Root Response to K+-Deprivation in Wheat (Triticum aestivum L.): Coordinated Roles of HAK Transporters, AKT2 and SKOR K+-Channels, and Phytohormone Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Plant Materials
2.1.2. Field Pot Experiment
2.1.3. Hydroponic Culture
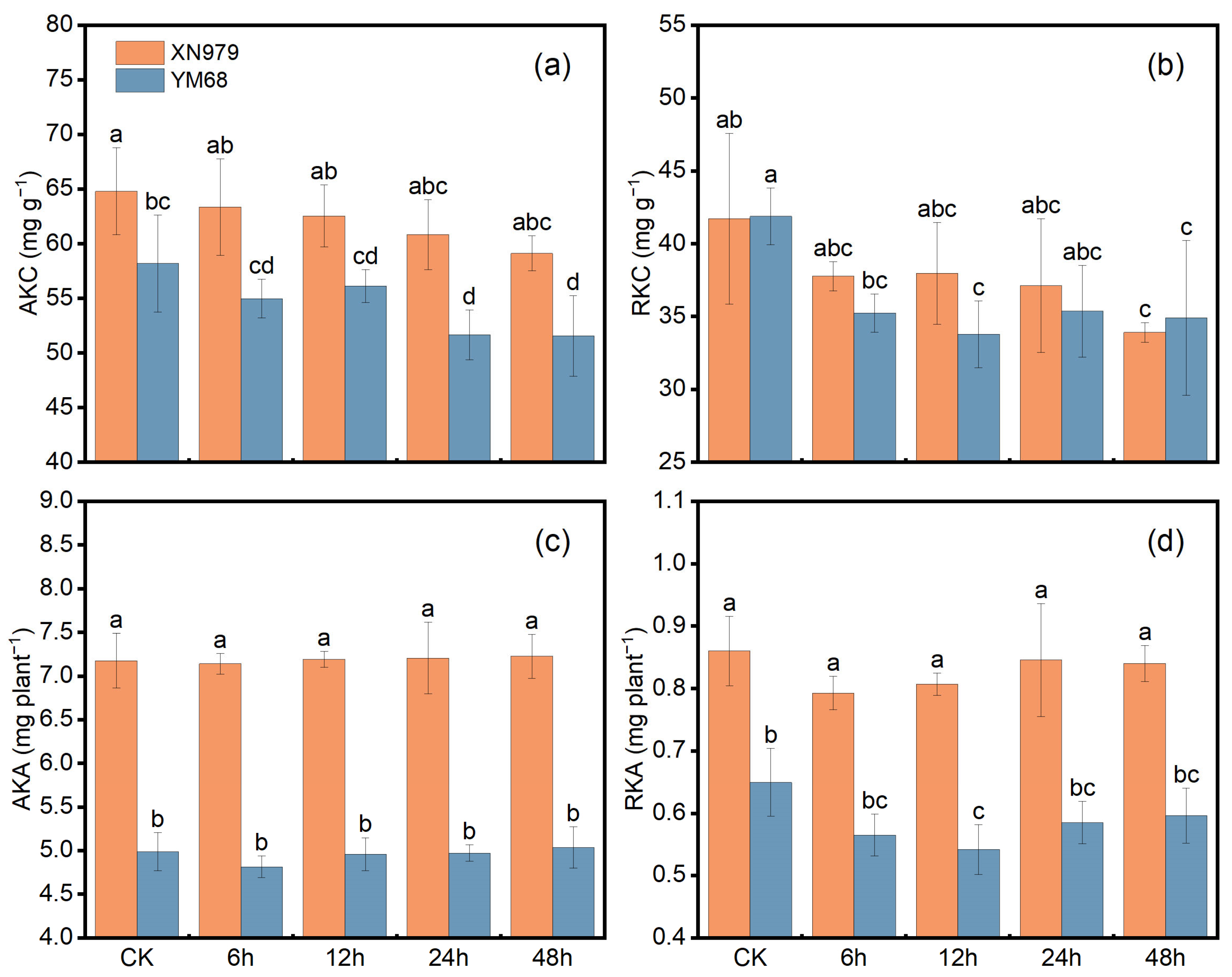
2.2. Measurement of K Uptake and Utilization-Related Indicators in Field Pot Experiment
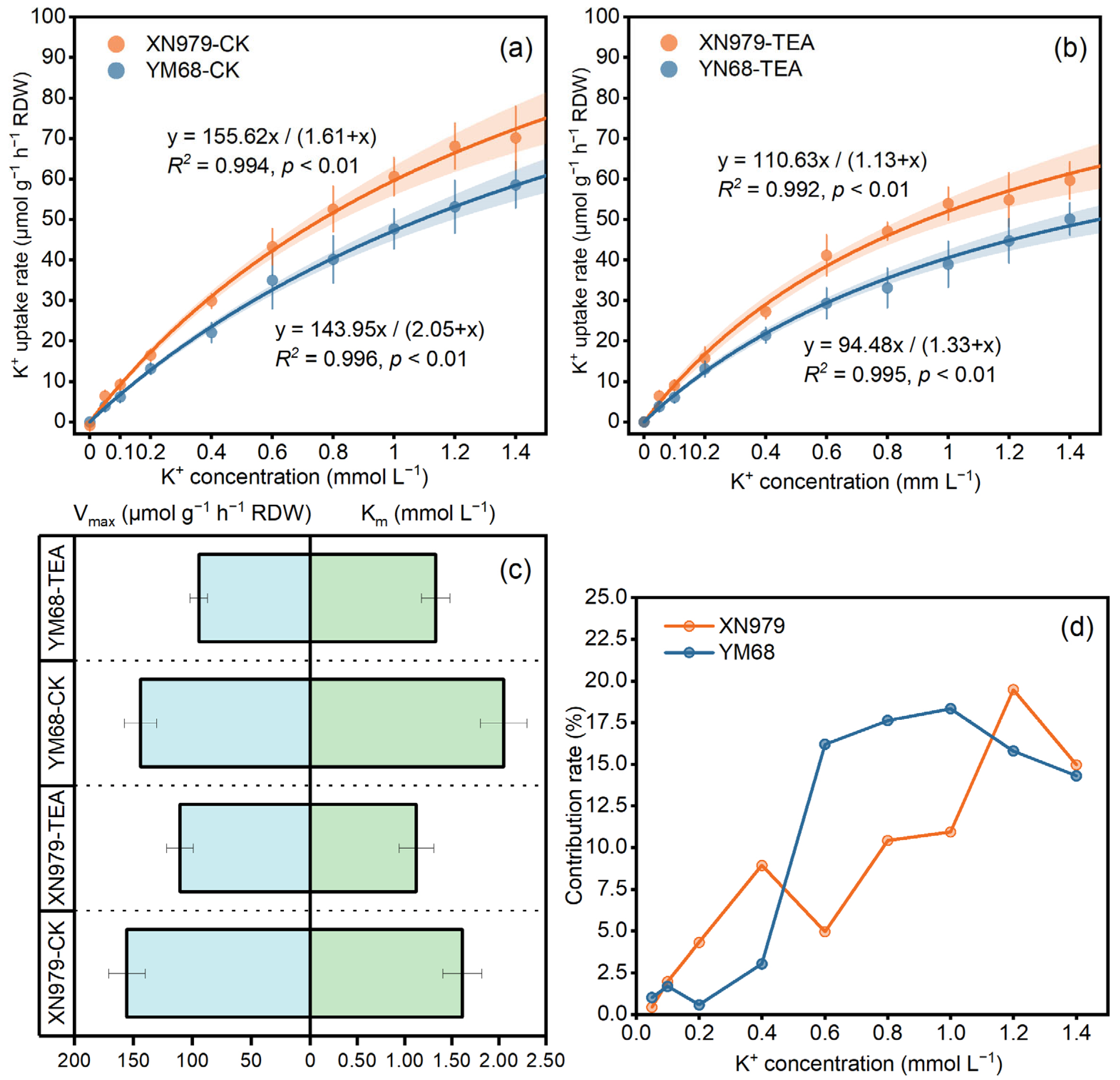
2.3. Measurement of K+ Uptake Kinetic Parameters
2.4. RNA Isolation, Library Preparation, and RNA-Seq
2.5. qRT-PCR Analysis
2.6. Net K+ Flux Measurement
2.7. Statistical Analysis
3. Results
3.1. K Uptake and Utilization-Related Indicators in Field Pot Experiment
3.2. K+ Uptake Kinetic Parameters
3.3. Growth Dynamics of the Aboveground Part and Root Under K+-Deprivation
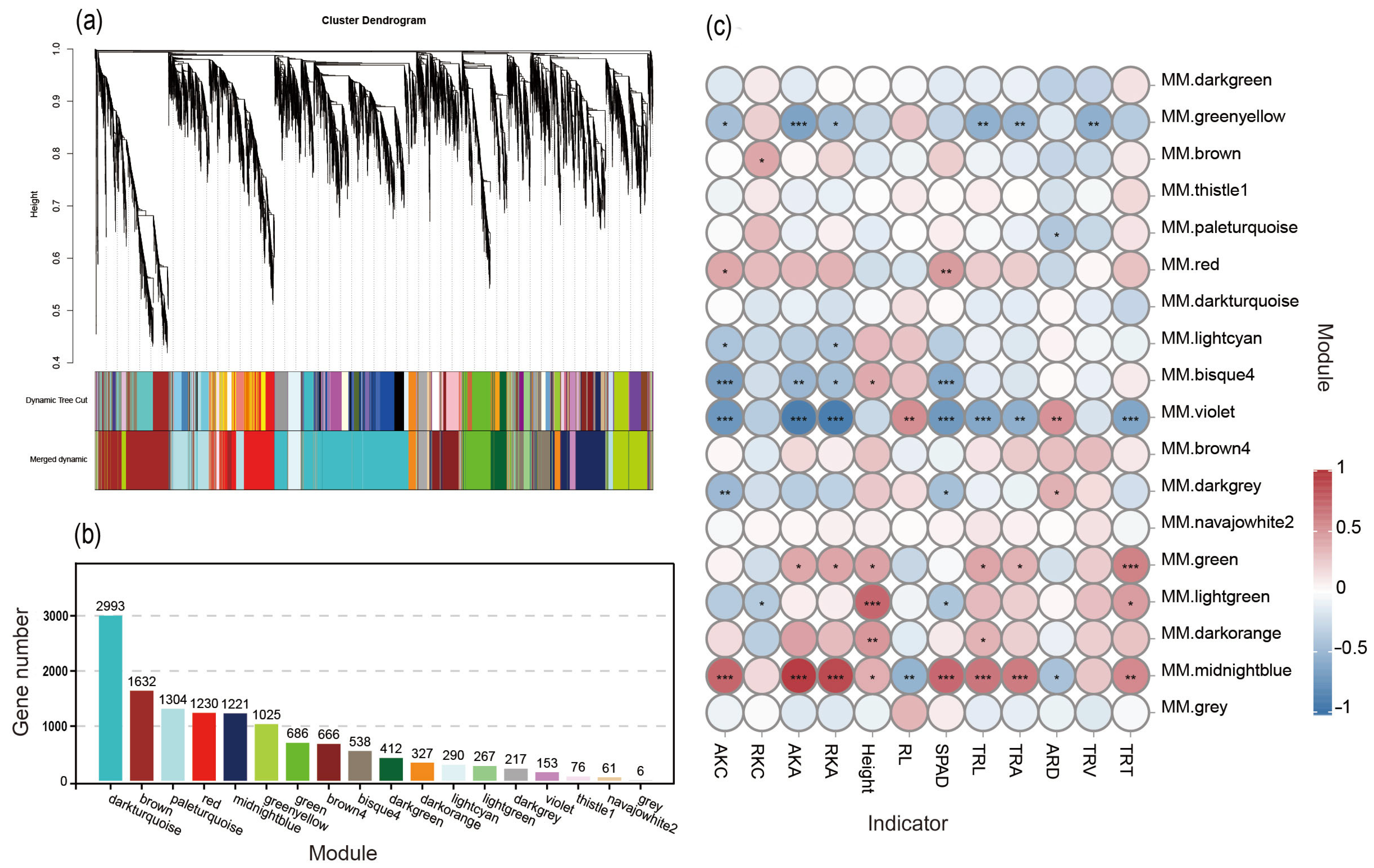
3.4. RNA Sequence Analysis Under K+-Deprivation
3.5. Gene Ontology (GO) Term Enrichment Analysis
3.6. WGCNA and Co-Expression Module GO Term Enrichment Analysis
3.7. DEGs Involved in K+-Channel and K+-Transporter
3.8. DEGs Involved in CBL-CIPK Signaling System
3.9. DEGs Involved in Phytohormone Biosynthesis and Signal Transduction
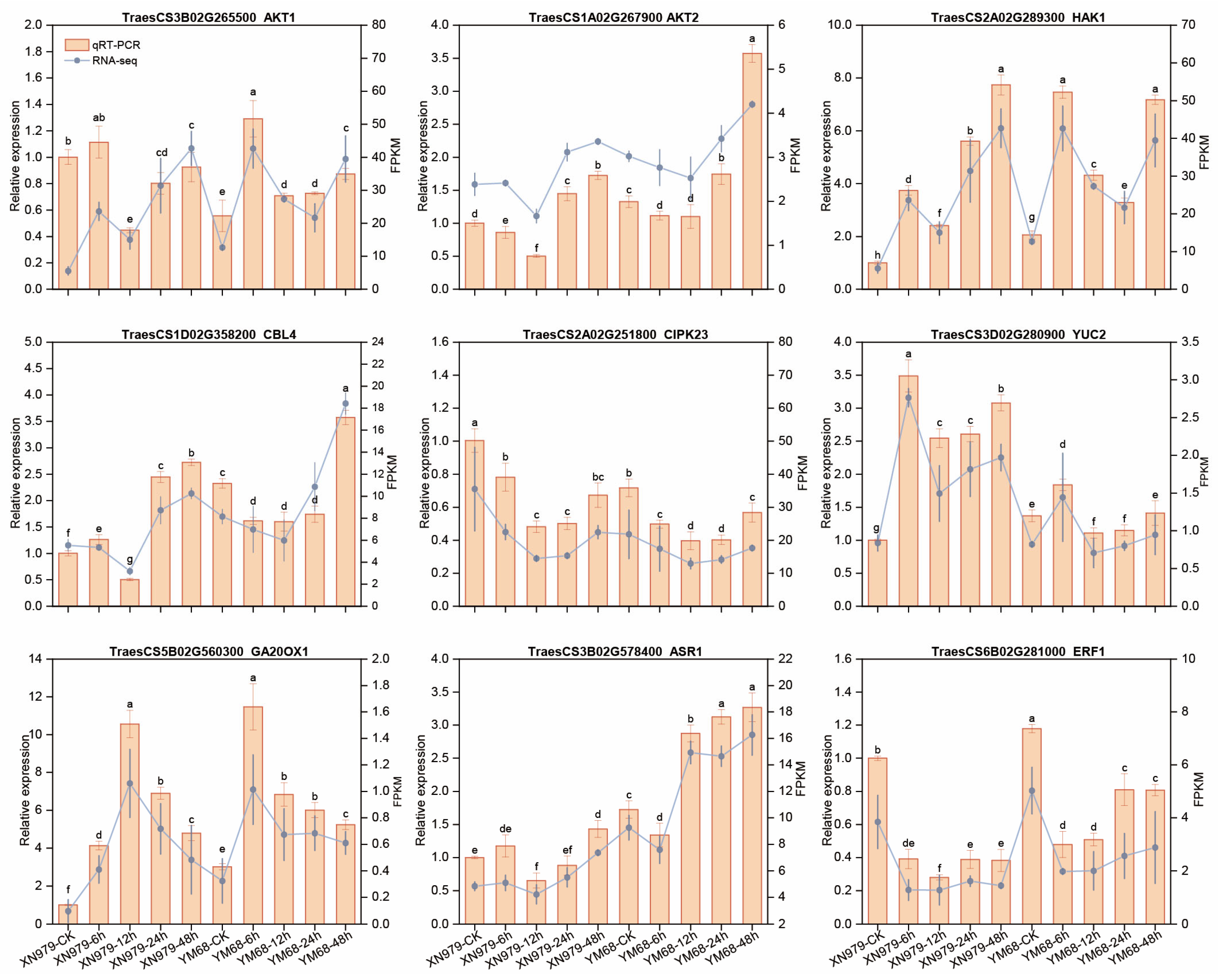
3.10. Validation of DEGs Under K+-Deprivation in XN979 and YM68
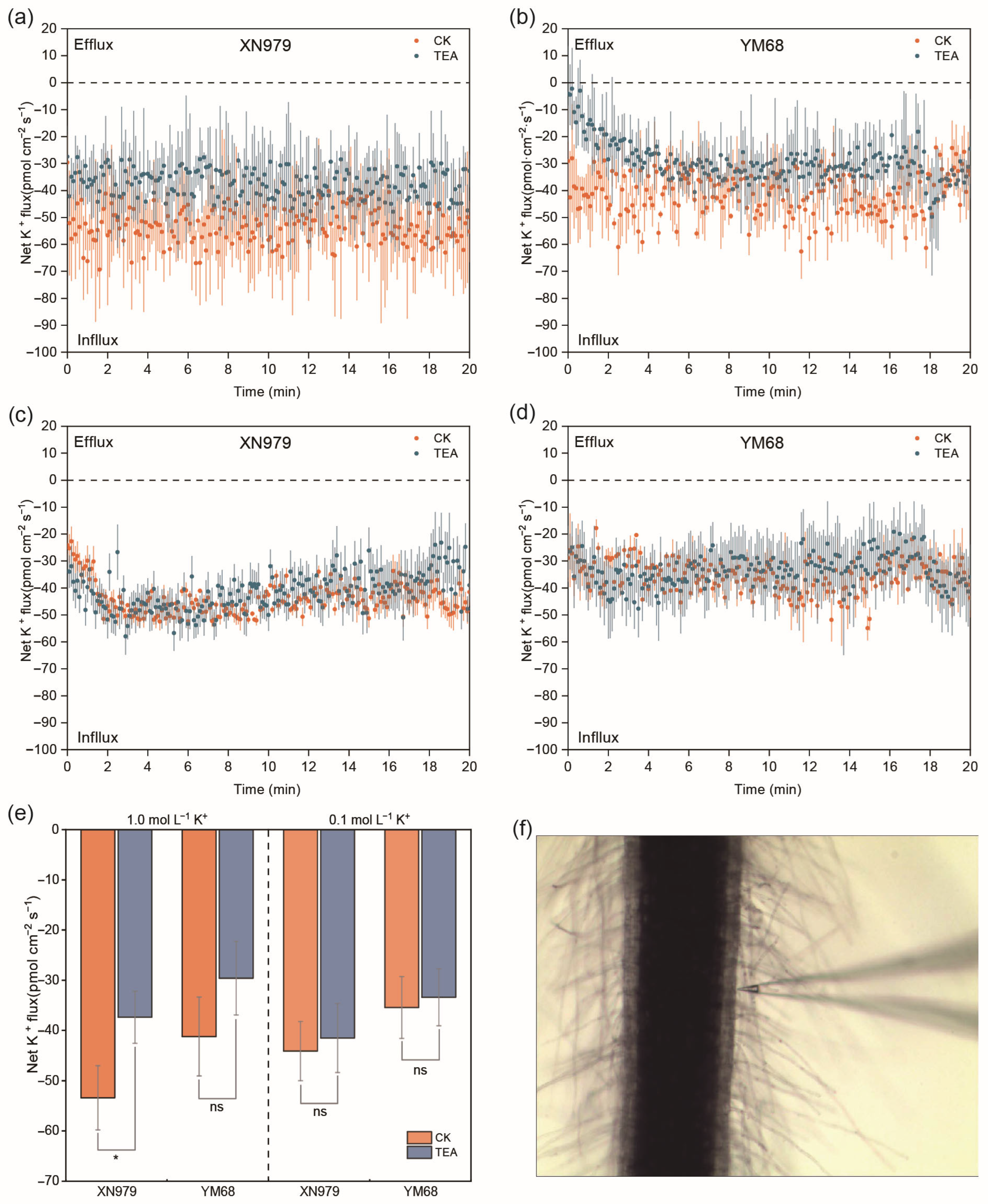
3.11. Net K+ Flux from Root Mature Zones
4. Discussion
4.1. Differential K+ Uptake Characteristics of Two Wheat Varieties
4.2. Response of Wheat Root K+ Transport to K+-Deprivation
4.3. Response of CBL-CIPK Signaling System and Phytohormones to K+-Deprivation in Wheat Root
4.4. Regulation of Wheat Root in Response to K+-Deprivation
5. Conclusions
- (1)
- The capacity for K+ uptake and utilization under low-K conditions differed significantly between the two wheat varieties. XN979 showed superior K+ uptake capacity and adaptability to external K+ concentration changes compared with YM68. The K+ uptake kinetics test, conducted by controlling the K+ concentration in culture solution, can effectively screen wheat varieties with efficient K+ uptake. Combining the selection of efficient K+ uptake wheat varieties with an application rate of 60 kg hm−2 of K fertilizer can significantly enhance K utilization efficiency in wheat.
- (2)
- Under various durations of K+-deprivation, two types of varieties with different K+ uptake capacities exhibited reduced AKT1 K+-channel-mediated K+ transport in roots, with the HAK family of K+-transporters becoming the primary mechanism for K+ uptake. The wheat variety with a higher K+ uptake capacity exhibited a higher abundance of significantly upregulated genes encoding root K+-transporters, potentially explaining its enhanced superior capacity to uptake K+ under K+-deprivation. Additionally, the AKT2 K+-channel in phloem cells and SKOR K+-channel in xylem cells were critical in maintaining K+ homeostasis in root cells during K+ deficiency. The CBL-CIPK signaling system and phytohormone signaling pathways played significant roles in modulating wheat root responses to K+-deprivation. The CBL-CIPK system influenced the K+ transport activities of both AKT1 and AKT2 and impacted GA synthesis. K+ deficiency also affected the synthesis and signal transduction of IAA, GA, and ABA. Notably, an upsurge in GA synthesis curbed the synthesis and accumulation of DELLA proteins in roots, stimulating root growth and facilitating branching. These findings highlight that wheat roots employ a complex array of regulatory and responsive mechanisms in the reaction to K+-deprivation, warranting further extensive research.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wieser, H.; Koehler, P.; Scherf, K.A. The Two Faces of Wheat. Front. Nutr. 2020, 7, 517313. [Google Scholar] [CrossRef]
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, 261. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Run, Y.; Cheng, X.; Dou, W.; Dong, Y.; Zhang, Y.; Li, B.; Liu, T.; Xu, H. Wheat potassium transporter TaHAK13 mediates K+ absorption and maintains potassium homeostasis under low potassium stress. Front. Plant Sci. 2022, 13, 1103235. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, Y.; Li, N.; Liu, Y.; Zhao, D.; Wei, B.; Wen, X. Effects of the Foliar Application of Potassium Fertilizer on the Grain Protein and Dough Quality of Wheat. Agronomy 2021, 11, 1749. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, W.; Ru, S.H.; Chen, X.P.; Xiao, K.; Zhang, X.; Assaraf, M.; Imas, P.; Magen, H.; Zhang, F. Effects of potassium fertilization on winter wheat under different production practices in the North China Plain. Field Crop. Res. 2013, 140, 69–76. [Google Scholar] [CrossRef]
- Zhan, A.; Zou, C.; Ye, Y.; Liu, Z.; Cui, Z.; Chen, X. Estimating on-farm wheat yield response to potassium and potassium uptake requirement in China. Field Crops Res. 2016, 191, 13–19. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Lebaudy, A.; Véry, A.A.; Sentenac, H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium Transport and Signaling in Higher Plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Chérel, I.; Lefoulon, C.; Boeglin, M.; Sentenac, H. Molecular mechanisms involved in plant adaptation to low K+ availability. J. Exp. Bot. 2014, 65, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Hiya, H.J.; Nakashima, Y.; Takeuchi, A.; Nakamura, T.; Nakamura, Y.; Murata, Y.; Munemasa, S. Outward-rectifying potassium channels GORK and SKOR function in regulation of root growth under salt stress in Arabidopsis thaliana. J. Plant Physiol. 2024, 302, 154322. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, P.J.M.; Gao, J.; Mol, S.; Zwart, N.; Zhang, H.; Li, K.W.; de Boer, A.H. The Arabidopsis GORK K+-channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol. Biochem. 2018, 125, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, W.H.; Wang, Y. Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in Arabidopsis response to low K+ stress. J. Integr. Plant Biol. 2017, 59, 895–909. [Google Scholar] [CrossRef]
- Feng, X.; Liu, W.; Cao, F.; Wang, Y.; Zhang, G.P.; Chen, Z.; Wu, F.B. Overexpression of HvAKT1 improves barley drought tolerance by regulating root ion homeostasis and ROS and NO signaling. J. Exp. Bot. 2020, 71, 6587–6600. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, S.Y.; Li, J.L.; Wang, S.F.; Wang, J.; Hao, D.L.; Su, Y.H. The rectification control and physiological relevance of potassium channel OsAKT2. Plant Physiol. 2021, 187, 2296–2310. [Google Scholar] [CrossRef]
- Cai, K.; Zeng, F.; Wang, J.; Zhang, G.P. Identification and characterization of HAK/KUP/KT potassium transporter gene family in barley and their expression under abiotic stress. BMC Genom. 2021, 22, 317. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, X.; Mao, W.; Zhang, X.; Chen, S.; Zhan, K.; Bi, H.; Xu, H. Genome-wide identification and analysis of HAK/KUP/KT potassium transporters gene family in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2018, 19, 3969. [Google Scholar] [CrossRef]
- Li, L.; Kim, B.G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Qi, G.; Xu, Z.J.; Wu, W.H.; Wang, Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef]
- Ragel, P.; Ródenas, R.; García-Martín, E.; Andrés, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martínez, V.; Pardo, J.M.; Quintero, F.J.; et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ Uptake in Arabidopsis roots. Plant Physiol. 2015, 169, 2863–2873. [Google Scholar] [PubMed]
- Lara, A.; Ródenas, R.; Andrés, Z.; Martínez, V.; Quintero, F.J.; Nieves-Cordones, M.; Botella, M.A.; Rubio, F. Arabidopsis K+ transporter HAK5-mediated high-affinity root K+ uptake is regulated by protein kinases CIPK1 and CIPK9. J. Exp. Bot. 2020, 71, 5053–5060. [Google Scholar] [CrossRef]
- Kim, M.J.; Ciani, S.; Schachtman, D.P. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol. Plant 2010, 3, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell Online 2009, 21, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; González-Guzmán, M.; Rodriguez, L.; Peirats-Llobet, M.; Pizzio, G.A.; Fernández, M.A.; De Winne, N.; De Jaeger, G.; Dietrich, D.; Bennett, M.J.; et al. PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of Abscisic Acid signaling in root. Plant Physiol. 2012, 161, 931–941. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Inahashi, H.; Nishizawa, N.K.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 20770–20775. [Google Scholar] [CrossRef]
- Hetherington, F.M.; Kakkar, M.; Topping, J.F.; Lindsey, K. Gibberellin signaling mediates lateral root inhibition in response to K+-deprivation. Plant Physiol. 2021, 185, 1198–1215. [Google Scholar] [CrossRef]
- Liang, B.; Cao, J.; Wang, R.; Fan, C.; Wang, W.; Hu, X.; He, R.; Tai, F. ZmCIPK32 positively regulates germination of stressed seeds via gibberellin signal. Plant Physiol. Biochem. 2023, 199, 107716. [Google Scholar] [CrossRef]
- Ruan, L.; Zhang, J.; Xin, X.; Zhang, C.; Ma, D.; Chen, L.; Zhao, B. Comparative analysis of potassium deficiency-responsive transcriptomes in low potassium susceptible and tolerant wheat (Triticum aestivum L.). Sci. Rep. 2015, 5, 10090. [Google Scholar] [CrossRef]
- Zhang, B.; Thornburg, T.E.; Liu, J.; Xue, H.; Fontana, J.E.; Wang, G.; Li, Q.; Davis, K.E.; Zhang, Z.; Liu, M.; et al. Potassium deficiency significantly affected plant growth and development as well as microRNA-mediated mechanism in wheat (Triticum aestivum L.). Front. Plant Sci. 2020, 11, 01219. [Google Scholar]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The physiology of channel-mediated K+ acquisition in roots of higher plants. Physiol. Plant. 2014, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; He, X.; Liu, D.; Li, J.J.; Zhao, X.; Li, B.; Tong, Y.; Zhang, A.; Li, Z.S. Major quantitative trait loci for seminal root morphology of wheat seedlings. Mol. Breed. 2012, 30, 139–148. [Google Scholar] [CrossRef]
- Percell, W.M.; Howard, D.D.; Essington, M.E. Relationship between total and extractable cotton leaf potassium in studies involving soil and foliar applied treatments. Commun. Soil Sci. Plant Anal. 1995, 26, 3121–3131. [Google Scholar] [CrossRef]
- Claassen, N.; Barber, S.A. A method for characterizing the relation between nutrient concentration and flux into roots of intact plants. Plant Physiol. 1974, 54, 564–568. [Google Scholar] [CrossRef]
- Renkema, H.; Koopmans, A.; Hale, B.; Berkelaar, E. Thallium and potassium uptake kinetics and competition differ between durum wheat and canola. Environ. Sci. Pollut. Res. 2015, 22, 2166–2174. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-Induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.H.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.; Zhou, M.; Venkataraman, G.; et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Zang, F.; Zhang, M.; Zhou, Q.; Wang, X.; Zhong, Y.; Huang, M.; Dai, T.; Jiang, D.; Cai, J. Effect of sulfur and potassium foliar applications on wheat grain protein quality. Field Crops Res. 2024, 319, 109639. [Google Scholar] [CrossRef]
- Manning, D.A.C. Mineral sources of potassium for plant nutrition. A review. Agron. Sustain. Dev. 2010, 30, 281–294. [Google Scholar] [CrossRef]
- Sedri, M.H.; Roohi, E.; Niazian, M.; Niedbała, G. Interactive Effects of Nitrogen and Potassium Fertilizers on Quantitative-Qualitative Traits and Drought Tolerance Indices of Rainfed Wheat Cultivar. Agronomy 2022, 12, 30. [Google Scholar] [CrossRef]
- He, B.; Xue, C.; Sun, Z.; Ji, Q.; Wei, J.; Ma, W. Effect of different long-term potassium dosages on crop yield and potassium use efficiency in the maize-wheat rotation system. Agronomy 2022, 12, 2565. [Google Scholar] [CrossRef]
- Mahiwal, S.; Pandey, G.K. Potassium: A vital nutrient mediating stress tolerance in plants. J. Plant Biochem. Biotechnol. 2022, 31, 705–719. [Google Scholar] [CrossRef]
- Chachar, K.M.; Chachar, Q.; Chachar, N.A.; Chachar, S.; Chachar, M.H. Genotypic variation for potassium uptake and utilization efficiency in Wheat (Triticum aestivum L.). Int. J. Agric. Technol. 2015, 11, 839–853. [Google Scholar]
- Xu, K.; Zhao, Y.; Yu, Y.; Sun, R.; Wang, W.; Zhang, S.; Yang, X. Proteomic Analysis of Roots Response to Potassium Deficiency and the Effect of TaHAK1-4A on K+ Uptake in Wheat. Int. J. Mol. Sci. 2022, 23, 13504. [Google Scholar] [CrossRef]
- Niu, F.; Cui, X.; Yang, B.; Wang, R.; Zhao, P.; Zhao, X.; Zhang, H.; Fan, X.; Li, Y.; Deyholos, M.K.; et al. WRKY6 transcription factor modulates root potassium acquisition through promoting expression of in Arabidopsis. Plant J. 2024, 118, 1652–1667. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, M.; Jia, Y.; Yang, F.; Zhang, Y.; Xu, X.; Li, X.; Yang, F.; Lei, J.; Wang, Y.; et al. Structural basis for the activity regulation of a potassium channel AKT1 from Arabidopsis. Nat. Commun. 2022, 13, 5682. [Google Scholar] [CrossRef]
- Sandmann, M.; Kamil, S.; Pawel, G.; Erwan, M.; Marcio, R.; Gomez-Porras, J.L.; Wendy, G.; Guedes, C.L.G.; Ramírez-Aguilar, S.J.; Ann, C.T.; et al. The K+ battery-regulating Arabidopsis K+ channel AKT2 is under the control of multiple post-translational steps. Plant Signal. Behav. 2011, 6, 558–562. [Google Scholar] [CrossRef]
- Michard, E.; Dreyer, I.; Lacombe, B.; Sentenac, H.; Thibaud, J.B. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. Cell Mol. Biol. 2005, 44, 783–797. [Google Scholar] [CrossRef]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K.; Corratgé-Faillie, C.; Offenborn, J.N.; Lacombe, B.; Dreyer, I.; Thibaud, J.B.; et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011, 21, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, Q.; Zhao, P.; Eickelkamp, A.; Ma, C.; He, G.; Li, F.; Wallrad, L.; Becker, T.; Li, Z.; et al. The potassium channel GhAKT2bD is regulated by CBL–CIPK calcium signalling complexes and facilitates K+ allocation in cotton. FEBS Lett. 2022, 596, 1904–1920. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, L.; Luan, S. Intracellular K+ sensing of SKOR, a Shaker-type K+ channel from Arabidopsis. Plant J. Cell Mol. Biol. 2006, 46, 260–268. [Google Scholar] [CrossRef]
- Li, W.; Xu, G.; Alli, A.; Yu, L. Plant HAK/KUP/KT K+ transporters: Function and regulation. Semin. Cell Dev. Biol. 2018, 74, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ankit, A.; Kamali, S.; Singh, A. Genomic & structural diversity and functional role of potassium K+ transport proteins in plants. Int. J. Biol. Macromol. 2022, 208, 844–857. [Google Scholar]
- Chen, J.S.; Wang, S.T.; Mei, Q.; Sun, T.; Hu, J.T.; Xiao, G.S.; Chen, H.; Xuan, Y.H. The role of CBL–CIPK signaling in plant responses to biotic and abiotic stresses. Plant Mol. Biol. 2024, 114, 53. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.-M.; Guerrero-Galán, C.; Ortega-Villaizán, A.G.; Ortiz-García, P.; Scholz, S.S.; Ramos, P.; Sakakibara, H.; Kiba, T.; Ludwig-Müller, J.; Krapp, A.; et al. The calcium sensor CBL7 is required for Serendipita indica-induced growth stimulation in Arabidopsis thaliana, controlling defense against the endophyte and K+ homoeostasis in the symbiosis. Plant Cell Environ. 2022, 45, 3367–3382. [Google Scholar] [CrossRef]
- Chen, X.; Luo, M.; Mo, C.; Li, W.; Ji, Y.; Xie, Q.; Jiang, X. MeCIPK10 regulates the transition of the K+ transport activity of MeAKT2 between low- and high-affinity molds in cassava. J. Plant Physiol. 2022, 280, 153861. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Wu, G.; Wang, H.; Chen, Y.; Wei, J. HbCIPK2, a novel CBL-interacting protein kinase from halophyte Hordeum brevisubulatum, confers salt and osmotic stress tolerance. Plant Cell Environ. 2012, 35, 1582–1600. [Google Scholar] [CrossRef]
- Du, X.; Yu, R.; Shi, C.; Wang, Y.; Meng, R.; Shi, W.; Jin, M.; Wei, X.; Sun, T. Comparative transcriptomics reveals osmotic and ionic stress key genes contributing to the difference in the salinity tolerance of two pak choi cultivars. Environ. Exp. Bot. 2021, 191, 104621. [Google Scholar] [CrossRef]
- Steinhorst, L.; Mähs, A.; Ischebeck, T.; Zhang, C.; Zhang, X.; Arendt, S.; Schültke, S.; Heilmann, I.; Kudla, J. Vacuolar CBL-CIPK12 Ca2+-Sensor-Kinase Complexes Are Required for Polarized Pollen Tube Growth. Curr. Biol. 2015, 25, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lan, W.; Chen, B.; Fang, W.L.; Luan, S. A Calcium Sensor-Regulated Protein Kinase, CALCINEURIN B-LIKE PROTEIN-INTERACTING PROTEIN KINASE19, Is Required for Pollen Tube Growth and Polarity. Plant Physiol. 2015, 167, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A crucial role of GA-regulated flavonol biosynthesis in root growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef]
- Moriconi, J.I.; Buet, A.; Simontacchi, M.; Santa-María, G.E. Near-isogenic wheat lines carrying altered function alleles of the Rht-1 genes exhibit differential responses to potassium deprivation. Plant Sci. 2012, 185–186, 199–207. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, Q.; Jia, Z.; Fang, Y.; Wang, Z.; Wang, J. Identification of TabZIP family members with possible roles in the response to auxin in wheat roots. Phytochemistry 2022, 196, 113103. [Google Scholar] [CrossRef]
- Lewis, D.R.; Negi, S.; Sukumar, P.; Muday, G.K. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 2011, 138, 3485–3495. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Shan, Y.; Sheng, H.; Feng, K. Membrane-Potential Alteration During K+ Uptake of Different Salt-Tolerant Wheat Varieties. Agric. Sci. China 2010, 9, 974–979. [Google Scholar] [CrossRef]
- Thabet, S.G.; Moursi, Y.S.; Sallam, A.; Karam, M.A.; Alqudah, A.M. Genetic associations uncover candidate SNP markers and genes associated with salt tolerance during seedling developmental phase in barley. Environ. Exp. Bot. 2021, 188, 104499. [Google Scholar] [CrossRef]
- Tian, Q.; Shen, L.; Luan, J.; Zhou, Z.; Guo, D.; Shen, Y.; Jing, W.; Zhang, B.; Zhang, Q.; Zhang, W. Rice shaker potassium channel OsAKT2 positively regulates salt tolerance and grain yield by mediating K+ redistribution. Plant Cell Environ. 2021, 44, 2951–2965. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Hu, N.; Yang, X.; Zhou, S.; Song, M.; Zhang, J.; Chen, X.; Du, X.; He, D. Root Response to K+-Deprivation in Wheat (Triticum aestivum L.): Coordinated Roles of HAK Transporters, AKT2 and SKOR K+-Channels, and Phytohormone Regulation. Agriculture 2025, 15, 993. https://doi.org/10.3390/agriculture15090993
Huang Y, Hu N, Yang X, Zhou S, Song M, Zhang J, Chen X, Du X, He D. Root Response to K+-Deprivation in Wheat (Triticum aestivum L.): Coordinated Roles of HAK Transporters, AKT2 and SKOR K+-Channels, and Phytohormone Regulation. Agriculture. 2025; 15(9):993. https://doi.org/10.3390/agriculture15090993
Chicago/Turabian StyleHuang, Yuan, Naiyue Hu, Xiwen Yang, Sumei Zhou, Miao Song, Jiemei Zhang, Xu Chen, Xihe Du, and Dexian He. 2025. "Root Response to K+-Deprivation in Wheat (Triticum aestivum L.): Coordinated Roles of HAK Transporters, AKT2 and SKOR K+-Channels, and Phytohormone Regulation" Agriculture 15, no. 9: 993. https://doi.org/10.3390/agriculture15090993
APA StyleHuang, Y., Hu, N., Yang, X., Zhou, S., Song, M., Zhang, J., Chen, X., Du, X., & He, D. (2025). Root Response to K+-Deprivation in Wheat (Triticum aestivum L.): Coordinated Roles of HAK Transporters, AKT2 and SKOR K+-Channels, and Phytohormone Regulation. Agriculture, 15(9), 993. https://doi.org/10.3390/agriculture15090993









