1. Introduction
Morchella spp. is a rare edible and medicinal fungus of Ascomycota, which is mainly divided into black type (
M. angusticeps) and yellow type (
M. esculenta). Its honeycomb fruiting body has unique morphological characteristics and is widely distributed in the northern temperate deciduous forest area [
1]. The sporophores of
Morchella spp. are rich sources of proteins, minerals, vitamins, and essential amino acids, conferring significant nutritional value. This species exhibits a range of beneficial properties, including immune enhancement, organ protection, anti-fatigue, anti-oxidation, lipid-lowering, and anti-tumour [
2]. Consequently,
Morchella spp. is considered a valuable mushroom for culinary and medicinal applications [
3]. Due to its considerable potential in the food and pharmaceutical industries, the market demand for
Morchella spp. continues to rise, exceeding the supply from wild sources. The widespread adoption of exogenous nutrient bag technology has facilitated the rapid expansion of cultivated
Morchella spp. production in China, particularly for the black series varieties. However, the limited availability of suitable cultivation areas presents challenges. To optimise land use efficiency and maximise economic returns, researchers and agricultural practitioners across China have explored and promoted
Morchella spp. rotation systems that incorporate various crops, including the grape [
4], ginger [
5], and mulberry trees [
6].
Intensive, continuous greenhouse cropping is a common strategy employed to maximise yields and economic returns. However, such highly intensive systems can lead to a decline in productivity over time and increase the risk of soil-borne pests and diseases [
7].
Morchella spp., as a soil-dependent fungus, is particularly vulnerable to complex soil conditions and diverse pest pressures encountered in open-field cultivation. These challenges, often intensified by continuous cropping practices, present significant obstacles to the sustainable development of the
Morchella spp. industry. For example, between 2020 and 2021, 80–90% of the cultivated areas in Chengdu Plain and Yunnan Gongshan Valley experienced total crop failures [
8]. Cropping systems such as rotation and intercropping effectively enhance multiple cropping indices and improve economic returns. However, the complex interactions between different crops introduce a range of uncertainties. Investigating these uncertainties is difficult due to several challenges, including determining the best way to balance soil fertility, the increased risk of insect infestations, and the limitations of crop rotation schedules. These challenges limit the widespread adoption and effective implementation of these potentially beneficial cropping systems.
Soil plays a crucial role in enhancing the physical and chemical properties of a substrate, including factors such as porosity, water retention, temperature, and nutrient availability [
9]. More importantly, soil microorganisms influence the mycelial growth and fruiting of
Morchella spp. [
10]. The mechanism of the continuous cropping obstacle of
Morchella spp. is very complex, which may be related to the imbalance of soil microbial flora and the increase in harmful microorganisms. It has been reported that the diseases caused by continuous cropping obstacles are generally closely related to the microbial community structure of the soil [
11]. The main reason for the high incidence of diseases caused by continuous cropping of crops is the reduction in beneficial microorganisms and the accumulation of pathogens [
12].
M. esculenta rotation and intercropping can allow other crops to decompose the secretions of the last
M. esculenta mycelium and establish a microbial flora suitable for the growth of
M. esculenta mycelium. However, due to the complexity of the microbial system, the interaction between different crops and
M. esculenta may form dominant colonies in the soil, thereby inhibiting the growth of other microorganisms, and the harmful microorganisms that feed on specific crop roots and
M. esculenta hyphae will also accumulate in large quantities, which may eventually lead to a decrease in microbial diversity. Therefore, different crops in rotation or intercropping systems may bring unpredictable changes to soil microbial communities. While previous research on soil microorganisms in
Morchella spp. cultivation has predominantly focused on bacteria, fungal diseases represent a more significant threat to the production of
Morchella spp. Studies have linked the incidence of
Morchella spp. canker disease to the presence of
Fusarium incarnatum and
F.equiseti in soils [
13]. Furthermore, He et al. [
14] identified
Paecilomyces penicillatus as the causative agent of white mould disease, a common disease of
Morchella spp. Research by Su Wenying [
15] and Huang Hui [
16] et al. revealed that
Diploöspora longispora is responsible for pileus rot and pythium disease. Therefore, analysing soil fungal community structures and diversity is crucial for identifying potential fungal pathogens, understanding their impact on edible mushroom growth and development, and ultimately addressing yield and quality limitations in
Morchella spp. cultivation.
Limited research has explored the effects of rotation and intercropping with different crops on the abundance and community structure of soil microorganisms in Morchella spp. cultivation. This study addresses this gap by investigating fungal diversity, community composition, and the presence of harmful taxa in Morchella spp. cultivation soils under different cropping patterns using high-throughput sequencing technology. Additionally, the physicochemical properties of the soils were analysed to identify potential correlations with microbial communities. This study aimed to identify disease-associated microorganisms in Morchella spp. cultivation soils and provide a theoretical basis for mitigating succession barriers in practical production.
2. Materials and Methods
2.1. Experimental Design and Sample Acquisition
The field experiment was conducted at Junlin Tianxia Ecological Farm in Pan’an County, Zhejiang Province, Eastern China (28.884132° N, 120.387460° E, elevation: 326 m). The area is sandy loam soil, and the air permeability and water permeability are good. Ginger, grape, and mulberry are planted all year round. We took measures according to local conditions. Morchella spp. was cultivated in natural soil, in the spaces between grape vines, and among mulberry trees within the same greenhouse plot on 15 October 2023. Following the harvest on 30 April 2024, ginger was planted in some of the fields. Soil samples were collected five months after sowing, in 2024. The local temperature was 27 °C, the air humidity was 70%, and the soil humidity was 80% before the commencement of the next cropping cycle. Specifically, soil samples were collected from five distinct experimental treatments: natural soil (NS, uncultivated control), fallow soil (FS, post-Morchella sextelata cultivation), ginger rotation soil (GRS, Morchella spp. rotated with ginger), vine intercropping soil (VIS, Morchella spp. intercropped with grapevines), and mulberry tree intercropping soil (MTIS, Morchella spp. intercropped with mulberry trees). For each treatment, five sampling points were randomly selected within the plot following a five-point sampling method. At each point, surface soil was carefully removed using a sterilised shovel, and soil samples were collected from a depth of 3–5 cm. The collected soils were thoroughly mixed and divided into two portions. A portion (approximately 300 g) was used to analyse the physicochemical properties of the soil. The other portion was sieved through a 2 mm mesh, and 50 g of the screened soil was immediately transferred into sterilised tubes. These tubes were labelled and promptly placed in a container with dry ice to preserve the samples. All the soil samples were transported to the laboratory without delay. The soil samples in sterile tubes were then stored at −80 °C for DNA extraction. Subsequently, the samples were flash-frozen in liquid nitrogen and sent to Shanghai Donghuan Biotech Co., Ltd. (Shanghai, China) for NovaSeq PE250 sequencing. In order to reduce the experimental error, the soil physical and chemical properties and high-throughput sequencing were repeated six times. The total number of sequencing samples was 30.
2.2. Test of Physical and Chemical Properties of Covering Soils
The soil chemical properties were determined following established NY/T and LY/T reference methods [
17,
18]. Soil organic carbon content was measured using the potassium dichromate oxidation–external heating method. The total salt content was determined by the deionised water extraction–mass method. Total nitrogen (TN) was analysed using an elemental analyser. Soil pH and available phosphorus (AP) were measured using the sodium bicarbonate (NaHCO
3) extraction–molybdenum-antimony (Mo-Sb) colourimetric method. Available potassium (AK) was determined by ammonium acetate extraction and flame photometry. Ammonium nitrogen content was determined using potassium chloride solution extraction–indophenol blue colourimetry, and nitrate nitrogen content was measured using potassium chloride solution extraction–double wavelength colourimetry.
2.3. DNA Extraction and ITS High-Throughput Sequencing
The soil genomic DNA was extracted using the FastDNATMSpin Kit (MP Biomedicals, Santa Ana, CA, USA) for Soil. DNA fragment size was assessed using agarose gel electrophoresis (AGE). Additionally, DNA concentration and purity were measured using a NanoDrop One spectrophotometer (Thermo Fisher, Waltham, MA, USA) to ensure that the samples met the quality requirements for sequencing. The qualified samples were then sent to Shanghai Donghuan Biotech Co., Ltd. for NovaSeq PE250 sequencing. The ITS region was amplified using the primers ITS1 (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′). PCR conditions: pre-denaturation at 98 °C for 30 s; denaturation at 98 °C for 10 s, renaturation at 58 °C for 30 s, and extension at 72 °C for 1 min, 28 cycles; and extension at 72 °C for 2 min.
2.4. Data Analysis and Visualisation
To investigate species diversity within the samples, the filtered sequence data were analysed using Illumina NovaSeq 6000 (San Diego, CA, USA). The sequence underwent a series of processing steps, including filtering, quality control, chimaera removal, integration of double-end sequences, and noise reduction using DADA2. This process generated Amplicon Sequence Variants (ASVs), which represent feature information. The specific processing steps are as follows: remove reads containing low-quality bases (mass value ≤ 15) exceeding a certain proportion (default set to 40 bp); remove reads with a certain proportion of N bases (default is set to 10 bp); and remove reads that overlap with the Adapter exceeding a certain threshold (default is set to 10 bp). For taxonomic classification, the UNITE reference database (version 8.0) was clustered at 99% similarity and used to train a classifier. Subsequently, analyses of relative abundance, alpha diversity, beta diversity, and intergroup differences were conducted to determine species richness and evenness within the samples, as well as to identify shared and unique species among the different samples or groups. In addition, differences in community structures among various samples and groups were analysed and visualised using principal coordinates analysis (PCoA) and the unweighted pair group method with an arithmetic mean (UPGMA) sample clustering tree. To further investigate community structural differences among different groups, statistical analysis methods such as permutational analysis of variance (PERMANOVA) using the Adonis test and analysis of similarities (ANOSIM) were employed. The linear discriminant analysis effect size (LEfSe) was used to identify differentially abundant taxa. ITS raw data have been uploaded to NCBI, and the accession number is PRJNA1234878.
3. Results
3.1. Diversity of Fungal Community in Soils Covered with Morchella spp. Under Different Cropping Patterns
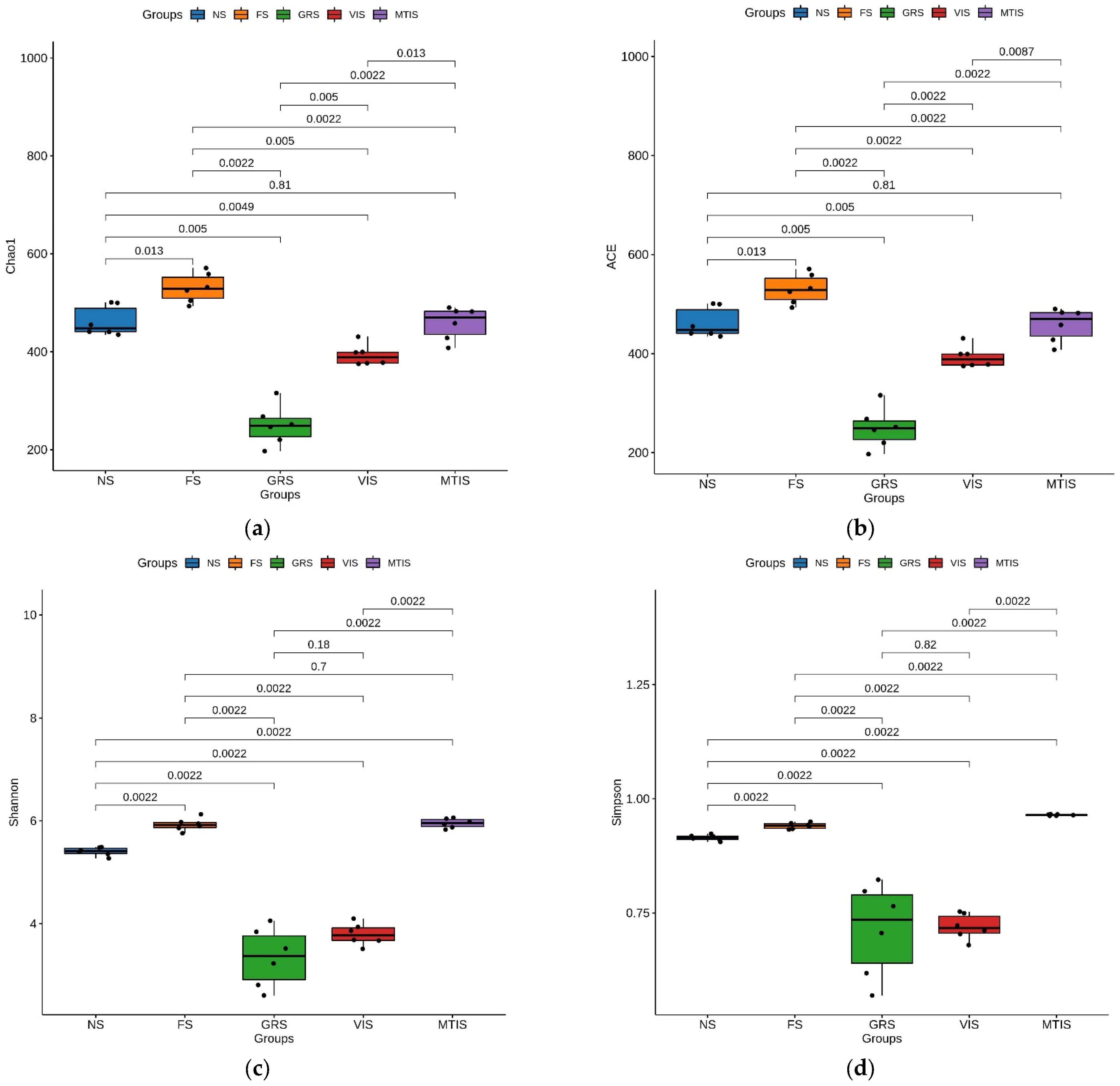
An alpha (α) diversity analysis was performed to assess the microbial diversity within individual samples, providing insights into the abundance and diversity of microbial communities by evaluating a single sample’s composition. The Chao1 and Ace indices measure species richness, whereas the Shannon and Simpson indices quantify species diversity. It can be seen from
Figure 1 that in the planting system, the fungal diversity of GRS was the lowest and FS was the highest, as shown by Chao1, ACE, Shannon, and Simpson indices.
3.2. The Number of Soil Fungal Communities Covered with Morchella spp. Under Different Cropping Patterns
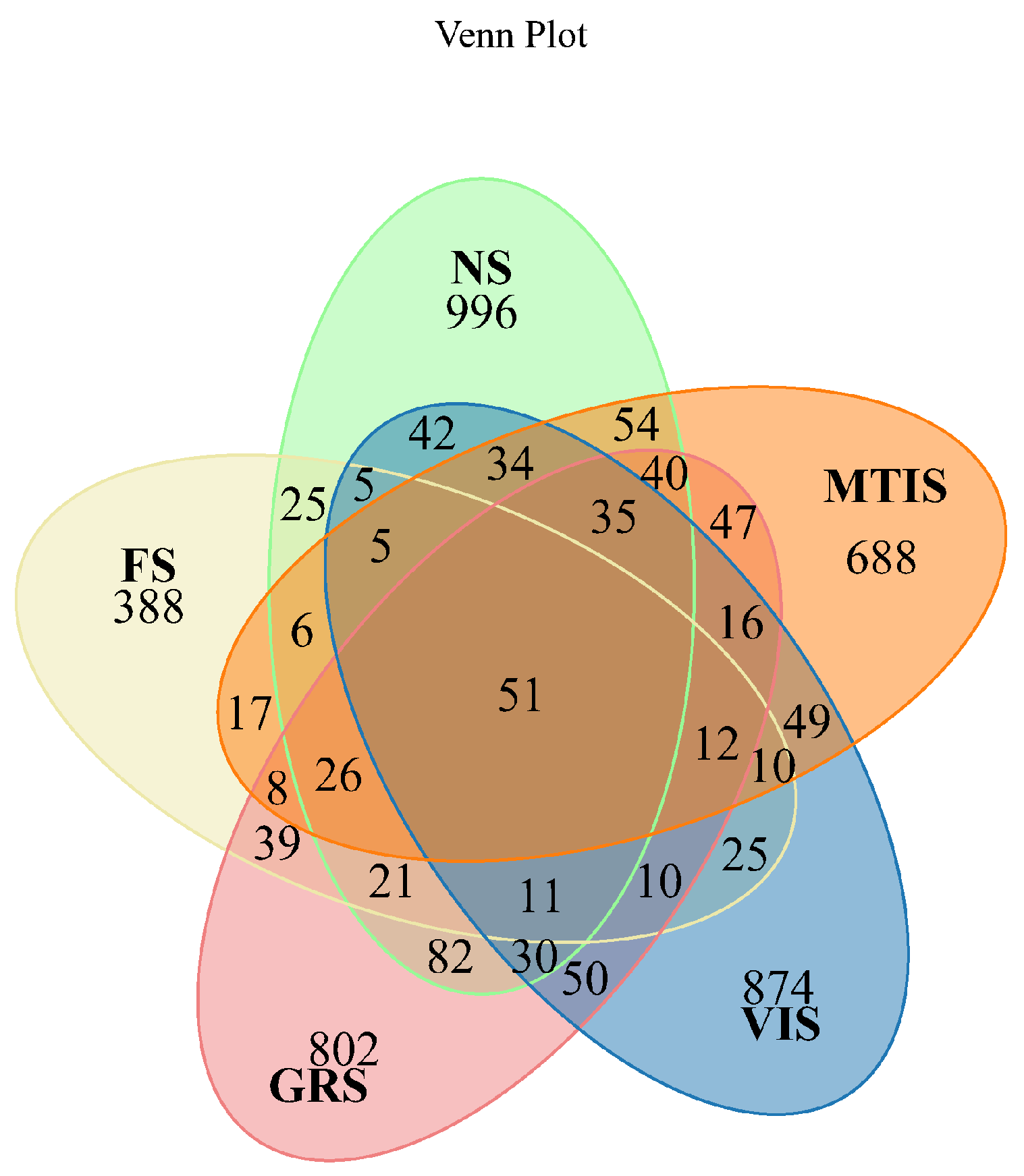
A total of 5759 operational taxonomic units (OTUs) were identified across the NS, FS, GRS, VIS, and MTIS groups, and their species distribution was analysed using a Venn diagram. The number of OTUs detected in the NS, FS, GRS, VIS, and MTIS was 1463, 659, 1280, 1259, and 1098, respectively. Among these, 996 OTUs were unique to NS, 388 to FS, 802 to VIS, and 688 to MTIS. Additionally, 51 OTUs were common to all five samples, representing a core set of microbial taxa shared across all the cropping systems.
As shown in
Figure 2, the number of OTUs in the soil samples followed the following order: NS > GRS > VIS > MTIS > FS. This pattern indicates that fungal community abundance is highest in NS, whereas soils under different cropping patterns exhibit varying degrees of decline in fungal abundance. Notably, FS displays the lowest fungal community abundance among all the samples.
3.3. Fungal Community Structure and β Diversity of Soils Cultivated with Morchella spp. Under Different Cropping Patterns
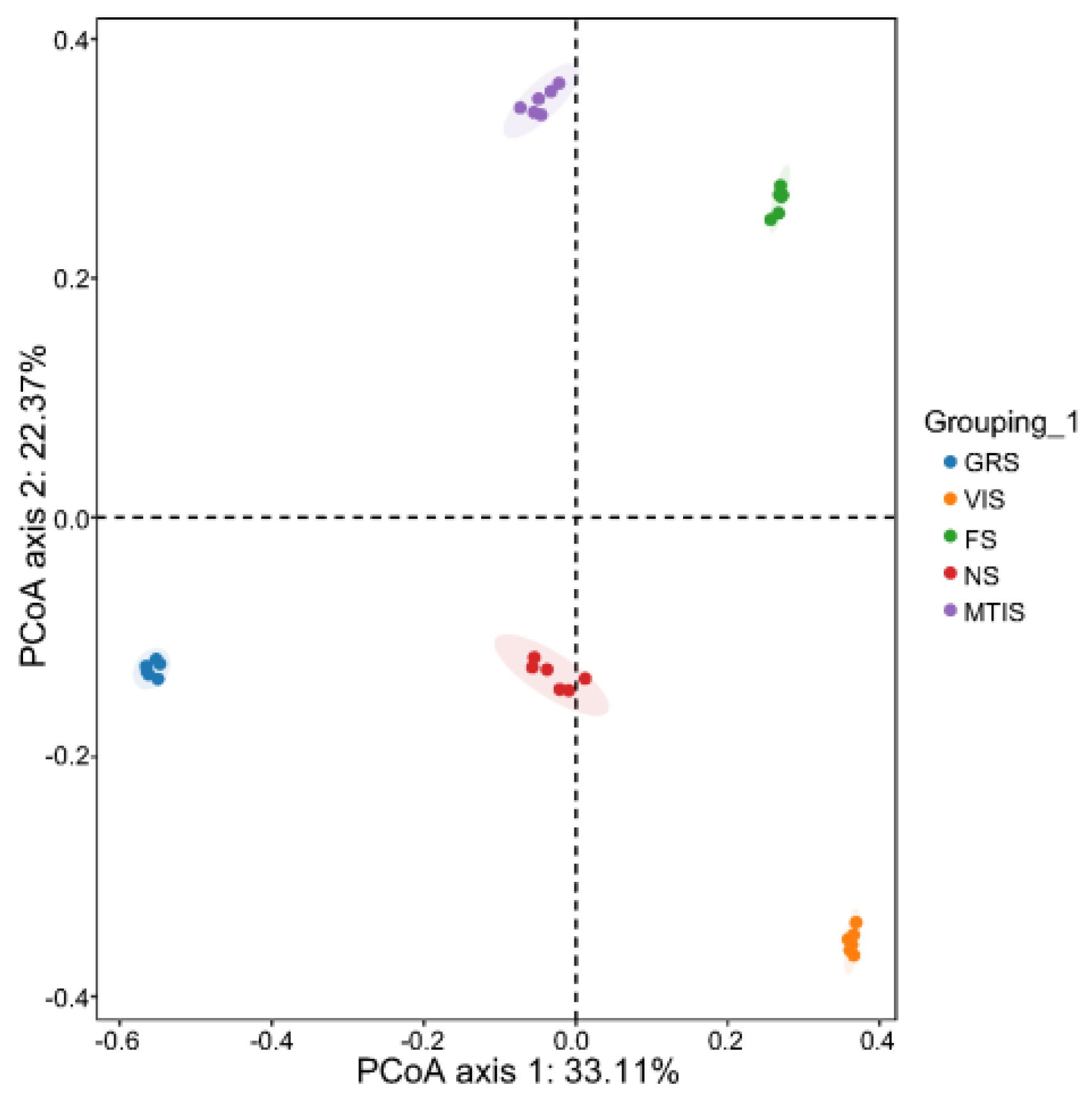
The fungal community structure of the soils cultivated with
Morchella spp. was analysed using PCoA based on Bray–Curtis distance (
Figure 3). In PCoA plots, greater distances between sample points indicate greater dissimilarity in fungal community structure and diversity. Conversely, shorter distances reflect more similar fungal communities. The results show that the principal coordinate axes PC1 and PC2 account for 33.11% and 22.37% of the variation in fungal community structure, respectively. The analysis reveals that the FS, GRS, VIS, and MTIS occupy distinct quadrants and are located far from the NS. This spatial distribution indicates significant differences in fungal community structure and diversity among the soils cultivated with
Morchella spp. under different cropping patterns. It is hypothesised that these differences arise due to the selective influence of root systems from different crops on soil fungal species.
3.4. UPGMA Clustering of Fungal Community in Soils Cultivated with Morchella spp. Under Different Cropping Patterns
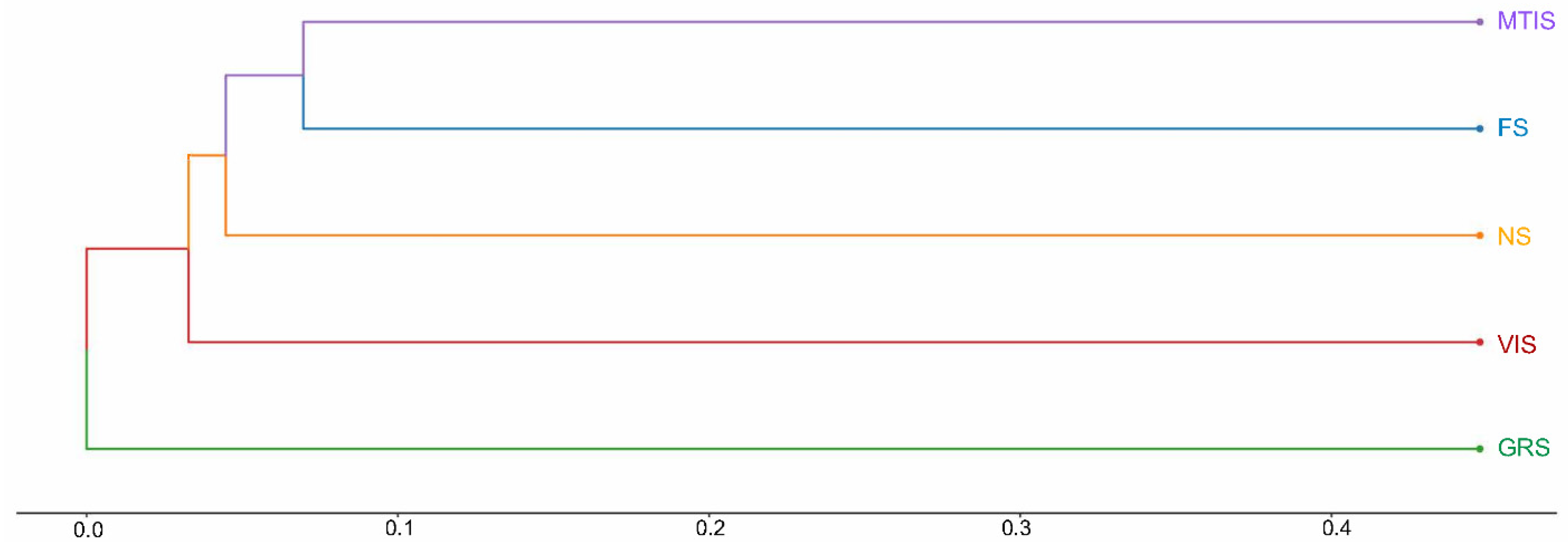
Figure 4 illustrates that the FS and MTIS cluster within the same branch, indicating the shortest distance between them. This close clustering reflects a high degree of similarity in their fungal community structures. However, FS and MTIS were positioned far from the other soil samples, indicating significant differences in fungal community composition compared to the remaining groups.
3.5. Fungal Community Differences in Soils Cultivated with Morchella spp. Under Different Cropping Patterns
To provide a comprehensive understanding of the fungal community structure in the soils cultivated with
Morchella spp., the fungal communities were analysed at various taxonomic levels. As shown in
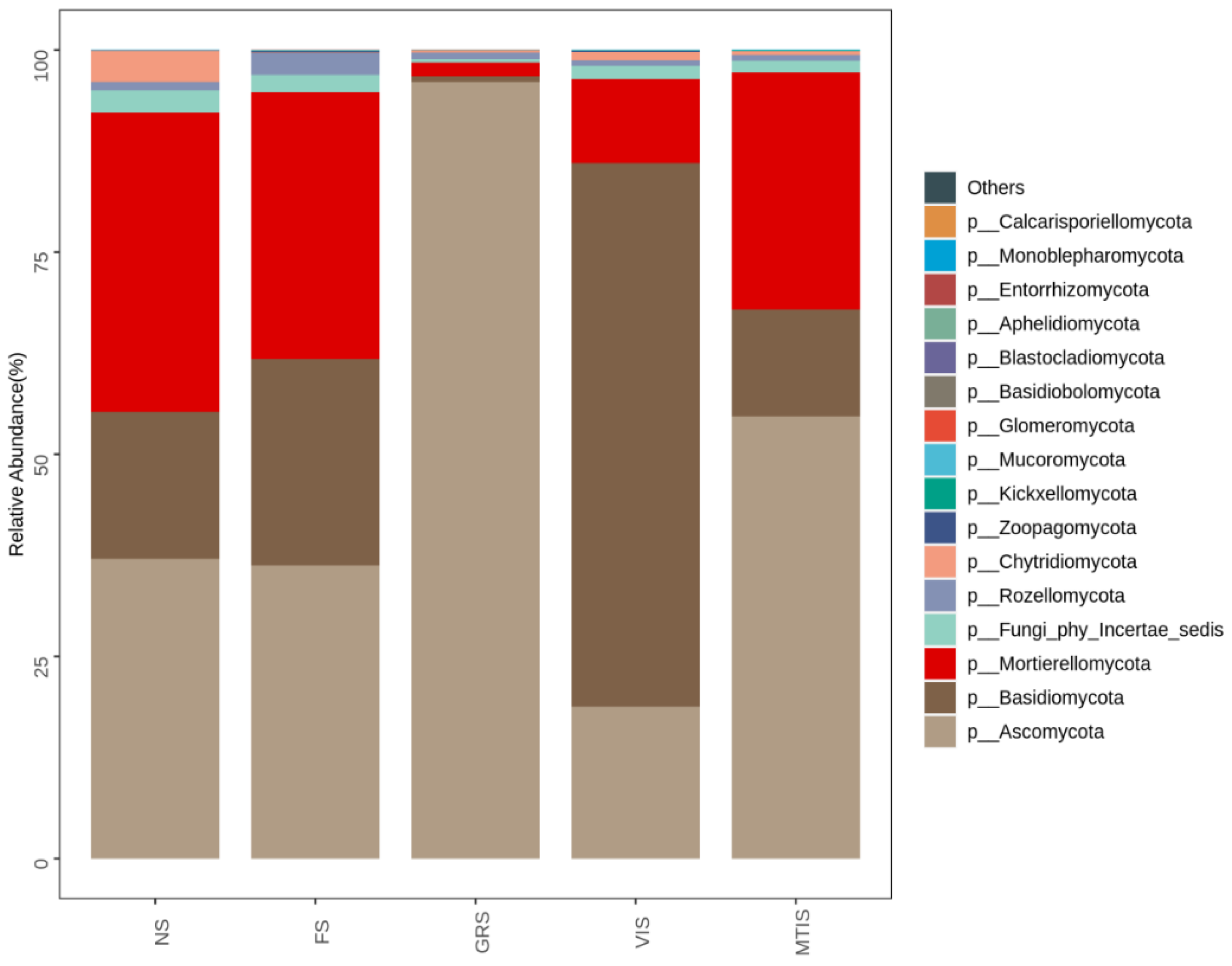
Figure 5, at the phylum level,
Ascomycota,
Basidiomycota, and
Mortierellomycota were the three dominant phyla across all the soil samples. Specifically,
Ascomycota exhibited the highest relative abundance in GRS (96.05%), while
Basidiomycota had the lowest relative abundance (0.69%). Conversely, in VIS, the relative abundance of
Ascomycota was the lowest (18.78%) and that of
Basidiomycota was the highest (67.23%). The FS group, when compared to the control (NS), displayed similar relative abundances of these dominant phyla.
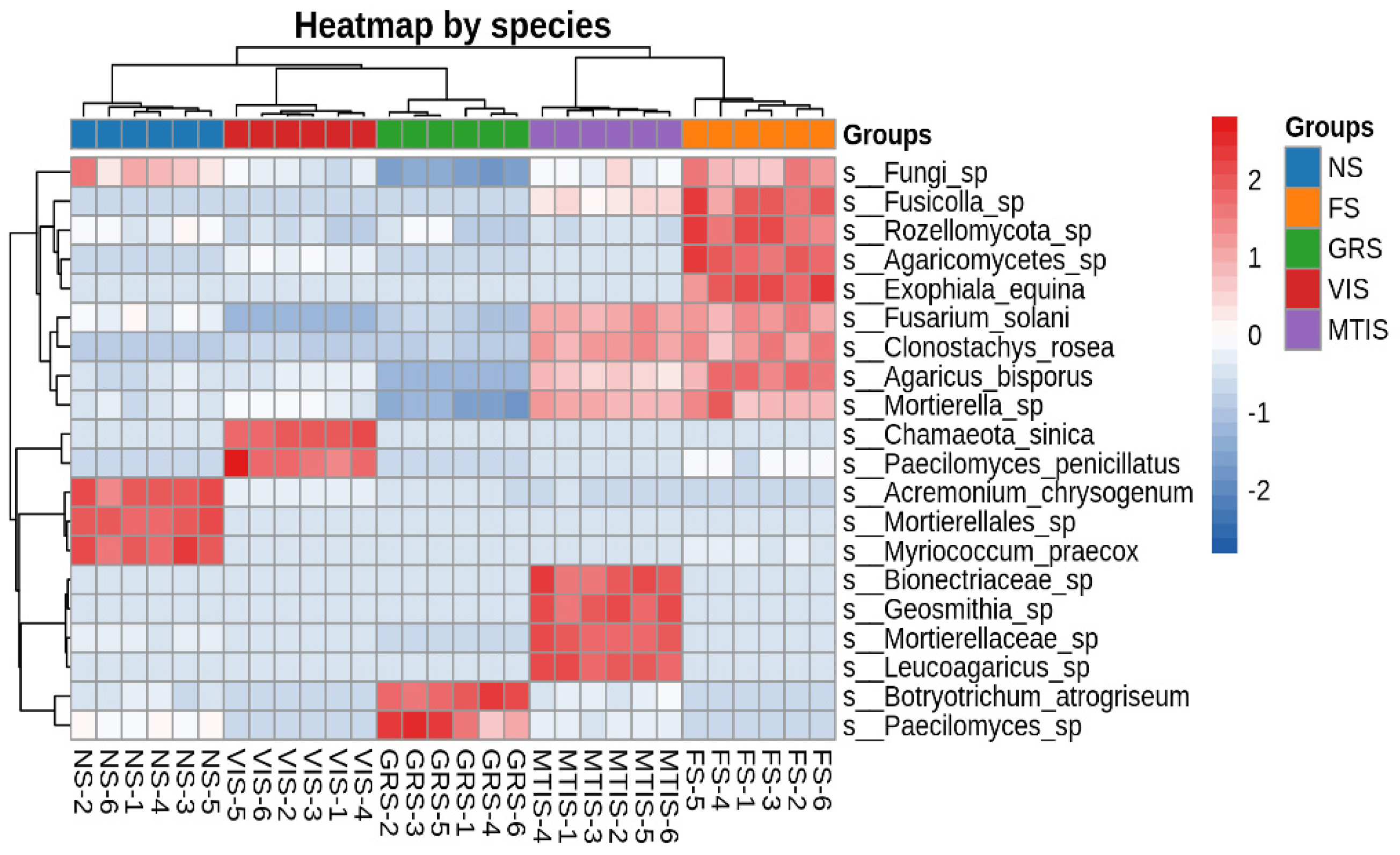
At the species level, as depicted in
Figure 6,
Acremonium_chrysogenum,
Mortierellales, and
Myriococcum praecox were the dominant species in NS. The heatmap reveals significant changes in the fungal community structures among the soil samples. Notably, specific trends in relative abundance were observed:
Paecilomyces penicillatus exhibited increased abundance in VIS, while
Botryotrichum atrogriseum and
Paecilomyces sp. increased in GRS. Similarly,
Fusarium solani and
Mortierella sp. showed higher relative abundances in MTIS and FS, respectively.
3.6. LEfSe Analysis of Fungal Communities in Soils Cultivated with Morchella spp. Under Different Cropping Patterns
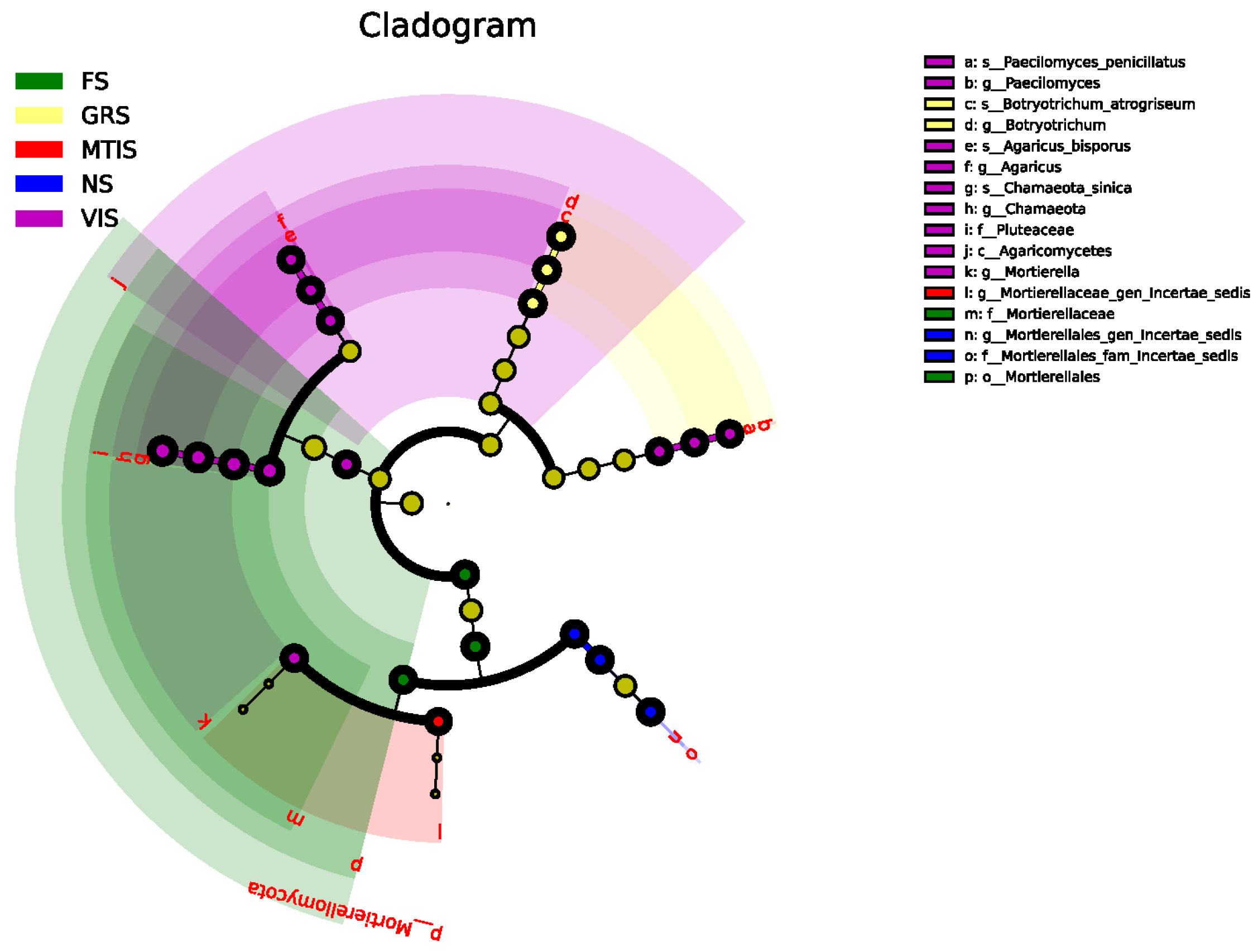
A circular tree map generated through the LEfSe analysis was used to visualise intergroup differences in fungal community composition. The concentric circles, arranged from the innermost to the outermost regions, represent taxonomic ranks. As shown in
Figure 7, certain taxa exhibited notably high relative abundances in specific soil types:
Mortierellales_fam_Incertae_sedis in NS,
Mortierellales in FS,
Botryotrichum and
Botryotrichum atrogriseum in GRS, and
Mortierellaceae_gen_Incertae_sedis in MTIS. In VIS, relatively high abundances of
Paecilomyces sp.,
Agaricus,
Pluteaceae, and
Mortierella were observed.
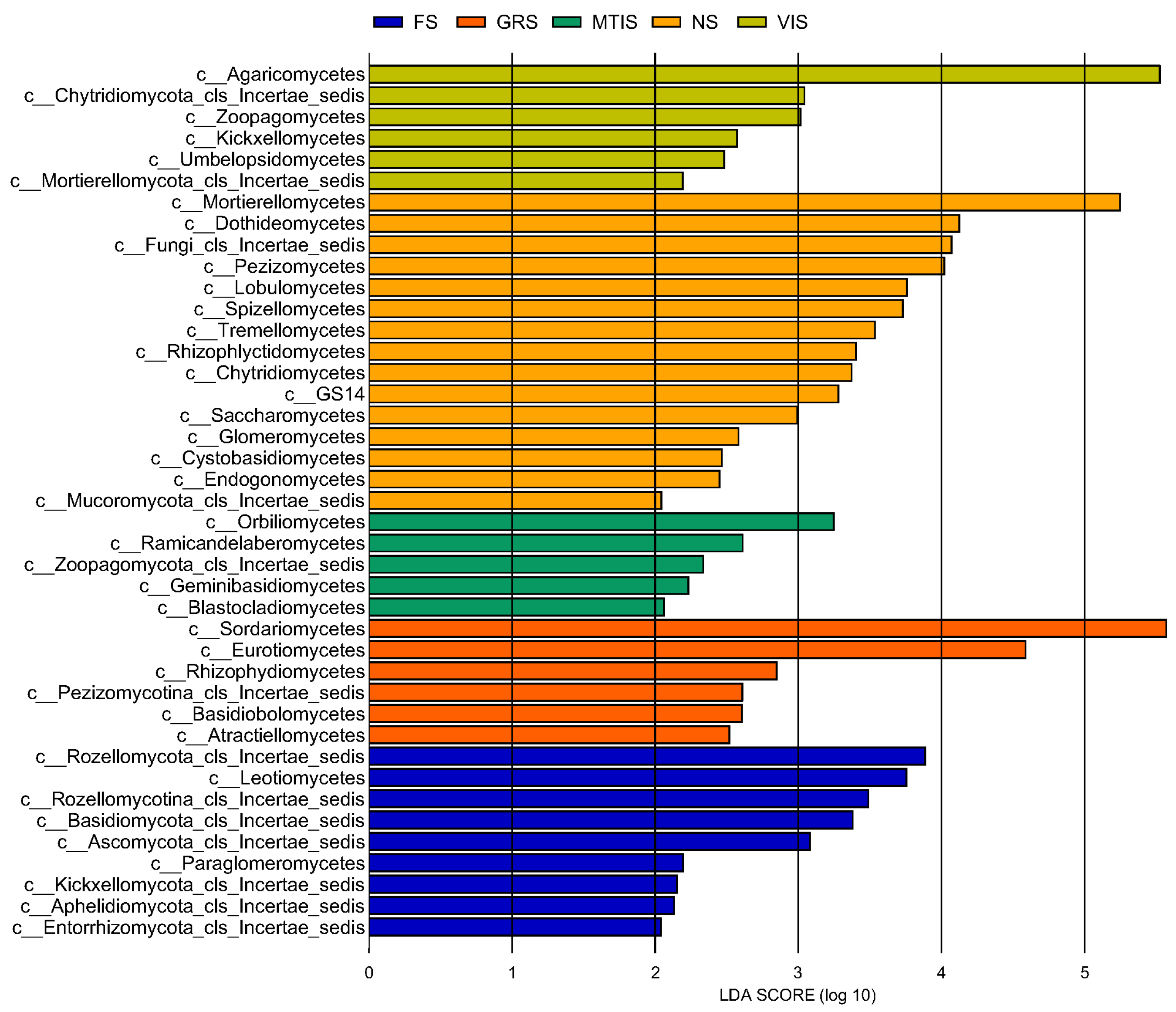
LEfSe was used to assess the differential effects of various treatments on the relative abundance of individual fungal taxa. A linear discriminant analysis (LDA) based discrimination bar chart was used to identify the key taxa (species with an LDA score ≥ 6) across various treatments. Higher LDA scores indicate a stronger influence of different treatments on the relative abundance of specific species within the fungal community.
At the class level, the
t-test analysis (
Figure 8) revealed that
Mortierellomycetes was the dominant class in the NS, significantly differentiating its fungal community structure from the other samples. This class exhibited a high degree of influence (LDA score = 5). In the FS group,
Rozellomycota, Leotiomycetes, and
Eurotiales were identified as the key taxa driving significant differences among the treatments.
Sordariomycetes and
Eurotiomycetes strongly influenced (LDA score = 4) community differences in GRS.
Orbiliomycetes and
Ramicandelaberomycetes were the primary fungal classes responsible for the significant differences between the MTIS and the other soil samples. Finally, VIS was significantly differentiated from the other soil samples by
Aricomycetes.
3.7. Potential Function Prediction of Fungi in Soils Cultivated with Morchella spp. Under Different Cropping Patterns
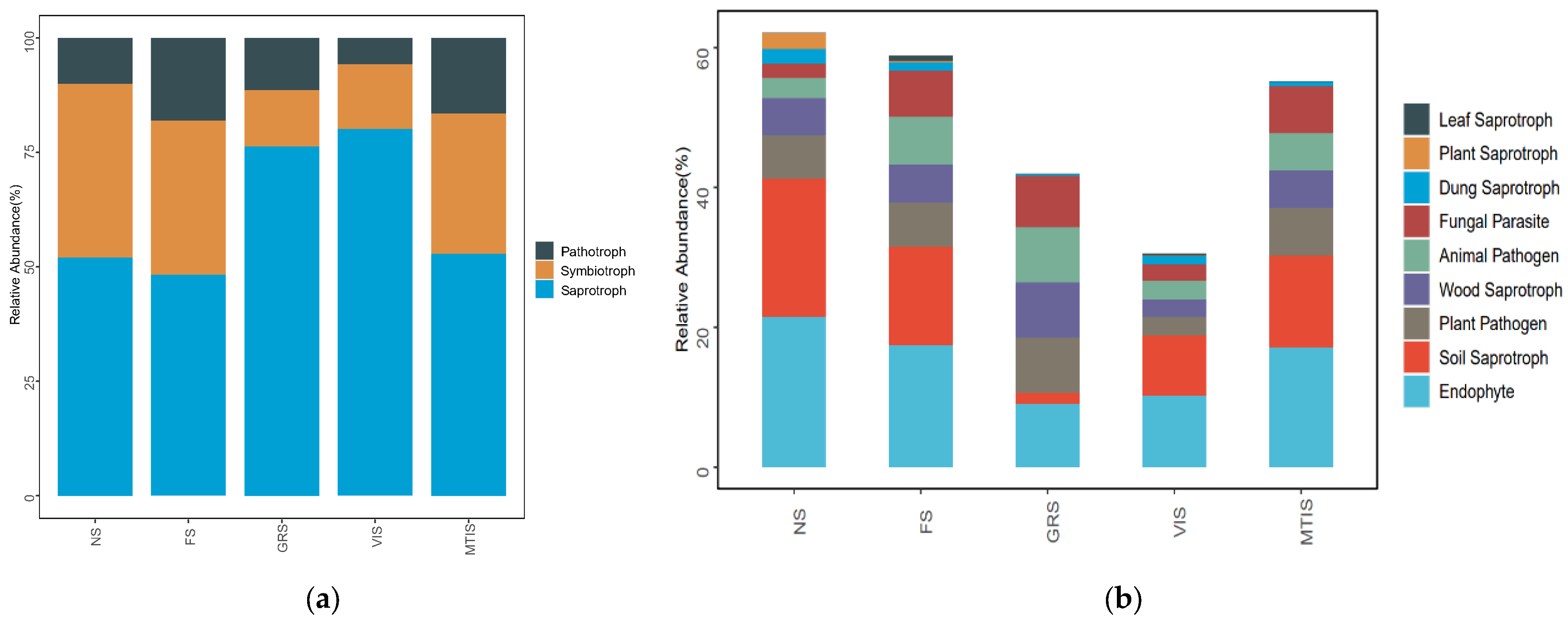
To further investigate the functional roles of microbial communities in soils under crop rotation with Morchella spp., data with a confidence level of “Possible” were excluded, while data classified as “Highly Probable” and “Probable” were retained for analysis. FUNGuild was then used to analyse the functional profiles of the fungal communities before and after planting. The reliability of the data was ensured by confidence screening. FUNGuild was used to realise functional annotation. Combined with abundance statistics and intergroup comparison, the effects of crop rotation on soil fungal functional communities, especially the dynamic changes in saprophytic and pathological microorganisms, were revealed.
As illustrated in
Figure 9a, the fungal communities in the soils associated with
Morchella spp. were predominantly composed of saprophytic fungi, while symbiotic and pathogenic fungi represented the smallest proportion of the community. Notably, the most significant differences in fungal types were observed between GRS and VIS. In both soil types, saprophytic fungi were predominant, with relative abundances of 76.33% and 80.20%, respectively. The proportion of pathological nutrition type is the lowest and their relative abundance is lower than 15%.
It can be seen from
Figure 9b that the saprophytic nutrition type, soil rot fungi, coprotrophic fungi, and plat rot fungi in all the soil samples cultivated with
Morchella spp. decreased continuously compared with those in NS. Pathogenic fungi constituted the smallest proportion, with relative abundances below 15% in both soil types.
3.8. Effects of Soil Physical and Chemical Properties on Fungal Community Structure Under Different Cropping Patterns
As shown in
Table 1, significant differences were observed in the ammonium nitrogen, nitrate nitrogen, AK, and total salt content between NS and all the soil samples associated with
Morchella spp., except for VIS. Specifically, GRS exhibited the lowest pH but the highest levels of organic matter, TN, ammonium nitrogen, nitrate nitrogen, AP, AK, and total salt. In contrast, VIS had the highest pH but the lowest organic matter, TN, ammonium nitrogen, nitrate nitrogen, and total salt levels. FS recorded the lowest values for AP and AK among the soil types analysed. Notably, ammonium nitrogen and nitrate nitrogen levels in FS, GRS, and MTIS were significantly higher than those in NS. Specifically, ammonium nitrogen concentrations in FS, GRS, and MTIS were 2.85, 3.85, and 1.78 times higher, respectively, than those in NS, while nitrate nitrogen levels were 11.89, 20.93, and 9.31 times higher, respectively. The increase in nitrate nitrogen is more pronounced than that in ammonium nitrogen in these samples. However, VIS showed a different trend. While ammonium nitrogen increased slightly, nitrate nitrogen
decreased to the lowest level (11.66 mg/kg) observed across all the soil samples.
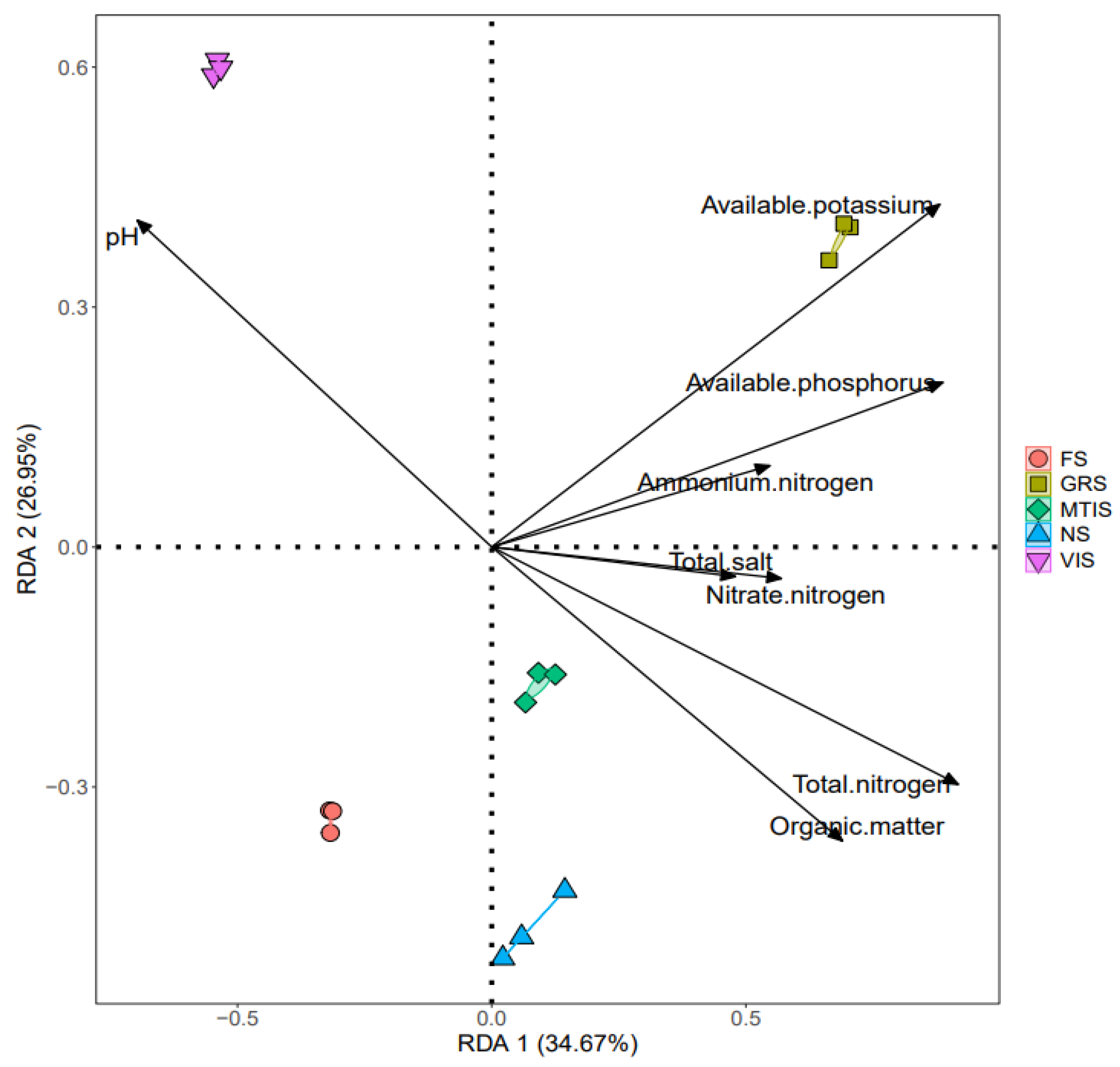
To investigate the influence of soil physicochemical properties on fungal community composition under the different cropping patterns, all the measured soil properties were included in a redundancy analysis (RDA). As shown in
Figure 10, pH emerged as a significant factor influencing fungal community structure, whereas AK, AP, and ammonium nitrogen primarily influenced the fungal community in GRS. In MTIS, the total salt content, organic matter, TN, and nitrate nitrogen were identified as significant drivers of fungal community variation. Collectively, these measured soil properties explained 61.62% of the observed variation in fungal community composition across the different soil samples.
Pearson correlation analysis was performed to investigate the relationships between soil physicochemical properties and fungal community α-diversity (
Table 2). The pH of all the samples, except VIS, was below 7.5. Ammonium nitrogen, nitrate nitrogen, AP, and AK showed negative correlations with fungal community α-diversity. Specifically, AP showed strong negative correlations with Chao1 (–0.79,
p < 0.01), ACE (−0.79,
p < 0.01), and Shannon indices (−0.57,
p < 0.05). Similarly, AK exhibited strong negative correlations with Chao1 (−0.80,
p < 0.01), ACE (−0.80,
p < 0.01), and Shannon indices (−0.53,
p < 0.05). These results indicate that fungal community abundance and diversity decreased with increasing AP and AK levels.
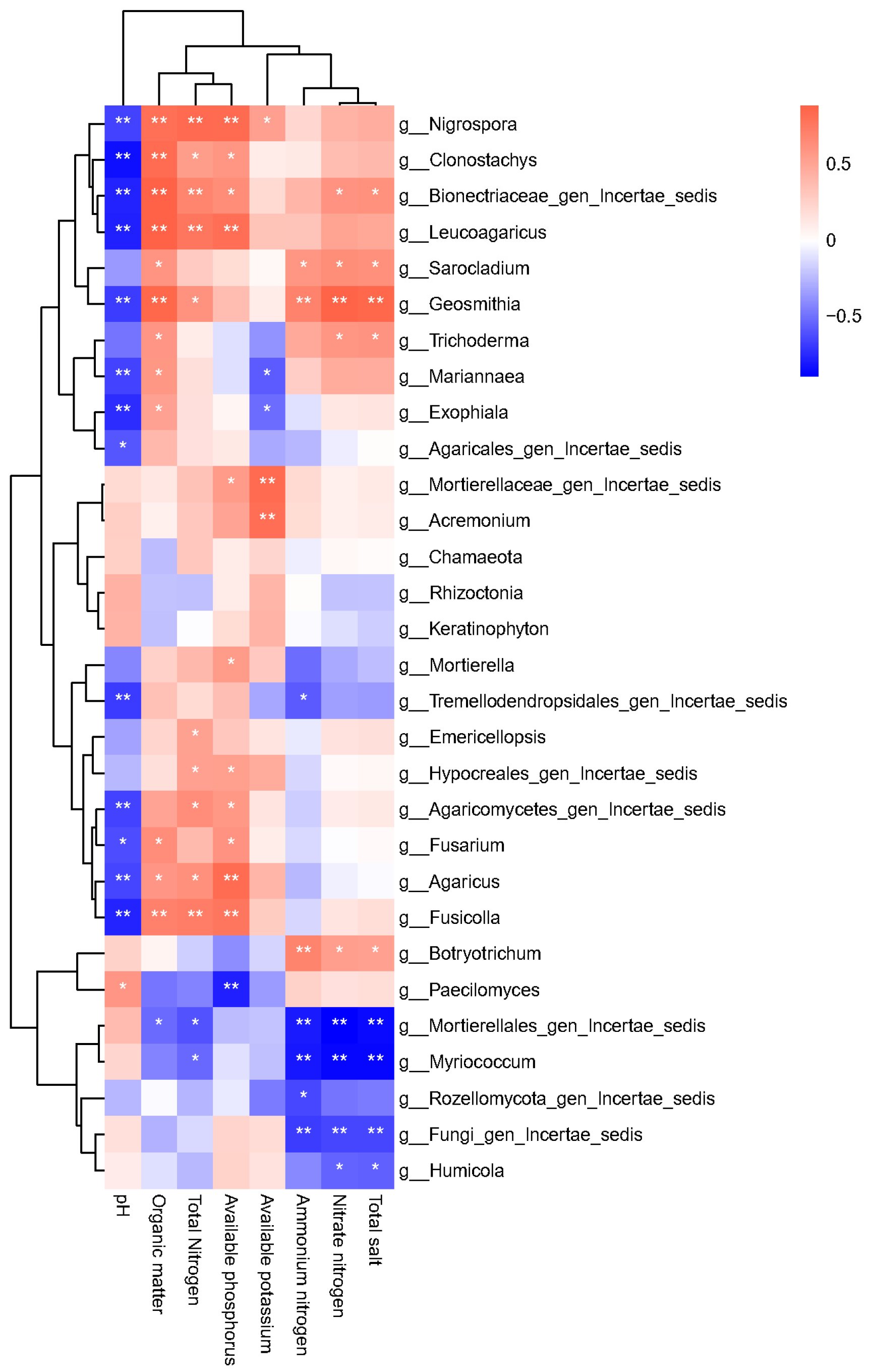
Furthermore, a Spearman correlation analysis was conducted to examine the relationships between the 30 most abundant fungal genera and soil physicochemical properties. The results presented in
Figure 11 indicate that 27 genera exhibit significant correlations with eight soil properties. Specifically,
Nigrospora,
Clonostachys,
Bionectriaceae, and
Leucoagaricus showed negative correlations with pH, whereas organic matter, total nitrogen, and AP were positively correlated with pH.
Fusarium exhibited a negative correlation with pH.
4. Discussion
Morchella spp. is an indigenous fungal species whose mycelium is densely distributed in soil, resembling a mushroom bed [
19]. Soil serves as a crucial growth medium for
Morchella spp., providing an essential environment for its life cycle. Rich organic matter and diverse microbial communities within soil contribute to increased yields of
Morchella spp. and support ecological restoration and sustainable development [
20]. However, inappropriate cropping practices during soil management have led to the emergence of rotation barriers for
Morchella spp. These unsuitable rotations intensify the incidence of plant diseases and pests, significantly affecting both yield and quality [
21]. Common canker and white mould disease are among the most prevalent fungal infections affecting
Morchella spp. cultivation. Therefore, this study investigated soils cultivated with
Morchella spp. under different cropping patterns using NS as a control. The characteristics of the fungal communities in these soil samples, along with the factors influencing them, were investigated using high-throughput sequencing technology with multiple ecological and statistical correlation analyses. The findings of this research provide a theoretical basis for understanding the causes and potential pathogenic mechanisms of fungal diseases affecting
Morchella spp. under different cropping patterns. Li Xuesong et al. found that the dominant microbial groups in the soil of diseased
Morchella spp. were
Paecilomyces,
Fusarium,
Trichoderma, etc., which were all related groups of macrofungal pathogens. [
22]
The results revealed a significant decrease in fungal community diversity in all the
Morchella spp.-associated soil samples (VIS, GRS, MTIS, and FS) compared with the unplanted control (NS). This reduction in diversity may be attributed to
Morchella spp. becoming the dominant organism during its growth, thereby suppressing the diversity of other background microorganisms. This observation is consistent with previous studies [
23,
24]. Prior research has also reported that approximately 98% of the fungi in soil ecosystems belong to the phyla
Ascomycota and
Basidiomycota, with
Ascomycota being more abundant than
Basidiomycota [
25]. At the phylum level, fungal community structures vary significantly among different cropping patterns. However,
Ascomycota,
Basidiomycota, and
Mortierella are the dominant fungal taxa in all the groups. At the phylum level, the distribution of soil fungi under different cropping patterns is characterised by the presence of common species. However, at the genus level, the fungal community structure and functional types exhibited significant variation. Previous studies have identified fungi that cause physiological diseases in
Morchella spp. rotation, including
Paecilomyces penicillatus, Fusarium solani [
12],
Mortierella [
26], and
Botryotrichum_atrogriseum [
27]. An analysis of the genus level showed that the pathogenic fungi showed a specific enrichment of cultivation patterns: the relative abundance of
Paecilomyces_penicillatus in grape intercropping soil was higher, which may lead to a high incidence of white mould disease by destroying the mycelial structure through infection; the co-occurrence of
Botryotrichum_atrogriseum and
Paecilomyces_sp in ginger rotation soil was significantly increased, which may aggravate rhizosphere micro-damage through synergistic effect. The synergistic pathogenic mode is highly similar to the pathogen interaction network in the continuous cropping obstacle of Panax notoginseng [
28]. These findings further verify the applicability of the “pathogen complex” theory in the continuous cropping system of edible fungi. In both FS and MTIS, functional prediction analysis further revealed an increase in pathogenic fungi, particularly animal and plant pathogens, in all the soil samples except VIS compared with natural soil (NS). This trend was especially pronounced in GRS. The abundance of pathogenic fungi exerts stress on rhizosphere cells, affecting the growth of aerial plant parts and leading to significant rotation barriers.
Yinglian et al. [
29] found that alterations in soil physicochemical properties are a key factor that contributes to crop rotation challenges. The occurrence of
Morchella spp. is closely linked to soil mineral element content. Zhao Yongchang et al. [
30] investigated mineral element concentrations in soils from the origin of
Morchella spp. and concluded that high levels of phosphorus and potassium are essential for its growth. In this study, FS exhibited the lowest levels of AP and AK, indicating that the application of phosphorus and potassium fertilisers in later stages could help maintain the nutrient balance and support
Morchella spp. cultivation. The research by Tan Hao’s team revealed the complex regulation mechanism of nitrogen forms in the cultivation system of Morchella. It was found that in the conventional cultivation system, the ratio of ammonium nitrogen to nitrate nitrogen was significantly negatively correlated with yield, which may be due to the fact that nitrate nitrogen was more conducive to maintaining soil pH stability and promoting the absorption of trace elements by mycelia. It is worth noting that except for the grape intercropping system, the increase in nitrate nitrogen in the other cultivation systems was significantly higher than that of ammonium nitrogen, but there was a unique reverse response in the grape intercropping system. For this special phenomenon, it is speculated that it may be due to the secretion of specific phenolic acids (such as phlorizin, caffeic acid, etc.) by grape roots. Based on the above findings, it is recommended to implement a nitrate nitrogen supplementation strategy in the grape intercropping system, which can not only improve the imbalance of nitrogen forms, but also alleviate the continuous cropping obstacles.
5. Conclusions
This study integrated the analysis of fungal diversity and soil physicochemical properties to further elucidate the factors driving fungal community changes. The results indicate that soil pH has a significant impact on fungal community structure. Weakly acidic soil conditions are generally considered favourable for the growth of harmful fungi, such as
Fusarium, while alkaline soils tend to inhibit their growth [
31]. This phenomenon is closely related to the pH-dependent physiological characteristics of fungi: the activity of pathogenic factors such as pectinase and cellulose produced by
Fusarium spp. is significantly enhanced in a weakly acidic environment [
32]. In addition, the alkaline environment induces the proliferation of chitinase-producing bacteria to form biological antagonism [
33]. The correlation analysis between soil fungi and chemical elements indicated a significantly negative correlation between
Fusarium and pH. This observation aligns with previous studies. The Pearson correlation analysis between the α-diversity of fungal communities and soil physicochemical properties revealed that the pH of all the soil samples, excluding VIS, was below 7.5. It is speculated that the increase in the
Fusarium solani content in MTIS and FS is related to pH. This verifies the research by Song et al. (2022) on the correlation between
Morchella spp. disease and soil acidification [
34]. It is recommended to apply lime (CaCO
3) in the mulberry intercropping system to increase the pH to the range of 7.2–7.5, which can not only inhibit the growth of
Fusarium, but also maintain the neutral microenvironment required by
Morchella spp. so as to achieve the synergistic effect of disease prevention and control and yield improvement. Tang Jie et al. [
35] reported that
Morchella spp. continuously accumulates secondary metabolites by absorbing soil mineral elements, which promotes its growth while altering the structure of soil microorganisms, thereby influencing its yield. Variations in soil organic matter, nitrogen, and other nutrients can lead to differences in the relative abundance of fungal phyla, subsequently causing changes in the fungal community structure [
36]. These findings align with the conclusions of this study, which demonstrated significant variations in the organic matter, TN, ammonium nitrogen, nitrate nitrogen, AP, AK, and total salt content under the different cropping patterns. Such differences may substantially impact the fungal community. These research results will contribute to a deeper understanding of soil microorganisms in
Morchella spp. cultivation.
Given that many fungal species are also pathogenic, the disease management of
Morchella spp. cultivation is challenging. The established fungal diseases are often difficult to treat, highlighting the critical importance of preventative measures [
37]. This study employed high-throughput amplicon sequencing and bioinformatics analysis to investigate the relationship between fungal diversity and the physicochemical properties of soil cultivated with
Morchella spp. By analysing these data, the study aimed to identify the potential disease pressures on
Morchella spp. under different cropping regimes. Furthermore, the research sought to identify the potential fungal pathogens of
Morchella spp., providing a basis for understanding disease development and mechanisms. These findings can contribute to improved disease management strategies and promote the sustainable development of the
Morchella spp. industry.