Abstract
In vineyard mulching research, using biodegradable liquid mulch represents a novel and environmentally conscious approach to mulching. In comparison, grapevine branch return has been identified as the most effective mulching method. The effects of in-row mulching with two materials, biodegradable liquid film (BLF) and grapevine branches (GBM), on soil properties and microbial communities in the vineyard were assessed using a one-way horizontal block test with tillage as a control. The results indicated that the application of mulching resulted in a reduction in soil bulk weight; an increase in soil moisture; an enhancement in soil organic matter; and a notable elevation in soil nutrients content compared to the control treatment. Both mulching techniques increased the abundance and diversity of soil microorganisms, strongly correlated with soil physicochemical properties. The correlation analysis demonstrated that total organic carbon (TOC); total nitrogen (TN); total potassium (TK); nitrate nitrogen (NN); and available phosphorus (AP) had the most significant impact on shaping the microbial community, exhibiting a positive correlation with microbial diversity. Additionally, soil nutrients were identified to exert a more pronounced influence on the composition of the bacterial community.
1. Introduction
The practice of orchard mulching effectively alleviates surface runoff [1,2], prevents soil erosion and nutrient loss [3], improves soil fertility [4], changes the soil structure [5], reduces water evaporation, and maintains soil moisture. Mulching also has the effect of improving the microenvironment within the orchard, increasing the structural and functional diversity of soil microorganisms [6] and promoting microbial metabolism and reproduction [7]. Mulching treatment can improve the water retention capacity of soil, increase soil enzyme activity [8], improve the soil structure, significantly reduce the soil volume weight, increase soil porosity, improve soil permeability (which benefits root growth) [9], optimize the development of shallow and lateral roots, and increase root density and growth [9]. It can also increase the content of soil nutrients in the short term [10] and increase the soil carbon–nitrogen ratio [11]. Mulching treatment results in the advancement of grape phenology, increases the fruit setting rate, and makes fruit mature earlier [12]. It can also increase the chlorophyll content of grape leaves, enhance the rate of photosynthesis and rate of color change of grapes, and promote fruit maturity [13], all of which increase the fruit yield and quality.
Recently, there has been a notable increase in interest in sustainable and environmentally conscious agricultural practices as applied to grapevines, coinciding with the broader context of ecological transition [14,15,16]. Grape branch mulching (GBM), defined as the practice of covering soil surfaces with a specified depth of branches, has proven an efficacious method for returning branches to agricultural fields [17]. It has been observed that GBM can markedly enhance crop yields and precipitation utilization, control weed growth, optimize the soil structure, maintain moisture, moderate temperatures, and alter biodiversity [18,19,20]. A synthesis of prior research indicates that grapevine branch return represents the most efficacious mulching method when compared to other forms of mulching, including mulch, straw mulch, and grass mulch [21,22].
The preliminary research indicates that biodegradable liquid film (BLF) has the potential to serve as an effective alternative material in place of plastic mulch [23]. This approach has already been employed in numerous countries, including Norway, Japan, China, and Spain, as a means of preventing pollution from plastic residues [24,25,26,27]. The BLF is a new crop management technique for moisture retention, which can be broken down naturally by sunlight and soil micro-organisms, without damage to the ecosystem. The BLFs act as binders, stabilizing soil aggregates, binding soil particles together to form aggregates, reducing surface damage and soil wind erosion, improving the soil structure, regulating soil’s physical and chemical properties, promoting crop growth and development, promoting microbial growth and proliferation, and promoting the conversion and accumulation of soil organic matter to improve soil fertility [28,29]. The BLF material used in this trial is an environmentally friendly, highly adhesive soil structure regulator. Once sprayed onto the soil surface, it rapidly forms a multimolecular network membrane that seals the pores on the soil surface and minimizes soil water evaporation without affecting rainfall infiltration.
In this study, GBM and BLF were used as mulches, taking tillage as a control, in order, to investigate the changes in soil microbial communities and the influencing factors under the different treatments, through the determination of various physico-chemical soil properties and 16S rRNA and ITS rRNA gene sequencing.
2. Materials and Methods
2.1. Site Information and Sampling Collection
The experiments were carried out in a flat orchard in Shengtang Winery in the middle of the Guanzhong Plain, Yangling, Shaanxi, China (34°27′ N; 108°8′ E) in the years 2021 and 2022. The site exhibits the typical semi-humid and semi-arid climate of the warm temperate zone of East Asia. Its altitude is 514 m, its mean annual temperature is 15.12 °C, its mean annual rainfall is 664 mm, its frost-free period is 211 d, its annual average sunshine duration is 2163.8 h, and its annual total solar radiation is 480.79 kJ/cm2 (data from China Statistical Yearbook). The soils in the orchard exhibited a relatively uniform composition, predominantly loam.
The material used in this experiment was Vitis vinifera L. cultivar ‘MeiLi’ selected and bred by the College of Enology, Northwest Agriculture and Forestry University. The vines were planted in 2008 in Shengtang Vineyard, with a spacing of 1.0 m between vines and 2.5 m between rows and a row length of 90 m. A single hedge frame system was applied, and single stems and double arms with long and short branches were mixed and trimmed.
The in-row mulching treatment was implemented prior to germination in the month of March of each year. The treatments were comprised of three distinct methodologies: biodegradable liquid mulch film (BLF), grapevine branch mulching (GBM), and tillage, which served as the control. A single-factor, level-block design with three replicates was employed. The experimental area was divided into three distinct blocks, each serving as a replicate. Each experimental unit, or block, consisted of three treatments, each occupying two rows with 90 vines per row. In order to circumvent the effects of wind drift and edge effects, the treatments were arranged in nonadjacent rows. Within the control rows, the soil was cultivated to keep it weed-free until harvest. The BLF material with a humic acid content of 121.6 g/kg, purchased from Shaanxi Kerui Company, was applied to the soil in the BLF-treated rows. Soil sprayed with BLF is black and becomes lighter as the BLF degrades. The soil was covered with segments of broken grapevines in the GBM-treated rows.
Soil moisture content and bulk density were determined while harvesting the fruit. In addition, surface soil samples (0–20 cm) from five randomly selected locations in each treatment were also collected and mixed. Samples were divided into two parts; one part was moistened and stored in a −80 °C freezer for the determination of soil microorganisms, and the other part was air-dried and passed through a steel sieve for the determination of soil nutrients.
2.2. The Determination of Soil Characteristics
Soil temperature, soil moisture content, bulk density, total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), total potassium (TK), ammonium nitrogen (AN), nitrate nitrogen (NN), available phosphorus (AP) and available potassium (AK) content were analyzed. Measurements of TN, TP, and TK were made following the methods of [30], using the H2SO4-H2O2 digestion method with modifications. Measurements of available nutrients were based on the methods of [30]. Among them, AN and NN were determined using the KCl extraction method, AP was measured by the NaHCO3 dissolution method, and AK was analyzed via the NH4OAc extraction method. Soil temperatures were monitored at a depth of 20 cm using temperature and humidity loggers (RC-4HC (Jiangsu Jingchuang Electric Co., Jiangsu, China)). A total organic carbon analyzer (TOC-L03030135) was used to determine the TOC. TN, TP, TK, AN, NN, AP, and AK contents were determined by an AA3 continuous flow analyzer (Flowsys03030402).
2.3. Soil DNA Extraction and Illumina Sequencing
Microbial DNA was extracted with HiPure Soil DNA Kits (Magen, Guangzhou, China) in accordance with the manufacturer’s protocols. Amplification and sequencing of the V3—V4 regions of the bacterial 16S rRNA gene used primers 341F (CCTACGGGGNGGCWGCAG) and 806R (GGACTACHVGGGTATCTAAT) [31]. And amplification of the ITS1 region of the fungal DNA gene used primer pairs ITS1F (CTTGGTCATTTAGAGGAAGTAA) and ITS2 (GCTGCGTTCTTCATCGATGC) [32]. Purification was performed using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions, and quantification was performed using the ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, CA, USA). Purified amplicons were subjected to double-end sequencing (PE250) on the Illumina platform according to standard practices.
2.4. Statistical Analysis
The data on soil characteristics were organized in Microsoft Office Excel 2017, analyzed using IBM SPSS Statistics 21, and plotted using GraphPad Prism 6. Prior to conducting a one-way ANOVA, the data were tested for normality and homogeneity of variance. The p-values of the data were all greater than 0.05. This indicated that the data were not statistically significant, followed a normal distribution, and exhibited an homogeneity of variance. The threshold for statistical significance was set at p < 0.05 for the one-way ANOVA and Duncan’s multiple comparison tests. The multiple comparison test was employed solely for the purpose of facilitating comparisons between treatments. The analysis of biological information was conducted utilizing Omicsmart (http://www.omicsmart.com accessed on 17 February 2025).
3. Results
3.1. Differences in Soil Characteristics Within Two Treatments and Control
In this experiment, the characteristics of the soil were observed under two mulch treatments (BLF and GBM) and a control in the years 2021 and 2022. There were significant differences between the treatments in the observed soil parameters. The GBM significantly reduced the soil bulk density and increased the soil moisture content. Soil TOC, TN, and TK content increased significantly under the two mulch treatments. However, the effect of the mulch on TP content differed between years. Of these, the TOC and TN content varied consistently across all three sites, with GBM > BLF > control. In addition, the overlay increased AN, NN, AP, and AK contents. Among them, the change pattern of AP content was consistent over the two years, showing GBM > BLF > control in Table 1.

Table 1.
Soil characteristics of two treatments (BLF and GBM) and control at harvest.
3.2. Differences in Soil Microbial Community Characteristics Within Two Treatments and Control
A total of 3,703,772 and 4,379,172 valid 16S rRNA and ITS rRNA sequences were obtained from 36 soil samples from 2021 and 2022. Among them, there were 1,849,980 16S sequences and 2,180,769 ITS sequences in 2021, and clusters with a 97% similarity were assigned to 82,421 bacterial and 14,788 fungal OTUs, respectively. And there were 1,853,729 16S sequences and 2,198,403 ITS sequences in 2022, and clusters with a 97% similarity were assigned to 84,366 bacterial and 17,139 fungal OTUs, respectively.
The number of bacterial core OTUs identified in 2021 in the three groups of soils totaled 2295, with 750, 839, and 443 unique OTUs in the BLF, GBM, and control soils, respectively (Figure 1a). Then, the fungal OTUs Venn diagram showed that there were 357 core OTUs in the three groups, and soils from the BLF, GBM, and control had 118, 153, and 221 unique OTUs, respectively (Figure 1b). The number of core OTUs identified in 2022 in the three groups of soils totaled 2427, with 550, 582, and 471 unique OTUs in the BLF, GBM, and control soils, respectively (Figure 1c). Then, the fungal OTUs Venn diagram showed that there were 515 core OTUs in the three groups, and soils from the BLF, GBM, and control had 140, 164, and 110 unique OTUs, respectively (Figure 1d).
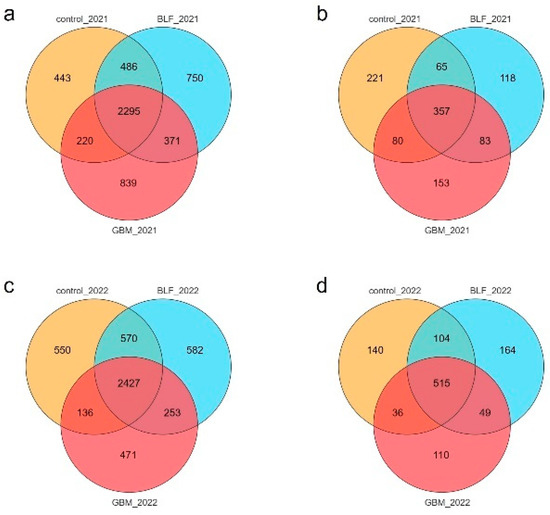
Figure 1.
Venn diagrams demonstrating the shared and unique bacterial and fungal species in different treatments. Notes: The number of bacterial species in 2021 (a), the number of fungal species in 2021 (b), the number of bacterial species in 2022 (c), and the number of fungal species in 2022 (d).
The NMDS plots based on confidence intervals (p < 0.05) revealed the clear separation of the three groups of soil samples, indicating significant differences in the bacteria and fungi floras of the three soil groups (Figure 2). The UPGMA-based clustering dendrograms revealed significant differences in bacterial and fungal communities between the three groups of soils (Figure 3), indicating that ground cover had a significant impact on soil microbial communities. As the number of sequences per sample increased, the sparse profile of the operational taxonomic units (OTUs) reached a plateau. This emphasizes that 40,000 and 60,000 sequences per sample were sufficient to characterize the soil bacterial and fungal communities in the studied soils (refer to Figure 4).
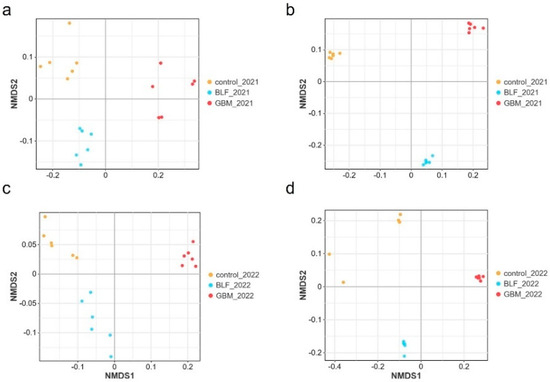
Figure 2.
NMDS analysis of soil microorganisms in different treatments. Notes: The bacterial species in 2021 (a), the fungal species in 2021 (b), the bacterial species in 2022 (c), and the fungal species in 2022 (d).
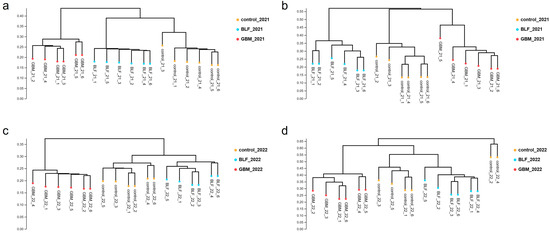
Figure 3.
UPGMA analysis of soil samples in different treatments. Notes: The bacterial species in 2021 (a), the fungal species in 2021 (b), the bacterial species in 2022 (c), and the fungal species in 2022 (d).
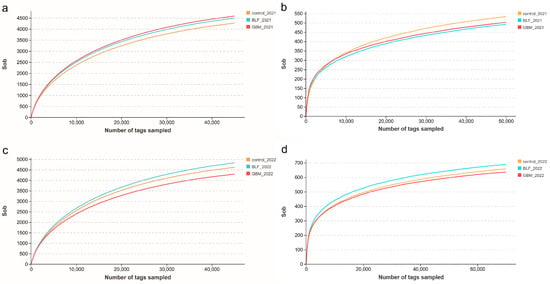
Figure 4.
Sparse curves of soil microbial OTUs. Notes: The bacterial species in 2021 (a), the fungal species in 2021 (b), the bacterial species in 2022 (c), and the fungal species in 2022 (d).
The presence of ground cover has been demonstrated to significantly enhance the diversity of bacterial communities. The control soil bacterial Simpson index, Shannon index, Chao 1 index, and ACE index exhibited the lowest values at 10.08, 0.9966, 4780.65, and 5100.1, respectively, in 2021. The highest bacterial Shannon and Simpson indices were observed in GBM, with values of 10.39 and 0.9978, respectively. The BLF exhibited the second-highest indices. In a similar manner, the Shannon index for bacteria in GBM in 2022 exhibited the highest value at 10.21. The BLF exhibited the highest Chao 1 index and ACE index, with values of 5471.6 and 5727.44, respectively. It is noteworthy that both BLF and GBM exhibited a Simpson’s index of 0.9974, which was considerably higher than that observed in the control group. The results demonstrated that GBM exhibited superior performance in terms of soil fungal community diversity. Nevertheless, the differential performance of fungi was not as favorable as that of bacteria in Table 2.

Table 2.
Soil microbial α-diversity index.
3.3. Differences in Microbial Community Composition Across Mulch Treatments
At the level of the phylum, operational taxonomic units (OTUs) were classified into different classes in order to identify the ten most prevalent bacterial gates in terms of relative abundance. The dominant bacterial phyla were Proteobacteria, Acidobacteriota, Bacteroidota, Planctomycetota, Actinobacteriota, Verrucomicrobiota, Chloroflexi, Myxococcota, and Gemmatimonadota in the two years. The most prevalent of these were the strains Proteobacteria and Acidobacteriota, which constituted 21.2% and 18.28% of all bacterial isolates in 2021 and 18.05% and 19.31% in 2022, respectively. The application of both mulch treatments resulted in a notable increase in the abundance of Planctomycetota and Myxococcota when compared to the control treatment. It is noteworthy that the relative abundance of Verrucomicrobiota exhibited an increase in 2021, followed by a decline in 2022. Conversely, Proteobacteria exhibited a decline in 2021, followed by an increase in 2022. The most prevalent phyla of fungi were Ascomycota, Mortierellomycota, Basidiomycota, Glomeromycota, Mucoromycota, Rozellomycota, Chytridiomycota, Zoopagomycota, and Kickxellomycota. The most dominant of these was Ascomycota, which accounted for 43.01% and 58.68% of all fungi in 2021 and 2022, respectively. Both types of soil mulch treatments increased the abundance of Basidiomycota and Rozellomycota and decreased the abundance of Chytridiomycota in comparison to the control. There was an inconsistent performance of Zoopagomycota and Mortierellomycota over the two years (Figure 5).
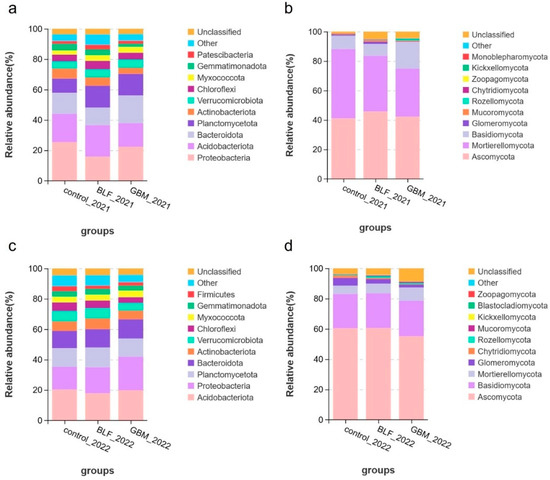
Figure 5.
Abundance at the microbial phylum level. Notes: The relative abundance of bacterial species in 2021 (a), the relative abundance of fungal species in 2021 (b), the relative abundance of bacterial species in 2022 (c), and the relative abundance of fungal species in 2022 (d).
Figure 6 depicts the top ten most prevalent genera in terms of relative abundance. The most prevalent bacterial genera were RB41, Flavobacterium, Sphingomonas, MND1, Pirellula, Nitrospira, Chryseolinea, and Bryobacter. The most dominant of these was RB41, which accounted for 4.54% and 5.8% of all bacteria in 2021 and 2022, respectively. The abundance of MND1 and Chryseolinea colonies exhibited a tendency to increase, while that of Bryobacter exhibited a tendency to decrease, in both ground cover soils. In comparison to the control group, the relative abundance of Pirellula and Nitrospira exhibited an upward trend in 2021, while that of Sphingomonas demonstrated a downward trend. In contrast, the relative abundance of Pirellula and Nitrospira exhibited a downward trend in 2022, while that of Sphingomonas demonstrated an upward trend. The most prevalent genera of fungi were Mortierella, Tetracladium, Acremonium, Solicoccozyma, Fusarium, and Alternaria. The abundance of Tetracladium, Solicoccozyma, and Alternaria exhibited an increasing trend, whereas that of Acremonium exhibited a decreasing trend in both ground cover soils. While the abundance of Mortierella exhibited a decline in 2021, it demonstrated an increase in 2022.
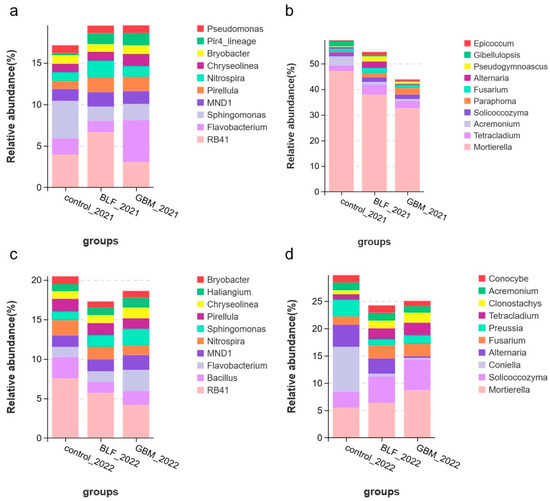
Figure 6.
Abundance at the microbial genus level. Notes: The relative abundance of bacterial species in 2021 (a), the relative abundance of fungal species in 2021 (b), the relative abundance of bacterial species in 2022 (c), and the relative abundance of fungal species in 2022 (d).
3.4. Correlations Between Soil Characteristics and Soil Microbial Communities
The correlation between soil properties and microbial community diversity is illustrated in Figure 7. The Simpson’s index of soil bacteria was found to be positively correlated with soil water content, total organic carbon (TOC), total nitrogen (TN), total potassium (TK), available nitrogen (AN), nitrate nitrogen (NN), available phosphorus (AP), and available potassium (AK) and negatively correlated with soil bulk density and total phosphorus (TP). The diversity in soil fungal communities exhibited a comparable pattern of change. The Shannon index and Simpson index demonstrated a decline with an increase in TP, while an increase was observed with an increase in TOC, TN, TK, NN, and AP.
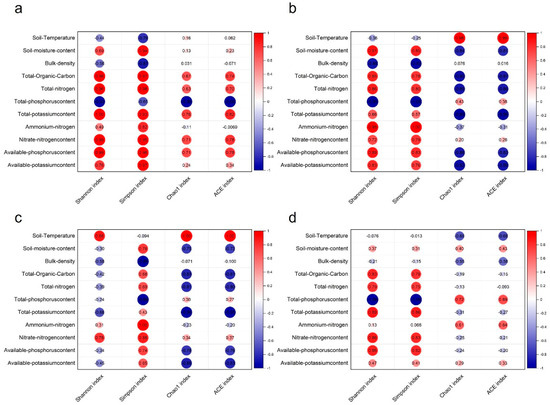
Figure 7.
Correlation analysis of soil physicochemical and diversity indices. Notes: The bacterial species in 2021 (a), the fungal species in 2021 (b), the bacterial species in 2022 (c), and the fungal species in 2022 (d).
The composition of soil microbial communities is the result of a complex interplay between multiple environmental factors and is not determined by a single factor alone. To quantify the relative contribution of different environmental factors to the observed variation in microbial community composition, the Variable Partitioning Analysis (VPA) method was employed. The results demonstrated that these 11 variables collectively explained the observed variation in microbial communities in soil. The combined two-year results demonstrated that soil moisture content, AK, TN, TOC, and AP exerted a more pronounced influence on soil bacteria, whereas TP, AP, and NN exhibited a more pronounced influence on soil fungi. Notably, soil temperature exhibited no discernible effect on the fungal community (Figure 8).
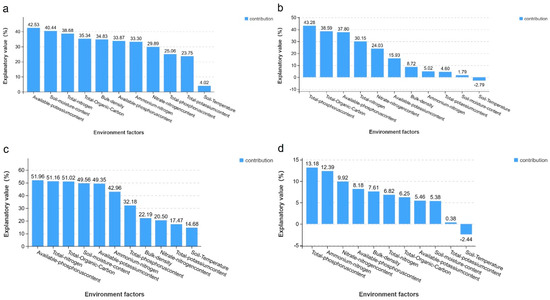
Figure 8.
Quantifying the relative contribution of different soil characteristics to changes in microbial community composition using the VPA method. Notes: The bacterial species in 2021 (a), the fungal species in 2021 (b), the bacterial species in 2022 (c), and the fungal species in 2022 (d).
A correlation analysis was conducted between soil characteristics and microbial communities (Figure 9). Among the bacteria, Gammaproteobacteria, Blastocatellia, Gemmatimonadetes, Polyangia, Acidobacteriae, Nitrospiria, OM190, Acidimicrobiia, bacteriap25, Methylomirabilia, Thermoleophilia, and Bacilli exhibited the greatest sensitivity to environmental factors. Among the bacteria, Blastocatellia exhibited a significantly negative correlation with soil water content, total organic carbon (TOC), total nitrogen (TN), total potassium (TK), available nitrogen (AN), nitrate nitrogen (NN), available phosphorus (AP), and available potassium (AK), while exhibiting a significantly positive correlation with soil capacity. The TOC, TN, AP, and AK exhibited a notable negative impact on Gemmatimonadetes. Conversely, Polyangia demonstrated a significant and positive correlation with soil water content, TOC, TN, TK, AN, NN, AP, and AK. However, it exhibited a notable negative correlation with bulkiness and TP. Furthermore, OM190 demonstrated an increasing trend with increasing TOC, TN, NN, and AP content. Conversely, it exhibited a decreasing trend with an increase in TP content. The water content, TOC, TN, and AK exhibited a significant negative effect on Thermoleophilia. The Bacilli exhibited a significant negative correlation with AN and NN, and a significant positive correlation with soil bulkiness. Among the fungal groups, those belonging to Tremellomycetes, Glomeromycetes, Mucoromycetes, and Microbotryomycetes were particularly susceptible to environmental influences. Among the fungi, Tremellomycetes demonstrated an increase in abundance with an increase in soil water content, total organic carbon (TOC), total nitrogen (TN), available nitrogen (AN), available phosphorus (AP), and available potassium (AK). In contrast, Glomeromycetes exhibited a positive correlation with bulk density and a negative correlation with water content and AK. Mucoromycetes demonstrated a decrease in abundance with an increase in total phosphorus (TP) content. Microbotryomycetes had a significant positive effect on TP and a significant negative effect on TOC, TN, AP, and AK.
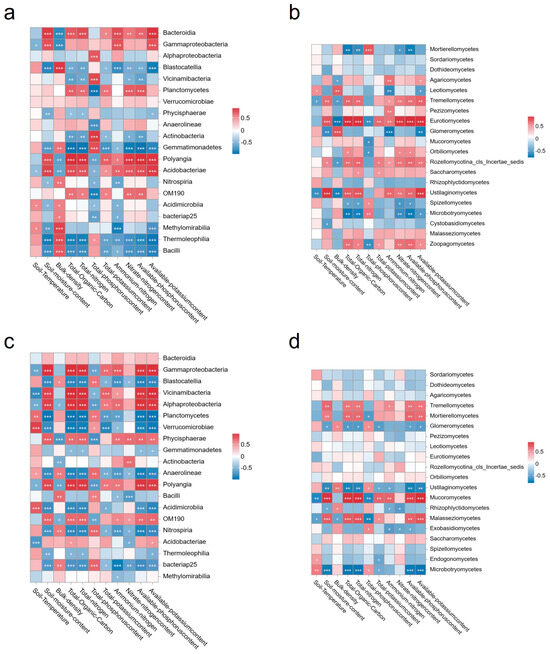
Figure 9.
Correlation between soil physicochemical and microbial communities. Notes: The number of bacterial species in 2021 (a), the number of fungal species in 2021 (b), the number of bacterial species in 2022 (c), and the number of fungal species in 2022 (d). * means the two indicators are significantly different at the 0.05 level (p < 0.05); ** means the two indicators are extremely significantly different at the 0.01 level (p < 0.01); *** means the two indicators are extremely significantly different at the 0.01 level (p < 0.001).
4. Discussion
4.1. Mulching Practices Contribute to Soil Quality
The practice of mulching can alter the physicochemical environment of the soil, enhance soil properties, and facilitate plant growth. It is a crucial strategy for soil and water conservation in agricultural and horticultural settings [33]. Soil bulk density serves as an indicator of soil health and compaction. It has a significant impact on soil porosity, the efficacy of plant nutrients, and soil microbial activity. Consequently, it serves as a crucial indicator of soil degradation [34]. The present study found that GBM significantly reduced the soil bulk density, which is in agreement with the findings of [35]. Soil moisture represents the primary source of water for plants, existing in soil pores in both liquid and vapor form. The process of mulching can influence the rate of evaporation and transpiration, which in turn maintains soil moisture [36]. These findings are in alignment with the results of the present study. Soil organic matter is widely acknowledged as an effective indicator of soil health and fertility. Additionally, nitrogen (N), phosphorus (P), and potassium (K) are essential mineral nutrients required for optimal grape growth. The application of mulching resulted in an increase in soil organic matter and nutrient element content. In the BLF treatment, humic acid substances were directly added to the soil, while in the GBM treatment, humus was formed through the degradation of grapevine branches. Both approaches regulated the dissolution and transport of nutrients [37], thereby improving the efficiency of nutrient utilization [38]. Furthermore, using GBM was observed to be more effective than BLF. Both GBM and BLF mulches contain organic matter. The primary source of organic matter input during GBM cover is the decomposition of plant residue. The decomposition of grapevine branches may facilitate the accumulation of soil nitrogen (N) by reducing the leaching of nitrate (NO3-N) from the soil [39,40]. Consequently, the implementation of GBM coverage in this study yielded a favorable outcome with respect to the most TN, NN, and AN content. BLF contains humic acid-like substances that also act as a source of organic matter for the soil. Humic acid is one of the main components of soil organic matter (SOM). Directly incorporating humic acid into the soil as an SOM supplement is an important aspect of sustainable agriculture [41]. Nevertheless, BLF materials are subject to degradation over time and do not offer the same level of coverage as GBM. In conclusion, the application of organic mulching materials has been demonstrated to enhance the soil ecology and increase soil nutrients in a manner that is consistent with the findings of previous studies [42,43,44].
4.2. Effects of Mulching Treatments on the Diversity and Composition of Bacterial and Fungal Communities
Alpha diversity is a measure of the diversity of microorganisms within a sample and plays a pivotal role in maintaining soil quality and the functioning of ecosystems [45,46]. Herein, the BLF and GBM treatments resulted in an increase in bacterial and fungal community alpha diversity, which may be associated with the observed rise in soil organic matter. The application of BLF enhances the diversity of microbial communities in rhizosphere soil [47]. Organic matter constitutes an essential resource for microbial survival, and an elevation in soil organic carbon is known to enhance the diversity and composition of both bacterial and fungal soil communities [48,49]. Furthermore, the stable and moist environment of the soil provides an optimal environment for the growth of microorganisms [50,51]. Additionally, the elevation in soil moisture levels within the BLF and GBM applications influenced microbial diversity, including bacteria and fungi. The practice of tillage has been observed to impede microbial growth in soil, resulting from the loss of soil organic carbon and the direct disturbance to the microbial population.
The relative abundance of dominant gates varies contingent on the specific material utilized for the cover. The most prevalent bacterial phyla were Proteobacteria and Acidobacteriota. The relative abundance of Planctomycetota and Myxococcota was higher in BLF and GBM than in the control. Planctomycetota are oligotrophic bacteria that are capable of processing carbon sources that are otherwise difficult to utilize. They are also the dominant nitrogen-fixing bacteria in soil [52,53,54]. The microorganism is capable of secreting β-glucosidase and xylanase, which are involved in the breakdown of plant residues [55]. The majority of Myxococcota exhibit a comprehensive array of genes pertaining to glycolysis and the tricarboxylic acid (TCA) cycle. Additionally, these organisms are renowned for their prolific synthesis of bioactive secondary metabolites [56,57,58]. Myxococcota are known to exhibit a predatory or carrion lifestyle, characterized by the ability to swarm [59]. The humic acid analogues present in the BLF material, in association with the rotting grapevine branches subjected to GBM treatment, both constituted an optimal environment for the survival of the Myxococcota. The most abundant fungus was identified as Ascomycota. The relative abundance of Basidiomycota and Rozellomycota was observed to be higher than in the control in BLF and GBM, while the relative abundance of Chytridiomycota was found to be lower than in the control. The phylum Basidiomycota is a significant component of the fungal kingdom, representing 28–40% of fungal diversity and exhibiting a proclivity for oligotrophic environments [60,61,62,63,64]. Basidiomycota are likely to be a significant contributor to the decomposition of wood and apoplastic material, including the degradation of various components of wood that play a pivotal role in carbon recovery [65,66]. It was thus demonstrated that the mulching measures employed in this experiment resulted in an increase in the abundance of Basidiomycota. Chytridiomycota represents the sister group to Monoblepharidomycota and Neocallimastigomycota [52]. As saprophytes, members of the Chytridiomycota are capable of decomposing chitin and keratin while also facilitating the accumulation of soil nitrogen [67]. It is hypothesized that Rozellomycota may have evolved from an ancestor with an almost complete set of classical fungal-specific traits as divergent fungi. It is most notable that the members are characterized by a conspicuous absence of chitin cell walls during the process of food uptake [68]. Rozellomycota are capable of acquiring nutrients through the process of phagocytosis [69]. The presence of humic acids in BLF and nutrients resulting from the degradation of grapevine branches creates an environment conducive to the growth of Rozellomycota. Additionally, Rozellomycota is an intracellular parasitic bacterium that proliferates as naked protoplasts within the host [70]. They are capable of attaching to the surface of host cells and forming tubular structures with the objective of penetrating and entering host cells. In addition, a subset of these species are even able to ingest host organelles through a process called phagocytosis [71]. This may be the reason why the abundance of Rozellomycota and Chytridiomycota developed in opposite directions under cover conditions. Furthermore, the soil microbial response to ground cover exhibited interannual variability. The results of this experiment demonstrated that certain microorganisms exhibited divergent behaviors over a two-year period following the covering. These included Verrucomicrobiota and Proteobacteria in the bacterial kingdom and Zoopagomycota and Mortierellomycota in the fungal kingdom.
At the level of the genus, it was observed that there was a higher relative abundance of MND1 and Chryseolinea in both the BLF and GBM treatments in comparison to the control treatment. This finding may have beneficial implications for soil health and plant growth. MND1, a significant microbial taxon that plays a pivotal role in nitrification, is a member of the Nitrosomonadaceae, as well as a key genus in the process of nutrient cycling [72]. The elevated NN content observed in this experiment may be attributable to the ammonia oxidation (AMO) effect of MND1. It can be reasonably deduced that Chryseolinea plays a significant role in the conversion of precursors to humus. There is a possibility that it is responsible for the biological processes of biodegradation and humification of lignocellulose [73,74]. Increased Chryseolinea provides nutritional support for plant growth. Bryobacter belongs to the phylum Acidobacteria, strictly aerobic, slow-growing, chemoautotrophic, living in acidic wetlands and soils [75]. Bryobacter shows chemo-organotrophic activity and is able to use sugars, polysaccharides, and organic acids as a source of energy [76]. Dimethylsulfonylpropionate, sulfonates, and carbon monoxide can be used to obtain energy from members of the genus Acidibiotic [77]. In this experiment, the decrease in the abundance of Bryobacter under cover conditions could be related to an increase in the content of AN. Tetracladium is a filamentous bacterium that is widespread in both aquatic ecosystems and terrestrial habitats, with the main drivers of the composition of the community being crop management practices and soil nutrients [78,79,80]. It is a widespread root-colonizing endophyte with a strong preference for roots [80]. Tetracladium was found to coexist with root pathogens and correlated positively with crop yield, suggesting potential plant health benefits [80,81]. The cover conditions in this experiment increased the abundance of Tetracladium and had a positive effect on the health and yield of the grapes. Solicoccozyma is a yeast that has been isolated from soil and is biologically degradable [82]. It was involved in the degradation of humic acids and in the rot of vine branches under the BLF and GBM treatments, respectively. A wide range of saprophytic and pathogenic species belong to the genus Alternaria [83]. Coverage increases Alternaria’s abundance. However, studies have shown that its ability to produce a large number of mycotoxins has become a major concern for food safety [84].
In summary, micro-organisms are forced to adapt to new habitats as a result of anthropogenic soil disturbance, which alters the balance of ecosystems [85]. The tillage and cover treatments in this study acted as disturbances and therefore had an impact on soil nutrients and micro-organisms. Mineral elements and organic matter in the soil are the necessary conditions for the life activities of micro-organisms, the production of metabolites that are returned to the soil. Inevitably, the plant root system is involved in this process. Autotrophic bacteria compete with the plant’s root system for mineral elements to produce organic matter, saprophytic bacteria break down humic acids and grapevine shoots to provide nutrients for the root system, and parasitic bacteria complement the root system. We therefore analyzed the relationship between nutrient elements and microorganisms in the soil.
4.3. Environmental Factors Affecting the Soil Microorganisms
In light of the profound influence soil management exerts upon soil microbial communities, there is mounting evidence indicating that agricultural practices, including but not limited to fertilization, tillage, crop rotation, and cover crops, have the capacity to modify soil microbial communities, which subsequently impact an agroecosystem’s functionality [86]. Soil physicochemical parameters exert a significant influence on soil microbial diversity [87,88]. Our findings indicate that different soil physicochemical factors exert varying effects on soil microbial diversity. In particular, soil TOC, TN, TK, NN, and AP were identified as the most significant in shaping the microbial community, exhibiting the strongest positive correlation with microbial diversity. The present study revealed a positive correlation between soil TOC and TN and the majority of microorganisms. The total organic carbon (TOC) enters the soil through the decomposition of a variety of plant and animal residues, root secretions, and living and dead microorganisms, as well as soil biota, thereby serving as a primary source of energy for microorganisms inhabiting the soil [89]. As a ubiquitous macronutrient essential for the sustenance of plant life, soil nitrogen plays a pivotal role in plant growth and metabolic processes [90,91,92]. It is typically the case that biological processes, such as the decomposition of plant litter and the secretion of root exudates, are the primary sources of soil organic carbon (SOC) and total nitrogen (TN) [93,94]. This experimental investigation demonstrates that both humic acid present in the BLF material and organic matter derived from grapevine branches in GBM were instrumental in providing and enriching C and N. The results of the correlation analysis indicated that the levels of Gammaproteobacteria, Blastocatellia, Gemmatimonadetes, Polyangia, Acidobacteriae, Nitrospiria, OM190, Acidimicrobiia, bacteriap25, Methylomirabilia, Thermoleophilia and Bacilli in bacteria and Tremellomycetes, Glomeromycetes, Mucoromycetes, and Microbotryomycetes in fungi were significantly influenced by soil nutrient content. Prior research has demonstrated that the structure of soil microbial communities is subject to a multitude of influences, including tillage practices, fertilization, vegetation genotypes, land use patterns, and geographic locations [95,96,97]. At the same time, changes in the soil microbial community structure are determined by changes in soil physicochemical properties under different mulching regimes. Changes in the soil microbial community induced by different types of ground cover management were found to be related to soil organic matter input, nutrient efficiency, and fruit tree growth [98]. Effective nitrogen plays an important role in the fungal composition of the soil [99]. However, correlations between soil properties and microbial communities vary between soil systems. For example, significant correlations have been reported between soil actinomycetes and ascomycetes and soil OM, TN, and TP [100]. Soil TP was positively correlated with Actinobacteria and negatively correlated with Thick-Walled Bacteria [101]. Consistent with the results of previous studies, the correlation analysis in this study revealed a complex relationship between the soil microbial community’s composition and diversity and changes in the soil’s physicochemical properties, with soil nutrients having a more significant effect on the bacterial community’s composition. However, the relationships and interaction mechanisms between microbial community characteristics and soil physicochemical properties, as well as agricultural management practices, need to be further investigated due to the complexity of the soil environment and microbial community composition and function.
5. Conclusions
The present study employed a series of soil physicochemical and microbiological analyses, to evaluate the impact of diverse mulch treatments (BLF and GBM) on vineyard soils during the harvest period across two vintages. The results obtained were then compared with those from a control treatment, which served to provide a baseline for comparison. The findings of the study indicated that the application of mulching reduced the bulk density, increased the moisture content, enhanced the organic matter and elevated the nutrient content in the soil. Furthermore, the nutrient content was observed to be higher in GBM than in BLF. Microbial growth was stimulated by the introduction of mulch treatment, which provided additional fresh substrate. The abundance and diversity of soil micro-organisms, closely related to the physicochemical properties of the soil, increased with both types of cover. A heterogeneous soil environment for microbial growth should be created by the differences in nutrient levels produced by different mulching methods. The composition and diversity of the soil microbial community and changes in the physical and chemical properties of the soil have a complex relationship, in which soil nutrients have a more significant effect on the composition of the bacterial community.
Author Contributions
Conceptualization, X.D. and T.L.; methodology, X.D., Y.D. and X.H.; software, X.D.; validation, X.D. and T.L.; formal analysis, X.D.; investigation, Y.D.; resources, X.D. and T.L.; data curation, X.D.; writing—original draft preparation, X.D.; writing—review and editing, X.D. and T.L.; visualization, X.D.; supervision, H.W.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research and Application of Key Technologies for the Sustainable Development of the Wine Industry, grant number LYNJ202110.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All the data of this study have been largely presented in the graphs and charts in this article. All the authors are aware of and agree to the open publication and submission of the article.
Acknowledgments
We would like to thank the Shaanxi Kerui Company for providing the biodegradable liquid film.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- López-Vicente, M.; García-Ruiz, R.; Guzmán, G.; Vicente-Vicente, J.L.; Van Wesemael, B.; Gómez, J.A. Temporal stability and patterns of runoff and runon with different cover crops in an olive orchard (SW Andalusia, Spain). CATENA 2016, 147, 125–137. [Google Scholar] [CrossRef]
- Novara, A.; Cerdà, A.; Gristina, L. Sustainable vineyard floor management: An equilibrium between water consumption and soil conservation. Curr. Opin. Environ. Sci. Health 2018, 5, 33–37. [Google Scholar] [CrossRef]
- García-Díaz, A.; Bienes, R.; Sastre, B.; Novara, A.; Gristina, L.; Cerdà, A. Nitrogen losses in vineyards under different types of soil groundcover. A field runoff simulator approach in central Spain. Agric. Ecosyst. Environ. 2017, 236, 256–267. [Google Scholar] [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripoche, A.; Valdés-Gómez, H.; Metay, A. Management of service crops for the provision of ecosystem services in vineyards: A review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Peregrina, F.; Pérez-Álvarez, E.; Colina, M.; García-Escudero, E. Cover crop and tillage influence soil organic matter and nitrogen availability in a semi-arid vineyard. Arch. Agron. Soil Sci. 2012, 58, 1–8. [Google Scholar] [CrossRef]
- Capó-Bauçà, S.; Marqués, A.; Llopis-Vidal, N.; Bota, J.; Baraza, E. Long-term establishment of natural green cover provides agroecosystem services by improving soil quality in a Mediterranean vineyard. Ecol. Eng. 2019, 127, 285–291. [Google Scholar] [CrossRef]
- Celette, F.; Findeling, A.; Gary, C. Competition for nitrogen in an unfertilized intercropping system: The case of an association of grapevine and grass cover in a Mediterranean climate. Eur. J. Agron. 2009, 30, 41–51. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.; Zhang, P.; Hakeem, A.; Song, H.; Yu, M.; Wang, F. Effects of Different Mulching Practices on Soil Environment and Fruit Quality in Peach Orchards. Plants 2024, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Zhang, Y.; Feng, H.; Zhang, W.; Siddique, K.H.M. Response of plastic film mulched maize to soil and atmospheric water stresses in an arid irrigation area. Eur. J. Agron. 2024, 154, 127080. [Google Scholar] [CrossRef]
- Luo, C.-L.; Zhang, X.-F.; Duan, H.-X.; Mburu, D.M.; Ren, H.-X.; Kavagi, L.; Dai, R.-Z.; Xiong, Y.-C. Dual plastic film and straw mulching boosts wheat productivity and soil quality under the El Nino in semiarid Kenya. Sci. Total Environ. 2020, 738, 139808. [Google Scholar] [CrossRef]
- Muscas, E.; Cocco, A.; Mercenaro, L.; Cabras, M.; Lentini, A.; Porqueddu, C.; Nieddu, G. Effects of vineyard floor cover crops on grapevine vigor, yield, and fruit quality, and the development of the vine mealybug under a Mediterranean climate. Agric. Ecosyst. Environ. 2017, 237, 203–212. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Feng, H. Colored Plastic Film Mulching Regulates Light Quality and Sucrose Metabolism in Wine Grape in an Arid Desert Oasis. J. Plant Growth Regul. 2023, 42, 7705–7714. [Google Scholar] [CrossRef]
- McIntosh, H.; Guédot, C.; Atucha, A. Plastic mulches improve yield and reduce spotted-wing drosophila in primocane raspberry. Sci. Hortic. 2023, 320, 112203. [Google Scholar] [CrossRef]
- Burns, K.N.; Bokulich, N.A.; Cantu, D.; Greenhut, R.F.; Kluepfel, D.A.; O’Geen, A.T.; Strauss, S.L.; Steenwerth, K.L. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by vineyard management. Soil Biol. Biochem. 2016, 103, 337–348. [Google Scholar] [CrossRef]
- Fraga, H.; Santos, J.A. Vineyard mulching as a climate change adaptation measure: Future simulations for Alentejo, Portugal. Agric. Syst. 2018, 164, 107–115. [Google Scholar] [CrossRef]
- Lamine, C. Transition pathways towards a robust ecologization of agriculture and the need for system redesign. Cases from organic farming and IPM. J. Rural Stud. 2011, 27, 209–219. [Google Scholar] [CrossRef]
- Cabrera-Pérez, C.; Llorens, J.; Escolà, A.; Royo-Esnal, A.; Recasens, J. Organic mulches as an alternative for under-vine weed management in Mediterranean irrigated vineyards: Impact on agronomic performance. Eur. J. Agron. 2023, 145, 126798. [Google Scholar] [CrossRef]
- Maienza, A.; Baronti, S.; Cincinelli, A.; Martellini, T.; Grisolia, A.; Miglietta, F.; Renella, G.; Stazi, S.R.; Vaccari, F.P.; Genesio, L. Biochar improves the fertility of a Mediterranean vineyard without toxic impact on the microbial community. Agron. Sustain. Dev. 2017, 37, 47. [Google Scholar] [CrossRef]
- Pou, A.; Mairata Pons, A.; Rodrigo, E.; Labarga Varona, D.; Escudero, E.; Huete, J.; Martínez-Vidaurre, J. Effects of Organic Mulches on the Soil Temperature, Humidity and CO2 Emissions. Int. J. Environ. Sci. Nat. Res. 2021, 29, 556265. [Google Scholar] [CrossRef]
- Shi, C.H.; Wang, X.Q.; Jiang, S.; Zhang, L.Q.; Luo, J. Revealing the role of the rhizosphere microbiota in reproductive growth for fruit productivity when inorganic fertilizer is partially replaced by organic fertilizer in pear orchard fields. Microb. Biotechnol. 2023, 16, 1373–1392. [Google Scholar] [CrossRef]
- Cerdà, A.; Rodrigo-Comino, J.; Giménez-Morera, A.; Novara, A.; Pulido, M.; Kapović-Solomun, M.; Keesstra, S.D. Policies can help to apply successful strategies to control soil and water losses. The case of chipped pruned branches (CPB) in Mediterranean citrus plantations. Land Use Policy 2018, 75, 734–745. [Google Scholar] [CrossRef]
- Yu, C.; Wang, X.; Hu, B.; Yang, C.; Sui, N.; Liu, R.; Meng, Y.; Zhou, Z. Effects of wheat straw incorporation in cotton-wheat double cropping system on nutrient status and growth in cotton. Field Crops Res. 2016, 197, 39–51. [Google Scholar] [CrossRef]
- Duan, X.; Yan, Y.; Han, X.; Wang, Y.; Li, R.; Gao, F.; Zhang, L.; Wei, R.; Li, H.; Wang, H. Effects of Biodegradable Liquid Film on the Soil and Fruit Quality of Vitis Franco-american L. Hutai-8 Berries. Horticulturae 2022, 8, 418. [Google Scholar] [CrossRef]
- Marí, A.I.; Pardo, G.; Cirujeda, A.; Martínez, Y. Economic Evaluation of Biodegradable Plastic Films and Paper Mulches Used in Open-Air Grown Pepper (Capsicum annum L.) Crop. Agronomy 2019, 9, 36. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G.; Gontard, N.; Gastaldi, E. Performance and environmental impact of biodegradable polymers as agricultural mulching films. Chemosphere 2016, 144, 433–439. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Cheng, M.; Zhang, F.; Wang, X.; Fan, J.; Wu, L.; Fang, D.; Zou, H.; Xiang, Y. Optimal drip fertigation management improves yield, quality, water and nitrogen use efficiency of greenhouse cucumber. Sci. Hortic. 2019, 243, 357–366. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Fan, B.; Zheng, X.; Zhang, J.; Li, W.; Guo, L. Effects of mulching biodegradable films under drip irrigation on soil hydrothermal conditions and cotton (Gossypium hirsutum L.) yield. Agric. Water Manag. 2019, 213, 477–485. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Šimůnek, J.; Shi, H.; Hu, Q.; Zhang, Y. Evaluating the effects of biodegradable and plastic film mulching on soil temperature in a drip-irrigated field. Soil Tillage Res. 2021, 213, 105116. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, J.; Zhu, Y.; Fang, Y.; Cong, R.; Li, X.; Ren, T. Rapeseed as a previous crop reduces rice N fertilizer input by improving soil fertility. Field Crops Res. 2022, 281, 108487. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus subtilis Improves Immunity and Disease Resistance in Rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- da Luz, F.B.; Castioni, G.A.F.; Tormena, C.A.; dos Santos Freitas, R.; Carvalho, J.L.N.; Cherubin, M.R. Soil tillage and machinery traffic influence soil water availability and air fluxes in sugarcane fields. Soil Tillage Res. 2022, 223, 105459. [Google Scholar] [CrossRef]
- Shahzad, D.K. Effect of Mulch on Soil Physical Properties and N, P, K Concentration in Maize (Zea mays) Shoots under Two Tillage Systems. Int. J. Agric. Biol. 2009, 11, 119–124. [Google Scholar]
- Jiménez, M.N.; Pinto, J.R.; Ripoll, M.A.; Sánchez-Miranda, A.; Navarro, F.B. Impact of straw and rock-fragment mulches on soil moisture and early growth of holm oaks in a semiarid area. CATENA 2017, 152, 198–206. [Google Scholar] [CrossRef]
- Roulia, M. Humic Substances: Importance for Agriculture, Affinity and Interactions with Soil Amendments and Pollutants. Agronomy 2024, 14, 382. [Google Scholar] [CrossRef]
- Wang, D.; Xiaoguang, C.; Zhonghou, T.; Ming, L.; Rong, J.; Aijun, Z.; and Zhao, P. Application of humic acid compound fertilizer for increasing sweet potato yield and improving the soil fertility. J. Plant Nutr. 2022, 45, 1933–1941. [Google Scholar] [CrossRef]
- Tejada, M.; Benítez, C. Effects of Crushed Maize Straw Residues on Soil Biological Properties and Soil Restoration. Land Degrad. Dev. 2014, 25, 501–509. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Yan, C.; Liu, E.; Chen, B. Soil nitrogen and its fractions between long-term conventional and no-tillage systems with straw retention in dryland farming in northern China. Geoderma 2016, 269, 138–144. [Google Scholar] [CrossRef]
- Nieweś, D.; Biegun, M.; Huculak-Mączka, M.; Marecka, K.; Kaniewski, M.; Zieliński, J.; Hoffmann, J. Extraction of humic acid from peat and lignite and the thermal behavior of their mixtures with ammonium nitrate. J. Therm. Anal. Calorim. 2023, 148, 13175–13188. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Li, M.; Yang, Y.; Li, F.-M. Does long-term plastic film mulching really decrease sequestration of organic carbon in soil in the Loess Plateau? Eur. J. Agron. 2017, 89, 53–60. [Google Scholar] [CrossRef]
- Gu, C.; Liu, Y.; Mohamed, I.; Zhang, R.; Wang, X.; Nie, X.; Jiang, M.; Brooks, M.; Chen, F.; Li, Z. Dynamic Changes of Soil Surface Organic Carbon under Different Mulching Practices in Citrus Orchards on Sloping Land. PLoS ONE 2016, 11, e0168384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.K.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef] [PubMed]
- Lemanceau, P.; Maron, P.-A.; Mazurier, S.; Mougel, C.; Pivato, B.; Plassart, P.; Ranjard, L.; Revellin, C.; Tardy, V.; Wipf, D. Understanding and managing soil biodiversity: A major challenge in agroecology. Agron. Sustain. Dev. 2015, 35, 67–81. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Ren, H.; Islam, M.S.; Wang, H.; Guo, H.; Wang, Z.; Qi, X.; Zhang, S.; Guo, J.; Wang, Q.; Li, B. Effect of Humic Acid on Soil Physical and Chemical Properties, Microbial Community Structure, and Metabolites of Decline Diseased Bayberry. Int. J. Mol. Sci. 2022, 23, 14707. [Google Scholar] [CrossRef]
- Cai, W.; Li, Y.; Wang, P.; Niu, L.; Zhang, W.; Wang, C. Revealing the relationship between microbial community structure in natural biofilms and the pollution level in urban rivers: A case study in the Qinhuai River basin, Yangtze River Delta. Water Sci. Technol. 2016, 74, 1163–1176. [Google Scholar] [CrossRef][Green Version]
- Zumsteg, A.; Luster, J.; Göransson, H.; Smittenberg, R.H.; Brunner, I.; Bernasconi, S.M.; Zeyer, J.; Frey, B. Bacterial, Archaeal and Fungal Succession in the Forefield of a Receding Glacier. Microb. Ecol. 2012, 63, 552–564. [Google Scholar] [CrossRef]
- Kabiri, V.; Raiesi, F.; Ghazavi, M.A. Tillage effects on soil microbial biomass, SOM mineralization and enzyme activity in a semi-arid Calcixerepts. Agric. Ecosyst. Environ. 2016, 232, 73–84. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Change Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, L.; Rariz, G.; Martínez-Pereyra, A.; Fernández-Scavino, A. Endophytic diazotrophic communities from rice roots are diverse and weakly associated with soil diazotrophic community composition and soil properties. J. Appl. Microbiol. 2024, 135, lxae157. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, K.; Singh, S.; Nain, L.; Shukla, P. Molecular Detection and Environment-Specific Diversity of Glycosyl Hydrolase Family 1 β-Glucosidase in Different Habitats. Front. Microbiol. 2016, 7, 1597. [Google Scholar] [CrossRef]
- Berleman, J.E.; Kirby, J.R. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol. Rev. 2009, 33, 942–957. [Google Scholar] [CrossRef]
- Herrmann, J.; Fayad, A.A.; Müller, R. Natural products from myxobacteria: Novel metabolites and bioactivities. Nat. Prod. Rep. 2017, 34, 135–160. [Google Scholar] [CrossRef]
- Li, L.; Huang, D.; Hu, Y.; Rudling, N.M.; Canniffe, D.P.; Wang, F.; Wang, Y. Globally distributed Myxococcota with photosynthesis gene clusters illuminate the origin and evolution of a potentially chimeric lifestyle. Nat. Commun. 2023, 14, 6450. [Google Scholar] [CrossRef]
- Dawid, W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 2000, 24, 403–427. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mikryukov, V.; Anslan, S.; Bahram, M.; Khalid, A.N.; Corrales, A.; Agan, A.; Vasco-Palacios, A.-M.; Saitta, A.; Antonelli, A.; et al. The Global Soil Mycobiome consortium dataset for boosting fungal diversity research. Fungal Divers. 2021, 111, 573–588. [Google Scholar] [CrossRef]
- Niskanen, T.; Lücking, R.; Dahlberg, A.; Gaya, E.; Suz, L.M.; Mikryukov, V.; Liimatainen, K.; Druzhinina, I.; Westrip, J.R.S.; Mueller, G.M.; et al. Pushing the Frontiers of Biodiversity Research: Unveiling the Global Diversity, Distribution, and Conservation of Fungi. Annu. Rev. Environ. Resour. 2023, 48, 149–176. [Google Scholar] [CrossRef]
- Baldrian, P.; Bell-Dereske, L.; Lepinay, C.; Větrovský, T.; Kohout, P. Fungal communities in soils under global change. Stud. Mycol. 2022, 103, 1–24. [Google Scholar] [CrossRef]
- He, M.-Q.; Zhao, R.-L.; Liu, D.-M.; Denchev, T.T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A.M.; Wedin, M.; McTaggart, A.R.; et al. Species diversity of Basidiomycota. Fungal Divers. 2022, 114, 281–325. [Google Scholar] [CrossRef]
- He, M.-Q.; Cao, B.; Liu, F.; Boekhout, T.; Denchev, T.T.; Schoutteten, N.; Denchev, C.M.; Kemler, M.; Gorjón, S.P.; Begerow, D.; et al. Phylogenomics, divergence times and notes of orders in Basidiomycota. Fungal Divers. 2024, 126, 127–406. [Google Scholar] [CrossRef]
- Oberwinkler, F. Evolutionary trends in Basidiomycota. Stapfia 2012, 96, 45–104. [Google Scholar]
- Yang, Z.L. Molecular techniques revolutionize knowledge of basidiomycete evolution. Fungal Divers. 2011, 50, 47–58. [Google Scholar] [CrossRef]
- Aislabie, J.; Deslippe, J.R. Soil microbes and their contribution to soil services. In Ecosystem Services in New Zealand—Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. [Google Scholar]
- James, T.Y.; Berbee, M.L. No jacket required--new fungal lineage defies dress code: Recently described zoosporic fungi lack a cell wall during trophic phase. Bioessays 2012, 34, 94–102. [Google Scholar] [CrossRef]
- Turner, M. The evolutionary tree of fungi grows a new branch. Nature 2011. [Google Scholar] [CrossRef]
- Voigt, K.; James, T.Y.; Kirk, P.M.; Santiago, A.L.C.M.d.A.; Waldman, B.; Griffith, G.W.; Fu, M.; Radek, R.; Strassert, J.F.H.; Wurzbacher, C.; et al. Early-diverging fungal phyla: Taxonomy, species concept, ecology, distribution, anthropogenic impact, and novel phylogenetic proposals. Fungal Divers. 2021, 109, 59–98. [Google Scholar] [CrossRef]
- Corsaro, D.; Walochnik, J.; Venditti, D.; Steinmann, J.; Müller, K.D.; Michel, R. Microsporidia-like parasites of amoebae belong to the early fungal lineage Rozellomycota. Parasitol. Res. 2014, 113, 1909–1918. [Google Scholar] [CrossRef]
- Yu, M.; Su, W.-q.; Huang, L.; Parikh, S.J.; Tang, C.; Dahlgren, R.A.; Xu, J. Bacterial community structure and putative nitrogen-cycling functional traits along a charosphere gradient under waterlogged conditions. Soil Biol. Biochem. 2021, 162, 108420. [Google Scholar] [CrossRef]
- Dong, S.; Wei, Y.; Yu, Q.; Gao, Y.; Chen, H.; Zhou, K.; Cheng, M.; Wang, B.; Wei, Y.; Hu, X. Inoculating functional bacteria improved the humification process by regulating microbial networks and key genera in straw composting by adding different nitrogen sources. Bioresour. Technol. 2024, 393, 130022. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Huang, X.; Min, H.; Wang, H.; Xie, Y.; Zou, H.; Qiao, C.; Wu, W. Different ratios of raw material triggered composting maturity associated with bacterial community co-occurrence patterns. Environ. Sci. Pollut. Res. 2023, 30, 62532–62543. [Google Scholar] [CrossRef]
- Ward, N.L.; Challacombe, J.F.; Janssen, P.H.; Henrissat, B.; Coutinho, P.M.; Wu, M.; Xie, G.; Haft, D.H.; Sait, M.; Badger, J.; et al. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl. Environ. Microbiol. 2009, 75, 2046–2056. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Pankratov, T.A.; Belova, S.E.; Kulichevskaya, I.S.; Liesack, W. Phylogenetic analysis and in situ identification of bacteria community composition in an acidic Sphagnum peat bog. Appl. Environ. Microbiol. 2006, 72, 2110–2117. [Google Scholar] [CrossRef]
- Mizuno, C.M.; Rodriguez-Valera, F.; Ghai, R. Genomes of planktonic Acidimicrobiales: Widening horizons for marine Actinobacteria by metagenomics. mBio 2015, 6, e02083-14. [Google Scholar] [CrossRef]
- Durán, P.; Barra, P.J.; Jorquera, M.A.; Viscardi, S.; Fernandez, C.; Paz, C.; Mora, M.d.l.L.; Bol, R. Occurrence of Soil Fungi in Antarctic Pristine Environments. Front. Bioeng. Biotechnol. 2019, 7, 28. [Google Scholar] [CrossRef]
- Bridge, P.D.; Newsham, K.K. Soil fungal community composition at Mars Oasis, a southern maritime Antarctic site, assessed by PCR amplification and cloning. Fungal Ecol. 2009, 2, 66–74. [Google Scholar] [CrossRef]
- Lazar, A.; Mushinski, R.M.; Bending, G.D. Landscape scale ecology of Tetracladium spp. fungal root endophytes. Environ. Microbiome 2022, 17, 40. [Google Scholar] [CrossRef]
- Picot, E.; Hale, C.C.; Hilton, S.; Teakle, G.; Schäfer, H.; Huang, Y.-J.; Perryman, S.; West, J.S.; Bending, G.D. Contrasting Responses of Rhizosphere Bacterial, Fungal, Protist, and Nematode Communities to Nitrogen Fertilization and Crop Genotype in Field Grown Oilseed Rape (Brassica napus). Front. Sustain. Food Syst. 2021, 5, 613269. [Google Scholar] [CrossRef]
- Stosiek, N.; Terebieniec, A.; Ząbek, A.; Młynarz, P.; Cieśliński, H.; Klimek-Ochab, M. N-phosphonomethylglycine utilization by the psychrotolerant yeast Solicoccozyma terricola M 3.1.4. Bioorg. Chem. 2019, 93, 102866. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Rajarammohan, S.; Mir, Z.A.; Bae, H. Revisiting Alternaria-host interactions: New insights on its pathogenesis, defense mechanisms and control strategies. Sci. Hortic. 2023, 322, 112424. [Google Scholar] [CrossRef]
- Pagaling, E.; Strathdee, F.; Spears, B.M.; Cates, M.E.; Allen, R.J.; Free, A. Community history affects the predictability of microbial ecosystem development. ISME J. 2014, 8, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Gould, T.J.; Sadowsky, M.J. Associations between soil bacterial community structure and nutrient cycling functions in long-term organic farm soils following cover crop and organic fertilizer amendment. Sci. Total Environ. 2016, 566–567, 949–959. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Dallaire, K.; Skousen, J. Early Tree Growth in Reclaimed Mine Soils in Appalachia USA. Forests 2019, 10, 549. [Google Scholar] [CrossRef]
- Lee, J.; Oh, Y.; Lee, S.T.; Seo, Y.O.; Yun, J.; Yang, Y.; Kim, J.; Zhuang, Q.; Kang, H. Soil organic carbon is a key determinant of CH4 sink in global forest soils. Nat. Commun. 2023, 14, 3110. [Google Scholar] [CrossRef]
- Sinfield, J.V.; Fagerman, D.; Colic, O. Evaluation of sensing technologies for on-the-go detection of macro-nutrients in cultivated soils. Comput. Electron. Agric. 2010, 70, 1–18. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Li, W. Predictive mapping of soil total nitrogen at a regional scale: A comparison between geographically weighted regression and cokriging. Appl. Geogr. 2013, 42, 73–85. [Google Scholar] [CrossRef]
- Bansod, S.J.; Thakare, S.S. Near Infrared Spectroscopy Based a Portable Soil Nitrogen Detector Design. Int. J. Comput. Sci. Inf. Technol. 2014, 5, 3953–3956. [Google Scholar]
- Luo, W.; Dijkstra, F.A.; Bai, E.; Feng, J.; Lü, X.-T.; Wang, C.; Wu, H.; Li, M.-H.; Han, X.; Jiang, Y. A threshold reveals decoupled relationship of sulfur with carbon and nitrogen in soils across arid and semi-arid grasslands in northern China. Biogeochemistry 2016, 127, 141–153. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, X.-B.; Zhang, S.-H.; Lu, H.-Y.; Shao, H. Soil nutrient stoichiometry on linear sand dunes from a temperate desert in Central Asia. CATENA 2020, 195, 104847. [Google Scholar] [CrossRef]
- Lienhard, P.; Tivet, F.; Chabanne, A.; Dequiedt, S.; Lelièvre, M.; Sayphoummie, S.; Leudphanane, B.; Prévost-Bouré, N.C.; Séguy, L.; Maron, P.-A.; et al. No-till and cover crops shift soil microbial abundance and diversity in Laos tropical grasslands. Agron. Sustain. Dev. 2013, 33, 375–384. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Shi, L.-L.; Mortimer, P.E.; Ferry Slik, J.W.; Zou, X.-M.; Xu, J.; Feng, W.-T.; Qiao, L. Variation in forest soil fungal diversity along a latitudinal gradient. Fungal Divers. 2014, 64, 305–315. [Google Scholar] [CrossRef]
- Yao, S.; Merwin, I.; Bird, G.; Abawi, G.; Thies, J. Orchard floor management practices that maintain vegetative or biomass groundcover stimulate soil microbial activity and alter soil microbial community composition. Plant Soil 2005, 271, 377–389. [Google Scholar] [CrossRef]
- Kamolmanit, B.; Vityakon, P.; Kaewpradit, W.; Cadisch, G.; Rasche, F. Soil fungal communities and enzyme activities in a sandy, highly weathered tropical soil treated with biochemically contrasting organic inputs. Biol. Fertil. Soils 2013, 49, 905–917. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, Y.; An, S. Bacterial Community Responses to Soils along a Latitudinal and Vegetation Gradient on the Loess Plateau, China. PLoS ONE 2016, 11, e0152894. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-c.; Wang, J.-n.; Guo, S.-h.; Hu, Y.-L.; Li, T.-t.; Mao, R.; Zeng, D.-H. Effects of salinization and crude oil contamination on soil bacterial community structure in the Yellow River Delta region, China. Appl. Soil Ecol. 2015, 86, 165–173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).