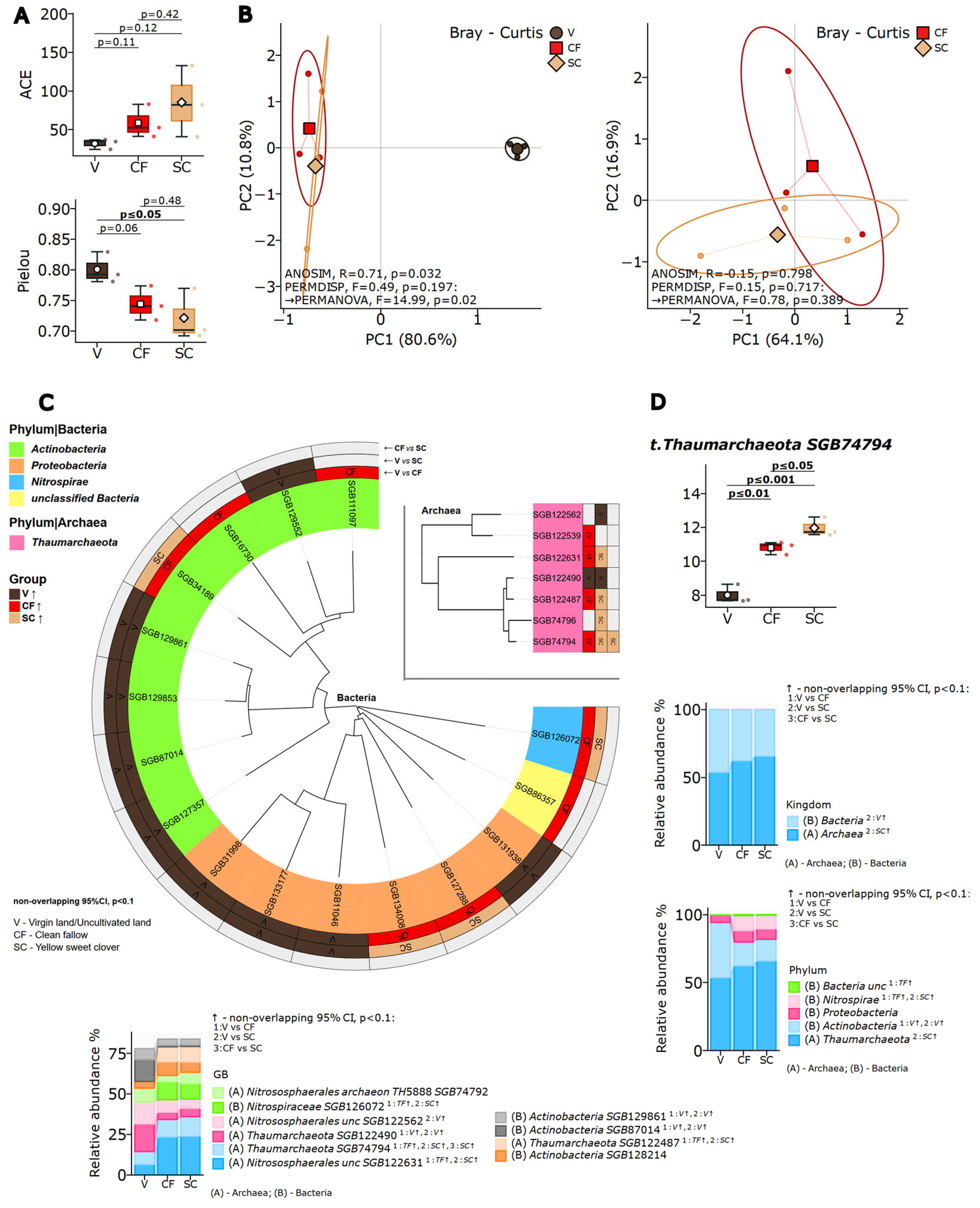
3.3. Alpha Diversity Patterns
Shotgun metagenomic sequencing yielded a total of 399 species-level genome bins (SGBs) across all samples, with an average sequencing depth of 23.9 million high-quality reads per sample (median Phred > Q30). Comparative analysis of alpha diversity indices (
Figure 2A) revealed distinct patterns in microbial community structure across three land-use systems. The Shannon diversity index for total microbial communities showed marginal differences (virgin steppe: 4.52 ± 0.21, sweet clover: 4.68 ± 0.18, clean fallow: 4.61 ± 0.15;
p = 0.082). However, when analyzed separately, archaeal communities demonstrated significant responses: archaeal Shannon diversity increased from 1.23 ± 0.14 in virgin steppe to 1.89 ± 0.22 in sweet clover plots (
p = 0.01), while bacterial Shannon indices remained stable (3.98 ± 0.19 vs. 4.05 ± 0.17,
p = 0.31).
Biomass incorporation of first-year sweet clover did not lead to statistically significant changes in overall microbial richness (ACE index, p > 0.05). However, Shannon diversity analysis showed a specific effect on archaeal communities: archaeal diversity increased significantly in the sweet clover treatment compared to the virgin steppe (p = 0.01). Bacterial diversity also tended to be higher in the sweet clover plots relative to the virgin soil, although this trend remained close to the threshold of statistical significance.
Pielou’s evenness index, measuring community balance through species abundance distribution, revealed that virgin steppe microbial communities maintained greater equilibrium with relatively similar species abundances. Variant SC showed a shift toward lower evenness, with a subset of taxa becoming more abundant, while others became rarer.
3.4. Beta Diversity and Community Structure
Beta diversity assessment revealed clear distinction between land-use systems (
Figure 2).
Table S1 presents the codes that are matched to the organisms to which the genomes belong. Virgin steppe soils (V) formed distinct clusters from all cultivated lands (CF and SC), while sweet clover and fallow showed overlapping community composition. Beta diversity assessment using Jaccard index confirmed group differences: ANOSIM test (R = 0.78,
p = 0.017), PERMANOVA test (F = 11.71,
p = 0.036), and marginal significance for homogeneity (PERMDISP test F = 1.42,
p = 0.052). Bray–Curtis beta diversity analysis yielded similar results with confirmed differences: ANOSIM test (R = 0.71,
p = 0.032), PERMANOVA test (F = 14.99,
p = 0.02), with uniform variation between groups (PERMDISP test F = 0.49,
p = 0.197).
3.5. Taxonomic Composition and Key Taxa
Taxonomic composition analysis at multiple hierarchical levels (
Figure 2C) revealed systematic shifts in community structure. At the phylum level, agricultural conversion increased the relative abundance of
Nitrospirae from 2.3% ± 0.4% in virgin steppe to 5.8% ± 0.7% in sweet clover and 5.6% ± 0.6% in clean fallow (
p < 0.01). Marker taxa identified through LEfSe analysis (
Figure 2D) demonstrated clear system-specific microbial signatures as detailed in Section LEfSe Biomarker Analysis.
Comprehensive taxonomic profiling identified a total of 399 microbial taxa across all samples, of which 157 showed differential abundance among land-use types at the exploratory threshold (FDR q < 0.25; see Limitations). These nitrite-oxidizing bacteria indicate enhancement of nitrogen cycling efficiency under cultivation.
Thaumarchaeota demonstrated pronounced system-specific responses in direct sweet clover-fallow comparisons, with selective enrichment of ammonia-oxidizing lineages under legume cultivation-specific Thaumarchaeota taxa (GGB53661_SGB74794 and GGB53661_SGB122487) showed significantly higher abundance in sweet clover systems compared to clean fallow (p ≤ 0.05).
LEfSe Biomarker Analysis
Linear discriminant analysis Effect Size (LEfSe) identified 23 biomarker taxa with LDA scores exceeding 2.0, revealing distinct microbial signatures across land management systems (
Figure 2D).
Sweet clover systems were characterized by enrichment of nitrogen-fixing symbionts. Mesorhizobium muleiense showed the highest discriminatory power (LDA = 4.12, p < 0.001), followed by Bradyrhizobium sp. URHD0069 (LDA = 3.87, p < 0.001). Additional plant-associated taxa included Phyllobacterium myrsinacearum (LDA = 3.45, p = 0.002) and Rhizobium leguminosarum (LDA = 3.22, p = 0.003).
Virgin steppe maintained a distinct actinobacterial signature. Nocardioides astragali served as the primary biomarker (LDA = 4.35, p < 0.001), accompanied by Arthrobacter sp. P2b (LDA = 3.98, p < 0.001) and Blastococcus sp. CT_GayMR19 (LDA = 3.76, p = 0.002). These taxa are associated with organic matter decomposition and stress tolerance in oligotrophic environments.
Clean fallow systems exhibited intermediate biomarker profiles with enrichment of opportunistic copiotrophs, including Bacillus subtilis (LDA = 3.54, p = 0.004) and Pseudomonas fluorescens (LDA = 3.21, p = 0.008), reflecting adaptation to periodic disturbance.
At the family level, Nitrososphaeraceae (LDA = 3.89, p < 0.001) and Nitrospiraceae (LDA = 3.67, p = 0.002) emerged as biomarkers for agricultural systems collectively, confirming enhanced nitrification capacity under cultivation.
3.6. Functional Profile Analysis
Functional analysis demonstrated that major metabolic restructuring occurs during transition from natural to agricultural systems, with direct sweet clover–fallow comparisons revealing only five significant functional differences (p < 0.05 after FDR correction), confirming metabolic convergence of agricultural systems. These five pathways were: polyisoprenoid biosynthesis (POLYISOPRENSYN-PWY), chorismate biosynthesis from 3-dehydroquinate (PWY-6163), Bifidobacterium shunt (P124-PWY), homolactate fermentation (ANAEROFRUCAT-PWY), and 4-oxopentanoate degradation (PWY-7948).
Quantitative functional profiling revealed 74 exploratory metabolic pathways with differential abundance after FDR correction (q < 0.25). Sweet clover systems showed 2.8-fold enrichment of amino acid biosynthesis pathways, including L-methionine biosynthesis III (log2FC = 2.14, q = 0.018) and L-lysine/threonine/methionine superpathway (log2FC = 1.87, q = 0.032). Virgin steppe maintained 69 pathways substantially enriched relative to agricultural systems (detected at <0.001% relative abundance in SC and CF soils), predominantly involving sulfur metabolism (PWY-801, log2FC = −1.68, q = 0.008) and coenzyme biosynthesis (COA-PWY-1, log2FC = −1.92, q = 0.006).
Nitrogen cycling genes showed treatment-specific enrichment: amoA gene abundance increased 4.5-fold in sweet clover (1.8 × 106 vs. 4.0 × 105 copies/g soil, p = 0.003), while nxrB genes increased uniformly 2.3-fold in both agricultural systems (p = 0.008). Functional gene diversity (Shannon index) decreased from 6.82 ± 0.15 in virgin steppe to 6.21 ± 0.18 in agricultural soils (p = 0.012), confirming functional homogenization.
Virgin steppe maintained 69 pathways substantially enriched relative to agricultural systems at the exploratory threshold (FDR q < 0.25; see Limitations), detected at <0.001% relative abundance in SC and CF soils. Among these, enhanced sulfur-containing amino acid metabolism (PWY-801: homocysteine-cysteine conversions, p ≤ 0.01) and coenzyme A biosynthesis (COA-PWY-1: coenzyme A biosynthesis III superpathway, p ≤ 0.01) met the stringent significance threshold. These pathways reflect complex metabolic networks of natural microbial consortia adapted to the oligotrophic conditions of steppe ecosystems.
Agricultural systems exhibited specialization in amino acid and vitamin biosynthesis, with both sweet clover and fallow systems significantly enhancing L-methionine biosynthesis pathways (HSERMETANA-PWY: L-methionine biosynthesis III, p ≤ 0.001), L-lysine/threonine/methionine biosynthesis superpathways (P4-PWY: L-lysine, L-threonine and L-methionine biosynthesis I superpathway, p ≤ 0.05), and menaquinol-8 synthesis (PWY-7992: menaquinol-8 biosynthesis III superpathway, p ≤ 0.01). This functional convergence indicates that agricultural management creates selective pressure favoring intensive protein metabolism and enhanced vitamin K biosynthesis.
Sweet clover-specific effects manifested in carbon-energy metabolism, including enhanced polyisoprenoid biosynthesis (POLYISOPRENSYN-PWY: polyisoprenoid biosynthesis, p ≤ 0.01) and aromatic metabolism pathway modification, particularly chorismate biosynthesis (PWY-6163: chorismate biosynthesis from 3-dehydroquinate, p ≤ 0.05). Conversely, clean fallow was characterized by enhancement of fermentative pathways, including the Bifidobacterium shunt (P124-PWY: Bifidobacterium shunt, p ≤ 0.05), homolactate fermentation (ANAEROFRUCAT-PWY: homolactate fermentation, p ≤ 0.01), and 4-oxopentanoate degradation (PWY-7948: 4-oxopentanoate degradation, p ≤ 0.01), reflecting adaptation to anaerobic conditions and organic residue degradation.
Functional specialization in the nitrogen cycle manifested through differential activation of coenzyme M biosynthesis pathways (P261-PWY: coenzyme M biosynthesis I, p ≤ 0.05), a critical cofactor in methanogenesis and anaerobic methane oxidation. Both agricultural systems showed enhancement of this pathway relative to virgin conditions, indicating modification of anaerobic microbial processes in cultivated soils.
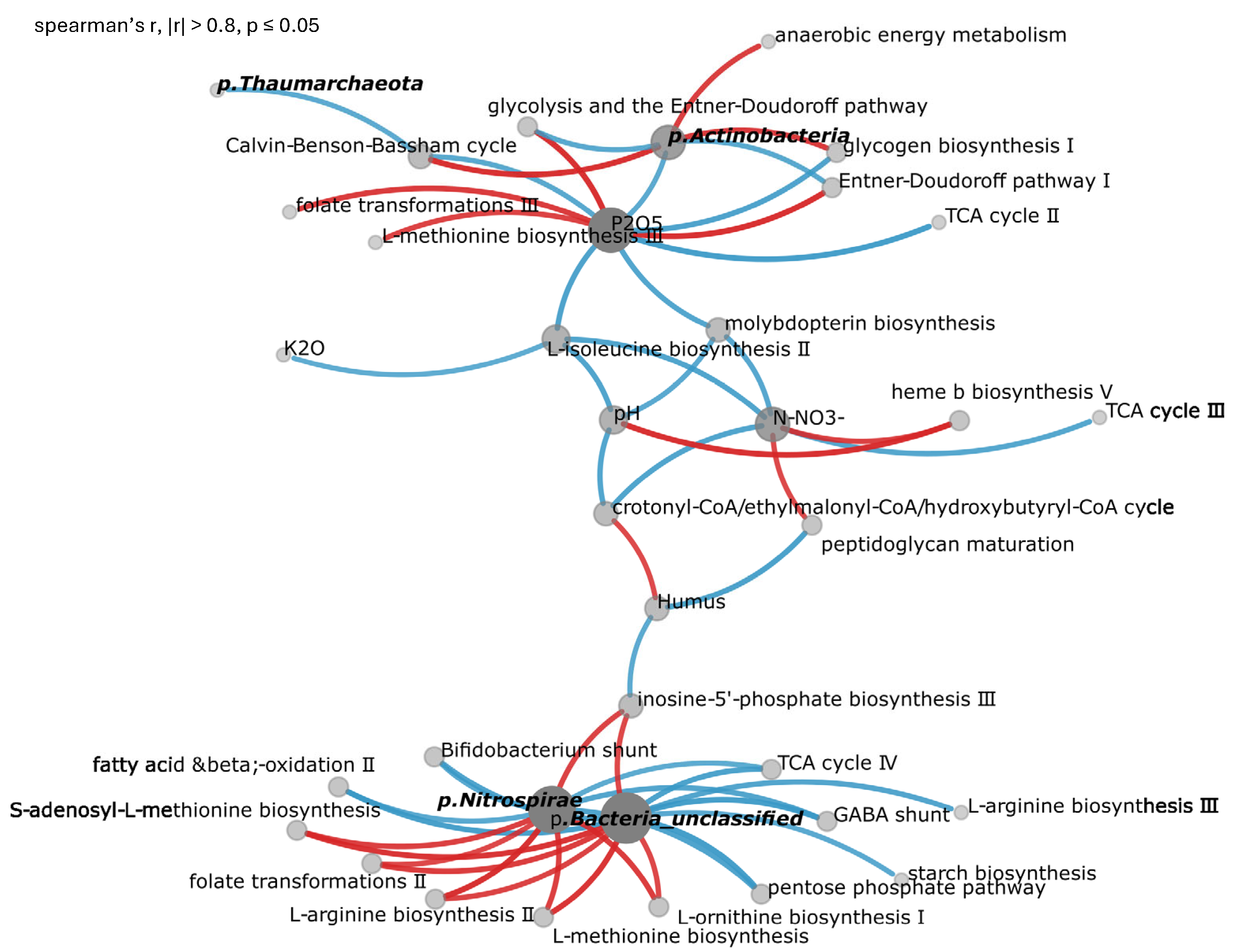
3.7. Correlation Analysis and Nutrient Controls
Correlation analysis of the combined dataset from sweet clover agroecosystems, virgin sites, and clean fallow identified phosphorus as the primary regulator of microbial metabolism, demonstrating significant correlations (r = 1.0,
p < 0.0001) with microbial glycolysis and L-methionine and folate metabolism pathways including PWY-8004: Entner-Doudoroff pathway I and GLYCOLYSIS-E-D: superpathway of glycolysis and the Entner-Doudoroff pathway (r = 0.95,
p < 0.0001), HSERMETANA-PWY: L-methionine biosynthesis III (r = 0.87,
p < 0.001), and 1CMET2-PWY: folate transformations III (r = 0.9,
p < 0.001) (
Figure 3). Soil organic matter demonstrated strong positive associations (r = 0.8–0.9,
p < 0.001) with central carbon metabolism networks across all studied land-use systems, particularly with pathways crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (r = 0.81,
p < 0.01).
Sweet clover systems were characterized by distinctive microbiome signatures with enrichment of nitrogen-fixing symbionts Mesorhizobium muleiense and Bradyrhizobium sp. URHD0069 (r = 0.8–0.9), coupled with activation of amino acid biosynthesis pathways including HSERMETANA-PWY (L-methionine biosynthesis III), ARGSYNBSUB-PWY (L-arginine biosynthesis II), and PWY-5101 (L-isoleucine biosynthesis II). Natural grassland communities maintained stable actinobacterial networks with key taxa Nocardioides astragali, Arthrobacter sp. P2b, and Blastococcus sp. CT_GayMR19, driving organic matter decomposition through pathways PWY-622 (starch biosynthesis) and GLYCOGENSYNTH-PWY (glycogen biosynthesis I).
3.8. Hierarchical Taxonomic Patterns
Taxonomic analysis across several hierarchical levels revealed that, at the family level, Phyllobacteriaceae and Nocardioidaceae served as key taxa (r = 0.8–1.0) linking soil chemistry with metabolic output. Class-level analysis identified Actinobacteria_CFGB52786 and Alphaproteobacteria as primary drivers of soil carbon cycling, while c_Nitrososphaeria drove nitrogen transformations through ammonia-oxidizing archaea, including Thaumarchaeota_GGB53661_SGB122487 and Nitrososphaerales_unclassified_GGB53670_SGB122539.
Correlation analysis revealed that elevated K2O, N-, and pH generated strong negative correlations (r = −0.7 to −0.9, p < 0.01) with essential metabolic pathways including PWY-7854 (crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA cycle), PWY-6823 (molybdopterin biosynthesis), and PWY-7383 (anaerobic energy metabolism).
4. Discussion
Our investigation of first-year sweet clover green manuring in alkaline steppe soils revealed unexpected patterns that challenge conventional assumptions about legume-based soil restoration. Despite successful establishment of nitrogen-fixing symbionts (
Mesorhizobium muleiense and
Bradyrhizobium sp.), sweet clover cultivation did not enhance soil nitrogen availability compared to clean fallow (9.1 vs. 10.5 mg NO
3-N/kg). Instead, we observed selective microbial restructuring with enhanced archaeal diversity but functional convergence between agricultural systems—only five metabolic pathways differed significantly between sweet clover and fallow treatments. Most critically, correlation analysis identified phosphorus, not nitrogen, as the primary controller of microbial metabolism (r = 1.0,
p < 0.0001), while elevated pH (9.0) and potassium (>1000 mg/kg) emerged as previously unrecognized constraints on soil biological processes.
| The Nitrogen Paradox: Why Green Manure Failed to Enhance N Availability? |
The lower nitrate content in sweet clover plots compared to fallow contradicts expected benefits of legume incorporation but aligns with nitrogen immobilization dynamics during early decomposition phases. When plant residues with C:N ratios exceeding 20:1 are incorporated, soil microorganisms temporarily sequester available nitrogen to metabolize carbon-rich substrates [
5]. We therefore hypothesize that our sampling at 50 days post-incorporation captured this immobilization phase, before net mineralization releases plant-available nitrogen—a process requiring extended periods in semi-arid conditions.
The strongly alkaline conditions (pH 9.0) compound this nitrogen limitation through multiple mechanisms. At pH > 8.5, nitrification rates decline substantially due to ammonia volatilization and enzymatic inhibition [
6]. Our enrichment of ammonia-oxidizing archaea (
Thaumarchaeota) represents an adaptive response, as these organisms possess superior ammonia affinity (Km = 133 nM) compared to bacterial nitrifiers (Km > 50 μM), allowing them to function under substrate limitation [
12]. However, even enhanced archaeal populations cannot fully compensate for pH-induced constraints on nitrogen cycling. This explains why both agricultural systems showed similar
Nitrospirae enrichment—cultivation itself, rather than specific management, drives selection for stress-tolerant nitrifiers [
13].
4.1. Taxonomic Restructuring: Nitrification Enhancement as Universal Agricultural Response
The enrichment of
Nitrospirae across both agricultural systems indicates that cultivation itself, rather than specific management practices, drives selection for specialized nitrifying communities. This universal enhancement aligns with recent studies showing that phylum
Nitrospirae represents a key component of soil microbial communities with enhanced activity in agricultural systems [
13,
41]. Contemporary metagenomic studies confirm the crucial role of
Nitrospira not only in nitrite oxidation but also in urea hydrolysis, providing ammonia for ammonia-oxidizing microorganisms [
42]. The discovery of complete ammonia oxidizers (comammox) within
Nitrospira further emphasizes their central role in nitrogen cycling across diverse agricultural environments [
13].
The selective enrichment of specific
Thaumarchaeota lineages (SGB74794 and GGB53661_SGB74794) in sweet clover systems represents a more targeted response to legume cultivation. These observations are fully supported by large-scale studies demonstrating that ammonia-oxidizing archaea (AOA) can outnumber ammonia-oxidizing bacteria 50-430-fold in fertile agricultural soils, with
Thaumarchaeota dominating nitrification processes [
10,
43]. Studies have shown that group I.1a
Thaumarchaeota are particularly active in agricultural soils and demonstrate autotrophic growth during ammonia oxidation [
44]. Stable isotope profiling confirms that soil
Thaumarchaeota perform autotrophic ammonia oxidation through the highly efficient 3-hydroxypropionate/4-hydroxybutyrate CO
2 fixation cycle [
11].
The contrasting patterns—universal
Nitrospirae enrichment versus selective
Thaumarchaeota response—suggest a two-tiered restructuring of nitrifying communities under cultivation. While both agricultural systems require enhanced nitrite oxidation capacity (explaining
Nitrospirae enrichment), only sweet clover provides the specific rhizosphere conditions that favor archaeal ammonia oxidizers. This specialization may reflect the superior ammonia affinity of
Thaumarchaeota (Km = 133 nM) [
12], allowing them to efficiently capture ammonia released during legume residue decomposition before volatilization losses occur at pH 9.0.
4.2. Phosphorus as the Master Regulator: Implications for Restoration
The emergence of phosphorus as the dominant factor controlling microbial metabolism fundamentally reframes our understanding of nutrient limitations in carbonate soils. The strong correlations between available P and essential pathways—peptidoglycan synthesis, nucleotide biosynthesis, and heme production—indicate that cellular growth and energy metabolism are P-limited rather than N-limited in these systems. With available P at 18.5 mg/kg in both agricultural treatments versus 4.7 mg/kg in virgin steppe, cultivation appears to mobilize phosphorus through enhanced weathering and organic acid production, independent of green manure addition.
In carbonate-rich soils, calcium-phosphate precipitation reduces P bioavailability by 10–100-fold compared to neutral soils [
18]. Our pH of 9.0 exacerbates this constraint, as phosphate solubility decreases exponentially above pH 7.5. The correlation analysis revealing P control over 12 major metabolic pathways suggests that microbial communities have adapted to extreme P limitation through metabolic downregulation. This finding aligns with recent evidence that P availability controls microbial carbon use efficiency and growth rates in alkaline soils [
19]. Without addressing phosphorus constraints—through acidification, organic acid application, or phosphate-solubilizing inoculants—green manure alone cannot restore soil biological function [
20].
4.3. Network Architecture and Nutrient Toxicity Thresholds
Beyond phosphorus limitation, our correlation analysis revealed unexpected antagonistic effects of elevated nutrients on microbial metabolism. The strong negative correlations between elevated K
2O (>1000 mg/kg), N-
, pH (9.0) and essential metabolic pathways—including crotonyl-CoA/ethylmalonyl-CoA cycles, molybdopterin biosynthesis, and anaerobic energy metabolism—indicate nutrient toxicity thresholds previously unrecognized in agricultural systems. These results align with recent studies showing that soil pH, available phosphorus, and NO
3-N content significantly influence nitrification and denitrification processes [
45,
46]. Studies demonstrate that excessive nitrogen fertilizer application in alkaline soils causes high nitrification rates and substantial nitrogen losses through N
2O emission and
leaching [
45,
46]. Metagenomic studies show that initial soil nutrient status and pH modulate fertilizer effects on microbial carbon and nitrogen cycling processes, confirming our observations on threshold effects [
47].
The correlation analysis further demonstrates that soil organic matter serves as a critical mediator of phosphorus effects, showing strong positive associations with central carbon metabolism pathways. This aligns with recent studies showing that soil phosphorus concentration strongly regulates microbial carbon and nitrogen metabolism [
48].
Metagenomic studies demonstrate that high soil phosphorus concentrations promote carbon fixation and methane oxidation processes, while phosphorus is a key element in regulating the genes associated with inorganic phosphorus solubilization and organic phosphorus mineralization [
49]. The tight coupling between P availability and carbon metabolism explains why organic matter additions alone may fail to restore soil function without addressing phosphorus constraints.
The specificity of sweet clover systems in establishing nitrogen-fixing symbiont networks (
Mesorhizobium muleiense, Bradyrhizobium sp. URHD0069) coupled with enhanced amino acid biosynthesis pathways provides insight into legume-microbe mutualism. Recent studies confirm the specificity of associations between legumes and rhizobia strains, demonstrating that nitrogen fixation efficiency depends on plant-microbe pair compatibility [
50]. Studies show that rhizosphere interactions between legumes and soil microorganisms enhance amino acid biosynthesis gene expression by 200–300% compared to non-legume systems [
51]. Large-scale metagenomic studies revealed global distribution of Deltaproteobacteria in nitrogen-fixing microbiomes, complementing our data on specific associations in sweet clover systems [
50]. Research in Agronomy shows the importance of plant–soil–microbe interactions in determining soil biological fertility through rhizosphere nutrient cycling changes [
52]. However, these beneficial associations appear insufficient to overcome the multiple constraints of alkaline chemistry and nutrient imbalances documented in our study.
These findings suggest potential nutrient imbalance effects that may influence conventional fertilization approaches in alkaline steppe soils. Rather than simple nutrient deficiency, these systems may exhibit a complex optimization problem where elevated concentrations of some nutrients (K, ) could negatively affect microbial processes while others (P) remain limiting. However, these correlative observations require experimental validation to confirm causal relationships. This suggests that precision nutrient management—targeting optimal rather than maximal levels—may be more effective than traditional blanket fertilization strategies.
The hierarchical taxonomic analysis revealed how community assembly operates across multiple organizational scales. At the family level,
Phyllobacteriaceae and
Nocardioidaceae emerged as keystone taxa bridging soil chemistry and metabolic function. Contemporary research confirms the key role of family
Phyllobacteriaceae in plant–microbe interactions and carbon metabolism [
53]. At the class level,
Actinobacteria and
Alphaproteobacteria drive carbon cycling, while
Nitrososphaeria (including
Thaumarchaeota groups) control nitrogen transformations. Analysis shows dominance of
Thaumarchaeota groups I.1a and I.1b in fertilized soils, consistent with our
Nitrososphaeria results [
54,
55].
This hierarchical organization demonstrates functional redundancy—multiple taxa at lower taxonomic levels can perform similar functions, explaining why agricultural systems maintain basic biogeochemical cycling despite dramatic taxonomic shifts. The integration of taxonomic and functional analyses in this study reveals functional convergence as a dominant pattern in agricultural microbiomes and identifies phosphorus as the primary regulator of microbial metabolism in alkaline steppe soils—insights with potential implications for understanding nutrient limitations in carbonate-rich agricultural systems globally. Microbiological indicators demonstrate high sensitivity and rapid response to environmental changes, making them valuable tools for assessing agricultural practice impacts on soil ecological functions [
56].
4.4. Functional Convergence: Agricultural Selection Overrides Management
The detection of only five differential metabolic pathways between sweet clover and fallow, despite distinct taxonomic composition, demonstrates that agricultural selection pressures override specific management effects. Both systems converged on enhanced amino acid biosynthesis (methionine, lysine, threonine), vitamin K production, and specialized carbon metabolism. This functional redundancy suggests that cultivation creates uniform selective pressures—alkalinity stress, nutrient imbalance, and reduced organic matter—that filter communities toward similar metabolic capabilities regardless of plant inputs [
3].
Virgin steppe maintained 69 unique pathways absent from agricultural soils, including complex sulfur metabolism and specialized coenzyme biosynthesis. This functional diversity loss represents the true cost of agricultural conversion, more significant than simple species loss [
57]. The enrichment of fermentation pathways in fallow (
Bifidobacterium shunt, homolactate fermentation) versus polyisoprenoid biosynthesis in sweet clover indicates subtle niche differentiation, but these differences are minor compared to the agriculture-steppe divide. This convergence explains why decades of research on green manures in alkaline soils show inconsistent results [
4]—the overriding constraints of pH and nutrient imbalance mask potential benefits.
4.5. Practical Implications for Steppe Agriculture
Our findings indicate that successful restoration of degraded steppe soils requires integrated management addressing multiple constraints simultaneously. Single-season sweet clover incorporation cannot overcome the combined limitations of phosphorus deficiency, pH stress, and 50% organic matter loss [
9]. The identification of putative nutrient stress thresholds—particularly potassium > 1000 mg/kg inhibiting essential metabolic pathways—suggests that conventional fertilization may actually impair soil biological recovery [
45].
For practical implementation, we recommend the following: (1) multi-year legume rotations to allow complete mineralization cycles; (2) co-application of phosphorus amendments or phosphate-solubilizing bacteria; (3) organic amendments to buffer pH and provide diverse carbon substrates; (4) reduced reliance on mineral fertilizers that may exceed toxicity thresholds. The 20–50-year timeline for organic matter recovery [
58] requires managing expectations and developing intermediate indicators of soil health improvement. Monitoring archaeal–bacterial ratios and functional gene abundance may provide earlier feedback than traditional soil tests [
59].
4.6. Future Research Priorities
This initial assessment raises critical questions requiring multi-year investigation. Time-series sampling across complete growing seasons would capture nitrogen mineralization dynamics and seasonal microbial succession. Metatranscriptomic analysis could distinguish between genetic potential and actual metabolic activity [
43], while stable isotope probing would trace nitrogen and carbon flow through microbial networks. Gross nitrogen transformation rates and enzyme activities remain essential for mechanistic understanding. Comparative studies across pH gradients (7.0–9.5) and phosphorus levels could identify threshold values for successful green manure implementation. Testing integrated amendments—legume + phosphorus + organic acids—may reveal synergistic effects masked in single-factor trials. Finally, developing region-specific microbial inoculants adapted to alkaline conditions could enhance green manure effectiveness where native communities are degraded beyond natural recovery capacity [
60].
4.7. Limitations
This study captures only initial decomposition (50 days post-incorporation), missing the complete nitrogen mineralization cycle and multi-year dynamics essential for evaluating green manure effectiveness. Shotgun metagenomics reveals genetic potential, not actual metabolic activity, while absence of enzyme assays, gross N transformation rates, and greenhouse gas measurements prevents mechanistic understanding. The liberal FDR threshold (p < 0.25) and four replications limit statistical power, while network correlations cannot establish causality. Site-specific conditions—pH 9.0, potassium > 1000 mg/kg, 50% organic matter loss—may not represent typical steppe soils. Comparing first-year sweet clover to established fallow introduces temporal asymmetry, while lack of untreated controls prevents separation of cultivation from green manure effects. Additionally, the virgin steppe reference site is located at a different coordinate than the agricultural treatments (SC and CF), and while all sites share similar soil type (southern carbonate chernozem) and climatic conditions, we cannot fully exclude potential site effects and pseudo-replication concerns inherent to this spatial design. Future studies should incorporate multiple virgin reference sites across the region to strengthen comparative inferences. These limitations indicate our findings should guide hypothesis generation rather than definitively conclude green manure ineffectiveness, emphasizing need for multi-year, multi-site validation with process-level measurements.