Phytotoxic Potential of Methyl 4-Hydroxyphenylacetate Against Ageratina adenophora (Spreng.): Mechanistic Insights and Implications for Sustainable Weed Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Compound Screening
2.2. Cell Viability and Apoptosis Detection
2.3. Stomatal Conductance and CO2 Assimilability Measurement
2.4. Hormone Detection
2.5. Statistical Analysis
3. Results
3.1. Compound Screening Results
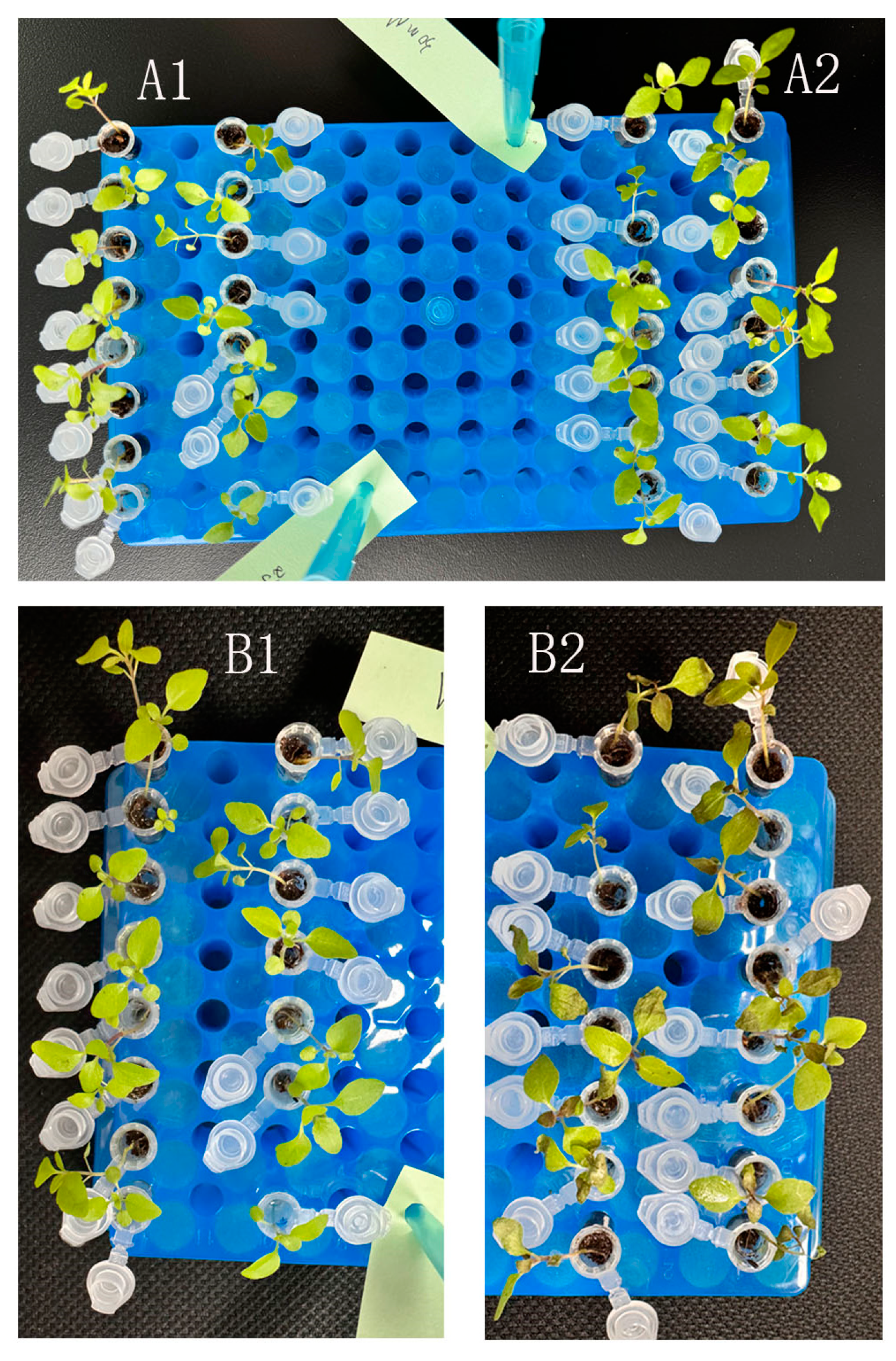
3.2. Cell Vitality and Apoptosis Time
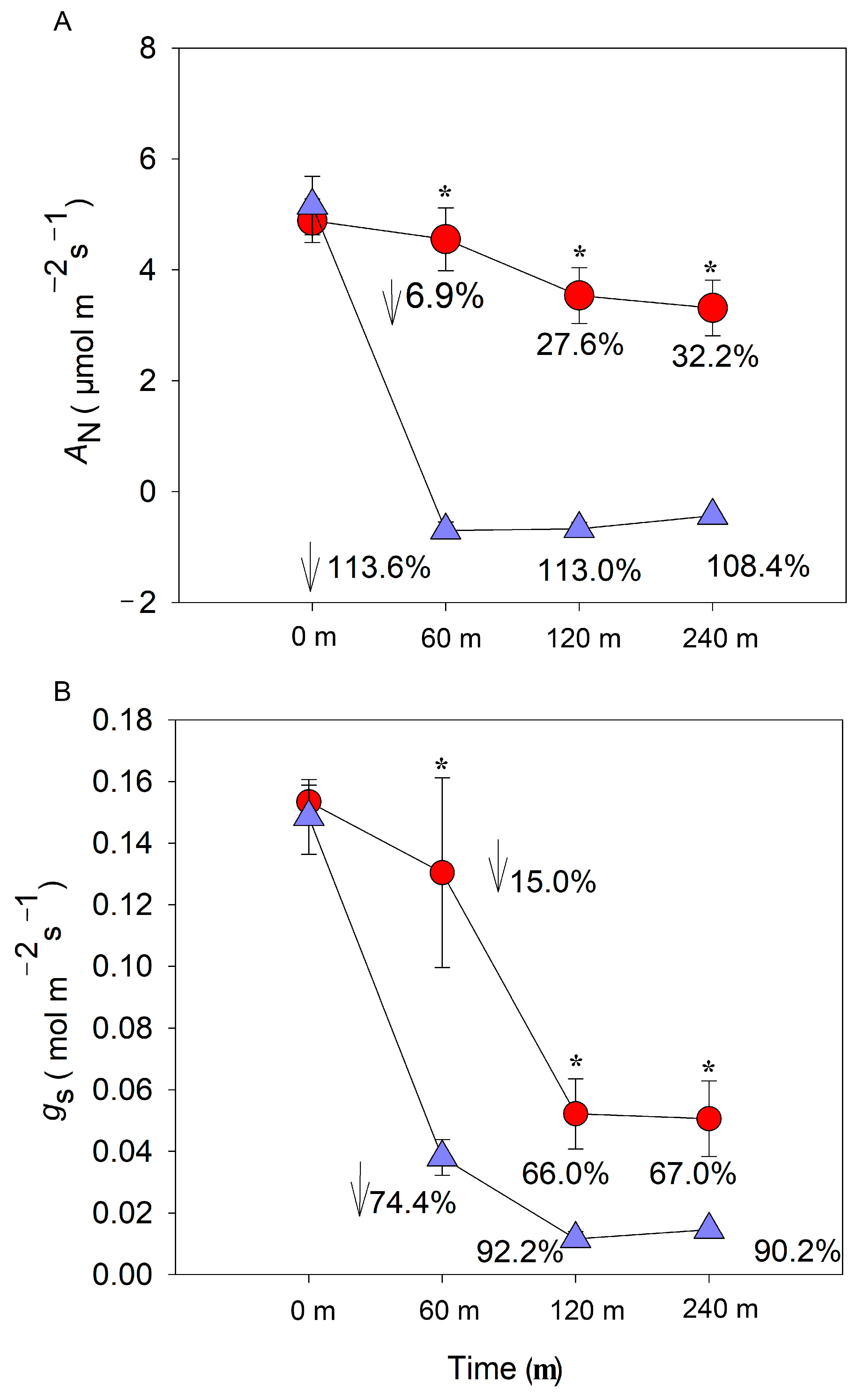
3.3. CO2 Assimilation Rate and Stomatal Conductance
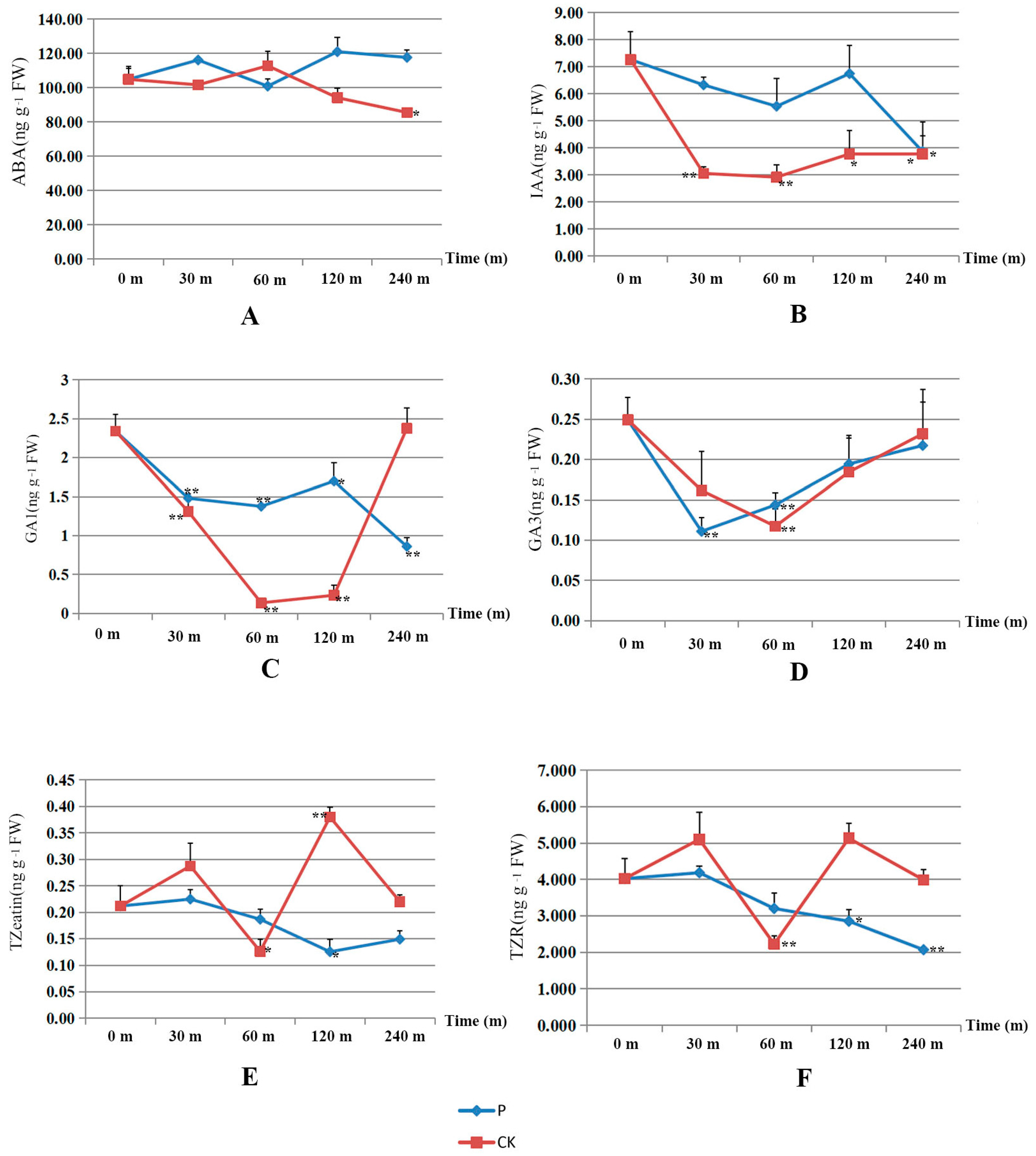
3.4. Hormonal Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Collingwood, Australia, 2001; pp. 239–242. [Google Scholar]
- Poudel, A.S.; Jha, P.K.; Shrestha, B.B.; Muniappan, R. Biology and management of the invasive weed Ageratina adenophora (Asteraceae): Current state of knowledge and future research needs. Weed Res. 2019, 59, 79–92. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, M.; Yu, L. Compositional and functional profiling of the rhizosphere microbiomes of the invasive weed Ageratina adenophora and native plants. PeerJ 2021, 9, e10844. [Google Scholar] [CrossRef] [PubMed]
- Heystek, F.; Wood, A.R.; Neser, S. Biological control of two Ageratina species (Asteraceae: Eupatorieae) in South Africa. Afr. Entomol. 2011, 19, 208–216. [Google Scholar] [CrossRef]
- Muniappan, R.; Raman, A.; Reddy, G.V.P. Ageratina adenophora (Sprengel) King and Robinson (Asteraceae). In Biological Control of Tropical Weeds Using Arthropods; Cambridge University Press: Cambridge, UK, 2009; pp. 63–73. [Google Scholar]
- Dong, S.K.; Cui, B.S.; Yang, Z.F. The role of road disturbance in the dispersal and spread of Ageratina adenophora along the Dian–Myanmar International Road. Weed Res. 2010, 48, 282–288. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.Z. Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Divers. Distrib. 2006, 12, 397–408. [Google Scholar] [CrossRef]
- Wan, F.; Liu, W.; Guo, J.; Qiang, S.; Li, B.; Wang, J.; Yang, G.; Niu, H.; Gui, F.; Huang, W.; et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel). Life Sci. 2010, 11, 1291–1298. [Google Scholar] [CrossRef]
- Gui, F.R.; Wan, F.H.; Guo, J.Y. Determination of the population genetic structure of the invasive weed Ageratina adenophora using ISSR-PCR markers. Russ. J. Plant Physiol. 2009, 56, 410–416. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Q.; Kang, Z.H. Advances in pyrimidiny (oxy) thiobenzoic acid herbicides. Plant Protect. 2018, 44, 22–28. [Google Scholar]
- Yi, X.; Chen, Y.; Shu, G. Biological function of Benzoic acid and advances of its application in livestock and poultry production. Siliao Gongye 2023, 44, 13–17. [Google Scholar]
- Pimjuk, P.; Noppawan, P.; Katrun, P. New furan derivatives from Annulohypoxylon spougei fungus. Asian Nat. Prod. Res. 2022, 24, 971–978. [Google Scholar] [CrossRef]
- Shen, S.; Li, W.; Wang, J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum 0312F1. Nat. Prod. Res. 2013, 22, 38. [Google Scholar]
- Cohen, S. Effects of plant protection chemicals on leaf gas exchange. J. Agric. Food Chem. 2004, 52, 901–905. [Google Scholar]
- Bertin, N.; Ledent, J.F. Effects of pesticide treatments on photosynthesis in maize. Agric. Ecosyst. Environ. 1998, 70, 1–12. [Google Scholar]
- Zhang, Y.; Xu, L. Effects of pesticides on stomatal behavior and plant growth. Agric. Sci. Technol. 2011, 9, 229–235. [Google Scholar]
- Wang, Y.; Li, Q. Interaction of plant growth regulators and environmental stress factors in plant growth and productivity. Front. Plant Sci. 2015, 6, 246. [Google Scholar]
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Photosynthesis in plants: A review of the Calvin cycle. Plant Physiol. 1971, 48, 295–310. [Google Scholar]
- Farquhar, G.D.; Sharkey, T.D. Stomatal control of photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Implications for climate change. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y. The effects of environmental factors on photorespiration and photosynthetic efficiency in plants. Environ. Exp. Bot. 2014, 105, 11–20. [Google Scholar]
- Hepler, P.K.; Gunning, B.E.S. Confocal fluorescence microscopy of plant cells. Protoplasma 1998, 201, 121–157. [Google Scholar] [CrossRef]
- Amos, W.B.; White, J.G. How the confocal laser scanning microscope entered biological research. Biol. Cell 2003, 95, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kamada-Nobusada, T.; Komatsu, H. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: An application for hormone profiling in Oryza sativa. Plant Cell Physiol. 2009, 50, 1201–1214. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, F.; Feng, Y.Q. Determination of cytokinins in plant samples by polymer monolith microextraction coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Methods 2010, 2, 1676–1685. [Google Scholar] [CrossRef]
- Mei, R.Q.; Nong, X.H.; Wang, B.; Sun, X.P.; Huang, G.L.; Luo, Y.P.; Zheng, C.J.; Chen, G.Y. A new phenol derivative isolated from mangrove-derived fungus Eupenicillium sp. HJ002. Nat. Prod. Res. 2020, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Du, J.; Pettit, R.K.; Knight, J.C.; Doubek, D.L. Antineoplastic agents. 575. The fungus Aspergillus phoenicis. Heterocycles 2009, 79, 909–916. [Google Scholar] [CrossRef]
- Ribeiro, T.A.N.; Sliva, L.R.; Junior, P.T.S.; Castro, R.N.; Carvalho, M.G. A new cyclopeptide and other constituents from the leaves of Zanthoxylum rigidum Humb. & Bonpl. ex Willd. (Rutaceae). Helv. Chim. Acta 2012, 95, 935–939. [Google Scholar]
- Winiewski, V.; Serain, A.F.; Sá, E.L.D.; Salvador, M.J.; Stefanello, M.É.A. Chemical constituents of Sinningia mauroana and screening of its extracts for antimicrobial, antioxidant and cytotoxic activities. Quím. Nova 2020, 43, 2. [Google Scholar] [CrossRef]
- Rösecke, J.; König, W. Odorous compounds from the fungus Gloeophyllum odoratum. Flavour Fragr. J. 2000, 15, 315–319. [Google Scholar] [CrossRef]
- Mendelsohn, B.A.; Ciufolini, M.A. Approach to tetrodotoxin via the oxidative amidation of a phenol. Org. Lett. 2009, 20, 4736–4739. [Google Scholar] [CrossRef]
- Müller, K.; Reindl, H.; Breu, K. Antipsoriatic anthrones with modulated redox properties. 5. Potent inhibition of human keratinocyte growth, induction of keratinocyte differentiation, and reduced membrane damage by novel 10-arylacetyl-1,8-dihydroxy-9(10H)-anthracenones. J. Med. Chem. 2000, 44, 814–821. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Chen, H.H.; Xing, S.T.; Yuan, W.; Wu, L.M.; Chen, X.; Zhan, C.G. Regioselective synthesis of 2- and 4-diarylpyridine ethers and their inhibitory activities against phosphodiesterase 4B. J. Mol. Struct. 2019, 1196, 455–461. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Sultana, N. Effects of herbicides on plant growth and photosynthesis. Weed Sci. 1983, 31, 59–62. [Google Scholar]
- Barrett, M.; Mudge, D. Herbicide-induced damage to the photosynthetic apparatus. Pest. Biochem. Physiol. 2002, 73, 41–49. [Google Scholar]
- Mishra, A. Effects of chemical treatments on photosynthetic activity and CO2 assimilation rate in leaves of Eupatorium adenophorum. Environ. Exp. Bot. 2013, 88, 19–26. [Google Scholar]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Munemasa, S.; Shimoishi, Y.; Uozumi, N. Regulation of stomatal closure and opening under environmental stress. Plant Cell Physiol. 2015, 56, 501–508. [Google Scholar]
- Cowan, I.R.; Farquhar, G.D. Stomatal function in relation to leaf metabolism and environment. Annu. Rev. Plant Physiol. 1977, 28, 47–70. [Google Scholar]
- Murch, S.J.; Saxena, P.K. Role of plant hormones in regulating the development of in vitro cultures of plants. Plant Growth Regulation 2000, 31, 1–14. [Google Scholar]
- Kim, Y.S.; Bressan, R.A. Signal transduction pathways in response to stress. Plant Cell Rep. 2009, 28, 1–8. [Google Scholar]
- Kearns, E.V.; Assmann, S.M. The guard cell-environment connection. Plant Physiol. 1993, 102, 711–715. [Google Scholar] [CrossRef]
- Assmann, S.M. Signal transduction in guard cells, Annu. Rev. Cell Biol. 1993, 9, 345–375. [Google Scholar] [CrossRef]
- Blatt, M.R.; Thiel, G. Hormonal control of ion channel gating. Annu. Rev. Plant Physiol. Mol. Biol. 1993, 44, 543–567. [Google Scholar] [CrossRef]
- Haswell, E.S.; Meyerowitz, E.M. Stomatal development and patterning. Curr. Opin. Plant Biol. 2006, 9, 64–69. [Google Scholar]
- Zhou, Y.; Zhang, H. Role of plant hormones in the regulation of water stress tolerance. J. Plant Growth Regul. 2009, 28, 125–132. [Google Scholar]
- Finkelstein, R.R.; Rock, C.D. Abscisic acid biosynthesis and response. Plant Cell 2002, 14, 15–28. [Google Scholar] [CrossRef]
- Montillet, J.L.; Leonhardt, N.; Mondy, S.; Tranchimand, S.; Rumeau, D.; Boudsocq, M. An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 2013, 11, e1001513. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Adachi, Y.; Munemasa, S.; Nakamura, Y.; Mori, I.C.; Murata, Y. Open stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant Cell Physiol. 2015, 56, 1239–1248. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615144. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Nir, I.; Shohat, H.; Panizel, I.; Olszewski, N.; Aharoni, A.; Weiss, D. The tomato DELLA protein PROCERA acts in guard cells to promote stomatal closure and reduce water loss under water deficiency. Plant Cell 2017, 29, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, R.S.; Sharma, S.; Yadav, V. Cytokinin-mediated regulation of abscisic acid biosynthesis and stomatal function in plants. Environ. Exp. Bot. 2020, 174, 104020. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Ding, X.; Yang, J.; Hussain, M.; Ruan, Y.; Gao, X.; Wu, G. Phytotoxic Potential of Methyl 4-Hydroxyphenylacetate Against Ageratina adenophora (Spreng.): Mechanistic Insights and Implications for Sustainable Weed Management. Agriculture 2025, 15, 824. https://doi.org/10.3390/agriculture15080824
Yang Z, Ding X, Yang J, Hussain M, Ruan Y, Gao X, Wu G. Phytotoxic Potential of Methyl 4-Hydroxyphenylacetate Against Ageratina adenophora (Spreng.): Mechanistic Insights and Implications for Sustainable Weed Management. Agriculture. 2025; 15(8):824. https://doi.org/10.3390/agriculture15080824
Chicago/Turabian StyleYang, Zhiyun, Xiao Ding, Junbo Yang, Mehboob Hussain, Yanan Ruan, Xi Gao, and Guoxing Wu. 2025. "Phytotoxic Potential of Methyl 4-Hydroxyphenylacetate Against Ageratina adenophora (Spreng.): Mechanistic Insights and Implications for Sustainable Weed Management" Agriculture 15, no. 8: 824. https://doi.org/10.3390/agriculture15080824
APA StyleYang, Z., Ding, X., Yang, J., Hussain, M., Ruan, Y., Gao, X., & Wu, G. (2025). Phytotoxic Potential of Methyl 4-Hydroxyphenylacetate Against Ageratina adenophora (Spreng.): Mechanistic Insights and Implications for Sustainable Weed Management. Agriculture, 15(8), 824. https://doi.org/10.3390/agriculture15080824






