Abstract
In the last decade, interest in the use of seaweed and seaweed-derived products in horticulture has grown due to their great potential as biostimulants for increasing yields and improving food quality in multiple crops. A greenhouse experiment was conducted to investigate the effects of the application of the green seaweed Ulva ohnoi (either as a seaweed suspension [SWS] or seaweed extract [SWE]) on the yield, size, shape, and nutritional quality (i.e., proximate composition and dietary antioxidant content) of tomato fruits (Solanum lycopersicum L. cv. Rio Fuego). A total of 36 tomato plants were potted individually and organized into three experimental groups: SWS (plants drenched with 250 mL of seaweed suspension [2.0%]), SWE (plants drenched with 250 mL of seaweed extract [0.2%]), and control (plants irrigated with water). Each treatment included three replications. The fruits harvested (66%) from SWS-treated plants were produced during the earliest harvest stages. In contrast, the fruits harvested from SWE-treated plants (82%) and control plants (77%) were produced during the late and very late harvest stages. Notably, SWS application significantly enhanced the number of fruits harvested per plant, average fruit weight, yield (kg/plant), number of seeds per fruit, and fruit size. Furthermore, tomato fruits from plants treated with either SWS or SWE exhibited higher percentages of protein, fat, crude fiber, dry matter, and total soluble solids, as well as lower acidity and reduced total carbohydrate content, compared to the control. The antioxidant metabolites in tomatoes, including lycopene, β-carotene, flavonoids, and phenolic acids, increased following the application of SWS and SWE, while anthocyanin and ascorbic acid contents increased only in SWS-treated plants. These results demonstrate that both forms of U. ohnoi application have biostimulating effects on tomato. In particular, the use of SWS shows great potential as a strategy to enhance tomato fruit productivity and quality in sustainable horticultural systems.
1. Introduction
In the 2030 Agenda for Sustainable Development, the United Nations proposed achieving global food security by ensuring access to sufficient quantities of food with adequate nutritional quality to support and improve human health [1]. Tomato (Solanum lycopersicum L.) is one of the most commonly consumed vegetables worldwide, and approximately 180 million tons of fresh tomatoes are produced each year [2]. Solanum lycopersicum cv. “Río Fuego” is a medium early variety of determinant growth; these compact growth habit plants are used in domestic gardens and commercial farming. The ‘Río Fuego’ variety is resistant to common diseases, adapts well to different growth environments, and exhibits notable production potential [3]. The tomato fruits from this variety are characterized as being firm, fleshy, medium-sized, deep red in color, and elongate shaped; they also show good transportability [4].
In addition to having a pleasant flavor and low acidity, tomatoes are functional foods, given the epidemiological evidence of their ability to reduce the risk of certain cancers [5]. Moreover, the nutritional and functional compounds (e.g., proteins, minerals, vitamins, dietary fibers, sugars, phytoalexins, glycoalkaloids, carotenoids, and phenolic compounds) found in tomatoes are highly beneficial to human health [6]. In particular, tomato fruits are rich sources of potent natural antioxidants, including ascorbic acid, lycopene, β-carotene, and polyphenols [5,7,8,9]. Therefore, the frequent consumption of tomatoes and tomato-based products can notably improve the intake of these beneficial nutraceutical compounds [5,10].
Worldwide, the organic production of tomatoes and other vegetables has increased by ~9% per year [11]. However, implementing sustainable agricultural practices that contribute to food security while minimizing the harmful effects that stem from the use of highly toxic agrochemicals and their residues to produce organic tomatoes requires notable investments [7,11]. To this end, organic inputs derived from local, biodegradable, and environmentally friendly raw materials offer promising alternatives to the excessive use of synthetic chemical products in horticulture [12,13]. In tomato cultivation, the application of seaweed plant biostimulants is a sustainable approach to improve the nutritional and functional characteristics of the resulting fruits that is growing in importance [14,15].
Seaweed-based products are increasingly being used in horticulture due to the wide range of bioactive compounds that result from the unique and diverse ecological conditions in which seaweeds have evolved [16]. Some studies have indicated that carbohydrates constitute the major component (%) of dry seaweed biomass, followed by minerals, proteins, lipids, and plant growth regulators and secondary metabolites, which are present in much smaller amounts [13,17,18]. These components affect plant metabolism and physiology, depend on the essential elements in soil, and improve the productivity of tomato plants [19,20]. Also, the components contained in seaweeds and their derivatives have been shown to improve fruit yield, firmness, flavor, external appearance, and nutritional and dietary antioxidant content, although these benefits may vary depending on genotype and growing conditions [7,12,14,19,20,21,22].
Currently, most commercial agricultural products derived from seaweed are liquid extracts from brown seaweeds [13]. However, the potential agricultural applications of green seaweed biomass in its natural form (i.e., powdered, granular, or fragmented), as well as derived bioproducts, have been poorly investigated and require further exploration, given their available biomass and overall accessibility [12]. In this context, seaweed extracts from Ulva spp. have been found to positively affect morphological and biochemical plant growth parameters, including germination and growth, as well as chlorophyll, carotenoid, sugar, protein, and lipid contents, with these improvements attributed to the presence of hormones, minerals, carbohydrates, and betaines in the extracts [15,23,24,25,26]. Kasim et al. [27] reported that an Ulva seaweed extract reduced drought-induced oxidative damage through activation of the antioxidative system and providing essential hormones and minerals for plant growth. Latique et al. [28] reported that extracts of Ulva rigida enhanced physiological and biochemical characteristics, including growth parameters, chlorophyll content, and antioxidant enzyme (i.e., SOD, ICDH, GR, PEPC, GDH, GPx, and GST) activity, as well as plant salt tolerance, by increasing non-antioxidant compound production and enhancing antioxidant enzyme activity.
Green seaweeds, such as Ulva species, are a renewable marine resource because they can grow massively under certain environmental conditions [12,23]. In particular, Ulva ohnoi has been observed to accumulate large amounts of biomass while forming green tides, making this Ulva species an attractive marine resource [29,30,31]. Indeed, U. ohnoi offers a simple and cost-effective means of obtaining novel and natural plant growth stimulators [32,33,34]. Furthermore, the growing commercial interest in U. ohnoi cultivation reflects its potential to achieve a high biomass, high productivity per unit area, and richness in bioactive compounds [35,36].
In the present study, we aimed to investigate the effects of an U. ohnoi treatment on tomato plant productivity and the quality attributes of tomato fruits harvested at the ripening stage (fully red skin). We hypothesized that applying U. ohnoi in the form of a suspended powder or aqueous extract to the growing substrate of tomato plants would differentially affect the availability and uptake of essential nutrients, ultimately leading to differences in the effectiveness of each U. ohnoi treatment on crop yield and quality.
2. Materials and Methods
2.1. Seaweed Suspension and Seaweed Extract Preparation
Ulva ohnoi M. Hiraoka & S. Shimada dry powdered biomass was provided by the Company Productos Marinos de las Californias S. de R.L. de C.V., located in Ensenada, Baja California, Mexico. A powdered seaweed suspension (SWS) (2.0%) was prepared according to the methods of Espinosa-Antón et al. [23], with modifications. Briefly, 5 g of the dry powder was added to 250 mL of distilled water and the suspension was homogenized for 15 min at 27 ± 2 °C before application. The method of Hernández-Herrera et al. [32] was used to prepare the liquid seaweed extract (SWE). Briefly, dry powder (0.5 g) was mixed with distilled water (250 mL) and the crude extract was autoclaved (121 °C) for 15 min at 1.5 kg cm−2. Then, the hot extract was filtered (Whatman No. 40 filter paper, Sigma–Aldrich, Merck KGaA, Darmstadt, Germany). The SWE (0.2%) was stored at −4 °C for at least 24 h before use.
The chemical composition of U. ohnoi SWS and SWE was determined with a Phytomonitor, available online: https://phytomonitor.com.mx/analisis-nutricion/ (accessed on 5 September 2024). The physicochemical characteristics of SWS and SWE are shown in Table 1.

Table 1.
Physicochemical characteristics of the Ulva onhoi seaweed suspension (SWS) and seaweed extract (SWE).
2.2. Plant Material and Crop Growing Conditions
Solanum lycopersicum L. cv. “Rio Fuego” seedlings, 15 d old, were transferred to individual plastic pots (5 L) with a substrate mixture of vermiculite (Termolita S.A., Santa Catarina, NL, Mexico), peat moss (Sunshine Mix 3™), and organic soil in a ratio of 1:1:1 (v/v). The tomato plants were fertilized with 250 mL of 20N-20P-20K (2 g L−1) soil drench solution (Peters Professional, Scotts-Sierra Horticultural Products Co., Marysville, CA, USA) at 2 week intervals and watered daily until the end of the experiment. The plants were maintained in greenhouse under natural light conditions. Average daily temperature (day: 30 ± 2 °C; night: 16 ± 2 °C) and relative humidity (day/night ~85%) were monitored throughout the experiment.
2.3. Experimental Design and Treatments
A total of 36 individually potted plants were randomly assigned to treatment groups (12 plants per treatment), arranged in three replicates (Figure 1a). The treatments included: (1) control plants irrigated with 250 mL of water, (2) SWS-treated plants drenched with 250 mL of seaweed suspension (2.0%), and (3) SWE-treated plants drenched with 250 mL of seaweed extract (0.2%). The SWS or SWE were applied directly to the substrate one week after transplanting (6 applications of SWS at 15 day intervals or 12 applications of SWE at 7 day intervals) and continued until one week prior to the first harvest. The greenhouse experiment was conducted until the harvest phase (91 to 121 d). The productive capacity of the tomato plants (i.e., fruit number and fruit weight per plant) were recorded at each harvest stage. At the end of the experiment, the quality attributes of the harvested tomato fruits (i.e., size, shape, and nutritional composition) were determined.

Figure 1.
(a) Arrangement of the experimental units in the greenhouse at the University Center for Biological and Agricultural Sciences (CUCBA), University of Guadalajara. (b) Distinction between (A) matured green, and (B) fruit at 75% ripeness.
2.4. Productivity and Fruit Size and Shape
Fruit harvesting began at 91 d and ended at 121 d. Tomatoes at 75% ripeness (Figure 1b) were harvested every five days and allowed to fully ripen to 100% under ambient conditions. In all, seven tomato harvests were performed during the experiment. At each harvest time, the fruits per plant were counted, weighed, and classified into one of four harvest stages according to the criteria of Mannino et al. [7]: early (91 d), intermediate (96–101 d), late (106–111 d), and very late (116–121 d). The productive capacity of the tomato plants was determined based on the number of fruits harvested per plant, average fruit weight per plant, yield per plant, and the number of seeds per fruit. The values obtained for each productivity variable were averaged to obtain the mean of the true replicate (n = 3).
To evaluate the external traits of the tomato fruits, 42 fruits (6 from each harvest) were randomly selected per treatment once they reached the maturity criterion. The polar length (stem-end to blossom-end length), equatorial length (circumference across the widest part of the fruit), and shape index (ratio of length to width) were measured using a digital caliper (HER-411, Electronic STEREN S. A. de C.V., Mexico City, Mexico). The values obtained for each morphometric variable were averaged to obtain the mean of the true replicate (n = 7). Additionally, fruits were graded based on their fresh weight according to the criteria of Ali et al. [20]: Grade A (large: >70 g), Grade B (medium: 30–70 g), and Grade C (small: <30 g). The percentage of fruits of each grade was calculated. The selected fruits were then used for nutritional quality analysis.
2.5. Nutritional Content of Tomato Fruits
The red-matured tomatoes were cut in half to remove the seeds and placenta. A subsample of the fruit was pulped to obtain a homogeneous tomato juice sample for each treatment. The total soluble solid (TSS, °Brix) content was measured with a portable refractometer, and pH was determined using an HI 2211 pH Meter (Hanna Instruments®, Mexico City, Mexico). Another subsample of tomato fruits was lyophilized and ground into a powder using a mortar and pestle. The powdered tomatoes were then stored at −80 °C until analysis of the proximate composition and dietary antioxidant content.
The protein, crude fiber, lipid, ash, carbohydrate, and dry matter content were determined following the methods of the Association of Official Analytical Chemists [37]. Protein was quantified using a nitrogen-to-protein conversion factor of 6.25 (method 954.04). Crude fiber and lipid content were determined via the Soxhlet extraction method (method 954.02). Ash content was determined by calcination at 550 °C (method 942.05). An indirect estimation of carbohydrate content (%) was performed (carbohydrate content (%) = 100 − [% protein + % fat]), and dry matter content (DM) was determined using the oven drying method [6]. Briefly, 15 g of chopped fruit was packed into foil trays and oven-dried at 90 °C for at least 48 h until the dried weight remained constant. Energy values were determined (fat conversion factor: 9 kcal g−1; protein and carbohydrate conversion factor: 4 kcal g−1), and the results are presented in kcal per 100 g DM [38].
Lycopene and β-carotene content in the tomato pulp were simultaneously estimated by spectrophotometry at 450 nm following the method of Nagata and Yamashita [39]. Lycopene content was expressed as µg lycopene per gram of dry matter (μg g−¹ DM); carotene content was expressed as μg of β-carotene equivalent per 100 g of dry matter (µg CE 100 g DM).
Total flavonoid content (flavones and flavanols) was determined according to a modified aluminum (III) chloride colorimetric method [40], with some modifications, and expressed in mg equivalents of quercetin per gram of dry matter (mg EQ g−¹ DM). Total anthocyanin content was determined by means of pH differences [41], with some modifications. The concentration of anthocyanins in the extract was expressed in mg equivalents of cyanidin-3-glucoside per gram of dry matter (EC3G g−¹ DM). Total polyphenol content was determined based on the colorimetric oxidation–reduction reaction [42], with some modifications. Absorbance was measured at 765 nm, and the results were expressed as mg gallic acid equivalents per gram of dry matter (mg GAE g−¹ DM). Total phenolic acid content was determined according to the methodology of Matkowski et al. [43], with some modifications. The method was based on the reaction of hydroxycinnamic acids in an acid medium with Arnow reagent. The content of phenolic acids was expressed as mg caffeic acid equivalents per gram of dry matter (mg CAE g−¹ DM). Total tannin content was determined by the precipitation of tannins with fraction V of SAB bovine serum albumin (Calbio-Chem) according to the method described by Ricco et al. [44]. The results were expressed as mg gallic acid equivalents per gram of dry matter (mg GAE g−¹ DM). Ascorbic acid content was determined using 2,6-dicholorophenol indophenols dye by titration, as described by the Association of Official Analytical Chemists [37]. All nutritional quality analysis were performed in triplicate (n = 3).
2.6. Statistical Analysis
The Cohen’s statistical power analysis of a one-way analysis of variance (ANOVA) (three treatment groups, effect size 0.5, confidence level (α) of 95%, and n = 12 [individuals per group]) was evaluated using the ‘pwr.anova.test’ function from the ‘pwr’ package in R software (version 4.4.3).
All data were evaluated for normality (Shapiro–Wilk test) and homoscedasticity (Levene test). Significant differences (p < 0.05) among treatments were determined using one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test. Values are presented in graphs and figures as means ± standard deviations, with n = 3 for fruit productivity and nutritional quality variables, and n = 7 for fruit morphometric variables.
A principal component analysis (PCA) and cluster analysis were performed on the normalized data to evaluate potential relationships between the chemical composition of the treatments (seaweed suspension, seaweed extracts, and control) and the yield, size, fresh weight, proximate composition (TSS, pH, protein, fat, fiber, carbohydrates, ash, and energy), and dietary antioxidant content (lycopene, carotene, phenols, flavonoids, anthocyanins, tannins, and ascorbic acid) of the tomato fruits. Statgraphics® Centurion XVI. II for Windows (StatPoint Technologies, Inc. The Plains, USA) was used to generate the cluster groups, PCA plots, and correlation values and to perform statistical analyses.
3. Results
3.1. Yield, Size, and Shape of Tomato Fruits
In this study, 91-day-old plants treated with either the U. ohnoi SWS or SWE produced fruits in four ripening stages (unripe, mature green, 50% ripe, and 75% ripe), and the fruits were harvested at the 75% ripening stage. A total of seven harvests were performed over four harvest stages: early, intermediate, late, and very late.
The fruits produced by SWS-treated plants (66%) were harvested at the early (91 d) and intermediate (96 to 101 d) harvest stages. The number of fruits harvested in these stages was significantly greater (p < 0.05) than the control (early stage: more than 2-fold greater; intermediate stage: 3-fold greater). These results suggest that SWS application reduced fruit ripening times (Figure 2). However, in the late and very late harvest stages (fourth to seventh harvests), the number of fruits harvested from SWS-treated plants was lower than the control (p < 0.05; Figure 2a). Nonetheless, the average weight of fruits produced by SWS-treated plants significantly increased in all seven harvests and remained consistent (60 to 70 g) compared to that of the control (22 to 60 g), with variations throughout the harvest period. Moreover, the weight of tomato fruits produced by SWS-treated plants was significantly (p < 0.05) greater during the first (1.4-fold), third (1.1-fold), fifth (1.2-fold), and sixth (1.5-fold) harvests compared to the control (Figure 2b).
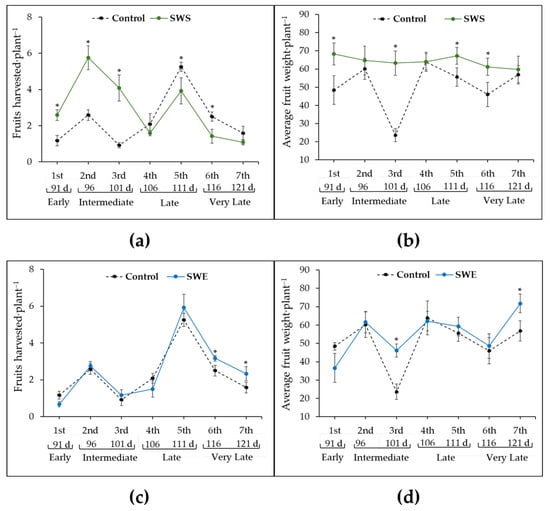
Figure 2.
Effect of seaweed suspension (SWS) and seaweed extract (SWE) from Ulva ohnoi on (a,c) the number of fruits harvested and (b,d) the fresh weight of fruits from 91 to 121-day-old tomato plants. The seven harvests (1st to 7th) were classified into four harvest stages: early (91 d), intermediate (96–101 d), late (106–111 d), and very late (116–101 d). Vertical bars show means ± standard deviations (n = 3, each replicate consisted of four plants). An asterisk (*) indicates significant differences with respect to the control group based on Dunnett’s multiple range test (p < 0.05).
In contrast, the number of fruits harvested from SWE-treated plants was similar to the control (at the early, intermediate and late harvest stages). In SWE-treated and control plants, 82% and 77% of the tomato fruits were collected during the late (106 to 111 d) and very late (116 to 121 d) harvest stages. The SWE-treated plants produced significantly more fruits during the sixth and seventh harvests than the control (p < 0.05; Figure 2c). Finally, the fruits produced by SWE-treated plants weighed significantly more than those produced by control plants (third harvest: 2-fold greater; seventh harvest: 1.2-fold greater; p < 0.05; Figure 2d).
Differences in the productive capacity of the tomato plants were observed within the harvest period (91 to 121 d) and are summarized in Table 2. Compared with the control, treatment with SWS or SWE increased the total number of harvested fruits (75% ripeness) by 28% and 6%, respectively. Furthermore, the application of SWS significantly (p < 0.05) increased the number of harvested fruits per plant (35.7%), average fruit weight per plant (26.1%), yield per plant (47.6%), and number of seeds per fruit (14.5%) compared with the control plants. In contrast, the application of SWE did not significantly affect the other variables associated with the productive capacity of the tomato plants when compared to the control (Table 2).

Table 2.
Productivity of tomato plants treated with Ulva ohnoi seaweed suspension (SWS) and seaweed extract (SWE) during the harvest period (91–121 d).
Regarding the external characteristics of the fruit, the application of SWS significantly (p < 0.05) enhanced the polar length (12.8%) and equatorial diameter (10%) of the tomato fruits (Table 3). In contrast, the external characteristics of the fruits from plants treated with SWE were similar to those of the control plants (Table 3). As expected, the fruit shape index was not affected by SWS or SWE treatments and remained similar to that of the control group (Table 3). Thus, the treatments preserved the typical shape of the tomato variety.

Table 3.
Morphometric characteristics of harvested tomato fruits from plants treated with Ulva ohnoi seaweed suspension (SWS) or seaweed extract (SWE).
In addition, the tomato fruits were classified into grade categories based on fresh weight. The harvested tomato fruits from control plants were classified as 24% Grade A (big > 70 g), 71% Grade B (medium 30−70 g), and 5% Grade C (small < 30 g) (Figure 3a). The plants treated with SWS exhibited the highest percentages of fruits classified as Grade A (39%) and Grade B (61%) (Figure 3b). In contrast, 30% of fruits produced by SWE-treated plants were classified as Grade A, and 69% of fruits were classified as Grade B (Figure 3c).
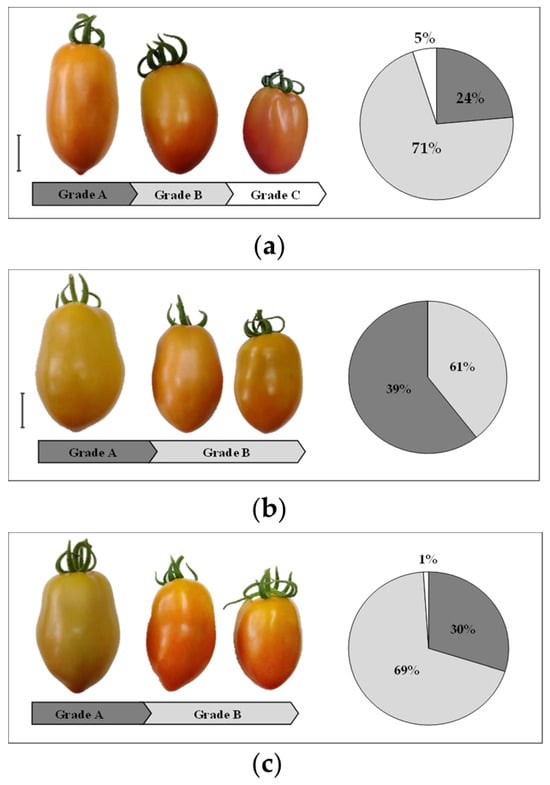
Figure 3.
The percentage (%) of tomatoes in each grade category based on fresh weight from (a) control plants, (b) plants treated with seaweed suspension (SWS), and (c) plants treated with seaweed extract (SWE). Grade A (big: >70 g, dark gray), Grade B (medium: 30−70 g, medium gray), and Grade C (small: <30 g, white). Bar = 5 cm.
3.2. Nutritional Analysis
The SWS- and SWE-treated plants produced fruits with significant variations in proximate composition (Table 4). Specifically, SWS treatment resulted in tomato fruits with significantly (p < 0.05) higher protein (10.12 g 100 g−1 DM), fat (0.70 g 100 g−1 DM), crude fiber (16.9 g 100 g−1 DM), dry matter (6.13 g 100 g−1 DM), and ash (8.7 g 100 g−1 DM) contents. Similarly, SWE treatment resulted in tomato fruits with significantly (p < 0.05) higher protein, fat, crude fiber, and dry matter (10.26, 0.81, 16.44 and 5.79 g 100 g−1 DM; respectively) contents. In addition, fruits harvested from SWS- and SWE-treated plants contained significantly (p < 0.05) fewer total carbohydrates (Table 4).

Table 4.
Proximate composition and energy content of tomato fruits from plants treated with Ulva ohnoi seaweed suspension (SWS) or seaweed extract (SWE).
Tomato fruits harvested from SWS- and SWE-treated plants exhibited significant (p < 0.05) differences in TSS (°Brix), exhibiting enrichment (5.10 and 4.64, respectively) compared with the control plants (3.78). Also, the pH of tomato fruits was significantly (p < 0.05) more acidic from SWS- and SWE-treated plants (pH 4.43 and 4.50, respectively) compared with those of the control (pH 5.12). The energy content of the tomato fruits from SWS-treated plants was similar to that of the control plants; however, SWE-treated plants produced fruits with comparatively less energy content (Table 4).
The analysis of lipophilic antioxidants in this study focused on two key carotenoid fractions: lycopene and β-carotene. Both SWS- and SWE-treated plants produced tomato fruits with significantly (p < 0.05) higher levels of lycopene and β-carotene than the control (Table 5). Lycopene content ranged from 611.99 and 657.45 µg g−¹ DM in SWE- and SWS-treated plants, respectively, whereas the control pants exhibited a value of 561.42 µg g−¹ DM. In addition, β-carotene content was significantly greater in tomatoes harvested from SWS- and SWE-treated plants (83.87 to 100.33 µg CE 100 g−¹ DM) compared with the control (76.83 µg to 83.87 µg CE 100 g−¹ DM).

Table 5.
Antioxidant metabolites contained in tomato fruits of plants treated with Ulva onhoi seaweed suspension (SWS) and seaweed extract (SWE).
The levels of hydrophilic antioxidants in tomato fruits from SWS- and SWE-treated plants were significantly (p < 0.05) different compared to the control (Table 5). The flavonoid content in fruits from SWS- and SWE-treated plants was 1.16 and 1.28 mg QE g−¹ DW, respectively, which was more than double that of fruits from control plants (0.54 mg QE g−¹ DM). Also, the total anthocyanin content in tomato fruits from SWS-treated plants was 8.11 mg CGE g−¹ DM, representing a 2.7-fold increase compared to the control. Conversely, the tomatoes from SWE-treated plants exhibited significantly lower anthocyanin content (1.55 mg 100 g−1 DM) compared to the control (2.97 mg 100 g−1 DM).
The analysis of proanthocyanin content in the fruit revealed no significant differences between the treatments and the control. On the other hand, the total phenol content in fruits harvested from SWS- and SWE-treated plants was significantly lower (2.3 and 2.6 mg GAE g−¹ DM, respectively) compared to the control (3.1 mg GAE g−¹ DM). In the case of phenolic acids, tomatoes from SWS- and SWE-treated plants exhibited levels of 0.84 and 0.73 mg CAE g−¹ DM, respectively, which were nearly double the levels found in the fruits from control plants (0.41 mg CAE g−¹ DM). The tannin content in tomatoes from SWS-treated plants was 2.72 mg GAE g−¹ DM, representing a significant (p < 0.05) 1.26-fold increase compared to the control. In contrast, SWS treatment resulted in fruits with a tannin content (2.18 mg GAE g−¹ DM) similar to that of the control (2.16 mg GAE g−¹ DM). Finally, total ascorbic acid content showed a significant (p < 0.05) 1.2-fold increase in tomato fruits from SWS-treated plants (400.24 mg 100 g−¹ DM), while a decrease was observed in SWE-treated plants (228.43 mg 100 g−¹ DM) compared to the control (349.33 mg 100 g−¹ DM). This increase in ascorbic acid levels in tomatoes from SWS-treated plants may be linked to the lower pH (4.43) of the fruit juice.
3.3. Understanding Treatment–Variable Interactions Through Hierarchical Clustering and PCA
By relating the chemical composition of the treatments (SWS, SWE, and control) with the growth and productivity (yield, size, and fresh weight), nutritional properties (TSS [°Brix], pH, protein, fat, fiber, carbohydrates, ash, and energy), and dietary antioxidants (lycopene, carotene, phenols, flavonoids, anthocyanins, tannins, and ascorbic acid) of tomato fruits via a PCA, two factors explained 93.4% of the total variation (Figure 4a). Factor 1 (PC1) explained 76.04% of the variation and was negatively correlated with anions, cations, microelements in Ulva ohnoi, yield, lycopene content, and TSS (°Brix) and positively correlated with carbohydrates, pH, and phenol content of tomato fruits. Factor 2 (PC2) explained 17.38% of the variation and was negatively correlated with ascorbic acid, anthocyanins, number of seeds per fruit, and energy content and positively correlated with carotenoids, tannins, flavonoids, and protein in tomato fruits (Figure 4a).
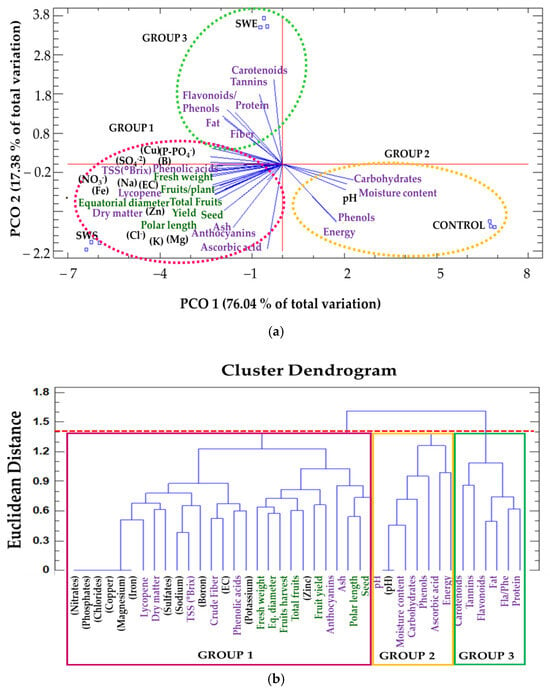
Figure 4.
Principal component analysis (PCA) and hierarchical clustering used to understand treatment–variable relationships between Ulva ohnoi seaweed suspension (SWS) and seaweed extract (SWE) and tomato fruit productivity and quality. (a) The data were analyzed via a principal component analysis (PCA). The lines originating from the central point of the biplots indicate the positive or negative correlations of different variables; their closeness indicates the correlation strength with a particular treatment. The variables include productivity (green), proximate composition, dietary antioxidants, energy content (purple) of the tomato fruits, and the chemical composition of treatments (abbreviations of capital letters in black). (b) The mean values of different parameters were clustered into three groups: group 1 (SWS) is highlighted by the purple outline, group 2 corresponds to the control (pink), and group 3 corresponds to SWE (green).
The data were plotted according to PC1 and PC2. Clearly separate clusters were identified based on the physicochemical variables of the different treatments and productivity, nutritional, and dietary antioxidant values. The first group, which was composed of SWS, had higher values of chemical characteristics (anions, cations, and microelements) in the seaweed suspension compared to the group composed of SWE. The plants in this group (SWS) had a higher yield, larger fruit, and greater fresh weight. Also, the tomato fruits had a higher content of dietary antioxidants (lycopene and anthocyanins). The second group comprised of the control treatment (water) and was mainly characterized by high carbohydrates, ascorbic acid, phenols, and moisture content in the tomato fruits. The third group was composed of SWE, which was characterized by high levels of carotenoids, flavonoids, tannins, protein, and fat (Figure 4a). A dendrogram classification based on the composition of the treatments with growth, productivity, nutritional properties, and dietary antioxidants revealed three groups: (1) SWS, (2) control, and (3) SWE with similar chemical characteristics at a Euclidean distance of 1.4 (Figure 4b).
4. Discussion
Tomato is a highly important horticultural crop due to its nutritional, nutraceutical, and economic value [12]. Tomatoes are also valuable sources of bioactive compounds that promote consumer health due to their unique phytochemical profile [7]. Currently, the main challenge in tomato production is optimizing plant development and productivity to meet the growing market demand for quantity and quality, while minimizing the use of synthetic chemical inputs [7]. Thus, there is a demand for alternative products derived from natural sources that can reduce the ripening time, enhance crop yield and fruit organoleptic properties, and protect the agroecosystem [11].
In this context, seaweeds and their by-products offer an economically viable option to promote plant growth and development, enhance soil fertility, improve nutrient use efficiency, and enhance crop quality and yield [13,18]. Additionally, seaweed-based products provide a safer and more sustainable approach to crop production due to their high biodegradability and low toxicity [6]. However, the effects of applying green seaweed-based biostimulants, such as SWS or SWE, on the quality of tomato fruits grown under standard conditions have been scarcely documented in the literature. Therefore, this study evaluated whether treatment with Ulva ohnoi SWS and SWE could positively impact the agronomic traits of tomato plants and the proximate composition and antioxidant content of their fruits. The results indicated that the application of SWS improved the productive response of tomato plants by accelerating overall ripening and increasing fruit production in the early harvest stages. Moreover, SWS treatment increased the number, size, fresh weight, and yield of the tomato fruits. This productive plant response is related to the chemical compounds present in SWS and SWE and the higher concentrations of chemical compounds present in SWS (Table 1; Figure 4), which may have positively influenced the absorption and assimilation of essential nutrient during the vegetative growth stage of the crop and their subsequent transport, enhancing plant development and hastening the time needed for flowering, fruiting, and harvesting. Contrary to the results obtained with the application of SWE from U. ohnoi (no improvement in the productive response of the tomato plants), Abu et al. [12] demonstrated that a seaweed extract of Ulva reticulata performed a dual function: it improved the vegetative growth of the plant and promoted early flowering and fruiting. According to Zodape et al. [45], better yields can be attributed to the improved uniformity of the fruit set and fruit weights following an improvement in the establishment of the plant canopy, light inception, and nutrient use through the application of seaweed-based products.
Other studies have also suggested that enhanced yield attributes in tomato plants treated with seaweed extracts were partly due to increased nutrient availability and uptake [11,18,19,33], as well as improved photosynthetic capacity and the efficient translocation of assimilates to the fruit set points [46,47,48]. In addition, supplying adequate amounts of Na and K to the growing substrate of tomato plants through SWS application can help maintain adequate water balance in the plants, thereby significantly improving fruit yield [49].
In this study, SWS was found to contain a higher concentration of all the chemical compounds analyzed (anions, cations, and microelements), which was nearly twice the concentration in SWE (Table 1). One possible explanation of these results could be the extraction temperature (121 °C), which influences the quantity and types of organic compounds present, such as polysaccharides, minerals, vitamins, oils, fats, acids, antioxidants, pigments, and hormones. Indeed, high extraction temperatures can degrade some biologically active compounds, while allowing inorganic compounds that are more temperature-resistant (e.g., minerals) to be released. This likely explains the differences observed in the productive responses of tomato plants to both treatments. The amounts of chemical compounds in SWE, which was applied during early crop development, may have been insufficient to significantly impact the productivity variables of the tomato plants. Furthermore, the soil application of SWE was relatively more complex due to the buffering effect of the biological, chemical, and physical properties of the soil, which may limit its immediate effectiveness in plants [47,50]. However, a significant increase in tomato fruit number and fresh weight was observed in the later stages of the harvest period, indicating a cumulative treatment effect.
In addition to increasing crop productivity in a sustainable manner to ensure food security, it is critical to ensure predictable crop quality traits for consumer acceptance [51]. Tomato internal quality is related to chemical (soluble solid content and pH) and nutritional (mineral, dry matter, fiber, and protein content) attributes and, more recently, to its nutraceutical value (phenolic content and antioxidant capacity) [8,22,52]. To improve tomato fruit quality, new production methods, preferably those that are organic and non-conventional, are required [9]. In this regard, both SWS and SWE applications significantly improved the quality of the harvested tomatoes, as evidenced by their improved proximate chemical composition. Tomatoes from treated plants had higher protein, fat, crude fiber, dry matter, and total soluble solid (TSS) content, as well as lower acidity and reduced total carbohydrate content, compared to the control.
The application of SWS and SWE to 91-day-old tomato plants resulted in increased levels of nitrogen, phosphorus, and potassium in the foliar tissues (Supplementary Table S1), which are involved in photosynthetic processes and facilitate phloem translocation of photoassimilates to the ripening fruit [53,54,55]. This may explain the increase in TSS, protein, crude fiber, and dry matter in tomato fruits from SWS- and SWE-treated plants. The higher accumulation of total minerals (ash) in the fruits from these treatments could be a result of enhanced root system development and nutrient uptake, as previously discussed by Espinosa-Antón et al. [23].
The most notable and significant effects of treatment with SWS or SWE on tomato fruit quality were observed with TSS (°Brix) and pH values, which constitute the main commercial properties that affect the sensorial qualities of the product [51,56]. Total soluble solid content determines the quality of tomato concentrate, with decreased °Brix values associated with decreased product quality [57]. Increased TSS content (i.e., soluble sugars and organic acids) can positively affect fruit sweetness and flavor intensity [11]. In our study, SWS and SWE application enhanced the TSS content in tomatoes. Mzibra et al. [21] found that a higher TSS content was attributed to increased metabolic activity in the tomato fruit, which promoted the synthesis and accumulation of metabolites, such as glucose and organic acids, as a result of seaweed-based biostimulant treatments. Other studies have also reported that treatment with seaweed-derived products positively affects the sweetness of tomato fruit, as expressed by an improvement in TSS content [12,20,22].
In our study, SWS- and SWE- application did not increase value pH, so don't affect the quality of tomato juice (pH = 4.4; mean of two treatments). Similarly, Colla et al. [58], Rouphael et al. [59], and Cozzolino et al. [14] also reported no effects of biostimulant application on mean pH (4.49, 4.46 and 4.21), which was optimal for canning. In addition, the pH value of 4.4 in tomatoes harvested from SWS- and SWE-treated plants suggests improved aroma and flavor, as well as reduced susceptibility to contamination by thermophilic organisms during processing and preservation [60]. These pH values fall within the optimal range required for tomato fruits of good and acceptable quality [61].
Overall, the observed improvement in chemical quality attributes following the application of U. ohnoi SWS and SWE highlights the potential of this green seaweed to boost the postharvest shelf life of tomatoes [38]. The importance of consuming tomatoes as functional foods is related to their role as an important source of secondary metabolites and health-promoting compounds [52,62,63]. In this study, carotenoid (lycopene and β-carotene) and phenolic compound (phenolic acids and flavonoids) contents were significantly enhanced with the use of SWS and SWE treatments.
Carotenoids are natural pigments that affect the sensory and nutritional quality of tomatoes and are recognized for their potential health benefits [7,8]. Lycopene, in particular, is the most abundant carotenoid in ripe tomatoes and is considered a bioactive compound that helps reduce the risk of several chronic diseases, including cancer and cardiovascular disease [64]. Similarly, plant polyphenols are highly valued in the human diet for their exceptional antioxidant properties, which allow them to scavenge reactive oxygen species and help prevent chronic disorders associated with oxidative stress [65].
Environmental and agronomic factors can significantly influence the synthesis of secondary metabolites in plants [9,38,55]. For example, Subramaniyan et al. [22], Grabowska et al. [66] and Colla et al. [58] observed that seaweed extract-based biostimulants can positively affect the formation of these bioactive compounds, such as lycopene, improving the nutritional and sensory attributes of tomato fruits under different growing conditions. Also, in another study, Kocira et al. [67] concluded that the application of three different seaweed-based biostimulants from Ulva lactuca increased the yield of beans and their production of polyphenols and carotenoids.
The supply and availability of specific plant nutrients throughout the crop cycle is another key factor that influences the accumulation of these metabolites in edible fruits [51]. Javaria et al. [54], found that lycopene levels in tomatoes were significantly affected by potassium levels, likely due to the critical role of this nutrient in accelerating enzymatic activities that enhance lycopene production in the fruit. Likewise, Colla et al. [58] suggested a positive correlation between lycopene and potassium concentrations and reported that the application of plant biostimulants appeared to benefit lycopene content through increased nutrient uptake.
In the present study, we showed that the higher lycopene content found in SWS- and SWE-treated fruits could be related to the K concentration. Espinosa-Antón et al. [23] and Espinosa-Antón and Hernández-Herrera [15] demonstrated that U. onhoi, applied to tomato plants as a seaweed extract or as a dry powder, improved nutrient uptake and assimilation and enhanced K content in foliar tissue, with treated plants increasing the quantity of fruits. Thus, K could be involved in carotenoid biosynthesis by acting on the activity of enzymes (pyruvate kinase and phosphofructokinase) that regulate carbohydrate metabolism [68].
The benefits of SWS and SWE by increasing phytochemical compounds in tomatoes (i.e., lycopene) could be the result of the activation of molecular and physiological mechanisms. Ertani et al. [69,70] reported that carbon and nitrogen metabolism and photosynthesis might be modulated by biostimulants through the increased activity of enzymes involved in glycolysis, the Krebs cycle, and nitrate assimilation [71]. Furthermore, Li et al. [55] concluded that different application rates of inorganic phosphorus may have differential effects on fruit quality by activating or repressing metabolic pathways related to carotenoid and organic acid accumulation. Based on this information, the increased content of bioactive compounds and antioxidant molecules in tomatoes can be partially attributed to the optimal levels of these nutrients in SWS- and SWE-treated plants (Supplementary Table S1).
Although all plants in this study received supplemental NPK fertilization every 15 days, those treated with SWS and SWE showed higher foliar nutrient content compared to the control plants at 91 days old. These results suggest two possible mechanisms that could explain the effects of SWS and SWE treatments. First, the supply of essential macro- and micronutrients, as well as other natural substances with biostimulant properties, can directly affect physiological and biochemical processes in plants. Second, seaweed-based products can modify the biological and physicochemical properties of the substrate by promoting the activity of beneficial microorganisms, improving water retention, and increasing nutrient availability, all of which contribute to improved plant fitness.
We acknowledge that trace element contamination is concerning, although U. ohnoi shows promise as a biostimulant that may help to meet food security goals. Indeed, previous studies have noted that further ecotoxicological research is needed before biostimulants can be widely used for human nutrition or as biofertilizers in agriculture [72,73]. Bioaccumulation factor analyses are needed to determine the accumulation efficiency of plants for the elements in their environments [74]. Ulva species accumulate metals from the water column and sediment, with heavy metal concentrations varying among species and environmental conditions. In seaweeds and seaweed-derived foods, the limits established by international regulations, such as the European Commission, regarding the maximum permissible residue limits for As, Cd, Hg, and Pb are 3, 0.5, 0.5–1, and 5 mg kg−1 (d/w basis), respectively, [72].
According to Hashem et al. [75], green macroalgae should be utilized as biostimulants to ensure healthy plants and adequate fruit production, with the resulting products suitable for human consumption. Of note, the U. ohnoi biomass obtained from land-based ponds of commercial culture systems do not contain heavy metals. Hence, the SWS and SWE employed in the present study could be used as biostimulants without the risk of introducing heavy metals, allowing farmers to boost tomato plant growth and productivity while promoting the production of high-quality fruits. Furthermore, the application of SWS or SWE would not lead to the contamination of water sources close to farming areas, including rivers and groundwater, nor would their application disrupt ocean ecosystems by causing eutrophication, which is often associated with current farming practices [76].
Therefore, to advance our understanding of these relationships, more detailed experiments should be conducted to evaluate raw seaweeds and the preparation of seaweed-derived biostimulants to decrease heavy metal concentrations after heating with various methods (e.g., autoclave, microwave, and water bath) [77,78] and their effects on crops. These findings would allow us to expand our knowledge of the biostimulant potential of seaweeds and advance the development of eco-friendly approaches for sustainable agriculture.
5. Conclusions
The results of this study demonstrate the different beneficial effects of the application of the green seaweed U. ohnoi, either in its natural form as a suspended powder (SWS) or as a seaweed extract (SWE), on key variables related to tomato fruit productivity and quality, which can be attributed to variations in the chemical profiles of each presentation. The SWS-treatment proved to be highly beneficial, significantly reducing tomato ripening times and improving the productive capacity of the plants in terms of fruit quantity, yield, and size. In addition, tomato fruits from SWS- and SWE-treated plants exhibited improved quality attributes that play an important role in tomato selection. In general, both treatments derived from U. ohnoi demonstrated effectiveness equal or superior to that of chemical fertilization (NPK) (applied to the control group), suggesting that the application of these biostimulants constitutes an alternative and efficient solution to reduce the use of traditional chemical fertilizers while improving yield and fruit quality. Furthermore, our findings highlight the agricultural potential of SWS and SWE application to biofortify vegetable crops and produce high-quality foods enriched with essential nutrients and nutraccompounds. In particular, the use of SWS is likely a cost-effective method for local farmers to promote sustainable crop production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15070750/s1, Table S1: Number of fruits harvested and fruit fresh weight (g) produced by 91 to 121-day-old tomato plants treated with seaweed suspension (SWS) and seaweed extract (SWE) from Ulva ohnoi over a 30-day harvest period. Table S2: Nutrients and chlorophyll content in the leaf tissue of tomato plants (91-day-old) treated with seaweed suspension (SWS) and seaweed extract (SWE) from Ulva ohnoi.
Author Contributions
Conceptualization, R.M.H.-H. and A.A.E.-A.; methodology, S.F.V.-R. and R.M.H.-H.; software, R.M.H.-H.; validation, E.S.-P. and A.C.R.-A.; formal analysis, A.A.E.-A. and R.M.H.-H.; investigation, A.A.E.-A.; resources, R.M.H.-H., S.F.V.-R. and A.C.R.-A.; writing—original draft preparation, A.A.E.-A.; writing—review and editing, R.M.H.-H.; visualization, A.A.E.-A. and E.S.-P.; supervision, R.M.H.-H.; project administration, R.M.H.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by The Secretariat of Science, Humanities, Technology and Innovation (SECIHTI) [grant code 2021-000018-02NACF, awarded to A.A.E.-A.]. This work was financed through the Program to Support the Improvement in Production Conditions of Members of the S.N.I. and S.N.C.A.—(PROSNI 2024) of the University of Guadalajara.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge José Antonio Zertuche González and Andrea Liévana MacTavish for her valuable suggestions and English language review. The authors would like to thank all the reviewers who participated in the review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United Nations Organization. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 18 November 2024).
- Food and Agricultural Organization. Faostat. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 November 2024).
- Quiroga, M.R.; Rosale, E.M.; Rincón, E.P.; Hérnandez, G.E.; Garrido, R. Enfermedades causadas por hongos y nematodos en el cultivo de Tomate (Lycopericon esculentum Mill.). Chiapas, México. Rev. Mex. Fitopatol. 2007, 25, 114–119. [Google Scholar]
- Agroconsult Buinov. Digital Marketing From. Available online: https://agroconsult-buinov.com/en/produkt/tomato-rio-fuego/ (accessed on 10 March 2025).
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [PubMed]
- Pohl, A.; Kalisz, A.; Sekara, A. Seaweed extracts’ multifactorial action: Influence on physiological and biochemical status of Solanaceae plants. Acta Agrobot. 2019, 72, 1–11. [Google Scholar]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of Physico-Chemical and Organoleptic Fruit Parameters Relevant for Tomato Quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Jurić, S.; Vlahoviček-Kahlina, K.; Jurić, O.; Uher, S.F.; Jalšenjak, N.; Vinceković, M. Increasing the lycopene content and bioactive potential of tomato fruits by application of encapsulated biological and chemical agents. Food Chem. 2022, 393, 133341. [Google Scholar]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar]
- Paull, J. The Uptake of Organic Agriculture: A Decade of Worldwide Development. J. Soc. Dev. Sci. 2011, 2, 111–120. [Google Scholar]
- Abu, N.J.; Bujang, J.S.; Zakaria, M.H.; Zulkifly, S. Use of Ulva reticulata as a growth supplement for tomato (Solanum lycopersicum). PLoS ONE 2022, 17, e0270604. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plant 2021, 10, 1–27. [Google Scholar]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar]
- Espinosa-Antón, A.A.; Hernández-Herrera, R.M. Effects of green seaweed (Ulva onhoi) on the reproductive development of tomato (Solanum lycopersicum) plants. Acta Agrobot. 2024, 77, 193117. [Google Scholar]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Kaur, I. Seaweeds: Soil Health Boosters for Sustainable Agriculture. In Soil Health. Soil Biology; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 163–182. [Google Scholar]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar]
- Mzibra, A.; Aasfar, A.; Khouloud, M.; Farrie, Y.; Boulif, R.; Kadmiri, I.M.; Bamouh, A.; Douira, A. Improving Growth, Yield, and Quality of Tomato Plants (Solanum lycopersicum L.) by the Application of Moroccan Seaweed-Based Biostimulants under Greenhouse Conditions. Agronomy 2021, 11, 1373. [Google Scholar] [CrossRef]
- Subramaniyan, L.; Veerasamy, R.; Prabhakaran, J.; Selvaraj, A.; Algarswamy, S.; Karuppasami, K.M.; Thangavel, K.; Nalliappan, S. Biostimulation Effects of seaweed extract (Ascophyllum nodosum) on phytomorpho-physiological, yield, and quality traits of tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 348. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.A.; Zamora-Natera, J.F.; Zarazúa-Villaseñor, P.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Águila Alcántara, E.; Torres-Morán, M.I.; Velasco-Ramírez, P.; Hernández-Herrera, R.M. Application of Seaweed Generates Changes in the Substrate and Stimulates the Growth of Tomato Plants. Plants 2023, 12, 1520. [Google Scholar] [CrossRef]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar]
- Hamouda, M.M.; Saad-Allah, K.M.; Gad, D. Potential of Seaweed Extract on Growth, Physiological, Cytological and Biochemical Parameters of Wheat (Triticum aestivum L.) Seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 1818–1831. [Google Scholar]
- Whapham, C.A.; Blunden, G.; Jenkins, T.; Hankins, S.D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 1993, 5, 231–234. [Google Scholar]
- Kasim, W.A.; Ham, E.A.M.; Shams El-Din, N.G. Eskander S Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int. J. Agron. Agric. Res. 2015, 7, 173–189. [Google Scholar]
- Latique, S.; Mrid, R.B.; Kabach, I.; Kchikich, A.; Sammama, H.; Yasri, A.; Nhiri, M.; El Kaoua, M.; Douira, A.; Selmaoui, K. Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.). Agronomy 2021, 11, 265. [Google Scholar] [CrossRef]
- Hiraoka, M.; Shimada, S.; Uenosono, M.; Masuda, M. A new green-tide-forming alga, Ulva ohnoi Hiraoka et Shimada sp. nov. (Ulvales, Ulvophyceae) from Japan. Phycol. Res. 2004, 52, 17–29. [Google Scholar]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar]
- Kang, E.J.; Han, A.R.; Kim, J.H.; Kim, I.N.; Lee, S.; Min, J.O.; Nam, B.R.; Choi, Y.J.; Edwards, M.S.; Diaz-Pulido, G.; et al. Evaluating bloom potential of the green-tide forming alga Ulva ohnoi under ocean acidification and warming. Sci. Total Environ. 2021, 769, 144443. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar]
- Suchithra, M.R.; Muniswami, D.M.; Sri, M.S.; Usha, R.; Rasheeq, A.A.; Preethi, B.A.; Dineshkumar, R. Effectiveness of green microalgae as biostimulants and biofertilizer through foliar spray and soil drench method for tomato cultivation. S. Afr. J. Bot. 2022, 146, 740–750. [Google Scholar]
- Revilla-Lovano, S.; Sandoval-Gil, J.M.; Zertuche-Gonzalez, J.A.; Belando-Torrentes, M.D.; Bernardeau-Esteller, J.; Rangel-Mendoza, L.K.; Ferreira-Arrieta, A.; Guzmán-Calderón, J.M.; Camacho-Ibar, V.F.; Muñiz-Salazar, R.; et al. Physiological responses and productivity of the seaweed Ulva ohnoi (Chlorophyta) under changing cultivation conditions in pilot large land-based ponds. Algal Res. 2021, 56, 102316. [Google Scholar]
- Gupta, A.; Rakhasiya, B.; Depani, P.; Bhagiya, B.K.; Kaushik, A.; Bodar, P.A.; Mantri, V.A. Effect of Ascophyllum extract on cell division, proximate composition, antioxidant response and internal plant hormone composition in green seaweed Ulva ohnoi (Chlorophyta). J. Appl. Phycol. 2024, 36, 3623–3636. [Google Scholar]
- AOAC. Association of Official Analytical Chemists, Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Liava, V.; Chaski, C.; Añibarro-Ortega, M.; Pereira, A.; Pinela, J.; Barros, L.; Petropoulos, S.A. The effect of biostimulants on fruit quality of processing tomato grown under deficit irrigation. Horticulturae 2023, 9, 1184. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar]
- Cimpoiu, C.; Cristea, M.; Hosu, A.; Sandru, M.; Seserman, L. Antioxidant activity prediction and classification of some teas using neutral networks. Food Chem. 2011, 127, 1323–1328. [Google Scholar]
- Martínez-Cruz, N.; Arévalo-Niño, K.; Verde-Star, M.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Núñez-González, M.; Morales-Rubio, M. Antocianinas y actividad anti radicales libres de Rubusa denotrichus Schltdl (zarzamora). Rev. Mex. Cienc. Farm. 2011, 42, 66–71. [Google Scholar]
- Curifuta, M.; Vidal, J.; Sánchez-Venegas, J.; Contreras, A.; Salazar, L.; Alvear, M. The in vitro antifungal evaluation of a commercial extract of Chilean propolis against six fungi of agricultural importance. Cienc. Investig. Agrar. 2012, 39, 347–359. [Google Scholar]
- Matkowski, A.; Tasarz, P.; Szypuła, E. Antioxidant activity of herb extracts from five medicinal plants from Lamiaceae, subfamily Lamioideae. J. Med. Plant Res. 2008, 2, 321–330. [Google Scholar]
- Ricco, R.; Agudelo, I.; Garcés, M.; Evelson, P.; Wagner, M.; Gurni, A. Polifenoles y actividad antioxidante en Equisetum giganteum L. (Equisetaceae). Bol. Latinoam. Caribe Plantas Med. Aromát. 2011, 10, 325–332. [Google Scholar]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Yao, Y.; Wang, X.; Chen, B.; Zhang, M.; Ma, J. Seaweed extract improved yields, leaf photosynthesis, ripening time, and net returns of tomato (Solanum lycopersicum Mill.). ACS Omega 2020, 5, 4242–4249. [Google Scholar] [PubMed]
- Baghdadi, A.; Della Lucia, M.C.; Borella, M.; Bertoldo, G.; Ravi, S.; Zegada-Lizarazu, W.; Chiodi, C.; Pagani, E.; Hermans, C.; Stevanato, P.; et al. A dual-omics approach for profiling plant responses to biostimulant applications under controlled and field conditions. Front. Plant Sci. 2022, 13, 983772. [Google Scholar]
- Layek, J.; Dutta, S.K.; Krishnappa, R.; Das, A.; Ghosh, A.; Mishra, V.K.; Panwar, A.S.; Hazarika, S.; Devi, S.; Kumar, M.; et al. Productivity, quality and profitability enhancement of French bean, okra and tomato with seaweed extract application under North-Eastern Himalayan condition. Sci. Hortic. 2023, 309, 111626. [Google Scholar]
- Idowu, M.K.; Aduayi, E.A. Effects of Sodium and Potassium Application on Water Content and Yield of Tomato in Southwestern Nigeria. J. Plant Nutr. 2006, 29, 2131–2145. [Google Scholar]
- Mattner, S.W.; Wite, D.; Riches, D.A.; Porter, I.J.; Arioli, T. The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biol. Agric. Hortic. 2013, 29, 258–270. [Google Scholar]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic plant biostimulants and fruit quality—A review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef]
- Mengel, K.; Viro, M. Effects of potassium supply on the transport of photosynthates to the fruits of tomato (Lycopersicon esculentum). Physiol. Plant. 1974, 30, 295–300. [Google Scholar]
- Javaria, S.; Khan, M.Q.; Bakhsh, I. Effect of potassium on chemical and sensory attributes of tomato fruits. J. Anim. Plant Sci. 2012, 22, 1081–1085. [Google Scholar]
- Li, Z.; Qiu, Q.; Chen, Y.; Lin, D.; Huang, J.; Huang, T. Metabolite alteration in response to low phosphorus stress in developing tomato fruits. Plant Physiol. Biochem. 2021, 159, 234–243. [Google Scholar]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar]
- Kabaş, A.; Ercan, U.; Kabas, O.; Moiceanu, G. Prediction of Total Soluble Solids Content Using Tomato Characteristics: Comparison Artificial Neural Network vs. Multiple Linear Regression. Appl. Sci. 2024, 14, 7741. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar]
- Paulson, K.N.; Stevens, M.A. Relationships among titratable acidity, pH and buffer composition of tomato fruits. J. Food Sci. 1974, 39, 354–357. [Google Scholar]
- Jones, J.B., Jr. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar]
- Mirdehghan, S.H.; Valero, D. Bioactive compounds in tomato fruit and its antioxidant activity as affected by incorporation of Aloe, eugenol, and thymol in fruit package during storage. Int. J. Food Prop. 2017, 20, 1798–1806. [Google Scholar]
- Meng, F.; Li, Y.; Li, S.; Chen, H.; Shao, Z.; Jian, Y.; Liu, L.; Wang, Q. Carotenoid biofortification in tomato products along whole agro-food chain from field to fork. Trends Food Sci. 2022, 124, 296–308. [Google Scholar]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E.; Jezdinsky, A.; Kalisz, A.; Sekara, A. The effect of biostimulants on the quality parameters of tomato grown for the processing industry. Agrochimica 2015, 59, 203–217. [Google Scholar]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [PubMed]
- Bramley, P.M. Regulation of carotenoids formation during tomato fruit ripening and development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar]
- El-Mahrouk, M.E.; Dewir, Y.H.; Hafez, Y.M.; El-Banna, A.; Moghanm, F.S.; El-Ramady, H.; Mahmood, Q.; Elbehiry, F.; Brevik, E.C. Assessment of Bioaccumulation of Heavy Metals and Their Ecological Risk in Sea Lettuce (Ulva spp.) along the Coast Alexandria, Egypt: Implications for Sustainable Management. Sustainability 2023, 15, 4404. [Google Scholar] [CrossRef]
- Lee, K.J.; Kang, E.H.; Yoon, M.; Jo, M.R.; Yu, H.; Son, K.T.; Jeong, S.H.; Kim, J.H. Comparison of Heavy Metals and Arsenic Species in Seaweeds Collected from Different Regions in Korea. Appl. Sci. 2022, 12, 7000. [Google Scholar] [CrossRef]
- Aitta, A.; El-Ramady, H.; Alshaal, T.; El-Henawy, A.; Shams, M.; Talha, N.; Elbehiry, F.; Brevik, E.C. Seasonal and spatial distribution of soil trace elements around Kitchener drain in the northern Nile Delta, Egypt. Agriculture 2019, 9, 152. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy 2019, 9, 146. [Google Scholar] [CrossRef]
- Sekhouna, D.; Kies, F.; Elegbede, I.O.; Matemilola, S.; Zorriehzahra, J.; Hussein, E.K. Potential assay of two green algae Ulva lactuca and Ulva intestinalisas biofertilizers. Sustain. Agri. Food Environ. Res. 2021, 9, 567–580. [Google Scholar]
- Ersoy, B.; Yanar, Y.; Kucukgulmez, A.; Celik, M. Effects of four cooking methods on the heavy metal concentrations of sea bass fillets (Dicentrarchus labrax Linne, 1785). Food Chem. 2006, 99, 748–751. [Google Scholar] [CrossRef]
- Tengku Nur Alia, T.K.A.; Hing, L.S.; Sim, S.F.; Pradit, S.; Ahmad, A.; Ong, M.C. Comparative study of raw and cooked farmed sea bass (Lates calcarifer) in relation to metal content and its estimated human health risk. Mar. Pollut. Bul. 2020, 153, 111009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).