The Impact of the Natural Grass-Growing Model on the Development of Korla Fragrant Pear Fruit, as Well as Its Influence on Post-Harvest Sugar Metabolism and the Expression of Key Enzyme Genes Involved in Fruit Sugar Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Situation
2.2. Experimental Design
2.3. Determination of Sugar Content
2.3.1. Determination of Glucose Content
- (1)
- Sample processing
- (2)
- Sample Determination
2.3.2. Determination of Fructose Content
- (1)
- Sample Treatment
- (2)
- Sample Determination
2.3.3. Determination of Sucrose Content
- (1)
- Sample processing
- (2)
- Sample Determination
2.3.4. Determination of Sorbitol Content
- (1)
- Sample processing
- (2)
- Sample Determination
2.4. Determination of Enzyme Activities Related to Sugar Metabolism
- (1)
- Crude enzyme solution extraction
- (2)
- Sample Determination
2.5. Extraction of RNA from the Pulp and Peel of Korla Fragrant Pears and Digestion of DNA
2.6. qRT-PCR
2.7. Fuzzy Comprehensive Evaluation Method
- (1)
- Calculate the membership function.
- (2)
- Calculate the weight using the mutation coefficient method (analysis of the degree of correlation with gray theory).
- (3)
- Conduct a comprehensive evaluation of fruit quality based on the weighted coefficients, using Formula (2).
2.8. Modeling Algorithm
2.9. Data Analysis Methods
3. Results
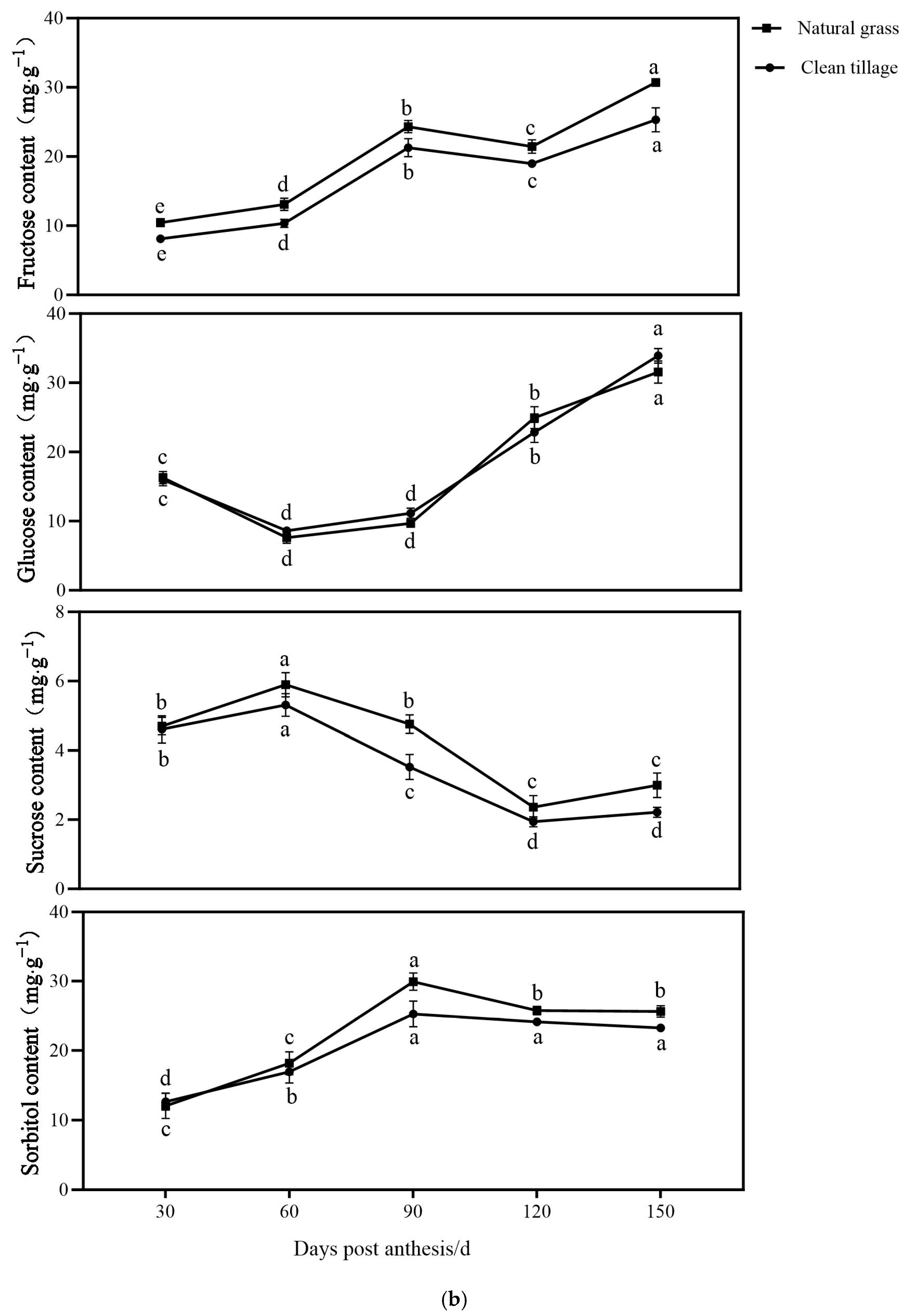
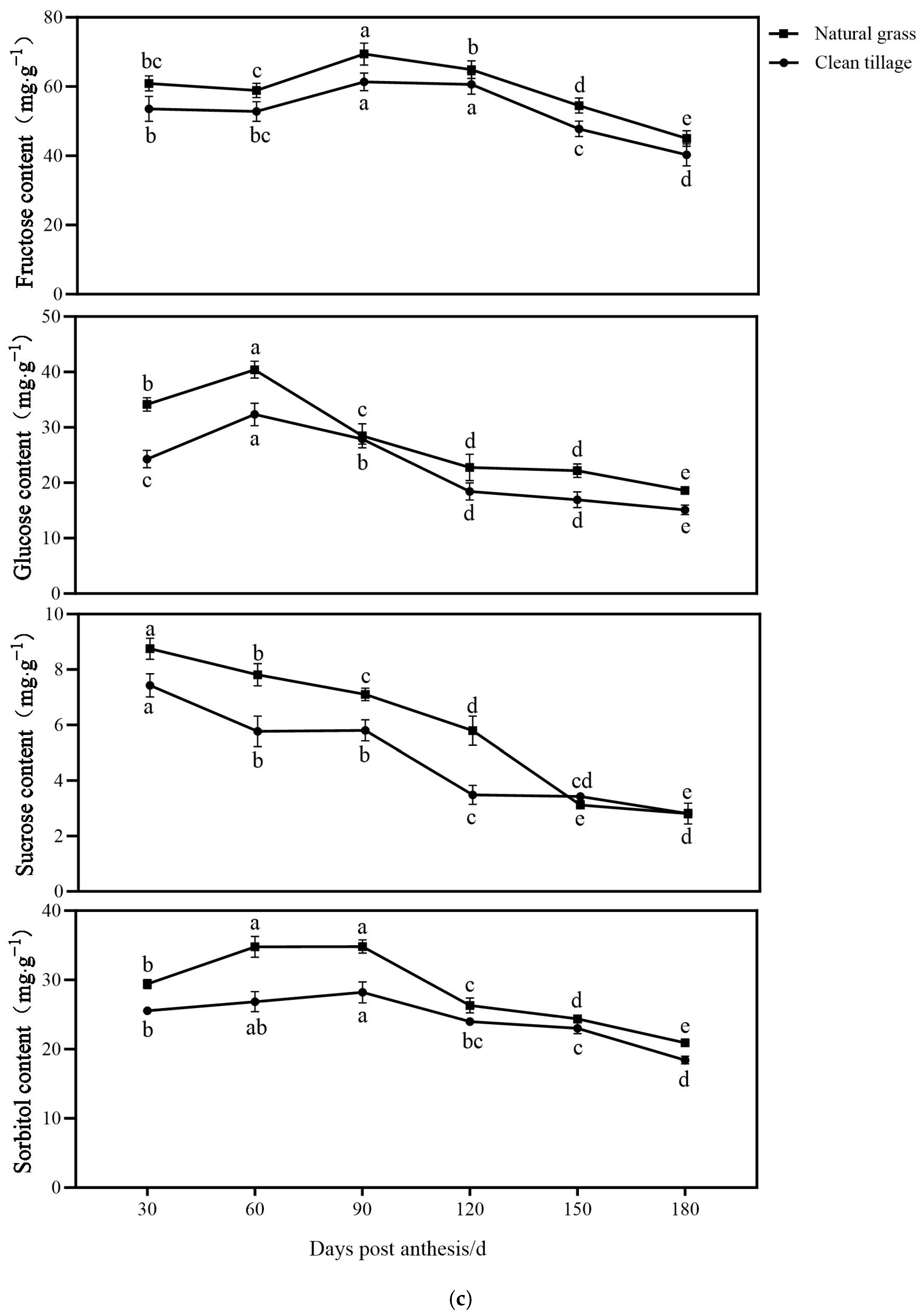
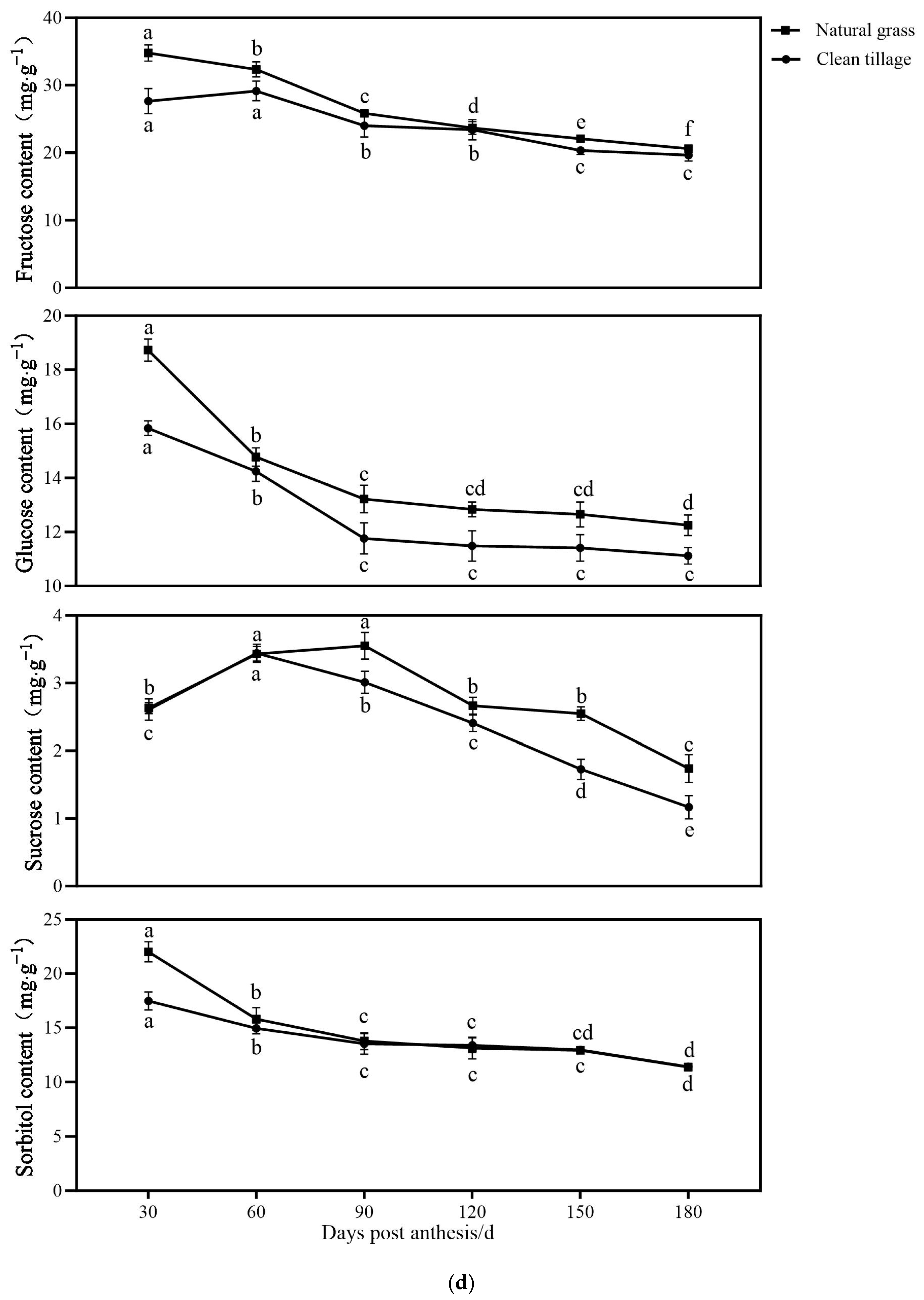
3.1. The Changes in the Fresh Weight of Flesh and Peel Sugar Components of Korla Fragrant Pears During Fruit Development and Storage Under Two Cultivation Patterns
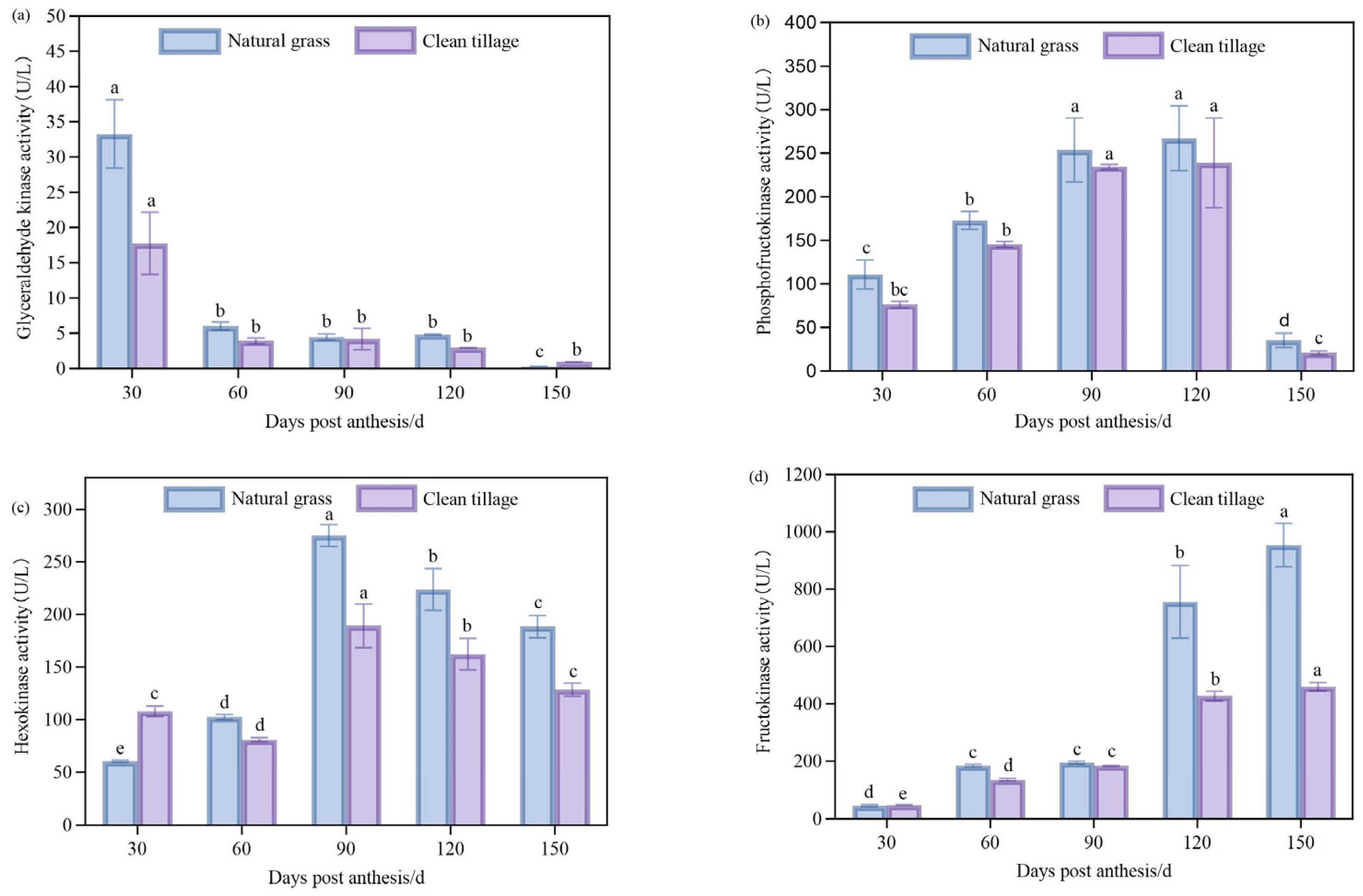
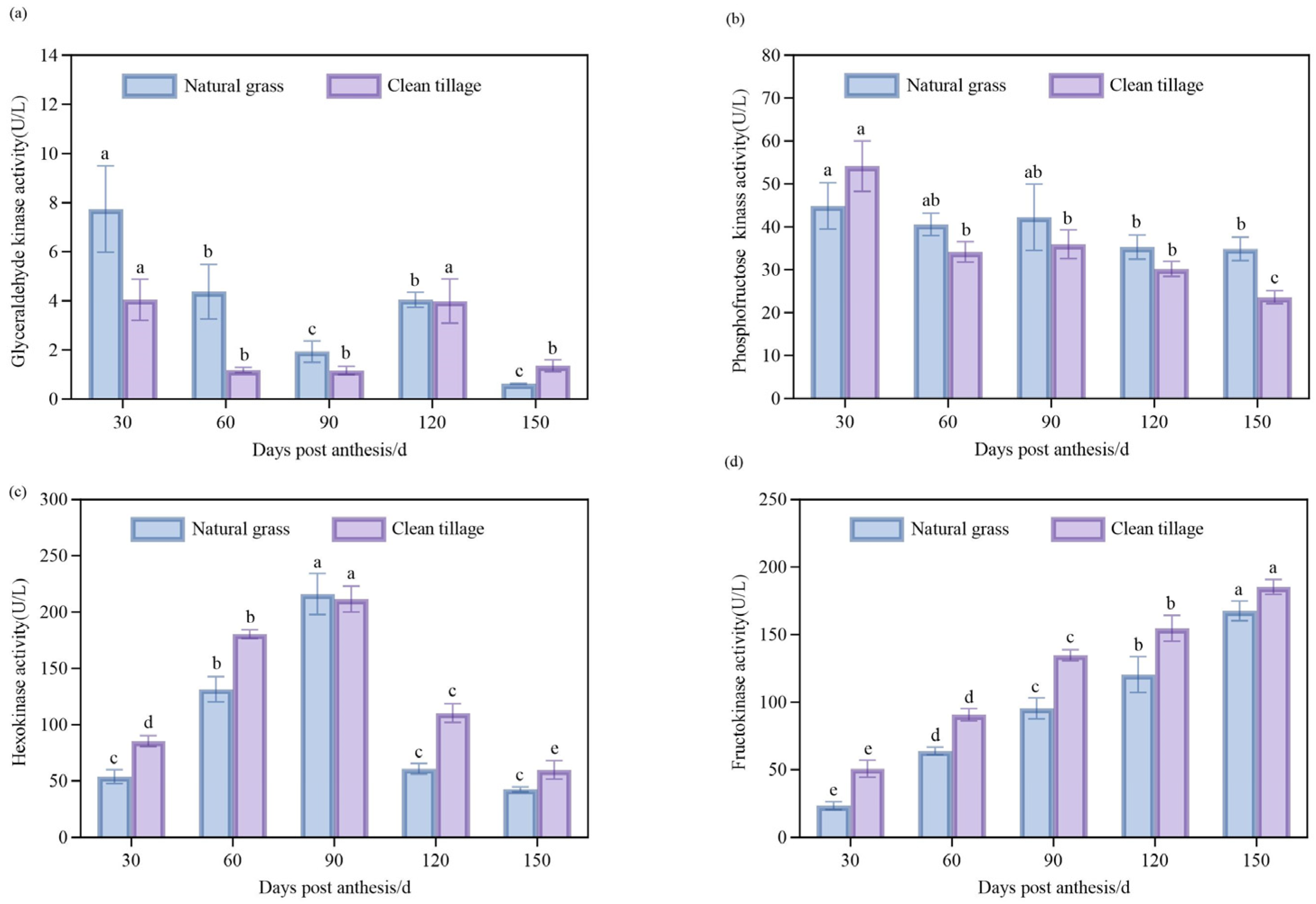
3.2. The Changes in the Enzyme Activities of the Pulp of Korla Fragrant Pears During Fruit Development Under Two Cultivation Modes
3.3. The Changes in the Activity of Enzymes in the Peel of Korla Fragrant Pears During Fruit Development Under Two Cultivation Modes
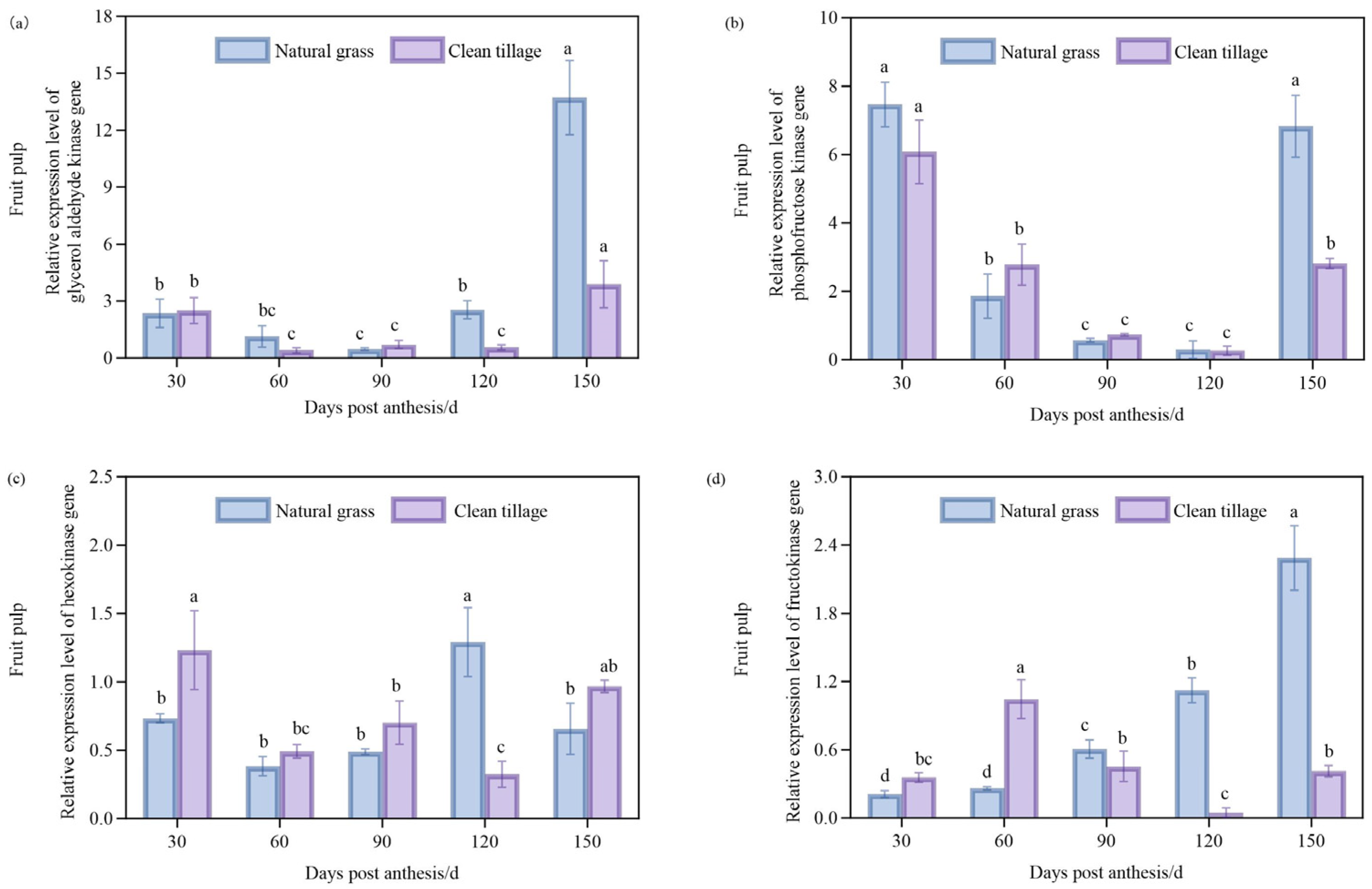
3.4. Changes in Enzyme Gene Expression in Pulp of Korla Fragrant Pear During Fruit Development Under Two Cultivation Modes
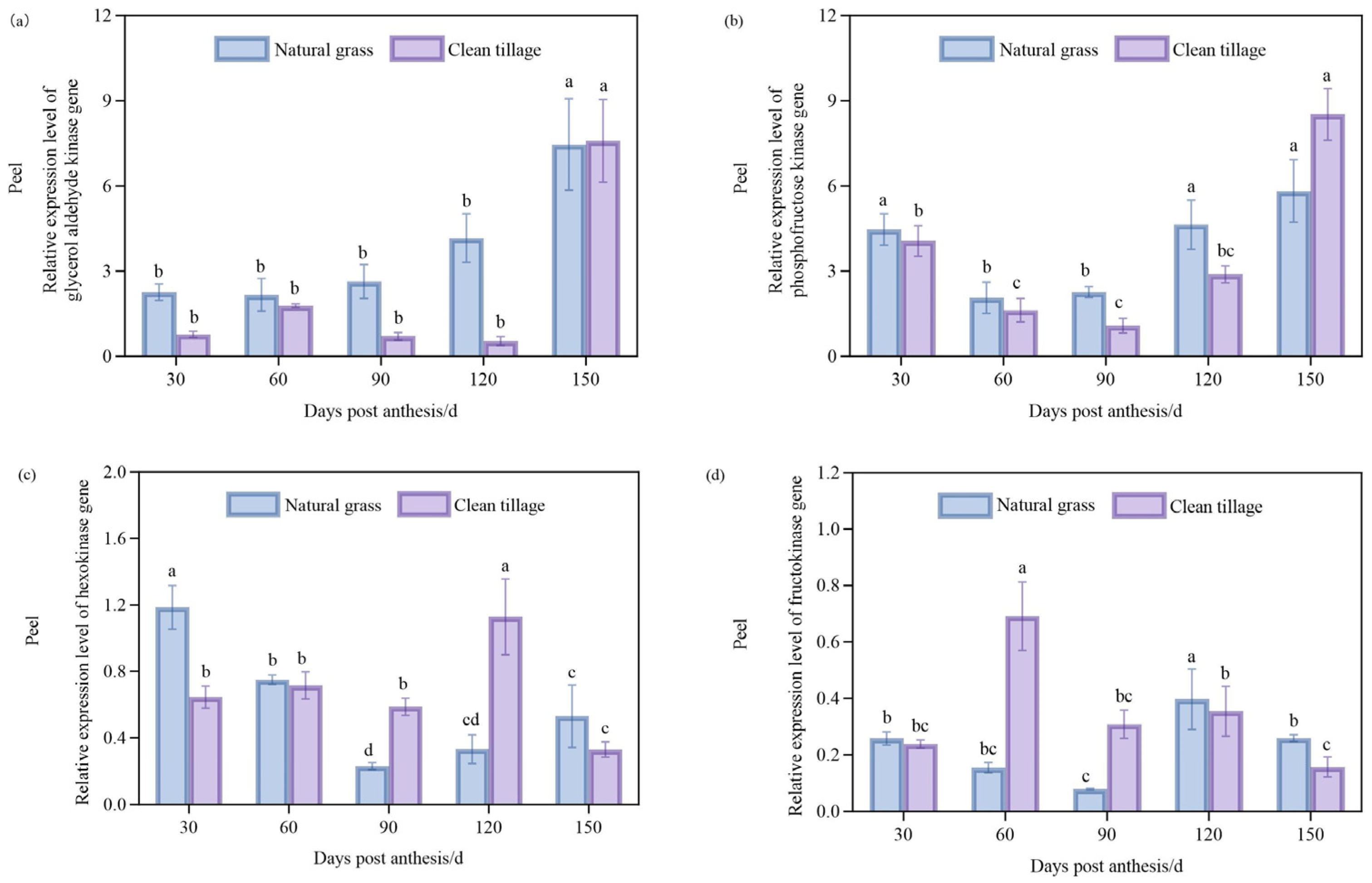
3.5. Changes in Peel Enzyme Gene Expression in Korla Fragrant Pear During Fruit Development Under Two Cultivation Modes
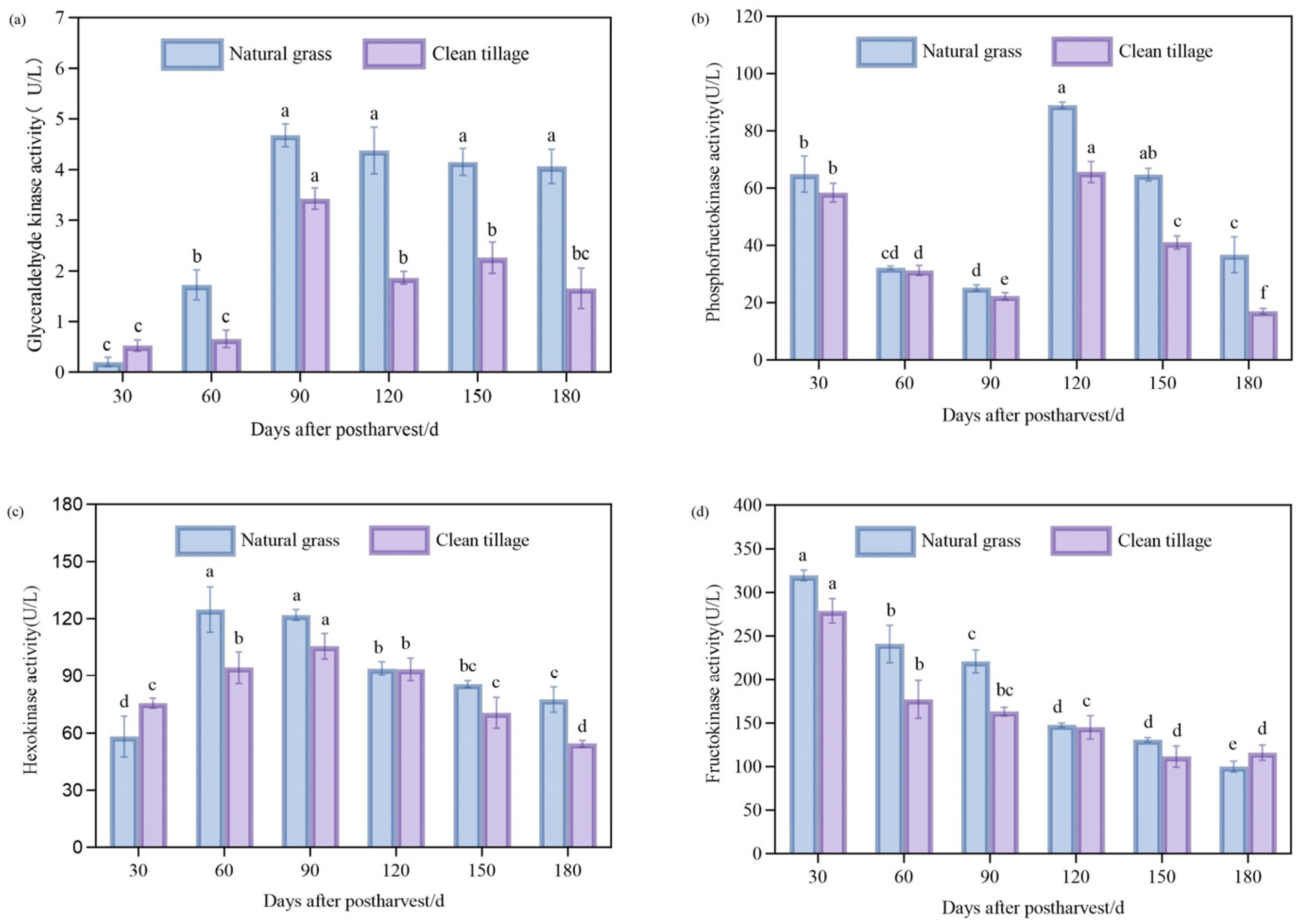
3.6. The Changes in the Enzyme Activity of the Pulp of Korla Fragrant Pears During Storage Under Two Cultivation Modes
3.7. The Changes in the Activity of Enzymes in the Peel of Korla Fragrant Pears During Storage Under Two Cultivation Modes
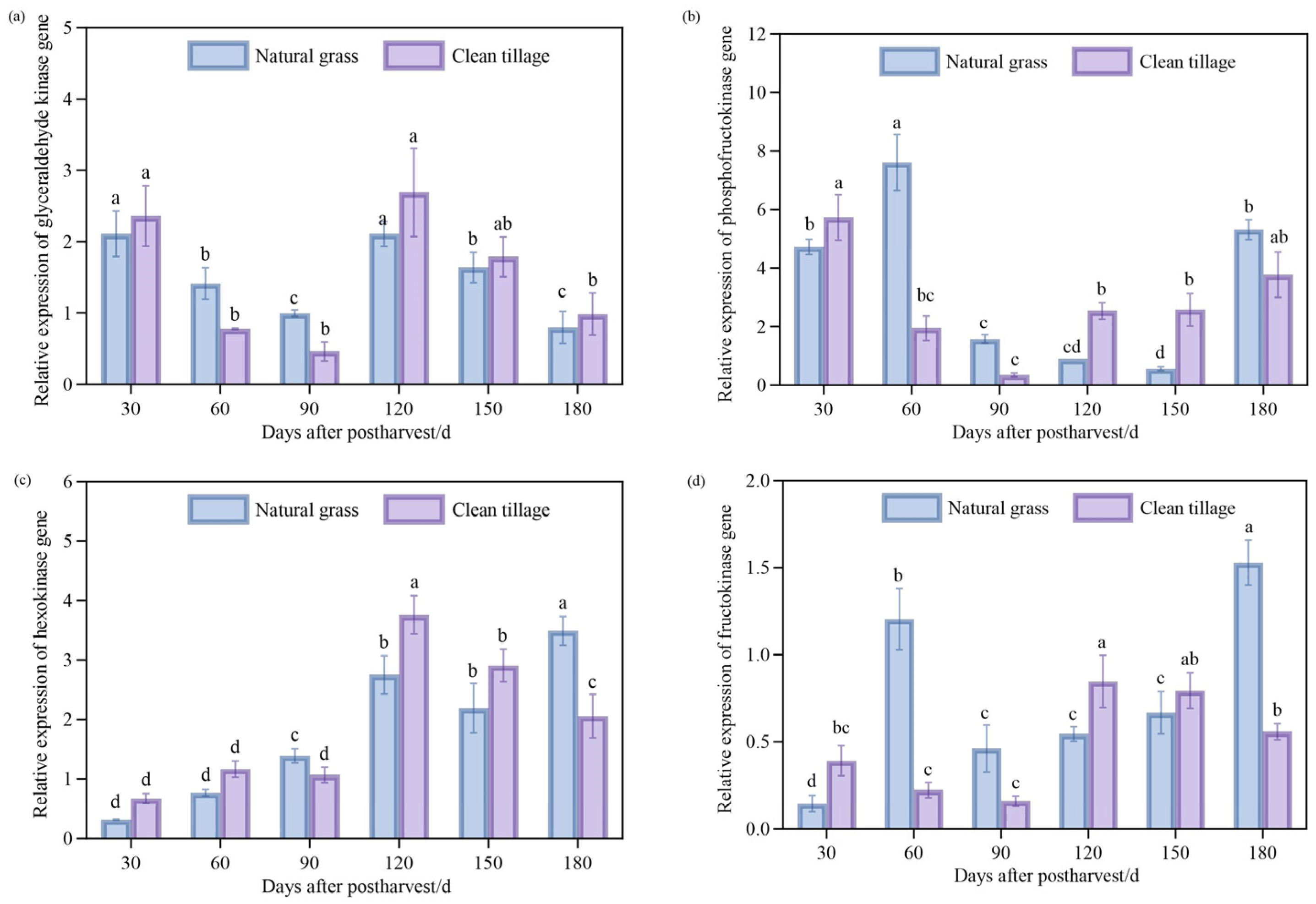
3.8. Changes in Enzyme Gene Expression in Pulp of Korla Fragrant Pear During Storage Under Two Cultivation Modes
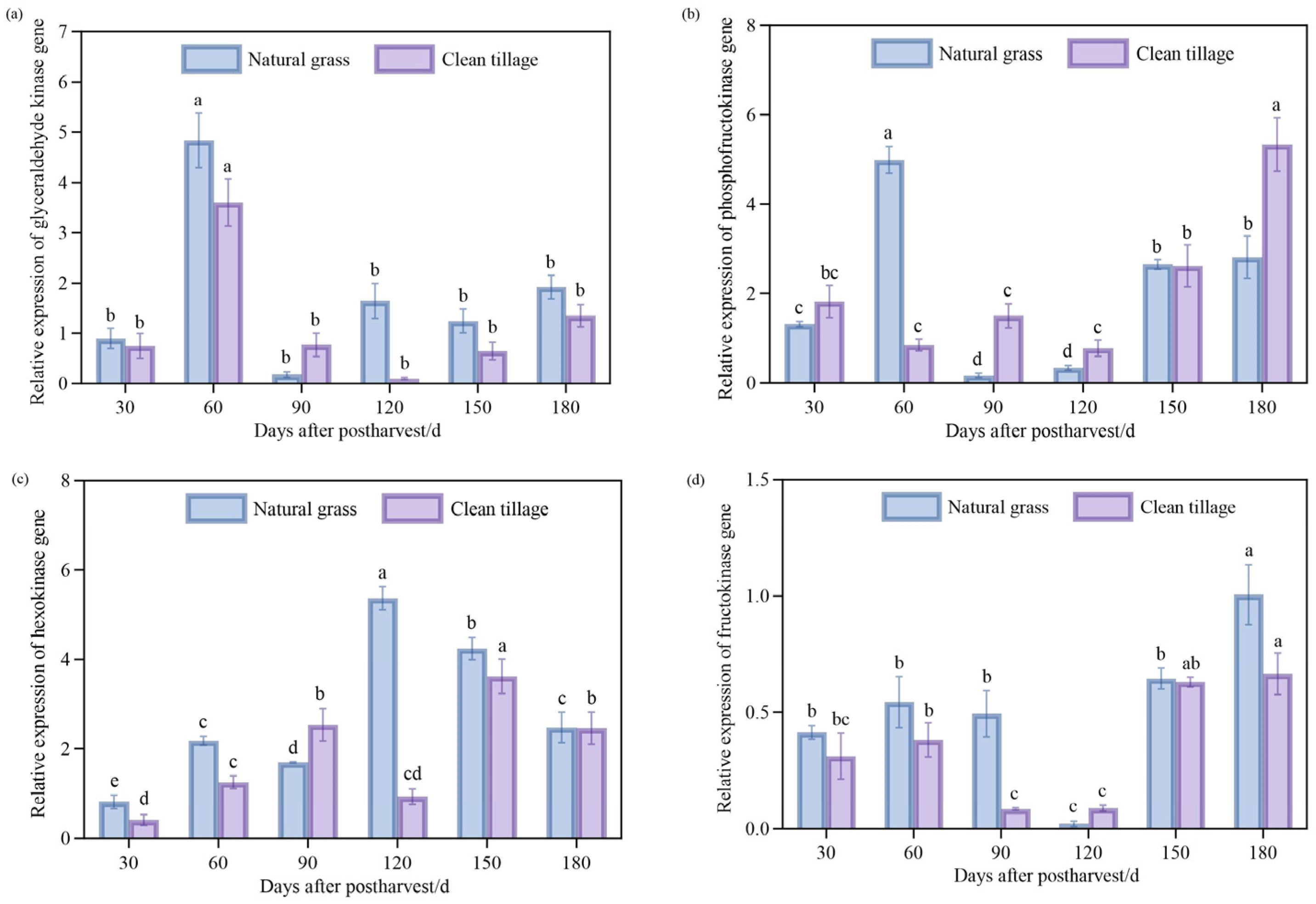
3.9. Changes in Peel Enzyme Gene Expression in Korla Fragrant Pear During Storage Under Two Cultivation Modes
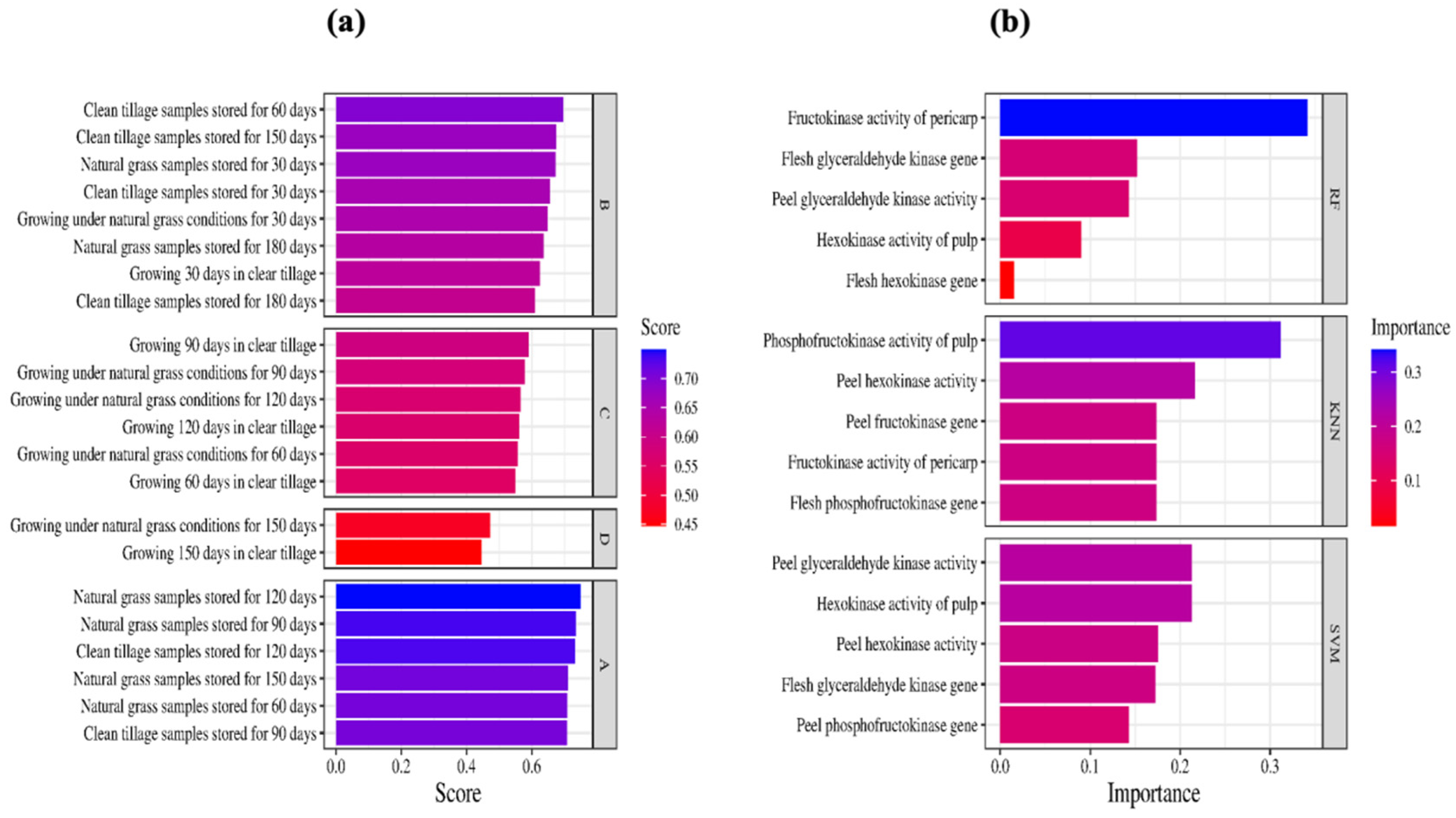
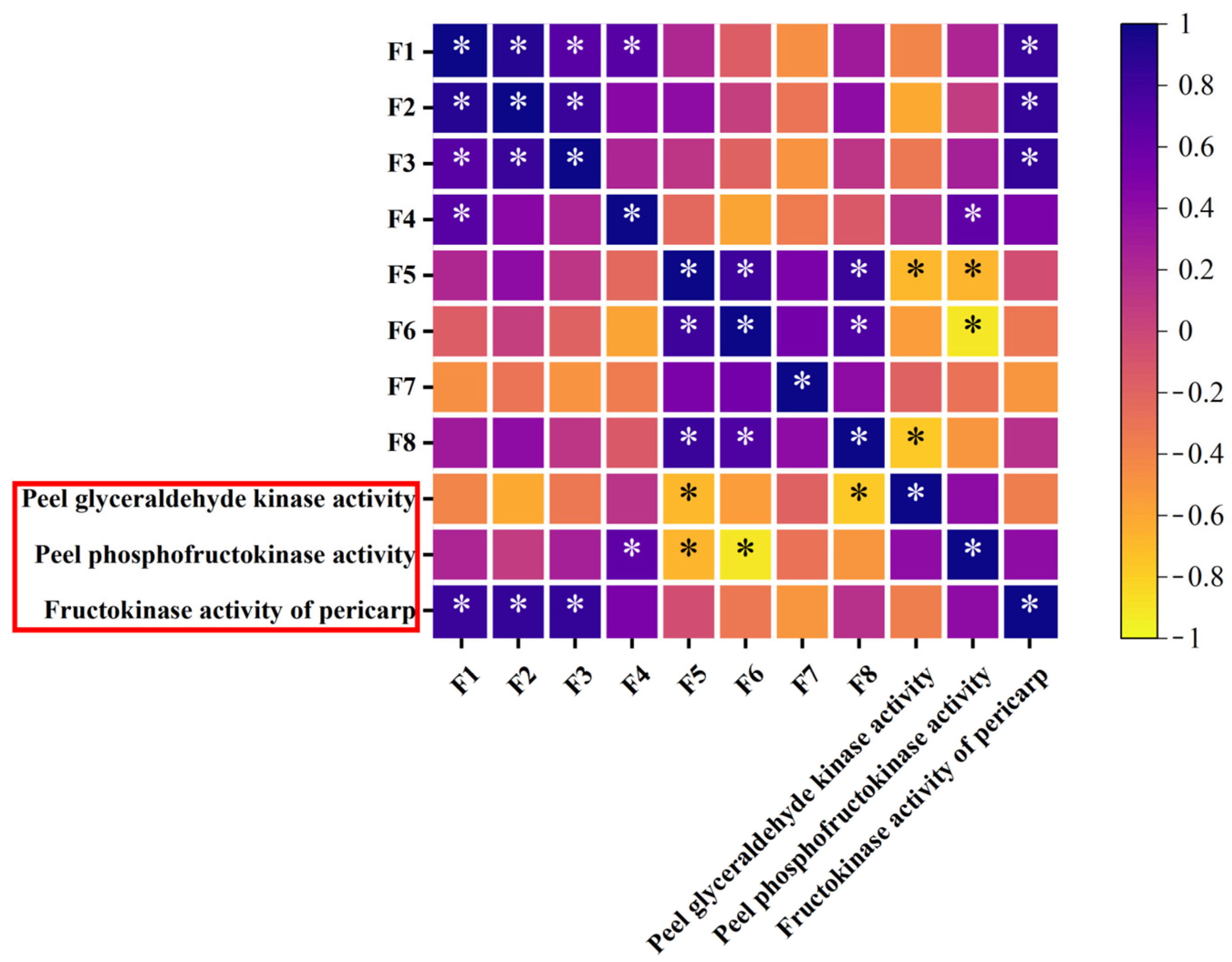
3.10. Screening of Key Factors Affecting Sugar Content in Korla Fragrant Pears
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GK | Glyceraldehyde kinase |
| PFK | Phosphofructokinase |
| HK | Hexokinase |
| FK | Fructokinase |
| RF | Random Forest |
| KNN | K-Nearest Neighbor |
| SVM | Support Vector Machine |
| PSO | Particle Swarm Optimization |
References
- Wang, M.; Li, Y.; Wang, Z.; Li, H.; Zuo, C.; Zhao, J.; Yang, Y.; Tu, K.; Lan, W.; Pan, L. Exploring the optical response of water status and light propagation in bruised ‘Korla’ fragrant pear tissues based on low-field nuclear magnetic resonance coupled with Monte Carlo simulation. Food Chem. 2025, 477, 143504. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, J.; Li, Y.; Lu, X.; Yuan, J.; Ye, J. Effects of Herbage on Ecological Environment and Photosynthetic Characteristics Fruit Quality of ‘Korla Fragrant Pear’. North Hortic. 2019, 22, 49–59. [Google Scholar]
- Wang, Y.; Ji, X.; Wu, Y.; Mao, Z.; Jiang, Y.; Peng, F.; Wang, Z.; Chen, X. Research progress of cover crop in Chinese orchard. Chin. J. Appl. Ecol. 2015, 26, 1892–1900. [Google Scholar]
- Huang, T.; Zheng, T.; Hong, P.; He, J.; Cheng, Y.; Yang, J.; Zhou, Y.; Wang, B.; Zhou, S.; Cheng, G.; et al. Sucrose synthase 3 improves fruit quality in grape. Plant Physiol. Biochem. 2025, 221, 109590. [Google Scholar] [CrossRef]
- Belmys, C.C.; Gilles, V.; Valentina, B.; Léa, R.; Zhanwu, D.; Pierre, V.; Mohamed-Mahmoud, M.; Sophie, C.; Annick, M.; Yves, G.; et al. Model-assisted comparison of sugar accumulation patterns in ten fleshy fruits highlights differences between herbaceous and woody species. Ann. Bot. 2020, 126, 455–470. [Google Scholar]
- Zhong, H.; Yadav, V.; Wen, Z.; Zhou, X.; Wang, M.; Han, S.; Pan, M.; Zhang, C.; Zhang, F.; Wu, X. Comprehensive metabolomics-based analysis of sugar composition and content in berries of 18 grape varieties. Front. Plant Sci. 2023, 14, 1200071. [Google Scholar] [CrossRef]
- Li, B.; Zhu, L.; Yang, N.; Qu, S.; Cao, W.; Ma, W.; Wei, X.; Ma, B.; Ma, F.; Fu, A.; et al. Transcriptional landscape and dynamics involved in sugar and acid accumulation during apple fruit development. Plant Physiol. 2024, 195, 2772–2786. [Google Scholar]
- Middendorf, B.J.; Prasad, P.V.V.; Pierzynski, G.M.; Foyer, C. Setting research priorities for tackling climate change. J. Exp. Bot. 2020, 71, 480–489. [Google Scholar] [CrossRef]
- Grzeczka, A.; Graczyk, S.; Kordowitzki, P. DNA Methylation and Telomeres—Their Impact on the Occurrence of Atrial Fibrillation during Cardiac Aging. Int. J. Mol. Sci. 2023, 24, 15699. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, H.; Chen, J.; Cao, X.; Du, R.; Ma, L.; Wang, N.; Zhu, Z.; Rao, J.; Wang, J.; et al. Releasing a sugar brake generates sweeter tomato without yield penalty. Nature 2024, 635, 647–656. [Google Scholar] [CrossRef]
- Tang, G.; Chen, G.; Ke, J.; Wang, J.; Zhang, D.; Liu, D.; Huang, J.; Zeng, S.; Liao, M.; Wei, X.; et al. The Annona montana genome reveals the development and flavor formation in mountain soursop fruit. Ornam. Plant Res. 2023, 3, 14. [Google Scholar]
- Jia, L.; Zhang, X.; Zhang, Z.; Luo, W.; Nambeesan, S.U.; Li, Q.; Qiao, X.; Yang, B.; Wang, L.; Zhang, S. PbrbZIP15 promotes sugar accumulation in pear via activating the transcription of the glucose isomerase gene PbrXylA1. Plant J. 2023, 117, 1392–1412. [Google Scholar] [PubMed]
- Ren, J.; Li, F.; Yin, C. Orchard grass safeguards sustainable development of fruit industry in China. J. Clean. Prod. 2023, 382, 135291. [Google Scholar]
- Yu, B.; Wang, L.; Zhang, J.; Lyu, D. Natural Grass Cultivation Management Improves Apple Fruit Quality by Regulating Soil Mineral Nitrogen Content and Carbon–Nitrogen Metabolism. Metabolites 2023, 13, 925. [Google Scholar] [CrossRef]
- Yu, B.; Qin, S.J.; Lyu, D.G. Responses of soil microorganisms, enzyme activities and nutrient contents to inter-row grass ploughing and returning to the field in a natural sod culture apple orchard. Chin. J. Appl. Ecol. 2023, 34, 145–150. [Google Scholar]
- Yu, J.; Liu, X.; Wang, W.; Zhang, L.; Wang, C.; Zhang, Q.; Wang, J.; Du, M.; Sheng, L.; Hu, D. MdCIbHLH1 modulates sugar metabolism and accumulation in apple fruits by coordinating carbohydrate synthesis and allocation. Hortic. Plant J. 2025, 11, 578–592. [Google Scholar]
- Yao, G.; Zhang, S.; Cao, Y.; Liu, J.; Wu, J.; Yuan, J.; Zhang, H.; Xiao, C. Characteristics of Components and Contents of Soluble Sugars in Pear Fruits from Different Species. Sci. Agric. Sin. 2010, 43, 4229–4237. [Google Scholar]
- Qing, Y.W.; Ye, S.; Da, W.; Lan, L.J.; Zheng, Y.X.; Wen, J.L.; Wen, W.W. Research on Postharvest Physiology and Comprehensive Technologies for Quality Control of Korla Fragrant Pear. China Fruit Veg. 2018, 38, 1008–1038. [Google Scholar]
- Zhu, Y.; Zong, Y.; Wang, X.; Gong, D.; Zhang, X.; Zhang, F.; Prusky, D.; Bi, Y. Regulation of sucrose metabolism, sugar transport and pentose phosphate pathway by PacC in apple fruit colonized by Penicillium expansum. Food Chem. 2024, 461, 140863. [Google Scholar]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2021, 233, 373–389. [Google Scholar]
- Chen, J.; Liao, Q.; Huang, Y.; Chen, Q.; Zhang, W.; Tian, J. Effects of Different Cultivation Modes on Growth and Quality of Hongyang Kiwifruit. J. Nucl. Agric. Sci. 2023, 37, 1435–1441. [Google Scholar]
- Shu, X.; Ye, Y. Knowledge Discovery: Methods from data mining and machine learning. Soc. Sci. Res. 2023, 110, 102817. [Google Scholar] [PubMed]
- Dong, L.; Lei, G.; Huang, J.; Zeng, W. Improving crop modeling in saline soils by predicting root length density dynamics with machine learning algorithms. Agric. Water Manag. 2023, 287, 108425. [Google Scholar]
- Liu, X.; Wang, J.; Wang, H.; Huang, Y.; Ren, Z. Prediction of prunoideae fruit quality characteristics based on machine learning and spectral characteristic acquisition optimization. Food Control 2024, 165, 110627. [Google Scholar]
- Wittwer, C.T.; Vandesompele, J.; Shipley, G.L.; Pfaffl, M.W.; Nolan, T.; Mueller, R.; Kubista, M.; Huggett, J.; Hellemans, J.; Garson, J.A.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar]
- .Amsili, J.P.; van Es, H.M.; Schindelbeck, R.R. Pedotransfer Functions for Soil Protein Based on Random Forest Modeling for Routine Soil Health Analysis. Commun. Soil Sci. Plant Anal. 2025, 56, 1381–1393. [Google Scholar]
- He, S.-S.; Hou, W.-H.; Chen, Z.-Y.; Liu, H.; Wang, J.-Q.; Cheng, P.-F. Early warning model based on support vector machine ensemble algorithm. J. Oper. Res. Soc. 2024, 76, 411–425. [Google Scholar]
- Deng, N.; Xu, R.; Zhang, Y.; Wang, H.; Chen, C.; Wang, H. Forest biomass carbon stock estimates via a novel approach: K-nearest neighbor-based weighted least squares multiple birth support vector regression coupled with whale optimization algorithm. Comput. Electron. Agric. 2025, 232, 110020. [Google Scholar]
- Wei, J.; Zheng, Q.; Yan, W.; Li, H.; Chi, Z.; Jiang, B. Probabilistic analysis of blade flutter based on particle swarm optimization-deep extremum neural network. Int. J. Turbo Jet-Engines 2025, 42, 99–114. [Google Scholar]
- Yang, W.; Qian, D.; Qun, D.; Hui, Z.; Xiao, Z.; Hong, L. Expression analysis of genes related to sugar and acid metabolism during fruit development of ‘Shucuizao’ jujube. Acta Agric. Boreali-Occident. Sin. 2022, 31, 876–885. [Google Scholar]
- Hong, W.; Yan, W.; Xiao, X.; Lian, C. Effects of grass on apple yield and fruit quality in orchard. Friends Fruit Farmers 2022, 10, 4–5. [Google Scholar]
- Wei, H.; Xiang, Y.; Liu, Y.; Zhang, J. Effects of sod cultivation on soil nutrients in orchards across China: A meta -analysis. Soil Tillage Res. 2017, 169, 16–24. [Google Scholar]
- Chen, L.; Bao, Y.; He, X.; Yang, J.; Wu, Q.; Lv, J. Nature-based accumulation of organic carbon and nitrogen in citrus orchard soil with grass coverage. Soil Tillage Res. 2025, 248, 106419. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Hu, J.; Qu, X.; Ji, Q.; Zhuang, C.; Ren, Y.; Zhou, J.; Wei, T. Straw mulch and orchard grass mediate soil microbial nutrient acquisition and microbial community composition in Ziziphus Jujuba orchard. Plant Soil 2025, 1–17. [Google Scholar] [CrossRef]
- Crittenden, S.J.; de Goede, R.G.M. Integrating soil physical and biological properties in contrasting tillage systems in organic and conventional farming. Eur. J. Soil Biol. 2016, 77, 26–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X. Soil pore structure may affect microbial communities involved in carbon cycling during thaw slumps development. Appl. Soil Ecol. 2025, 206, 105821. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, X.; Yuan, X.; Wu, Q.; Chen, S.; Zou, Y.; Ma, F.; Li, C. Exogenous dopamine improves apple fruit quality via increasing flavonoids and soluble sugar contents. Sci. Hortic. 2021, 280, 109903. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Wang, F.; Gao, S.; Kang, H.; Ji, X.; Yao, Y. Postharvest changes in the phenolic and free volatile compound contents in Shine Muscat grapes at room temperature. Food Chem. 2025, 465, 141958. [Google Scholar] [CrossRef]
- Sun, S.; Sun, D.; Guo, L.; Cui, B.; Zou, F.; Wang, J.; Sun, C.; Zhu, Y.; Li, X. Structural and physicochemical properties of corn starch modified by phosphorylase b, hexokinase and alkaline phosphatase. Carbohydr. Polym. 2025, 349, 122979. [Google Scholar]
- Chen, J.; Zhang, L.; Zhao, Z.; Xu, J. Fruit Photosynthesis and Assimilate Translocation and Partitioning Their Characteristics and Role in Sugar Accumulation in Developing Citrus unshiu Fruit. Acta Bot. Sin. 2002, 44, 158–163. [Google Scholar]
- Tran, D.T.; Huh, J.-H. Building a model to exploit association rules and analyze purchasing behavior based on rough set theory. J. Supercomput. 2022, 78, 11051–11091. [Google Scholar] [CrossRef]
- Li, K.; ur Rahman, S.; Rehman, A.; Li, H.; Hui, N.; Khalid, M. Shaping rhizocompartments and phyllosphere microbiomes and antibiotic resistance genes: The influence of different fertilizer regimes and biochar application. J. Hazard. Mater. 2025, 487, 137148. [Google Scholar] [PubMed]
- Rehman, A.; Lin, J.C.W.; Heldal, I. Enhancing Psychologists’ Understanding Through Explainable Deep Learning Framework for ADHD Diagnosis. Expert Syst. 2024, 42, e13788. [Google Scholar]




| Name | Sequence (5′-3′) | Tm (°C) | Length (bp) |
|---|---|---|---|
| GADPH-F | GTGCCCACTGTTGATGTTTCC | 54.79 | 22 |
| GADPH-R | CCTTCTGACTCCTCCTTGATAGC | 55.13 | 24 |
| GK-F | GGTCTTCCTGTCATCGCATCCTTG | 54.10 | 25 |
| GK-R | TTACCGCTCAAACTACCGACAATCC | 54.52 | 26 |
| PFK-F | ATGTCCAGGTTCCGCTGCTT | 55.07 | 21 |
| PFK-R | ACTGGAACTGCCGTTGGGAA | 54.70 | 21 |
| HK-F | GAGCCTGGAGGTAGACGAGACAC | 54.06 | 24 |
| Algorithm | |
|---|---|
| RF | |
| SVM | |
| KNN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Wang, L.; Chen, Y.; Fan, W.; Wang, H.; Guo, K.; Tao, S.; Gong, X.; Bao, J. The Impact of the Natural Grass-Growing Model on the Development of Korla Fragrant Pear Fruit, as Well as Its Influence on Post-Harvest Sugar Metabolism and the Expression of Key Enzyme Genes Involved in Fruit Sugar Synthesis. Agriculture 2025, 15, 792. https://doi.org/10.3390/agriculture15070792
Yu M, Wang L, Chen Y, Fan W, Wang H, Guo K, Tao S, Gong X, Bao J. The Impact of the Natural Grass-Growing Model on the Development of Korla Fragrant Pear Fruit, as Well as Its Influence on Post-Harvest Sugar Metabolism and the Expression of Key Enzyme Genes Involved in Fruit Sugar Synthesis. Agriculture. 2025; 15(7):792. https://doi.org/10.3390/agriculture15070792
Chicago/Turabian StyleYu, Mingyang, Lanfei Wang, Yan Chen, Weifan Fan, Hao Wang, Kailu Guo, Shutian Tao, Xin Gong, and Jianping Bao. 2025. "The Impact of the Natural Grass-Growing Model on the Development of Korla Fragrant Pear Fruit, as Well as Its Influence on Post-Harvest Sugar Metabolism and the Expression of Key Enzyme Genes Involved in Fruit Sugar Synthesis" Agriculture 15, no. 7: 792. https://doi.org/10.3390/agriculture15070792
APA StyleYu, M., Wang, L., Chen, Y., Fan, W., Wang, H., Guo, K., Tao, S., Gong, X., & Bao, J. (2025). The Impact of the Natural Grass-Growing Model on the Development of Korla Fragrant Pear Fruit, as Well as Its Influence on Post-Harvest Sugar Metabolism and the Expression of Key Enzyme Genes Involved in Fruit Sugar Synthesis. Agriculture, 15(7), 792. https://doi.org/10.3390/agriculture15070792






