Abstract
The primary strategy for managing Nacobbus aberrans has traditionally relied on synthetic chemicals. However, increasing regulatory pressure on unsafe products has led to a growing research focus on nematicides. Despite this, chemical nematicides remain more effective than other control methods. Consequently, there is a pressing need to develop novel nematicides that are both effective and environmentally safer. This study aimed to evaluate the nematocidal efficacy of various synthetic molecules against the second-stage juveniles of N. aberrans, the false root-knot nematode. A total of fifty-eight synthetic derivatives were obtained and tested in vitro at a concentration of 500 µg/mL. The results identified the AGAz family as the most promising, with AGAz-3 (LC50: 52.7 µg/mL) and AGAz-4 (LC50: 103.22 µg/mL) surpassing the efficacy of chitosan. Our findings emphasize the strong potential of AGAz-3 and AGAz-4 as nematocidal agents, particularly for in situ applications in agricultural settings. Additionally, AGAz-3 demonstrates potential not only as a nematocidal agent but also as an incentive for related research exploring its analogs as effective ovicidal compounds and investigating its efficacy against other phytonematodes. Furthermore, compounds from the N-Sulfonyl-hydrazone and N-acyl-hydrazone series showed efficacy (>50%), warranting additional experiments to assess their effectiveness across the most important pest phytonematodes.
1. Introduction
Annual agricultural losses caused by phytoparasitic nematodes are estimated to reach up to USD 157 billion worldwide [1]. Among the significant nematodes, Nacobbus aberrans causes losses ranging from 30% to 80%. This nematode can infect up to 84 different plant species and is identified as a pest of quarantine significance in the United States. In Europe, it is listed on the A1 list (absent in the EPPO region) and is recommended for regulation [2]. N. aberrans typically occurs in higher population densities compared to Meloidogyne spp., another cecidogenous parasite [3]. The damage they cause manifests in both direct and indirect ways. Second-stage juveniles (J2) of N. aberrans infect plants and form syncytia to extract nutrients. Furthermore, the lesions created during infection render the plant vulnerable to attacks by other organisms such as viruses, bacteria, and fungi [4]. Most studies focus on M. incognita, the most important phytonematode worldwide. However, N. aberrans is also very significant due to its harmful impact and quarantine designation. It can even displace M. incognita when both are present in the same production system.
The primary strategy for managing N. aberrans has been the use of synthetic chemicals such as oxamyl, which have proven to be the most effective compared to novel alternatives due to their rapid effect, broad spectrum of activity, and other factors. However, several chemical nematocides have been restricted due to global regulations, citing their harmful effects on human health and the environment [5]. From 2019 to 2021, the Pesticide Action Network (PAN) added 94 new pesticides to the list of substances banned in various countries [6]. Recent research on nematicides has primarily been driven by increasing regulatory pressure on unsafe products. Current nematicides, including both fumigant and non-fumigant types, present significant environmental risks and are increasingly subjected to stringent regulatory controls. [7]. However, chemical nematocides continue to be more effective than other control methods [8]. According to Velasco-Azorsa [9], only two reports of natural compounds have been recorded as having the potential to control N. aberrans.
On the other hand, several synthetic compounds are still approved as nematocidal products in the southern zone of the European Union [10]. In 2020, the U.S. Environmental Protection Agency (EPA) registered fluazaindolizine, a new active ingredient nematocide for agricultural use [11]. Various authors advocate for the use of these products under an integrated pest management framework. Therefore, it is essential to research novel nematicides that are not only effective but also safer for the environment, soil, and human health. The development of such compounds is necessary to address the growing resistance and environmental concerns associated with current nematocides.
The common methods for identifying chemical nematocides include screening for nematocidal activity of commercial pesticides, such as fluopyram, and intermediate or lead compound derivatization, among others [1]. Novel synthetic compounds have been investigated as potential nematocidal agents, suggesting satisfactory results. For instance, Vázquez-Bravo et al. [12] achieved an over 80% mortality rate of N. aberrans J2 exposed in vitro to two ferrocenyl chalcones for 36 h. Velasco-Azorsa et al. [13] observed a nematostatic effect when they tested (para-bromobenzoate) and (ortho-nitrobenzoate) at 1000 µg mL−1. In another study, two trifluorobutene amide derivatives exhibited nematocidal activity, and the half-maximal lethal concentration (LC50) values were 2.02 mg L−1 and 0.76 mg L−1 when tested against M. incognita [14]. In 2023, Khan et al. [15] assessed the nematocidal potential of synthesized thiazine derivatives. Two of these compounds were effective against Caenorhabditis elegans, with half-maximal lethal dos (LD50) values estimated at 38.95 μg/mL and 38.21 μg/mL. In another study, the effectiveness of amide derivatives was assessed at 200 µg/mL against Aphelenchoides besseyi, and the results showed 80% efficacy, similar to fosthiazate, a commercial nematocide [16]. In the search for compounds with antiparasitic activity, our studies have yielded promising results (>60% efficacy) with a series of ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives tested in vitro against Trypanosoma cruzi trypomastigotes and Leishmania mexicana promastigotes [17]. In another study, Duque-Montaño et al. [18] observed in vitro antiamoebic activity against the Entamoeba histolytica HM1:IMSS strain. These findings led us to propose testing some of the synthesized compounds against the phytoparasitic nematode Nacobbus aberrans.
Although biological and natural nematocides have received considerable attention, chemical nematocides remain the main strategy for managing nematodes and are expected to maintain a significant market share in the future. Therefore, it is important to continue developing novel nematocides to expand the range of available alternatives. Thus, the objective of the present study was to assess the nematocidal effectiveness of different synthetic molecules against J2 of N. aberrans.
2. Materials and Methods
2.1. N-Sulfonyllhydrazone Series
Synthesis of compounds 1–5 (Figure 1): Benzenesulfonyl hydrazone derivatives were prepared by reacting to equimolar amounts of benzenesulfonyl hydrazide and diverse aldehyde derivatives (1 mmol: 1 mmol) using 30 mL of ethanol as a solvent and acetic acid drops as catalysts. Reagents and catalysts were added at room temperature. The reaction mixture was refluxed for 3–6 h with constant mixing, and progress was tracked by TLC. The product was isolated by recrystallization in ethanol or purification in column chromatography using n-hexane: ethyl acetate using 100 mL purification volumes with 2 mL polar gradient.
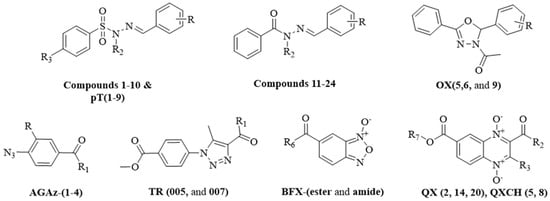
Figure 1.
General structure of compounds evaluated as potential nematocidal agents: N-acyl hydrazones, oxodiazoles, phenyl azides, triazoles, benzofuroxan-1-N-oxide esters, benzofuroxan-1-N-oxide amides, and quinoxaline-1,4-di-N-oxides.
Synthesis of compounds 6–10 (Figure 1): (E)-methyl 2-benzylidene-1-(phenylsulfonyl) hydrazinecarboxylate derivatives were obtained by initially reacting previously obtained benzenesulfonyl hydrazone derivatives (1 mmol) with dry triethylamine (3 mmol), which was added dropwise at 0 °C, using dichloromethane as a solvent and allowing the reaction to continue for 1 h. Subsequently, 1.5 mmol of methyl chloroformate was added dropwise and the reaction was permitted to continue for an additional 24–48 h at room temperature. The reaction mixture was purified by column chromatography using n-hexane: ethyl acetate using 100 mL purification volumes with a 2 mL polar gradient and crystallized using dichloromethane/n-Hexane (50:50).
2.2. N-Acylhydrazone Series
Synthesis of compounds 11–15 (Figure 1): N′-[(E)-phenylmethylidene]benzeneacyl hydrazone derivatives were prepared by reacting equimolar amounts of benzeneacyl hydrazide and diverse aldehyde derivatives (1 mmol: 1 mmol) using 30 mL of ethanol as a solvent and HCl drops (37%) as catalysts. Reagents and catalysts were added at room temperature. The reaction mixture was refluxed for 3–6 h with constant mixing, and progress was tracked by TLC. The product was isolated by purification in column chromatography using n-hexane: ethyl acetate using 100 mL purification volumes with a 2 mL polar gradient.
Synthesis of compounds 16–24 (Figure 1): (E)-methyl 1-benzoyl-2-benzylidenehydrazinecarboxylate derivatives were obtained by initially reacting previously obtained benzeneacyl hydrazone derivatives (1 mmol) with dry triethylamine (3 mmol), which were added dropwise at 0 °C, using dichloromethane as a solvent and allowing the reaction to continue for 1 h. Subsequently, 1.5 mmol of methyl chloroformate was added dropwise and the reaction was permitted to continue for an additional 24–48 h at room temperature. The reaction mixture was purified by column chromatography using n-hexane: ethyl acetate using 100 mL purification volumes with a 2 mL polar gradient and crystallized using dichloromethane/n-Hexane (50:50).
2.3. pT-Series
Synthesis of compounds pT 1–9 (Figure 1): (E)-N’-benzylidene-4-methylbenzenesulfonohydrazide derivatives were prepared by reacting to equimolar amounts of para-Toluenesulfonyl hydrazide and diverse aldehyde derivatives (1 mmol: 1 mmol) using 30 mL of ethanol as a solvent and acetic acid drops as catalysts. The reagents and catalysts were added at room temperature. The reaction mixture was refluxed for 3–6 h with constant mixing, and progress was tracked by TLC. The reaction product was isolated by recrystallization in EtOH.
2.4. Oxadiazole Series
Synthesis of compounds OX (Figure 1): 4-acetyl-1,3,4-oxadiazole derivatives (5, 6, and 12) were obtained by reacting diverse N-acyl hydrazone derivatives with 20 mL of acetic anhydride. The reaction mixture was refluxed for 1–2 h, and reaction progress was tracked by TLC. Precipitation of the product was induced by adding the reaction mixture to iced water; the precipitate was then filtered and washed with distilled water. The product was isolated by recrystallization from absolute ethanol.
2.5. Azide Series
Synthesis of compounds AGAz (Figure 1): Derivative AGAz-1 was synthesized by reacting 1 g of methyl-4-azidobenzoate with 1 mL of hydrazine monohydrate using methanol as a solvent. The reaction mixture was refluxed for 3 h. The product precipitated from the reaction mixture, and then was filtered and washed with cold methanol. Derivative AGAz-2 was synthesized by reacting 4-azidobenzoic acid with methanol as a solvent/reagent and sulfuric acid as the catalyst. The reaction mixture was refluxed for 4 h, and the solvent was then evaporated and purified by column chromatography. Derivatives AGAz-3, and AGAz-4 were synthesized by esterification of 4-azido-3-nitrobenzoic acid with methanol and ethanol, respectively, and then purified by column chromatography.
2.6. Triazole Series
Synthesis of compounds TR (Figure 1): Derivatives TR005, and TR007 were synthesized by the reaction of methyl-4-azidobenzoate with β-diketones under alkaline conditions achieved with sodium carbonate, using methanol as a solvent. The reaction mixture was refluxed for 1 h, and the product was purified by column chromatography.
2.7. Benzofuroxan-1-N-Oxide Series
Synthesis of compounds BFX (Figure 1): Synthesis of benzofuroxane esters: Methyl, ethyl, propyl, isopropyl, butyl, isobutyl, pentyl, and cyclohexyl esters of benzofuroxan-1-oxide were synthesized by the esterification of benzofuroxan-1-N-oxide-6-carboxylic acid. First, 1 g of benzofuroxan was reacted with 8 mL of the required alcohol. The reaction was catalyzed with 0.5 mL sulfuric acid, and the reaction mixture was refluxed for 4 h. The solvent was evaporated and purified by column chromatography.
Synthesis of benzofuroxane amides: The BFX-Ami series was synthesized by initial activation of the carboxylic acid of benzofuroxan-1-N-oxide-6-carboxylic acid using Mukaiyama reagent. To permit acid activation, 1 mmol of benzofuroxan-1-N-oxide-6-carboxylic acid was reacted with 3 mmol of Mukaiyama reagent and 6 mmol of triethylamine to catalyze the reaction, with the activation carried out at 0 °C using 20 mL of dichloromethane as a solvent. The activation reaction mixture was left for 2 h. Subsequently, a mixture of 10 mL of dichloromethane with 1.5 mmol of aniline derivative was added dropwise while maintaining the mixture at 0 °C. The reaction was maintained in an ice bath for an additional 2 h, after which the reaction was removed from the ice bath and permitted to react at room temperature overnight. The solvent was evaporated, and the product was purified by column chromatography.
2.8. Quinoxaline-1,4-di-N-oxide Series
Synthesis of compounds QX (Figure 1): The butyl and cyclohexyl quinoxaline-1,4-di-N-oxide series were synthesized from the reaction of 6-butyl ester benzofuroxan-1-oxide, and 6-cyclohexyl ester benzofuroxan-1-oxide, respectively, with diverse β-diketones using the Beirut reaction. First, 2 mmol of benzofuroxan ester was reacted with 4 mmol of corresponding β-diketone at 0 °C, and 1.5 mL of triethylamine was added dropwise. The reaction mixture was removed from the ice bath and allowed to react for 4–7 days; reaction progress was tracked using TLC. The product was isolated using column chromatography using n-hexane and ethyl acetate.
2.9. Obtaining Eggs and Juveniles of Nacobbus aberrans
Eggs were obtained from infected Capsicum annuum (pepper) cultivated at the Colegio de Postgraduados Campus Montecillo, Estado de México, following a standardized protocol. The roots were first washed with tap water and cut into 1–2 cm fragments, soaked in a 1.5% NaClO solution, and shaken for 3 min. The resulting suspension was filtered through a series of 200-, 350-, and 400-mesh sieves and rinsed with abundant distilled water to remove the chlorine. Eggs retained on the 400-mesh sieve were collected with a Pasteur pipette and suspended in 10 mL of distilled water, and then were subjected to centrifugation at 3000 rpm for three minutes.
The eggs were recovered by the centrifugal-flotation technique. The precipitate was resuspended in 5 mL of MgSO₄ solution (specific gravity 1.18) and centrifuged at 3000 rpm for 3 min. The upper phase was filtered through a 400-mesh sieve, and the retained eggs were thoroughly washed with distilled water (five times), with centrifugation between each wash [19]. The freshly collected eggs were then incubated in distilled water at 25 ± 1.0 °C for 8 days to obtain J2, following the method described by Vázquez-Sánchez et al. [20].
2.10. Nematocidal Activity of Compounds Against J2 of N. aberrans
This study employed a randomized experimental design. The tested compounds were dissolved in a 5% DMSO solution at a concentration of 1 mg/mL, with sonication for five minutes to aid dissolution. Initially, 2 mg of the compound was placed in a 1.5 mL Eppendorf tube, followed by the addition of 50 µL of pure DMSO. The mixture was vortexed, and distilled water was added to reach a final volume of 1 mL. The in vitro test was conducted using 96-well microtiter plates. Each well contained 50 µL of the corresponding treatment, 30 µL of chloramphenicol (at 333.33 µg/mL), and finally, 20 µL of distilled water containing 100 J2, which was added after the test chemical was introduced. The plates were sealed and incubated for 72 h in a humid chamber at room temperature. Subsequently, before assessing each well, 10 µL of 1 N NaOH was added. Later, a compound microscope (40× magnification) was employed to observe the twisted appearance of live larvae in response to the chemical stimulus, as described by Cruz-Arévalo et al. [21]. Each treatment was tested in triplicate, with DMSO 2.5% serving as the negative reference, while chitosan (6 mg/mL) was the commercial reference.
2.11. In Vitro Nematocidal Effectiveness of AGAz Family
The compounds were tested at a concentration of 1 mg/mL, dissolved in DMSO at 5%. In this bioassay, each treatment had four replicates. The experimental conditions were consistent across all assessments of the family’s compounds. The compounds AgAz3 and AgAz4 were evaluated in vitro at concentrations of 500, 250, 125, 62.5, 31.25, and 15.63 µg/mL, dissolved in 5% DMSO. Each treatment was tested in four replicates, and DMSO at 5% was used as a negative reference.
2.12. Statistical Analysis
All experiments were conducted using a completely randomized design. Data collection was performed using the KDsmart application [22]. Mortality was estimated for each replicate, and mean mortality was calculated as the average mortality across four replicates using R software (version 4.4.1). The dataset of phenyl azide derivatives was subjected to one-way ANOVA, and mean comparisons were carried out with the Tukey test through the agricolae package. Probit analysis was performed using the drc package to estimate the median lethal concentration (LC50) and LC90.
3. Results
3.1. Screening of the Nematocidal Effectiveness
A screening was conducted with fifty-eight synthetic compounds at 500 µg/mL (Table 1). Only seven of them had considerable effectiveness, with mortality rates up to 15%. According to the results, the compound with the highest effectiveness was AGAz-4 (Figure 2); therefore, it was decided to select four more compounds from this family, and a new mortality assay was carried out.

Table 1.
Mean mortality ± SD of Nacobbus aberrans J2 exposed in vitro against fifty-eight different synthetic molecules.
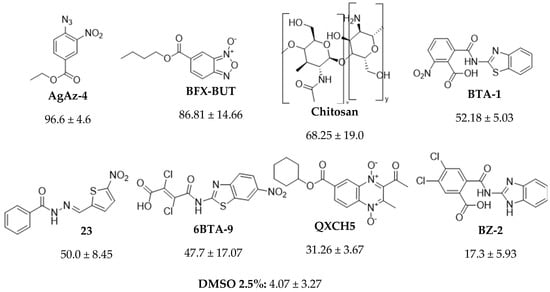
Figure 2.
Compounds with over 15% in vitro nematocidal effectiveness against juveniles of Nacobbus aberrans.
3.2. Nematocidal Effectiveness of AGAz Family Against Juveniles of Nacobbus aberrans
The nematocidal effectiveness of five compounds from the AGAz family was assessed against the J2 of N. aberrans. Among these, three compounds demonstrated high effectiveness, achieving mortality rates exceeding 90%. A Tukey test revealed that there were no significant differences in nematocidal effectiveness between AGAz-3, AGAz-4, and PAzBA (Figure 3). However, the remaining two compounds showed similar effectiveness to the 2.5% DMSO control. Median lethal concentration was measured with AGAz-3 and AGAz-4 compounds (Figure 4), where AGAz-3 was twice as toxic as AGAz4.
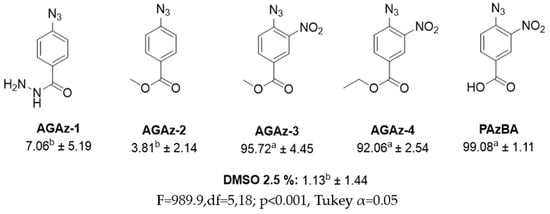
Figure 3.
Mean mortality ± SD of Nacobbus aberrans juveniles exposed in vitro to phenyl azide derivatives. Averages with different letters indicate significant.
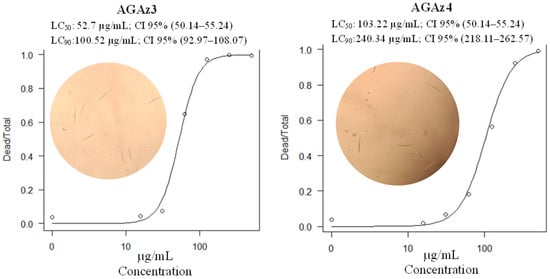
Figure 4.
Median lethal concentration of AGAz–3 and AGAz–4 tested in vitro against Nacobbus aberrans juveniles. Points in the graph represent the mean mortality of juveniles. CI = Confidence interval.
4. Discussion
Fifty-eight synthetic compounds were tested against the phytoparasitic nematode N. aberrans. Gomes et al. [23] tested the effectiveness of twenty-two compounds derived from norbornadiene, two of which achieved over 90% mortality against M. javanica. Another study reported the efficacy of several maleimide and succinimide derivatives against M. incognita, and at 100 µg/mL, they achieved up to 100% mortality under in vitro conditions [24]. Due to the high effectiveness of the AGAz-4 compound, we selected four additional compounds from the same family to screen their efficacy. The compounds were differentiated statistically into two groups: the most efficient, with mortality rates over 90% and no significant differences among them; and the least effective, which showed no significant difference compared to 5% DMSO. Comparing both groups, we observed that most effective compounds have a NO2 group in the ortho position. Apparently, cleavage of NO2 has a role in the effectiveness, as we can observe notably by comparing AGAz-2 and AGAz-3. The cuticle is probably the target of the compounds, and according to [25], this organ is the first barrier that a nematocide should beat to damage the nematode. Some xenobiotic compounds can permeate cell membranes and become toxic to organisms. The primary modes of action of commercial nematocidal compounds include acetylcholinesterase inhibition, nicotinic acetylcholine receptor agonist, tubulin polymerization inhibition, and gamma-aminobutyric acid receptor agonist [26].
Our results are similar to those obtained by Fráguas et al. [27], who tested the nematocidal effectiveness of four 4,5-dihydroisoxazole derivatives at 500 µg/mL. Two of their compounds were effective, achieving over 84% mortality of M. incognita J2, while against M. exigua, they achieved up to 63% mortality. In another study, the in vitro nematocidal activities of ascaridole derivatives were evaluated against M. incognita and Aphelenchoides besseyi. All compounds exhibited 100% of mortality against M. incognita at a concentration of 100 µg/mL, while two of them exerted the highest mortality against A. besseyi at a concentration of 200 µg/mL [28]. In another study, the nematocidal effectiveness of a coumarin derivative was observed, showing the lowest LC50 values against M. incognita (5.1 µmol/L), Ditylenchus destructor (3.7 µmol/L), Bursaphelenchus mucronatus (6.4 µmol/L), B. xylophilus (2.5 µmol/L), and A. besseyi (3.1 µmol/L) [29].
Since AGAz3 and AGAz4 demonstrated effectiveness in the experiments, we selected these compounds to determine their LC50 and LC90 values (Figure 4). AGAz3 was twice as effective as AGAz4, suggesting that both compounds are promising nematocide candidates. The LC50 and LC90 values of AGAz3 are not directly comparable to the results obtained by [30] using commercial nematocides such as methomyl (LC50: 5 µg/mL and, LC90: 15 µg/mL) and oxamyl (LC50: 4 µg/mL and, LC90: 11 µg/mL) against M. incognita. In another study, Yue et al. [31] tested the effectiveness of fluensulfone, avermectin, fluopyram, and fosthiazate, reporting EC50 values of 0.29, 0.68, 0.13, and 2.48 mg/L, respectively. The LC50 of AGAz3 is similar to the findings of Talavera-Rubia et al. [32], where milbemectin exhibited an LC50 of 30.3 µg/mL and an LC90 of 57.8 µg/mL against M. javanica. This difference in efficacy is likely attributed to the variation in nematode species, as our study focuses on N. aberrans, which makes direct comparisons with other studies difficult. Despite this, our findings emphasize the promising potential of AGAz3 and AGAz4 as nematocidal agents, particularly for in situ testing in agricultural contexts. Additionally, these results underline the strong potential of AGAz3, not only as a nematocidal agent, but also in research exploring its homologs as effective ovicidal compounds, as well as its efficacy against other phytonematode species.
5. Conclusions
Among five phenyl azide derivative compounds tested, three demonstrated nematocidal effectiveness against N. aberrans juveniles under in vitro conditions. AGAz3 demonstrated the best efficacy, with activity superior to chitosan, a commercial organic nematocidal ingredient. Despite this, the efficacy of these compounds remains lower than that of synthetic commercial products. To fully understand their potential, additional experiments are required to assess their effectiveness in situ, explore potential synergistic effects between AGAz3 and AGAz4, and evaluate their impact on other phytonematodes. Similarly, other compounds demonstrated nematocidal effectiveness, such as BFX-BUT, expanding the possibilities for investigating their potential as synergistic agents or their efficacy against other nematode species, including gastrointestinal and phytonematodes. This further exploration could help determine the broader applicability and utility of these derivatives in integrated pest management strategies.
Author Contributions
Conceptualization, J.C.-A., J.A.P.-A., L.A.-M., G.R. and E.O.-P.; methodology, J.C.-A., A.G.-G., E.O.-P., L.K.V.-J., T.D.-M., A.D.P.-G. and G.R.; software, J.C.-A., L.A.-M., G.R. and E.O.-P.; validation, L.A.-M., G.R. and E.O.-P.; formal analysis, J.C.-A., L.A.-M., G.R. and E.O.-P.; investigation, J.C.-A., A.G.-G., E.O.-P., L.K.V.-J., T.D.-M., A.D.P.-G. and G.R.; resources, L.A.-M. and G.R.; data curation, J.C.-A.; writing—original draft preparation, J.C.-A.; writing—review and editing, J.A.P.-A., L.A.-M., G.R. and E.O.-P.; visualization, L.A.-M., J.A.P.-A., G.R. and E.O.-P.; supervision, L.A.-M., G.R. and E.O.-P.; project administration, L.A.-M. and G.R.; funding acquisition, L.A.-M. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from project CBF2023-2024-387 of Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
We appreciate the support of Olga Gómez Rodríguez for donating the N. aberrans juveniles.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, J.; Li, Q.X.; Song, B. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef] [PubMed]
- EPPO. EPPO A1 List of Pests Recommended for Regulation as Quarantine Pests. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list (accessed on 17 April 2023).
- Gortari, M.C.; Hours, R.A. In Vitro Antagonistic Activity of Argentinean Isolates of Purpureocillium lilacinum on Nacobbus aberrans Eggs. Curr. Res. Environ. Appl. Mycol. 2019, 9, 164–174. [Google Scholar] [CrossRef]
- Tileubayeva, Z.; Avdeenko, A.; Avdeenko, S.; Stroiteleva, N.; Kondrashev, S. Plant-Parasitic Nematodes Affecting Vegetable Crops in Greenhouses. Saudi J. Biol. Sci. 2021, 28, 5428–5433. [Google Scholar] [CrossRef] [PubMed]
- Baazeem, A.; Alorabi, M.; Darwesh, H.; Alotaibi, S.S.; El-deen, A.N.; Iqbal, S.; Atif, S.; Naqvi, H. Biological Control of Root-Knot Nematode (Meloidogyne javanica) by Potential Antagonism of Endophytic Fungi Isolated from Taify Roses. J. King Saud Univ.-Sci. 2022, 34, 102329. [Google Scholar] [CrossRef]
- PAN International. PAN International Consolidated List of Banned Pesticides|PAN International. Pesticide Action Network International, 2022. Available online: https://pan-international.org/pan-international-consolidated-list-of-banned-pesticides/ (accessed on 17 April 2023).
- Desaeger, J.; Wram, C.; Zasada, I. New Reduced-Risk Agricultural Nematicides-Rationale and Review. J. Nematol. 2020, 52, e2020-91. [Google Scholar] [CrossRef]
- Antônio-Ebone, L.; Kovaleski, M.; Cardoso-Deuner, C. Nematicides: History, Mode, and Mechanism Action. Plant Sci. Today 2019, 6, 71–83. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Cruz-Santiago, H.; Cid del Prado-Vera, I.; Ramirez-Mares, M.V.; Gutiérrez-Ortiz, M.d.R.; Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Lira-de León, K.I.; Hernández-Carlos, B. Chemical Characterization of Plant Extracts and Evaluation of Their Nematicidal and Phytotoxic Potential. Molecules 2021, 26, 2216. [Google Scholar] [CrossRef]
- Sasanelli, N.; Konrat, A.; Migunova, V.; Toderas, I.; Iurcu-Straistaru, E.; Rusu, S.; Bivol, A.; Andoni, C.; Veronico, P. Review on Control Methods against Plant Parasitic Nematodes Applied in Southern Member States (C Zone) of the European Union. Agriculture 2021, 11, 602. [Google Scholar] [CrossRef]
- EPA. Pesticide Product Registration; Applications: New Active Ingredients; EPA: Washington, DC, USA, 2020; Volume 85.
- Vázquez-Bravo, J.; Aguilar-Marcelino, L.; Castañeda-Ramírez, G.S.; De Los Santos-Pérez, I.; Arroyo-Carmona, R.E.; Bernès, S.; Hernández-Pareja, U.; Gómez-Rodríguez, O.; Rosas-Saito, G.H. In Vitro Nematicidal Activity of Two Ferrocenyl Chalcones against Larvae of Haemonchus contortus (L3) and Nacobbus aberrans (J2). J. Helminthol. 2020, 94, e190. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Zeferino-Díaz, R.; Alvarado-Rodríguez, J.G.; López-Ruiz, H.; Rojas-Lima, S.; Flores-Castro, K.; del Prado-Vera, I.C.; Alatorre-Rosas, R.; Tut-Pech, F.; Carrillo-Benítez, M.G.; et al. Nematicidal Activity of Furanoeremophilenes against Meloidogyne incognita and Nacobbus aberrans. Pest Manag. Sci. 2022, 78, 2571–2580. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, R.; Li, Z.; Maienfisch, P.; Xu, X. Design, Synthesis and Nematicidal Activitives of Trifluorobutene Amide Derivatives against Meloidogyne incognita. Bioorganic Med. Chem. Lett. 2021, 40, 127917. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.U.; Sajid, M.; Obaidullah, A.J.; Rehman, W.; Faris Alotaibi, H.; Bibi, S.; Alanazi, M.M. Nematicidal Characterization of Newly Synthesized Thiazine Derivatives Using Caenorhabditis elegans as the Model Organism. ACS Omega 2023, 8, 20767–20778. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Huang, J.; Luo, Y.; Wang, S.; Wu, S.; Xing, Z.; Chen, J. Novel Amide Derivatives Containing an Imidazo[1,2-a]Pyridine Moiety: Design, Synthesis as Potential Nematicidal and Antibacterial Agents. Pestic. Biochem. Physiol. 2021, 175, 104857. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Rocha, J.C.; Sánchez-Torres, L.; Nogueda-Torres, B.; Segura-Cabrera, A.; García-Pérez, C.A.; Bocanegra-García, V.; Palos, I.; Monge, A.; Rivera, G. Anti-Trypanosoma Cruzi and Anti-Leishmanial Activity by Quinoxaline-7-Carboxylate 1,4-Di-N-Oxide Derivatives. Parasitol. Res. 2014, 113, 2027–2035. [Google Scholar] [CrossRef]
- Duque-Montaño, B.E.; Gómez-Caro, L.C.; Sanchez-Sanchez, M.; Monge, A.; Hernández-Baltazar, E.; Rivera, G.; Torres-Angeles, O. Synthesis and in Vitro Evaluation of New Ethyl and Methyl Quinoxaline-7-Carboxylate 1,4-Di-N-Oxide against Entamoeba Histolytica. Bioorganic Med. Chem. 2013, 21, 4550–4558. [Google Scholar] [CrossRef]
- Babaali, D.; Roeb, J.; Hammache, M.; Hallmann, J. Nematicidal Potential of Aqueous and Ethanol Extracts Gained from Datura stramonium, D. innoxia and D. tatula on Meloidogyne incognita. J. Plant Dis. Prot. 2017, 124, 339–348. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, M.; Medina-Medrano, J.R.; Cortez-Madrigal, H.; Angoa-Pérez, M.V.; Muñoz-Ruíz, C.V.; Villar-Luna, E. Nematicidal Activity of Wild Plant Extracts against Second-Stage Juveniles of Nacobbus aberrans. Nematropica 2018, 48, 136–144. [Google Scholar]
- Cruz-Arévalo, J.; Hernández-Velázquez, V.M.; Cardoso-Taketa, A.T.; González-Cortazar, M.; Sánchez-Vázquez, J.E.; Peña-Chora, G.; Villar-Luna, E.; Aguilar-Marcelino, L. Hydroalcoholic Extracts from Pleurotus ostreatus Spent Substrate with Nematocidal Activity against Nacobbus aberrans. Plants 2024, 13, 1777. [Google Scholar] [CrossRef]
- DArT. Introduction to the KDSmart Application. Available online: https://www.kddart.org/kdsmart.html (accessed on 18 November 2021).
- Gomes, A.C.S.; Demuner, A.J.; Alvarenga, E.S.; Gondim, J.P.E.; Fonseca, A.R.; Buonicontro, D.S.; Pilau, E.J.; Silva, E. Synthesis and Evaluation of Nematicidal Activity of Compounds Derived from Norbornadiene. J. Braz. Chem. Soc. 2020, 31, 1805–1814. [Google Scholar] [CrossRef]
- Eloh, K.; Demurtas, M.; Mura, M.G.; Deplano, A.; Onnis, V.; Sasanelli, N.; Maxia, A.; Caboni, P. Potent Nematicidal Activity of Maleimide Derivatives on Meloidogyne incognita. J. Agric. Food Chem. 2016, 64, 4876–4881. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G. Botanical Nematicides, Recent Findings. ACS Symp. Ser. 2014, 1172, 145–157. [Google Scholar] [CrossRef]
- Silvestre, A.; Cabaret, J. Nematode Parasites of Animals Are More Prone to Develop Xenobiotic Resistance than Nematode Parasites of Plants. Parasite 2004, 11, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Fráguas, R.M.; Costa, V.A.; Terra, W.C.; Aguiar, A.P.; Martins, S.J.; Campos, V.P.; Oliveira, D.F. Toxicities of 4,5-Dihydroisoxazoles against Root-Knot Nematodes and in Silico Studies of Their Modes of Action. J. Agric. Food Chem. 2020, 68, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.P.; Yeh, M.J.; Hsiao, W.F. Synthesis and Nematocidal Activity of Ascaridole Derivatives against Meloidogyne incognita and Aphelenchoides besseyi. J. Pestic. Sci. 2007, 32, 49–52. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.Z.; Sun, D.A.; Jin, H.; Guo, H.R.; Qin, B. Design and Synthesis of Novel Coumarin Analogs and Their Nematicidal Activity against Five Phytonematodes. Chin. Chem. Lett. 2016, 27, 375–379. [Google Scholar] [CrossRef]
- Desaeger, J.A.; Rivera, M.; Leighty, R.; Portillo, H. Effect of Methomyl and Oxamyl Soil Applications on Early Control of Nematodes and Insects. Pest Manag. Sci. 2011, 67, 507–513. [Google Scholar] [CrossRef]
- Yue, X.; Li, F.; Wang, B. Activity of Four Nematicides against Meloidogyne incognita Race 2 on Tomato Plants. J. Phytopathol. 2020, 168, 399–404. [Google Scholar] [CrossRef]
- Talavera-Rubia, M.; Vela-Delgado, M.D.; Verdejo-Lucas, S. Nematicidal Efficacy of Milbemectin against Root-Knot Nematodes. Plants 2020, 9, 839. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).