Crop Cultivation Reshapes Soil Microbiomes to Drive Heavy Metal Mobilization in Restored Mining Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Chemical Analysis
2.3. High-Throughput Sequencing of the V4 Region of 16S rRNA Genes
2.4. Statistical Analysis
3. Results
3.1. Geochemical Compositions in Farmland and Grassland Soils
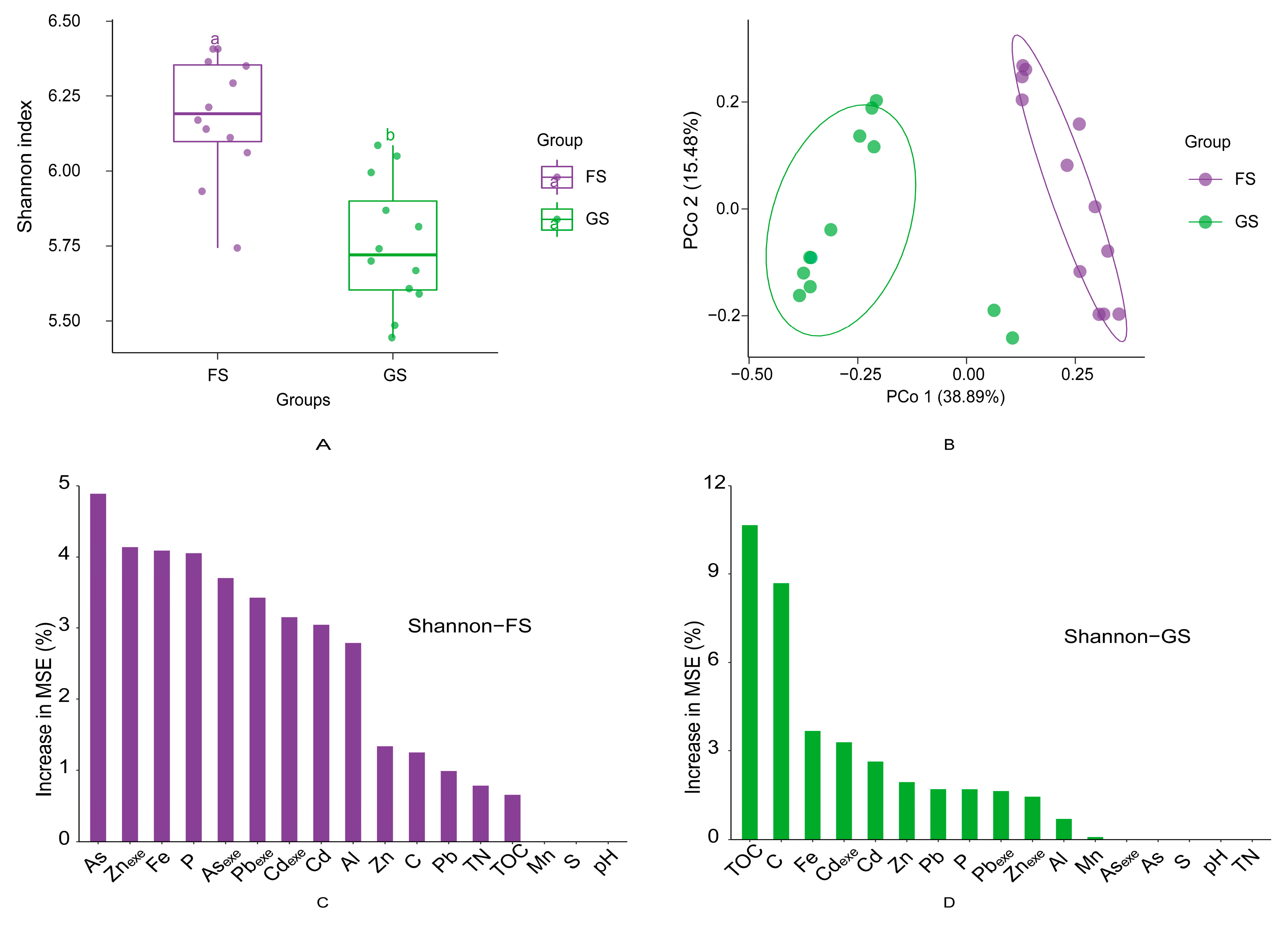
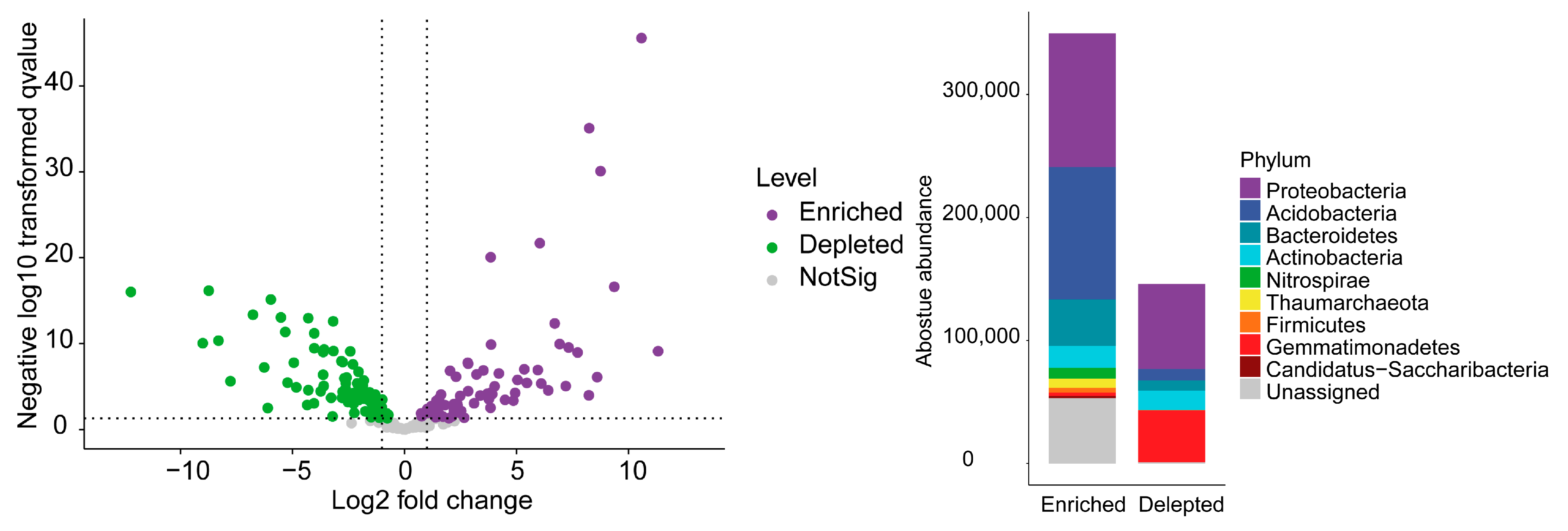
3.2. Microbial Diversity and Taxon Enrichment in the Farmland and Grassland Soils
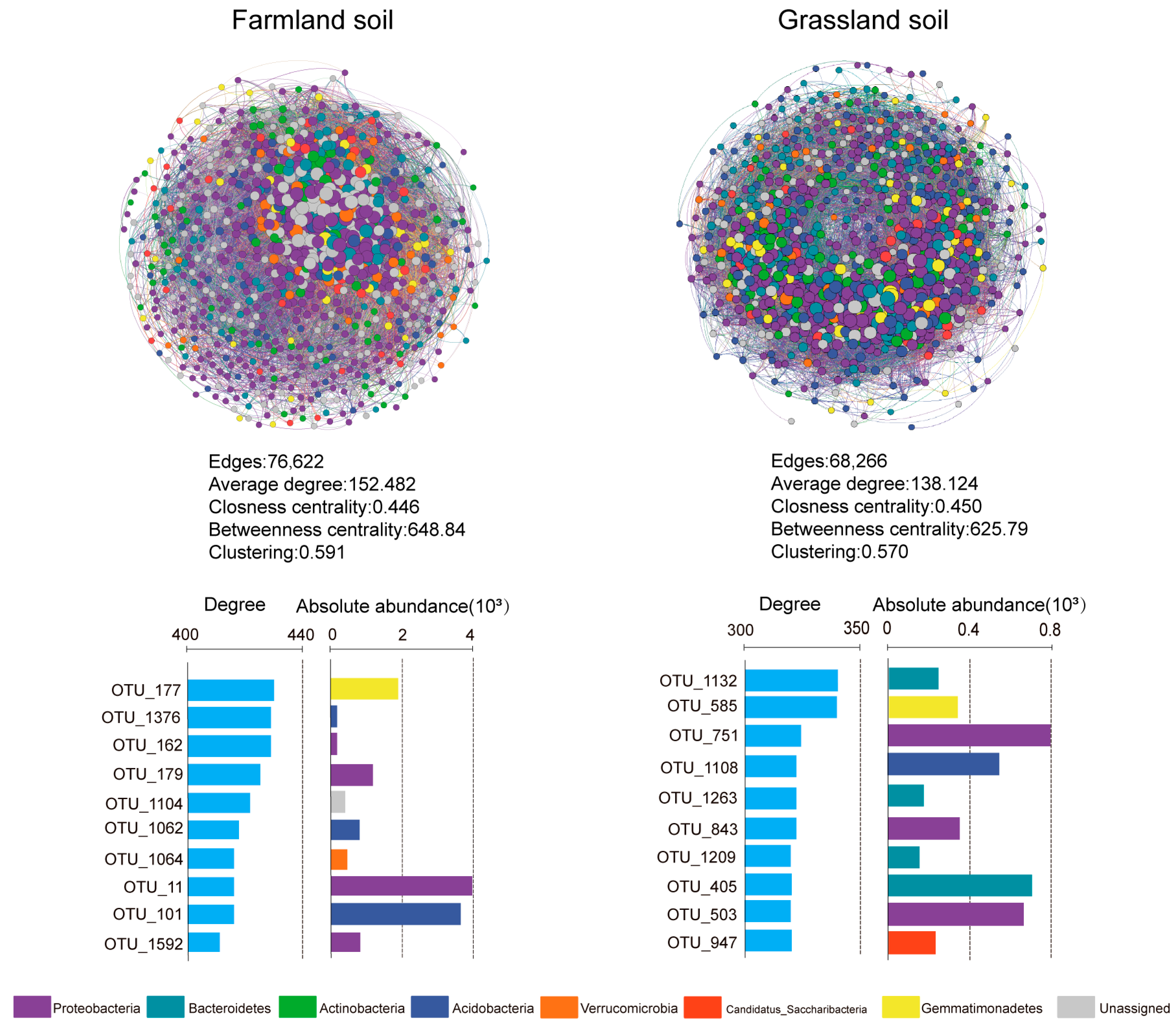
3.3. Microbiota Co-Occurrence Networks and Keystone Taxa in the Farmland and Grassland Soils
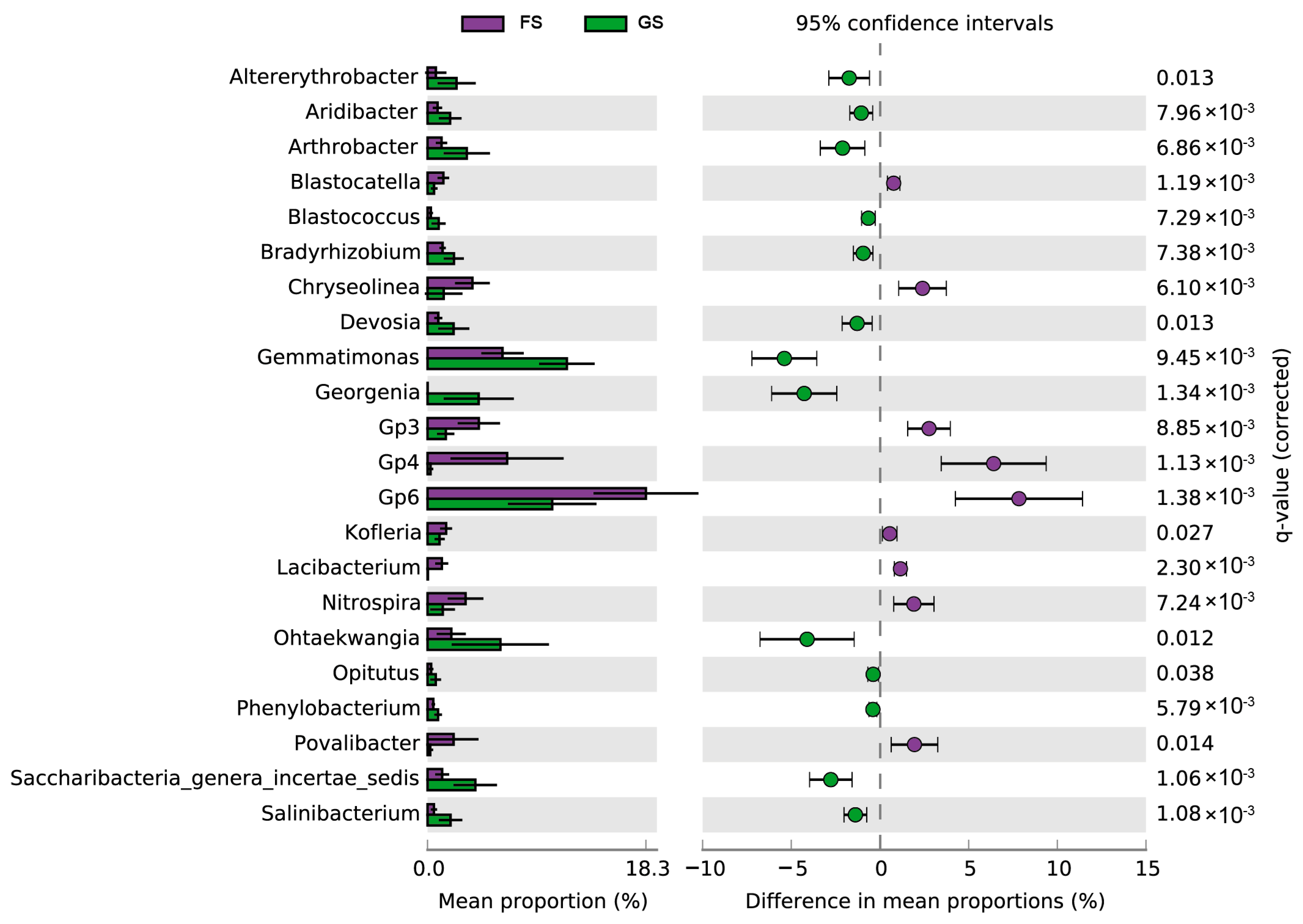
3.4. Distribution Pattern of Enriched Genera in the Farmland and Grassland Soils
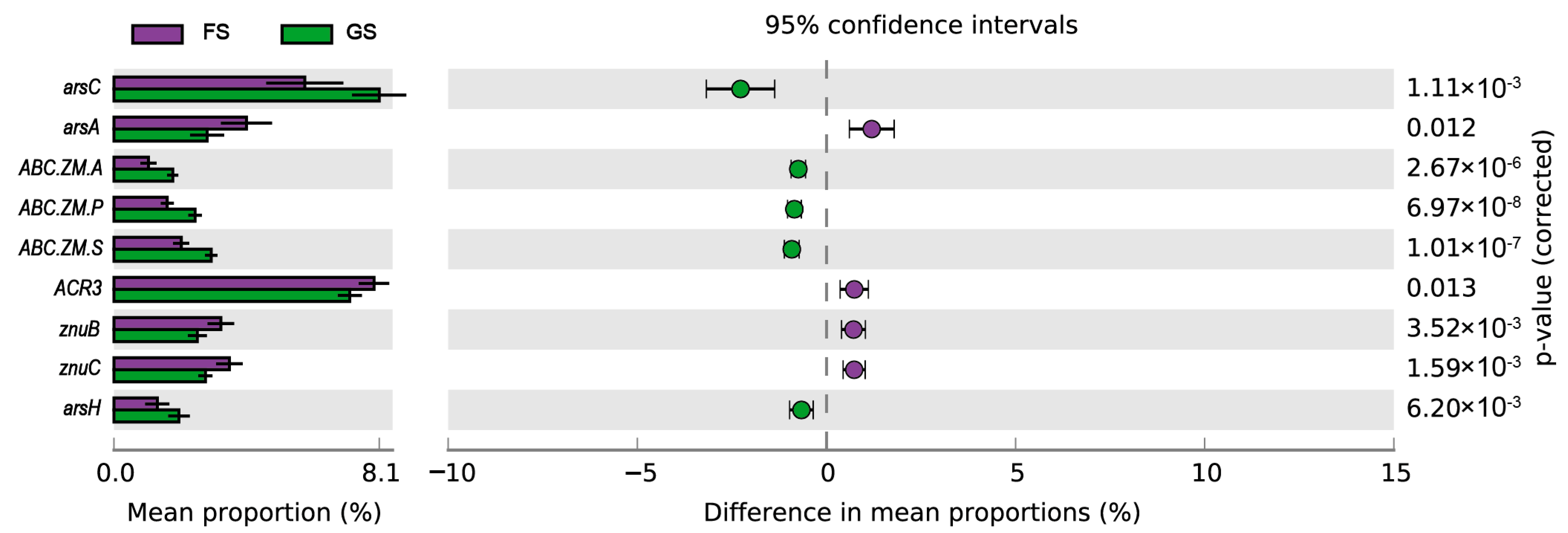
3.5. Predicted Metabolic Functions in the Farmland and Grassland Soils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Wen, X.; Zhou, J.; Lu, Z.; Li, X.; Yan, B. A critical review on the migration and transformation processes of heavy metal contamination in lead-zinc tailings of China. Environ. Pollut. 2023, 338, 122667. [Google Scholar] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.-Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil—Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar]
- Liu, X.; Bai, Z.; Shi, H.; Zhou, W.; Liu, X. Heavy metal pollution of soils from coal mines in China. Nat. Hazards 2019, 99, 1163–1177. [Google Scholar]
- Xiao, T.; Yang, F.; Li, S.; Zheng, B.; Ning, Z. Thallium pollution in China: A geo-environmental perspective. Sci. Total Environ. 2012, 421–422, 51–58. [Google Scholar]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Wen, T.; Meng, F.; Chen, G.; Qian, X. Influence of ferrous-metal production on mercury contamination and fractionation in farmland soil around five typical iron and steel enterprises of Tangshan, China. Ecotoxicol. Environ. Saf. 2020, 188, 109774. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, X.; Qi, L.; Li, L.; Guo, J.; Zhong, H.; Liu, J.; Huang, J. Nitrogen and Phosphorus Fertilizer Increases the Uptake of Soil Heavy Metal Pollutants by Plant Community. Bull. Environ. Contam. Toxicol. 2022, 109, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, M.; Li, J.; Chen, D.; Ye, T.; Wang, C.; Yan, F.; Qiu, Z. Soil microbial community variation in vanadium-enriched farmland surrounding vanadium titanomagnetite tailing in Southwest China. Appl. Soil. Ecol. 2024, 198, 105318. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Guo, Z.; Shi, C.; Wang, J. A Study of the Differences in Heavy Metal Distributions in Different Types of Farmland in a Mining Area. Bull. Environ. Contam. Toxicol. 2022, 109, 788–798. [Google Scholar] [CrossRef]
- Sun, W.; Sun, X.; Li, B.; Haggblom, M.M.; Han, F.; Xiao, E.; Zhang, M.; Wang, Q.; Li, F. Bacterial response to antimony and arsenic contamination in rice paddies during different flooding conditions. Sci. Total Environ. 2019, 675, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef] [PubMed]
- Lücker, S.; Wagner, M.; Maixner, F.; Pelletier, E.; Koch, H.; Vacherie, B.; Rattei, T.; Damsté, J.S.S.; Spieck, E.; Le Paslier, D.; et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 13479–13484. [Google Scholar] [CrossRef]
- Han, S.; Huang, Q.; Chen, W. Partitioning Nitrospira community structure and co-occurrence patterns in a long-term inorganic and organic fertilization soil. J. Soils Sediments 2021, 21, 1099–1108. [Google Scholar] [CrossRef]
- Wu, J.; Ma, C.; Li, F. Microbial community structure and function in paddy soil as affected by water-saving irrigation mode. Eur. J. Soil. Biol. 2022, 113, 103450. [Google Scholar] [CrossRef]
- de Menezes, A.B.; Prendergast-Miller, M.T.; Richardson, A.E.; Toscas, P.; Farrell, M.; Macdonald, L.M.; Baker, G.; Wark, T.; Thrall, P.H. Network analysis reveals that bacteria and fungi form modules that correlate independently with soil parameters. Environ. Microbiol. 2015, 17, 2677–2689. [Google Scholar] [PubMed]
- Li, Y.; Yang, R.; Häggblom, M.M.; Li, M.; Guo, L.; Li, B.; Kolton, M.; Cao, Z.; Soleimani, M.; Chen, Z.; et al. Characterization of diazotrophic root endophytes in Chinese silvergrass (Miscanthus sinensis). Microbiome 2022, 10, 186. [Google Scholar]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.-T.; Weigel, D.; Kemen, E.M. Microbial Hub Taxa Link Host and Abiotic Factors to Plant Microbiome Variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar]
- Bi, X.; Feng, X.; Yang, Y.; Li, X.; Sin, G.P.Y.; Qiu, G.; Qian, X.; Li, F.; He, T.; Li, P.; et al. Heavy metals in an impacted wetland system: A typical case from southwestern China. Sci. Total Environ. 2007, 387, 257–268. [Google Scholar]
- Bi, X.; Feng, X.; Yang, Y.; Qiu, G.; Li, G.; Li, F.; Liu, T.; Fu, Z.; Jin, Z. Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environ. Int. 2006, 32, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, Z.; Bi, X.; Han, Z.; Yu, G. Response of magnetic properties to heavy metal pollution in dust from three industrial cities in China. J. Hazard. Mater. 2013, 246–247, 189–198. [Google Scholar]
- Han, J.; Liang, L.; Zhu, Y.; Xu, X.; Wang, L.; Shang, L.; Wu, P.; Wu, Q.; Qian, X.; Qiu, G.; et al. Heavy metal(loid)s in farmland soils on the Karst Plateau, Southwest China: An integrated analysis of geochemical baselines, source apportionment, and associated health risk. Land Degrad. Dev. 2022, 33, 1689–1703. [Google Scholar]
- Liu, Y.; Xiao, T.; Ning, Z.; Li, H.; Tang, J.; Zhou, G. High cadmium concentration in soil in the Three Gorges region: Geogenic source and potential bioavailability. Appl. Geochem. 2013, 37, 149–156. [Google Scholar]
- Xiao, E.; Krumins, V.; Dong, Y.; Xiao, T.; Ning, Z.; Xiao, Q.; Sun, W. Microbial diversity and community structure in an antimony-rich tailings dump. Appl. Microbiol. Biot. 2016, 100, 7751–7763. [Google Scholar]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to Analyze 16S rRNA Gene Sequences from Microbial Communities. Curr. Protoc. Bioinform. 2011, 36, 10.17.1–10.17.20. [Google Scholar]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.-X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar]
- Sun, X.; Li, B.; Han, F.; Xiao, E.; Wang, Q.; Xiao, T.; Sun, W. Vegetation type impacts microbial interaction with antimony contaminants in a mining-contaminated soil environment. Environ. Pollut. 2019, 252, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Ju, F.; Hou, L.; Li, J.; Yang, X.; Wang, H.; Mulla, S.I.; Sun, Q.; Bürgmann, H.; Yu, C.-P. Strong impact of anthropogenic contamination on the co-occurrence patterns of a riverine microbial community. Environ. Microbiol. 2017, 19, 4993–5009. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Li, J.; Bu, P.; Lu, A.; Wang, H.; He, W.; Santos Bermudez, R.; Feng, J. Consecutive Fertilization-Promoted Soil Nutrient Availability and Altered Rhizosphere Bacterial and Bulk Fungal Community Composition. Forests 2024, 15, 514. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Xiao, T.; Krumins, V.; Wang, Q.; Häggblom, M.; Dong, Y.; Tang, S.; Hu, M.; Li, B.; et al. Response of Soil Microbial Communities to Elevated Antimony and Arsenic Contamination Indicates the Relationship between the Innate Microbiota and Contaminant Fractions. Environ. Sci. Technol. 2017, 51, 9165–9175. [Google Scholar]
- Xiao, E.; Wang, Y.; Xiao, T.; Sun, W.; Deng, J.; Jiang, S.; Fan, W.; Tang, J.; Ning, Z. Microbial community responses to land-use types and its ecological roles in mining area. Sci. Total Environ. 2021, 775, 145753. [Google Scholar] [CrossRef]
- Haque, S.; Srivastava, N.; Pal, D.B.; Alkhanani, M.F.; Almalki, A.H.; Areeshi, M.Y.; Naidu, R.; Gupta, V.K. Functional microbiome strategies for the bioremediation of petroleum-hydrocarbon and heavy metal contaminated soils: A review. Sci. Total Environ. 2022, 833, 155222. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Fang, Y.; Yin, H.; Fu, S.; Chang, S.X.; Cai, Y. Distinct effects of canopy vs understory and organic vs inorganic N deposition on root resource acquisition strategies of subtropical Moso bamboo plants. Sci. Total Environ. 2024, 927, 172424. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Davila Costa, J.S.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Pu, Z.; Krumins, V.; Dong, Y.; Li, B.; Hu, M. Paddy soil microbial communities driven by environment- and microbe-microbe interactions: A case study of elevation-resolved microbial communities in a rice terrace. Sci. Total Environ. 2018, 612, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhai, W.; Li, P.; Xiong, Y.; Li, H.; Liu, X.; Zhou, Z.; Li, B.; Wang, P.; Liu, D. Nitrogen fertiliser-domesticated microbes change the persistence and metabolic profile of atrazine in soil. J. Hazard. Mater. 2024, 469, 133974. [Google Scholar] [CrossRef]
- Xu, R.; Sun, X.; Häggblom, M.M.; Dong, Y.; Zhang, M.; Yang, Z.; Xiao, E.; Xiao, T.; Gao, P.; Li, B.; et al. Metabolic potentials of members of the class Acidobacteriia in metal-contaminated soils revealed by metagenomic analysis. Environ. Microbiol. 2022, 24, 803–818. [Google Scholar] [CrossRef]
- Lan, X.; Lin, W.; Ning, Z.; Su, X.; Chen, Y.; Jia, Y.; Xiao, E. Arsenic shapes the microbial community structures in tungsten mine waste rocks. Environ. Res. 2023, 216, 114573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, G.; Li, Z.; Xu, F.; Yang, N.; Sun, X.; Xu, R.; Sun, W. Effects of antimony on anaerobic methane oxidization and microbial community in an antimony-contaminated paddy soil: A microcosm study. Sci. Total Environ. 2021, 784, 147239. [Google Scholar] [CrossRef]
- Xu, R.; Li, B.; Xiao, E.; Young, L.Y.; Sun, X.; Kong, T.; Dong, Y.; Wang, Q.; Yang, Z.; Chen, L.; et al. Uncovering microbial responses to sharp geochemical gradients in a terrace contaminated by acid mine drainage. Environ. Pollut. 2020, 261, 114226. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Zhang, H.; Geng, R.; Huang, Y.; Yang, J.-Y.; Teng, Y. Ecological and Health Risks of Vanadium in the Biogeosphere. Rev. Environ. Contam. Toxicol. 2024, 262, 13. [Google Scholar]
- Gu, J.; Guo, F.; Lin, L.; Zhang, J.; Sun, W.; Muhammad, R.; Liang, H.; Duan, D.; Deng, X.; Lin, Z.; et al. Microbiological mechanism for “production while remediating” in Cd-contaminated paddy fields: A field experiment. Sci. Total Environ. 2023, 885, 163896. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, M.; Xu, R.; Lin, H.; Sun, X.; Xu, F.; Gao, P.; Kong, T.; Xiao, E.; Yang, N.; et al. Arsenic and antimony co-contamination influences on soil microbial community composition and functions: Relevance to arsenic resistance and carbon, nitrogen, and sulfur cycling. Environ. Int. 2021, 153, 106522. [Google Scholar] [PubMed]
- Guseva, K.; Darcy, S.; Simon, E.; Alteio, L.V.; Montesinos-Navarro, A.; Kaiser, C. From diversity to complexity: Microbial networks in soils. Soil Biol. Biochem. 2022, 169, 108604. [Google Scholar]
- Jing, X.; Chen, X.; Fang, J.; Ji, C.; Shen, H.; Zheng, C.; Zhu, B. Soil microbial carbon and nutrient constraints are driven more by climate and soil physicochemical properties than by nutrient addition in forest ecosystems. Soil Biol. Biochem. 2020, 141, 107657. [Google Scholar]
- Lan, X.; Ning, Z.; Jia, Y.; Lin, W.; Xiao, E.; Cheng, Q.; Cai, Q.; Xiao, T. The rhizosphere microbiome reduces the uptake of arsenic and tungsten by Blechnum orientale by increasing nutrient cycling in historical tungsten mining area soils. Sci. Total Environ. 2024, 924, 171429. [Google Scholar]
- Wang, X.; Teng, Y.; Wang, X.; Li, X.; Luo, Y. Microbial diversity drives pyrene dissipation in soil. Sci. Total Environ. 2022, 819, 153082. [Google Scholar]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar]
- Zhang, B.; Zhang, H.; He, J.; Zhou, S.; Dong, H.; Rinklebe, J.; Ok, Y.S. Vanadium in the Environment: Biogeochemistry and Bioremediation. Environ. Sci. Technol. 2023, 57, 14770–14786. [Google Scholar]
- Li, B.; Xu, R.; Sun, X.; Han, F.; Xiao, E.; Chen, L.; Qiu, L.; Sun, W. Microbiome–environment interactions in antimony-contaminated rice paddies and the correlation of core microbiome with arsenic and antimony contamination. Chemosphere 2021, 263, 128227. [Google Scholar] [CrossRef]
- Fei, Y.; Zhang, B.; He, J.; Chen, C.; Liu, H. Dynamics of vertical vanadium migration in soil and interactions with indigenous microorganisms adjacent to tailing reservoir. J. Hazard. Mater. 2022, 424, 127608. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, J.; Chen, J.; Chen, D.; Deng, Q. The role of available phosphorous in vanadate decontamination by soil indigenous microbial consortia. Environ. Pollut. 2021, 289, 117839. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Q.; Min, J.; Zhang, S.; Li, S.; Chen, Y.; Dai, J. Specific bacterial communities in the rhizosphere of low-cadmium and high-zinc wheat (Triticum aestivum L.). Sci. Total Environ. 2022, 838, 156484. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.-J.; Kim, Y.-J.; Cui, Y.; Nam, K.-H. Ecological network analysis reveals distinctive microbial modules associated with heavy metal contamination of abandoned mine soils in Korea. Environ. Pollut. 2021, 289, 117851. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, S.; Chen, Y.; Luo, J.; Hao, B.; Zhang, Z.; Yang, B.; Guo, W. Bio-organic fertilizer promoted phytoremediation using native plant leymus chinensis in heavy Metal(loid)s contaminated saline soil. Environ. Pollut. 2023, 327, 121599. [Google Scholar] [CrossRef]
- Gong, X.; Shi, W.; Zhang, Z.; Luo, M.; Zhang, B.; Wan, S.; Huang, J. Exploring the effects of zeolite, biochar, and diatomite as additives for enhancing heavy metals passivation and eliminating antibiotic resistance genes in composts during vermicomposting of dewatered sludge and green waste. J. Environ. Chem. Eng. 2024, 12, 112201. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, Q.; Jiang, Z.; Yan, H.; Nian, Y. Pollution characteristics and microbial community succession of a rural informal landfill in an arid climate. Ecotoxicol. Environ. Saf. 2023, 262, 115295. [Google Scholar] [CrossRef]
- Dia, A.; Lauga, B.; Davranche, M.; Fahy, A.; Duran, R.; Nowack, B.; Petitjean, P.; Henin, O.; Martin, S.; Marsac, R.; et al. Bacteria-mediated reduction of As(V)-doped lepidocrocite in a flooded soil sample. Chem. Geol. 2015, 406, 34–44. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Huang, H.; Yang, F. Deciphering soil bacterial community structure in subsidence area caused by underground coal mining in arid and semiarid area. Appl. Soil Ecol. 2021, 163, 103916. [Google Scholar] [CrossRef]
- Sun, X.; Song, B.; Xu, R.; Zhang, M.; Gao, P.; Lin, H.; Sun, W. Root-associated (rhizosphere and endosphere) microbiomes of the Miscanthus sinensis and their response to the heavy metal contamination. J. Environ. Sci. 2021, 104, 387–398. [Google Scholar] [CrossRef] [PubMed]
- de Chaves, M.G.; Silva, G.G.Z.; Rossetto, R.; Edwards, R.A.; Tsai, S.M.; Navarrete, A.A. Acidobacteria Subgroups and Their Metabolic Potential for Carbon Degradation in Sugarcane Soil Amended With Vinasse and Nitrogen Fertilizers. Front. Microbiol. 2019, 10, 1680. [Google Scholar] [CrossRef]
- Ke, T.; Zhang, D.; Guo, H.; Xiu, W.; Zhao, Y. Geogenic arsenic and arsenotrophic microbiome in groundwater from the Hetao Basin. Sci. Total Environ. 2022, 852, 158549. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Tang, Z.; Hou, B.; Ren, B.; Wang, Z.; Zhu, C.; Kelly, S.; Hursthouse, A. Microbial diversity in soils from antimony mining sites: Geochemical control promotes species enrichment. Environ. Chem. Lett. 2020, 18, 911–922. [Google Scholar] [CrossRef]
- Liu, C.; Lin, H.; He, P.; Li, X.; Geng, Y.; Tuerhong, A.; Dong, Y. Peat and bentonite amendments assisted soilless revegetation of oligotrophic and heavy metal contaminated nonferrous metallic tailing. Chemosphere 2022, 287, 132101. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Cheng, H.; Zhou, P.; Huo, Y.-Y.; Wang, C.-S.; Xu, X.-W. Complete genome sequence of the heavy metal resistant bacterium Altererythrobacter atlanticus 26DY36T, isolated from deep-sea sediment of the North Atlantic Mid-ocean ridge. Mar. Genom. 2015, 24, 289–292. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, R.; Sarkodie, E.K.; Tang, J.; Jiang, L.; Miao, B.; Liu, X.; Zhang, S. Insight into functional microorganisms in wet–dry conversion to alleviate the toxicity of chromium fractions in red soil. Front. Microbiol. 2022, 13, 977171. [Google Scholar] [CrossRef]
- Dary, M.; Chamber-Pérez, M.A.; Palomares, A.J.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Banasiewicz, J.; Rekosz-Burlaga, H.; Stępkowski, T. Anthyllis vulneraria and Lotus corniculatus on calamine heaps form nodules with Bradyrhizobium liaoningense-related strains harboring novel in Europe symbiotic nifD haplotypes. Appl. Soil Ecol. 2020, 151, 103539. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Wevar Oller, A.L.; Medina, M.I.; Agostini, E. Effect of arsenic on tolerance mechanisms of two plant growth-promoting bacteria used as biological inoculants. J. Environ. Sci. 2015, 33, 203–210. [Google Scholar] [CrossRef]
- CNEMC (the Chinese Environmental Monitoring Centre). The Background Values of Soil Elements in China; China Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Liao, X.; Xiao, J.; Jia, Y.; Lin, W.; Huang, Z.; Ning, Z.; Xiao, T.; Xiao, E. Crop Cultivation Reshapes Soil Microbiomes to Drive Heavy Metal Mobilization in Restored Mining Areas. Agriculture 2025, 15, 804. https://doi.org/10.3390/agriculture15080804
Lan X, Liao X, Xiao J, Jia Y, Lin W, Huang Z, Ning Z, Xiao T, Xiao E. Crop Cultivation Reshapes Soil Microbiomes to Drive Heavy Metal Mobilization in Restored Mining Areas. Agriculture. 2025; 15(8):804. https://doi.org/10.3390/agriculture15080804
Chicago/Turabian StyleLan, Xiaolong, Xinyin Liao, Jiaxin Xiao, Yanlong Jia, Wenjie Lin, Zhongwen Huang, Zengping Ning, Tangfu Xiao, and Enzong Xiao. 2025. "Crop Cultivation Reshapes Soil Microbiomes to Drive Heavy Metal Mobilization in Restored Mining Areas" Agriculture 15, no. 8: 804. https://doi.org/10.3390/agriculture15080804
APA StyleLan, X., Liao, X., Xiao, J., Jia, Y., Lin, W., Huang, Z., Ning, Z., Xiao, T., & Xiao, E. (2025). Crop Cultivation Reshapes Soil Microbiomes to Drive Heavy Metal Mobilization in Restored Mining Areas. Agriculture, 15(8), 804. https://doi.org/10.3390/agriculture15080804





