Dual Melatonin Enhances Coordination Between Carbon and Nitrogen Assimilation in Soybean
Abstract
1. Introduction
2. Material and Experimental Design
2.1. Plant Materials
2.2. Experimental Design
2.3. Melatonin (MT) Treatment
2.4. Sample Preparation for LC-MS
2.5. The Content of Components in the TCA Cycle
2.6. Statistical Analysis
3. Results
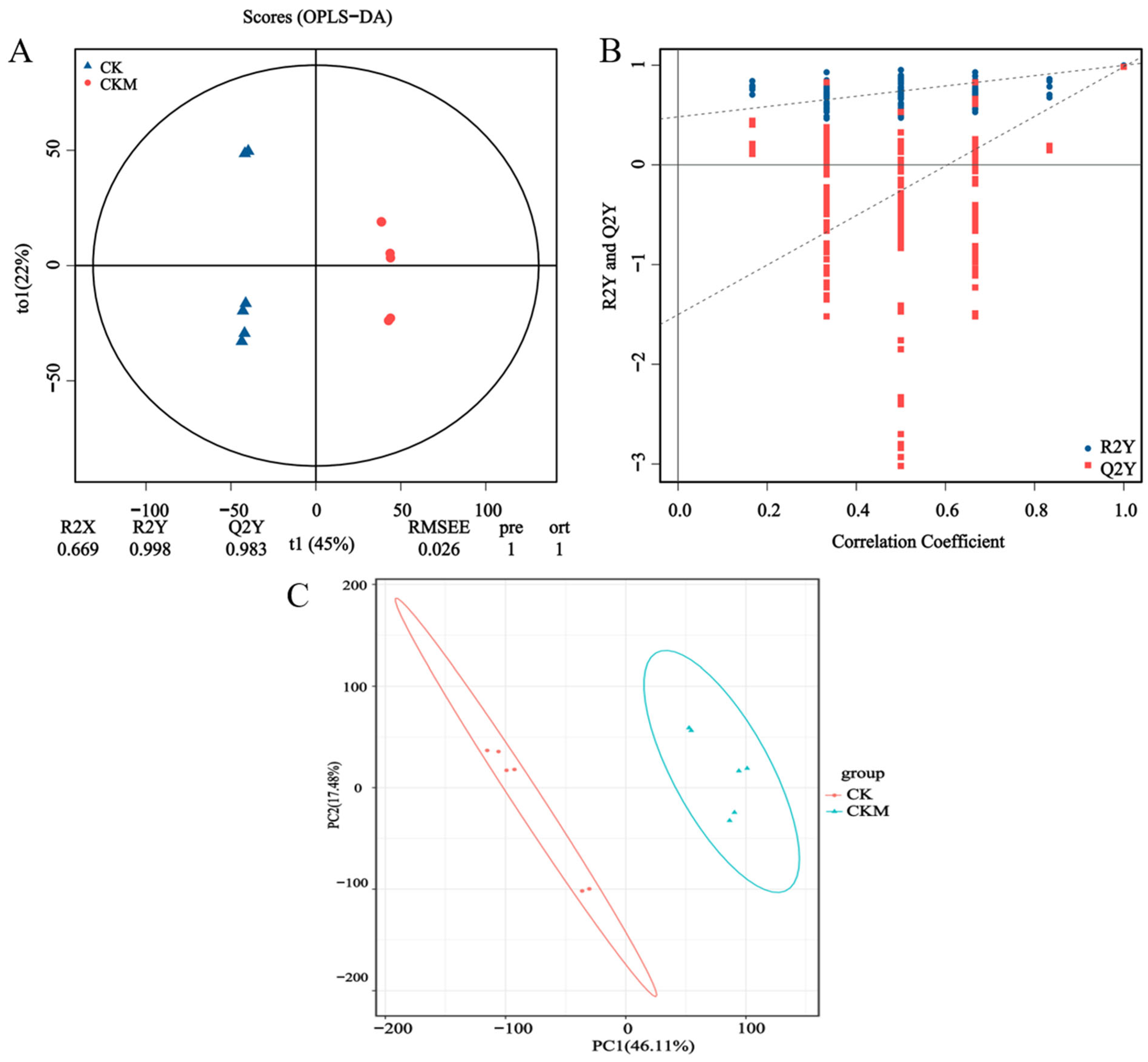
3.1. Metabolic Profiling and Quality Control
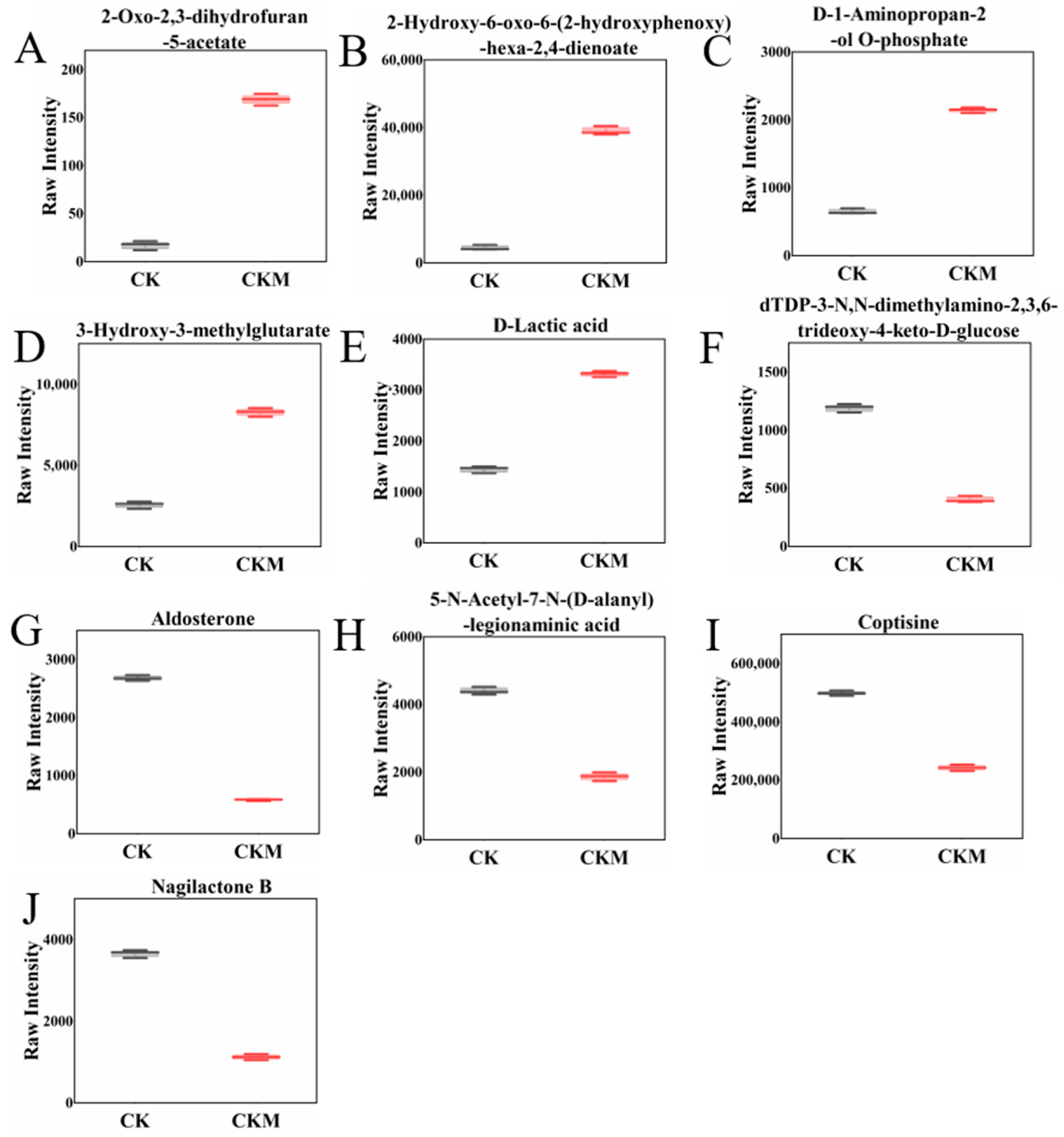
3.2. Analysis of the Differential Metabolites (DMs)
3.3. Metabolic Pathways Enrichment Analysis
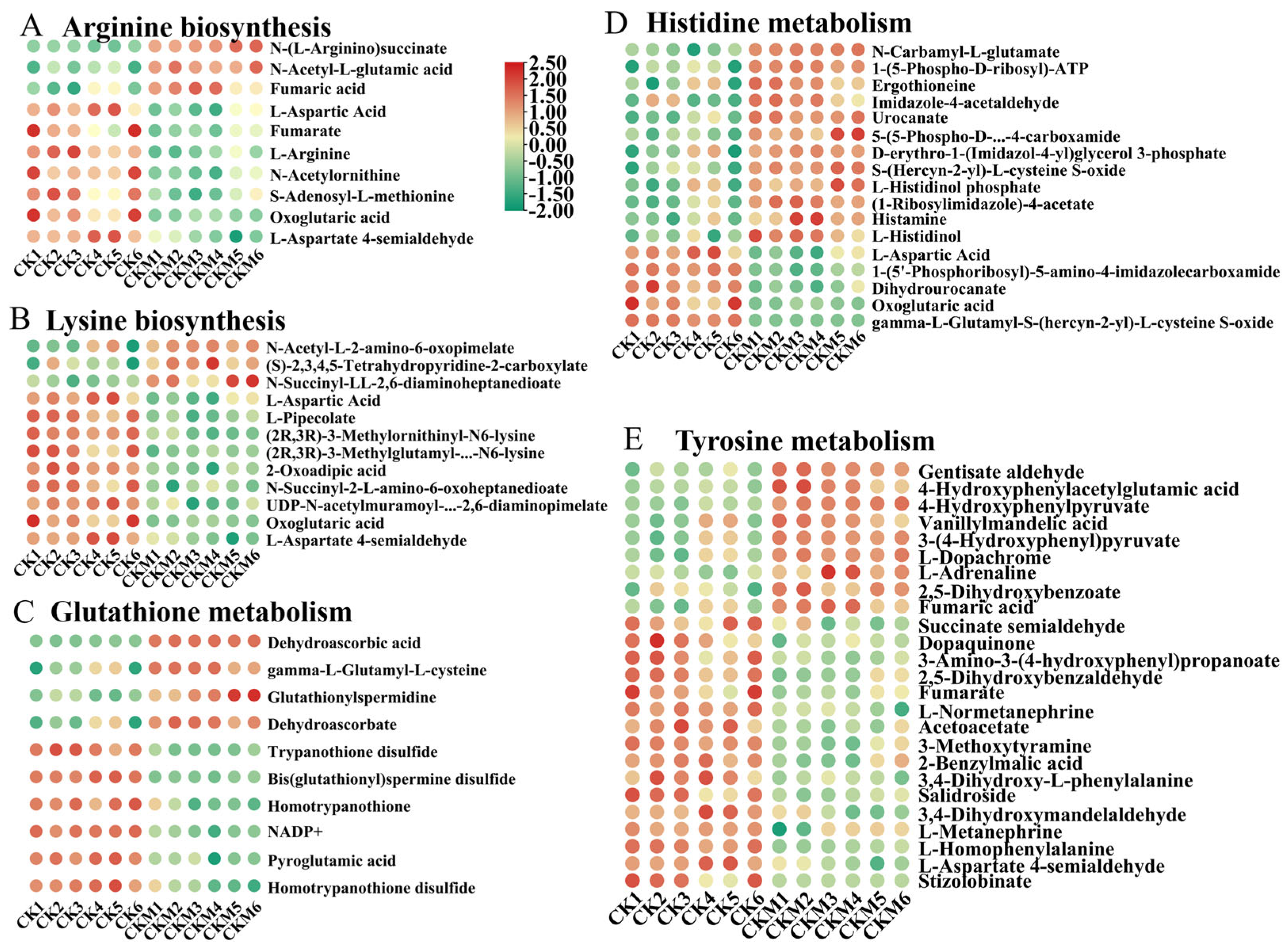
3.4. Amino Acid Metabolic Pathways Enrichment Analysis
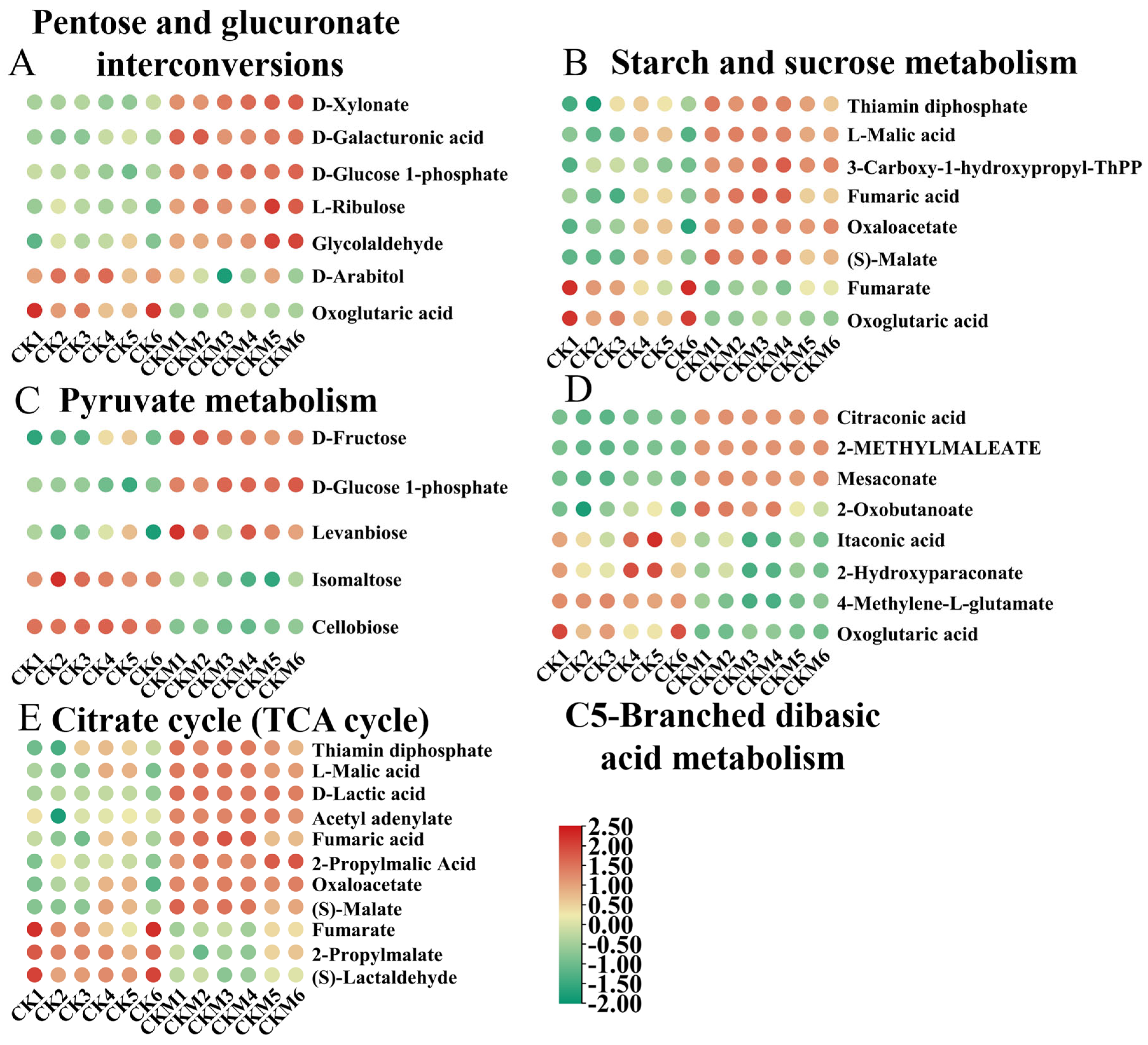
3.5. Carbohydrate Metabolites Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.-C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.-Q.; et al. Simultaneous Changes in Seed Size, Oil Content and Protein Content Driven by Selection of Sweet Homologues During Soybean Domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- FAO. Soybeans. Available online: https://www.fao.org/home/en/ (accessed on 1 August 2022).
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile Roles of Melatonin in Growth and Stress Tolerance in Plants. J. Plant Growth Regul. 2021, 41, 507–523. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by High Performance Liquid Chromatography-Mass Spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Flowering, Fruit Set and Fruit Ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wen, C.; Xu, W. Effects of Endogenous Melatonin Deficiency on the Growth, Productivity, and Fruit Quality Properties of Tomato Plants. Horticulturae 2023, 9, 851. [Google Scholar] [CrossRef]
- Samani, M.S.M.; Mansouri, H. The Novel Strategy for Enhancing Growth and Lipid Accumulation in Chlorella Vulgaris Microalgae Cultured in Dairy Wastewater by Monochromatic Leds and Melatonin. J. Appl. Phycol. 2022, 35, 593–601. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, S.; Wang, M.; Zeb, S.; Khan, M.N.; Chen, Y.; Zhu, G.; Zhu, Z. Enhancement of Sweetpotato Tolerance to Chromium Stress Through Melatonin and Glutathione: Insights into Photosynthetic Efficiency, Oxidative Defense, and Growth Parameters. Plant Physiol. Biochem. 2024, 208, 108509. [Google Scholar] [CrossRef]
- Tan, X.-L.; Fan, Z.-Q.; Zeng, Z.-X.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Su, X.-G.; Tao, N.-G.; Lakshmanan, P.; Chen, J.-Y.; et al. Exogenous Melatonin Maintains Leaf Quality of Postharvest Chinese Flowering Cabbage by Modulating Respiratory Metabolism and Energy Status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Che, J.; Wu, W.; Lyu, L.; Li, W. Lc–Ms and Gc–Ms Metabolomics Analyses Revealed That Different Exogenous Substances Improved the Quality of Blueberry Fruits Under Soil Cadmium Toxicity. J. Agric. Food Chem. 2023, 72, 904–915. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Guo, Q.; Xia, Y.; Jing, D.; Liang, G. Metabolomic Analyses Reveal Potential Mechanisms Induced by Melatonin Application for Tolerance of Water Deficit in Loquat (Eriobotrya japonica Lindl.). Sci. Hortic. 2022, 308, 111569. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and Signaling Aspects Underpinning the Regulation of Plant Carbon Nitrogen Interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Alamri, S.A.; Abd El-Mageed, T.A.; Abousekken, M.; Hashem, M. Role of Exogenous Nitrogen Supply in Alleviating the Deficit Irrigation Stress in Wheat Plants. Agric. Water Manag. 2018, 210, 261–270. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.; Gao, W.; Chen, Y.; Li, H.; Lawlor, D.W.; Paul, M.J.; Pan, W. Exogenous Trehalose Improves Growth Under Limiting Nitrogen Through Upregulation of Nitrogen Metabolism. BMC Plant Biol. 2017, 17, 247. [Google Scholar] [CrossRef]
- Erdal, S. Melatonin Promotes Plant Growth by Maintaining Integration and Coordination Between Carbon and Nitrogen Metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, X.; Ma, C.; Wang, Y.; Zhao, J. Melatonin Enhances Drought Stress Tolerance in Maize Through Coordinated Regulation of Carbon and Nitrogen Assimilation. Plant Physiol. Biochem. 2021, 167, 958–969. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R.; Li, S.; Ran, S.; Wang, J.; Zhou, Y.; Gao, H.; Zhong, F. The Mechanism of Melatonin Promotion on Cucumber Seedling Growth at Different Nitrogen Levels. Plant Physiol. Biochem. 2024, 206, 108263. [Google Scholar] [CrossRef]
- Zou, J.; Yu, H.; Yu, Q.; Jin, X.; Cao, L.; Wang, M.; Wang, M.; Ren, C.; Zhang, Y. Physiological and Uplc-Ms/Ms Widely Targeted Metabolites Mechanisms of Alleviation of Drought Stress-Induced Soybean Growth Inhibition by Melatonin. Ind. Crops Prod. 2021, 163, 113323. [Google Scholar] [CrossRef]
- Zhang, M.; He, S.; Zhan, Y.; Qin, B.; Jin, X.; Wang, M.; Zhang, Y.; Hu, G.; Teng, Z.; Wu, Y. Exogenous Melatonin Reduces the Inhibitory Effect of Osmotic Stress on Photosynthesis in Soybean. PLoS ONE 2019, 14, e0226542. [Google Scholar] [CrossRef]
- Wang, X.; Song, S.; Wang, X.; Liu, J.; Dong, S. Transcriptomic and Metabolomic Analysis of Seedling-Stage Soybean Responses to Peg-Simulated Drought Stress. Int. J. Mol. Sci. 2022, 23, 6869. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Li, K.; Dai, P.; Li, J.; Liang, B.; Sun, C.; Lin, X. Metabolomics Analysis Reveals Potential Mechanisms of Phenolic Accumulation in Lettuce (Lactuca sativa L.) Induced by Low Nitrogen Supply. Plant Physiol. Biochem. 2020, 158, 446–453. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. Tbtools-Ii: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Peng, J.T.; Klair, A.; Dickinson, A.J. Non-Canonical and Developmental Roles of the Tca Cycle in Plants. Curr. Opin. Plant Biol. 2023, 74, 102382. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. The Role of Tca Cycle Enzymes in Plants. Adv. Biol. 2023, 7, 2200238. [Google Scholar] [CrossRef]
- Yu, W.; Gong, F.; Cao, K.; Zhou, X.; Xu, H. Multi-Omics Analysis Reveals the Molecular Mechanisms of the Glycolysis and Tca Cycle Pathways in Rhododendron Chrysanthum Pall. Under Uv-B Stress. Agronomy 2024, 14, 1996. [Google Scholar] [CrossRef]
- Dellero, Y.; Berardocco, S.; Berges, C.; Filangi, O.; Bouchereau, A. Validation of Carbon Isotopologue Distribution Measurements by Gc-Ms and Application to 13c-Metabolic Flux Analysis of the Tricarboxylic Acid Cycle in Brassica Napus Leaves. Front. Plant Sci. 2023, 13, 885051. [Google Scholar] [CrossRef]
- Tcherkez, G.; Gauthier, P.; Buckley, T.N.; Busch, F.A.; Barbour, M.M.; Bruhn, D.; Heskel, M.A.; Gong, X.Y.; Crous, K.Y.; Griffin, K.; et al. Leaf Day Respiration: Low Co2 Flux but High Significance for Metabolism and Carbon Balance. New Phytol. 2017, 216, 986–1001. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, H.; Wang, J.; Wang, Y.; Zhang, R. Melatonin Enhances Drought Tolerance by Regulating Leaf Stomatal Behavior, Carbon and Nitrogen Metabolism, and Related Gene Expression in Maize Plants. Front. Plant Sci. 2021, 12, 779382. [Google Scholar] [CrossRef]
- Yu, G.; Chen, F.; Wang, Y.; Chen, Q.; Liu, H.; Tian, J.; Wang, M.; Ren, C.; Zhao, Q.; Yang, F.; et al. Exogenous Γ-Aminobutyric Acid Strengthens Phenylpropanoid and Nitrogen Metabolism to Enhance the Contents of Flavonoids, Amino Acids, and the Derivatives in Edamame. Food Chem. X 2022, 16, 100511. [Google Scholar] [CrossRef]
- Cao, L.; Zou, J.; Qin, B.; Bei, S.; Ma, W.; Yan, B.; Jin, X.; Zhang, Y. Response of Exogenous Melatonin on Transcription and Metabolism of Soybean under Drought Stress. Physiol. Plant. 2023, 175, e14038. [Google Scholar] [CrossRef]
- Nguyen, C.X.; Dohnalkova, A.; Hancock, C.N.; Kirk, K.R.; Stacey, G.; Stacey, M.G. Critical Role for Uricase and Xanthine Dehydrogenase in Soybean Nitrogen Fixation and Nodule Development. Plant Genome 2021, 16, e20171. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, J.; Martínez-Romero, E.; Montaño, N.M.; Camargo-Ricalde, S.L. Folates in Legume Root Nodules. Physiol. Plant. 2020, 171, 447–452. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Li, Q. Biosynthetic Regulatory Network of Flavonoid Metabolites in Stems and Leaves of Salvia Miltiorrhiza. Sci. Rep. 2022, 12, 18212. [Google Scholar] [CrossRef]
- You, W.; Zhang, J.; Ru, X.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. CaCl2 Promoted Phenolics Accumulation via the Cmcamta4-Mediated Transcriptional Activation of Phenylpropane Pathway and Energy Metabolism in Fresh-Cut Cantaloupe. Postharvest Biol. Technol. 2023, 206, 108263. [Google Scholar] [CrossRef]
- Ayan, A.K.; Radušienė, J.; Çirak, C.; Ivanauskas, L.; Janulis, V. Secondary Metabolites of Hypericum Scabrumandhypericum Bupleuroides. Pharm. Biol. 2009, 47, 847–853. [Google Scholar] [CrossRef]
- Gao, S.; Li, M.; Hu, Y.; Zhang, T.; Guo, J.; Sun, M.; Shi, L. Comparative Differences in Maintaining Membrane Fluidity and Remodeling Cell Wall Between Glycine Soja and Glycine Max Leaves Under Drought. Plant Physiol. Biochem. 2024, 109, 108545. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Li, M.; Li, M.; Hu, Y.; Zhang, T.; Shi, L. Membrane Lipid Phosphorus Reusing and Antioxidant Protecting Played Key Roles in Wild Soybean Resistance to Phosphorus Deficiency Compared with Cultivated Soybean. Plant Soil 2022, 474, 99–113. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kunert, K. The Ascorbate/Glutathione Cycle Coming of Age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in Plants: Novel Concepts, New Perspectives, and Outstanding Issues. Antioxid. Redox Signal. 2019, 32, 463–485. [Google Scholar] [CrossRef]
- Lima, V.F.; Freire, F.B.S.; Cândido-Sobrinho, S.A.; Porto, N.P.; Medeiros, D.B.; Erban, A.; Kopka, J.; Schwarzländer, M.; Fernie, A.R.; Daloso, D.M. Unveiling the Dark Side of Guard Cell Metabolism. Plant Physiol. Biochem. 2023, 201, 107862. [Google Scholar] [CrossRef]
- Robaina-Estévez, S.; Daloso, D.M.; Zhang, Y.; Fernie, A.R.; Nikoloski, Z. Resolving the Central Metabolism of Arabidopsis Guard Cells. Sci. Rep. 2017, 7, 8307. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.B.; Souza, L.P.; Antunes, W.C.; Araújo, W.L.; Daloso, D.M.; Fernie, A.R. Sucrose Breakdown Within Guard Cells Provides Substrates for Glycolysis and Glutamine Biosynthesis During Light-Induced Stomatal Opening. Plant J. 2018, 94, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Cui, C.; Shan, F.; Zhang, R.; Lyu, X.; Lyu, L.; Chang, H.; Yan, C.; Ma, C. Blue Light Regulates Cell Wall Structure and Carbohydrate Metabolism of Soybean Hypocotyl. Int. J. Mol. Sci. 2023, 24, 1017. [Google Scholar] [CrossRef]
- Peng, W.T.; Zhang, L.D.; Zhou, Z.; Fu, C.; Chen, Z.C.; Liao, H. Magnesium Promotes Root Nodulation Through Facilitation of Carbohydrate Allocation in Soybean. Physiol. Plant. 2018, 163, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade Effect on Carbohydrates Dynamics and Stem Strength of Soybean Genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Kobylińska, A.; Borek, S.; Posmyk, M.M. Melatonin Redirects Carbohydrates Metabolism During Sugar Starvation in Plant Cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef]
- Huber, S.C.; Israel, D.W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon Between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982, 69, 691–696. [Google Scholar] [CrossRef]
- Lal, M.A. Metabolism of Storage Carbohydrates. In Plant Physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer: Singapore, 2018; pp. 339–377. [Google Scholar]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S.; et al. Melatonin Enhances Plant Growth and Abiotic Stress Tolerance in Soybean Plants. J. Exp. Bot. 2014, 66, 695–707. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jin, X.; Zhang, Y. Dual Melatonin Enhances Coordination Between Carbon and Nitrogen Assimilation in Soybean. Agriculture 2025, 15, 681. https://doi.org/10.3390/agriculture15070681
Wang Y, Jin X, Zhang Y. Dual Melatonin Enhances Coordination Between Carbon and Nitrogen Assimilation in Soybean. Agriculture. 2025; 15(7):681. https://doi.org/10.3390/agriculture15070681
Chicago/Turabian StyleWang, Yanhong, Xijun Jin, and Yuxian Zhang. 2025. "Dual Melatonin Enhances Coordination Between Carbon and Nitrogen Assimilation in Soybean" Agriculture 15, no. 7: 681. https://doi.org/10.3390/agriculture15070681
APA StyleWang, Y., Jin, X., & Zhang, Y. (2025). Dual Melatonin Enhances Coordination Between Carbon and Nitrogen Assimilation in Soybean. Agriculture, 15(7), 681. https://doi.org/10.3390/agriculture15070681




