Establishing Monoxenic Culture of Arbuscular Mycorrhizal Fungus Glomus sp. Through In Vitro Root Organ Culture and Swietenia macrophylla King In Vitro Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Extraction and Identification of Glomerospores
2.3. Spore Sterilization
2.4. MSR Medium [26]
2.5. Bacterial Strain Propagation and Carrot Hairy Root Culture (Modified from [10,27,28])
2.6. Spore Inoculation and Culture Development on Bi-Compartmented Petri Dishes (Modified from [30,31])
2.7. Disinfection and Germination of Seeds
2.8. Monoxenic Culture of AM Inoculum Production by Root Organ Culture (ROC) (Modified from [35])
2.9. Assessment of Contamination and Spore Production
2.10. Thinning and Staining of Roots
2.11. Culture and Molecular Identification of Yeast
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ghorui, M.; Chowdhury, S.; Das, K.; Sunar, K.; Prakash, B. Optimizing factors for large-scale production of arbuscular mycorrhizal fungi consortia using root organ cultures. J. Biol. Methods 2023, 10, e99010006. [Google Scholar] [CrossRef]
- Powell, J.R.; Rilling, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. Tasley review. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, I.; Ruiz, M.; Fernández, C.; Peña, R.; Rodríguez, A.; Sanders, I.R. The in vitro mass-produced model mycorrhizal fungus, Rhizophagus irregularis, significantly increases yields of the globally important food security crop cassava. PLoS ONE 2013, 8, e70633. [Google Scholar] [CrossRef]
- Tiwari, P.; Adholeya, A. In vitro co-culture of two AMF isolates Gigaspora margarita and Glomus intraradices on Ri T-DNA transformed roots. FEMS Microbiol. Lett. 2002, 206, 39–43. [Google Scholar] [CrossRef] [PubMed]
- IJdo, M.; Cranenbrouck, S.; Declerck, S. Methods for large-scale production of AM fungi: Past, present, future. Mycorrhiza 2011, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Abdullah, S.N.A. Mass production techniques of arbuscular mycorrhizal fungi: Major advantages and disvantages: A Review. Biosci. Biotechnol. Res. Asia 2014, 11, 1199–1204. [Google Scholar] [CrossRef]
- White, P.R. The Cultivation of Animal & Plant Cells, 2nd ed.; Ronald Press: New York, NY, USA, 1963. [Google Scholar]
- Mosse, B.; Hepper, C.M. Vesicular-arbuscular infections in root organ cultures. Physiol. Plant Pathol. 1975, 5, 215–223. [Google Scholar] [CrossRef]
- Fernández, L.B.; Pergola, M.; Silvani, V.; Colombo, R.; Bompadre, J.; Godeas, A. Continuous and long-term monoxenic culture of the arbuscular mycorrhizal fungus Gigaspora decipiens in root organ culture. Fungal Biol. 2012, 116, 729–735. [Google Scholar]
- Bécard, G.; Fortin, J.A. Early events of vesicular arbuscular mycorrhizal formation on Ri T-DNA transformed roots. New Phytol. 1988, 108, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Declerck, S.; Strullu, D.G.; Plenchette, C. Monoaxenic culture of the intraradical forms of Glomus sp. Isolated from a tropical ecosystem: A proposed methodology for germplasm collection. Mycologia 1998, 90, 579–585. [Google Scholar] [CrossRef]
- Karandashov, V.; Kuzovkina, I.; Hawkins, H.J.; George, E. Growth and sporulation of the arbuscular mycorrhizal fungus Glomus caledonium in dual culture with transformed carrot roots. Mycorrhiza 2000, 10, 23–28. [Google Scholar] [CrossRef]
- Araújo Costa, F.; Meira Haddad, L.; Megumi Kasuya, M.; Campos Oton, W.; Dutra Costa, M.; Chaer Borge, A. In vitro culture of Gigaspora decipiens and Glomus clarum in transformed roots of carrot: The influence of temperature and pH. Acta Sci. Agron. 2013, 35, 315–323. [Google Scholar]
- Adholeya, A.; Tiwari, P.; Singh, R. Large-Scale Inoculum Production of Arbuscular Mycorrhizal Fungi on Root Organs and Inoculation Strategies. In In Vitro Culture of Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2005; pp. 315–333. [Google Scholar]
- Fortin, J.A.; Bécard, G.; Declerck, S.; Dalpe, Y.; St-Arnaud, M.; Coughlan, P.; Piche, Y. Arbuscular mycorrhiza on root-organ cultures. Can. J. Bot. 2002, 80, 1–20. [Google Scholar] [CrossRef]
- Drazhnikova, A.; Andrlanova, T.V. Strategy for conservation of arbuscular mycorrhizal fungi. In Proceedings of the Material of the International Scientific Practical Conference, Ternopil, Ukraine, 4–5 June 2020. [Google Scholar]
- Kobayashi, S. Landscape rehabilitation of degraded tropical forest ecosystems, case study of the CIFOR/Japan project in Indonesia and Peru. For. Ecol. Manag. 2004, 201, 13–22. [Google Scholar] [CrossRef]
- Navarro, C.; Hernández, G. Progeny test analysis and population differentiation of Mesoamerican Mahogany (Swietenia macrophylla). Agron. Costarric. 2004, 28, 37–51. [Google Scholar]
- Barstow, M.; Negrão, R. Swietenia macrophylla. The IUCN Red List of Threatened Species 2023: e.T32293A68104718. 2023. Available online: https://dx.doi.org/10.2305/IUCN.UK.2023-1.RLTS.T32293A68104718.en (accessed on 17 April 2024).
- Chaiyasen, A.; Douds, D.D.; Gavinlertvatana, P.; Lumyong, S. Diversity of arbuscular mycorrhizal fungi in Tectona grandis Linn.f. plantations and their effects on growth of micropropagated plantlets. New For. 2017, 48, 547–562. [Google Scholar] [CrossRef]
- Rodríguez-Morelos, V.H.; Soto-Estrada, A.; Pérez-Moreno, J.; Franco-Ramírez, A.; Díaz-Rivera, P. Arbuscular mycorrhizal fungi associated with the rhizosphere of seedlings and mature trees of Swietenia macrophylla (Magnoliophyta: Meliaceae) in Los Tuxtlas, Veracruz, México. Rev. Chil. De Hist. Nat. 2014, 87, 9. [Google Scholar] [CrossRef]
- Polo-Marcial, M.H.; Lara-Pérez, L.A.; Goto, B.T.; Noa-Carrazana, J.C.; Díaz-Fleischer, F.; Andrade-Torres, A. Tropical deciduous species under different land use retain a high glomerospores diversity and arbuscular and septate endophyte colonization. Nova Hedwig. Band 2022, 115 Heft 3–4, 487–517. [Google Scholar] [CrossRef]
- Polo-Marcial, M.H.; Solís-Ramos, L.Y.; Murillo-Cruz, R.; Ávila-Arias, C.; Andrade-Torres, A. Mycorrhizal and endophytic richness and colonization in Cedrela odorata L., in agroforestry systems and secondary forest from southeastern Costa Rica. Agrofor. Syst. 2023, 97, 647–658. [Google Scholar] [CrossRef]
- Błaszkowsk, J.; Glomeromycota, W. Szafer Institute of Botany; Polish Academy of Sciences: Warsaw, Poland, 2012. [Google Scholar]
- Schenck, N.C.; Pérez, Y. Manual for Identification of Vesicular Arbuscular Mycorrhizal Fungi. (INVAM). Synergistic; University of Florida: Gainesville, FL, USA, 1990. [Google Scholar]
- Strullu, D.G.; Romand, C. Méthode d’obtention d’ endomycorhizes a vesicules et arbuscules en condition axeniques. Comptes Rendus De Académie Sci. 1986, 303, 245–250. [Google Scholar]
- Mugnier, J.; Mosse, B. Vesicular–arbuscular infections in Ri T-DNA transformed roots grown monoxenically. Phytopathology 1987, 77, 1045–1050. [Google Scholar] [CrossRef]
- Srinivasan, M.; Kumar, K.; Kumutha, K.; Marimuthu, P. Establishing monoxenic culture of arbuscular mycorrhizal fungus Glomus intraradices through root organ culture. J. Appl. Nat. Sci. 2014, 6, 290–293. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- St-Arnaud, M.; Hamel, C.; Vimard, B.; Caron, M.; Fortin, J.A. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol. Res. 1996, 100, 328–332. [Google Scholar] [CrossRef]
- Dalpé, Y.; Seguin, S. A “paper bridge” system to improve in vitro propagation of arbuscular mycorrhizal fungi. Botany 2010, 88, 617–620. [Google Scholar] [CrossRef]
- Tennant, D. A Test of a Modified Line Intersect Method of Estimating Root Length. J. Ecol. 1975, 63, 995–1001. [Google Scholar] [CrossRef]
- Bago, B.; Azcón-Aguilar, C.; Piché, Y. Architecture and developmental dynamics of the external mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown under monoxenic conditions. Mycologia 1998, 90, 52–62. [Google Scholar] [CrossRef]
- Marturet, G.; Salazar, R.V.; Regales, B.; Betancourt, C. Efecto de los reguladores de crecimiento en la morfogénesis in vitro de caoba (Swietenia macrophylla king). Rev. Pittieria. 2016, 40, 164–173. [Google Scholar]
- Pérez-Moncada, U.A.; Ramírez-Gómez, M.M.; Núñez-Zarante, V.M.; Franco-Correa, M.; Roveda-Hoyos, G. Evaluación de un sistema para la micorrización in vitro en plantas de mora de castilla (Rubus glaucus, Benth). Univ. Sci. 2012, 17, 140–151. [Google Scholar] [CrossRef]
- Rosikiewicz, P.; Bonvin, J.; Snaders, I.R. Cost-efficient production of in vitro Rhizophagus irregularis. Mycorrhiza 2017, 27, 477–486. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 21 July 2024).
- Philips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorhizal for rapid assessement of infection. Trans. Br. Mycol. Soc. 1970, 55, 156–161. [Google Scholar] [CrossRef]
- Harrison, F.C. Rhodotorula truncatula, a new species of yeast-like organism. Can. J. Res. 1927, 2, 347–348. [Google Scholar]
- Danesh, Y.R.; Tufenkci, S. In vitro culturing of mycorrhiza and mycorrhiza like fungi. Int. J. Agric. Technol. 2017, 13, 1675–1689. [Google Scholar]
- Cranenbrouck, S.; Voets, L.; Bivort, C.; Renard, L.; Strullu, D.G.; Declerck, S. Methodologies for in Vitro Cultivation of Arbuscular Mycorrhizal Fungi with Root Organs. In In Vitro Culture of Mycorrhizas; Declerck, S., Fortin, J.A., Strullu, D.-G., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 4, pp. 341–375. [Google Scholar] [CrossRef]
- Eskandari, A.; Danesh, Y.R. Study on life cycle of arbuscular mycorrhizal fungus Glomus intraradices using in vitro culturing technique. J. Phytol. 2010, 2, 69–75. [Google Scholar]
- Mosse, B. The Establishment of Vesicular-Arbuscular Mycorrhiza under Aseptic Conditions. Microbiology 1962, 27, 509–520. [Google Scholar] [CrossRef]
- Raghavendra, K.; Nirmalnath, J.; Jagadeesh, K.S. Axenic Germination of Glomus intraradices in vitro. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2387–2396. [Google Scholar] [CrossRef]
- Aryal, H.P. A Protocol on in vitro Propagation of Arbuscular Mycorrhizal Fungi using Root Organ Culture Technique. Tribhuvan Univ. J. 2017, 31, 17–24. [Google Scholar] [CrossRef]
- Sawsan, A.E.E.; Abdullah, M.; Ali Hoda, H.; Senousy; Elsayed, S.; Razik, A. Production of Arbuscular Mycorrhizal Fungi using in vitro Root Organ Culture and Phenolic Compounds. J. Pure Appl. Microbiol. 2019, 13, 1985–1994. [Google Scholar]
- Zhang, L.; Fan, J.; Feng, G.; Declerck, S. The arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 43194 induces the gene expression of citrate synthase in the tricarboxylic acid cycle of the phosphate-solubilizing bacterium Rahnella aquatilis HX2. Mycorrhiza 2019, 29, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, S.; Godeas, A.; Scervino, J.M.; Sampedro, I.; Ocampo, J.A.; García-Romera, I. Interaction between the soil yeast Rhodotorula mucilaginosa and the arbuscular mycorrhizal fungi Glomus mosseae and Gigaspora rosea. Soil Biol. Biochem. 2003, 35, 701–707. [Google Scholar] [CrossRef]
- Nakayan, P.; Hameed, A.; Singh, S.; Young, L.; Hung, M.; Young, C. Phosphate-solubilizing soil yeast Meyerozyma guilliermondii CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant Soil 2013, 373, 301–315. [Google Scholar]
- Sarabia, M.; Cornejo, P.; Azcón, R.; Carreón-Abud, Y.; Larsen, J. Mineral phosphorus fertilization modulates interactions between maize, rhizosphere yeasts and arbuscular mycorrhizal fungi. Rhizosphere 2017, 4, 89–93. [Google Scholar] [CrossRef]
- Sampedro, I.; Aranda, E.; Scervino, J.M.; Fracchia, S.; García-Romero, I.; Ocapo, J.A.; Godeas, A. Improvement by soil yeasts of arbuscular mycorrhizal symbiosis of soybean (Glycine max) colonized by Glomus mosseae. Mycorrhiza 2004, 14, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Zou, Y.N.; Hashem, A.; Avila-Quezada, G.D.; Abd Allah, E.F.; Wu, Q.S. Rhizoglomus intraradices Is More Prominent in Improving Soil Aggregate Distribution and Stability Than in Improving Plant Physiological Activities. Agronomy 2023, 13, 1427. [Google Scholar] [CrossRef]
- Voets, L.; De la Providencia, I.E.; Fernández, K.; IJdo, M.; Cranenbrouck, S.; Declerck, S. Extraradical mycelium network of arbuscular mycorrhizal fungi allows fast colonization of seedlings under in vitro conditions. Mycorrhiza 2009, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Perera García, S.S.; Fernández Suárez, K.; Pérez Ortega, E.J.; Mujica Pérez, Y.; Pérez-Pérez, R.; Rodríguez Yon, Y.; Haesaert, G.; Perera García, S.S.; Fernández Suárez, K.; Pérez Ortega, E.J.; et al. In vitro propagation of a cuban Arbuscular Mycorrhizal Fungal strain and factible bacteria’s association. Rev. Colomb. De Biotecnol. 2022, 24, 36–45. [Google Scholar] [CrossRef]
- Cano, C.; Dickson, S.; González-Guerrero, M.; Bago, A. In vitro cultures open new prospects for basic research in arbuscular mycorrhizas. In Mycorrhiza; Varma, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 627–654. [Google Scholar]
- Calvet, C.; Camprubi, A.; Pérez-Hernández, A. Plant growth stimulation and root colonization potential of in vivo versus in vitro arbuscular mycorrhizal inocula. HortScience 2013, 48, 897–901. [Google Scholar] [CrossRef]
- Kokkoris, V.; Hart, M.M. The role of in vitro cultivation on symbiotic trait and function variation in a single species of arbuscular mycorrhizal fungus. Fungal Biol. 2019, 123, 732–744. [Google Scholar] [CrossRef]
- De Boulois, H.D.; Voets, L.; Delvaux, B.; Jakobsen, I.; Declerck, S. Transport of radiocaesium by arbuscular mycorrhizal fungi to Medicago truncatula under in vitro conditions. Environ. Microbiol. 2006, 8, 1926–1934. [Google Scholar] [CrossRef]
- Tiwari, P.; Adholeya, A. Host dependent differential spread of Glomus intraradices on various Ri T-DNA transformed roots in vitro. Mycol. Prog. 2003, 2, 171–177. [Google Scholar] [CrossRef]
- El Hilali, R.; Bouamri, R.; Crozilhac, P.; Calonne, M.; Symanczik, S.; Ouahmane, L.; Declerck, S. In vitro colonization of date palm plants by Rhizophagus irregularis during the rooting stage. Symbiosis 2021, 84, 83–89. [Google Scholar] [CrossRef]
- Declerck, S.; Strullu, D.G.; Plenchette, C. In vitro mass-production of the arbuscular mycorrhizal fungus, Glomus versiforme, associated with Ri T-DNA transformed carrot roots. Mycol. Res. 1996, 100, 1237–1242. [Google Scholar] [CrossRef]
- Voets, L.; Dupré de Boulois, H.; Renard, L.; Strullu, D.G.; Declerck, S. Development of an autotrophic culture system for the in vitro mycorrhization of potato plantlets. FEMS Microbiol. Lett. 2005, 248, 111–118. [Google Scholar] [CrossRef] [PubMed]
- de la Providencia, I.E.; Fernandez, F.; Declerck, S. Hyphal healing mechanism in the arbuscular mycorrhizal fungi Scutellospora reticulata and Glomus clarum differ in response to severe physical stress. FEMS Microbiol. Lett. 2007, 268, 120–125. [Google Scholar] [CrossRef]
- Declerck, S.; Bivort, C.; D’Or, D.; de Souza, F.A. Development of extraradical mycelium of Scutellospora reticulata under root-organ culture: Spore production and function of auxiliary cells. Mycol. Res. 2004, 108, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Rodríguez, T.; Dupré de Boulois, H.; Granet, F.; Gaurel, S.; Melgarejo, L.M.; Carron, M.P.; Declerck, S. In vitro mycorrhization of the rubber tree Hevea brasiliensis Müll Arg. Vitr. Cell. Dev. Biol. Plant 2013, 49, 207–215. [Google Scholar] [CrossRef]
- Ganoudi, M.; Calonne-Salmon, M.; Ibriz, M.; Declerck, S. In vitro mycorrhization of Argania spinosa L. using germinated seeds. Symbiosis 2021, 85, 57–68. [Google Scholar] [CrossRef]
- Bécard, G.; Piché, Y. Establishment of vesicular-arbuscular mycorrhiza in root organ culture: Review and proposed methodology. Method Microbiol. 1992, 24, 89–108. [Google Scholar]
- Vosátka, M.; Látr, A.; Gianinazzi, S.; Albrechtova, J. Development of arbuscular mycorrhizal biotechnology and industry: Current achievements and bottlenecks. Symbiosis 2012, 58, 29–37. [Google Scholar] [CrossRef]
- Oruru, M.B.; Njeru, E.M. Upscaling arbuscular mycorrhizal symbiosis and related agroecosystems services in smallholder farming systems. BioMed. Res. Int. 2016, 2016, 4376240. [Google Scholar] [CrossRef]
- Declerck, S.; Strullu, D.G.; Fortin, J.A. In Vitro Culture of Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
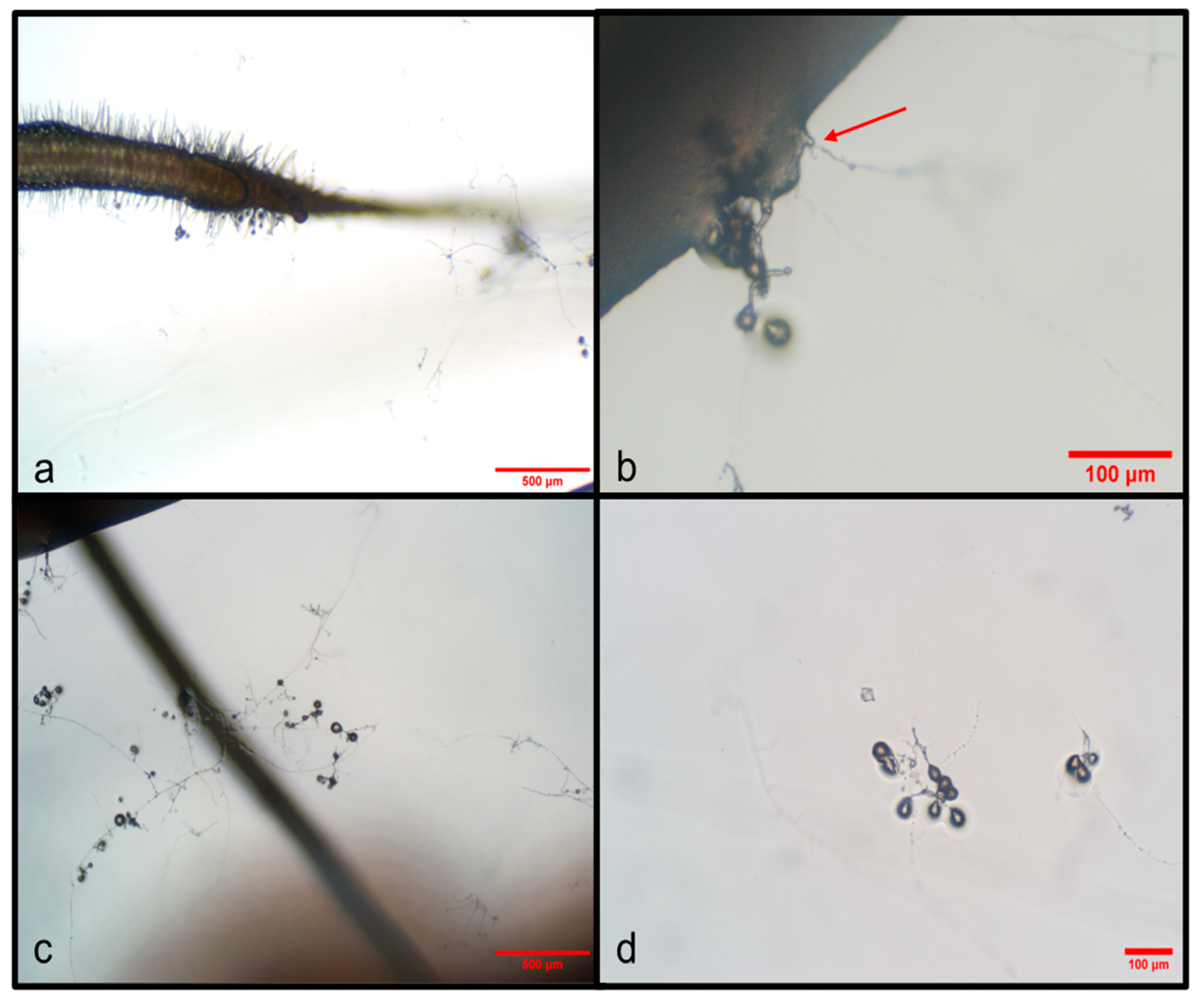

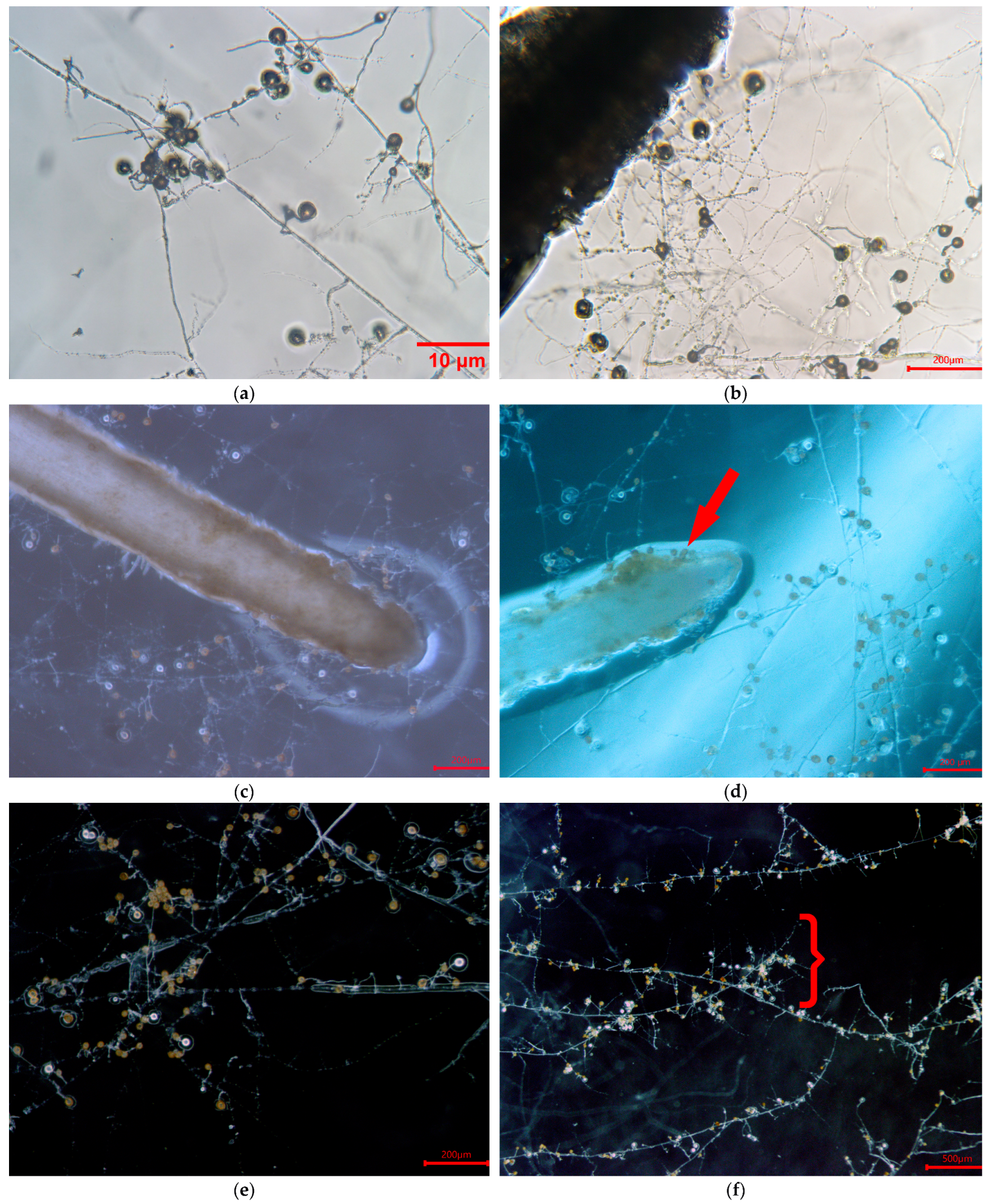
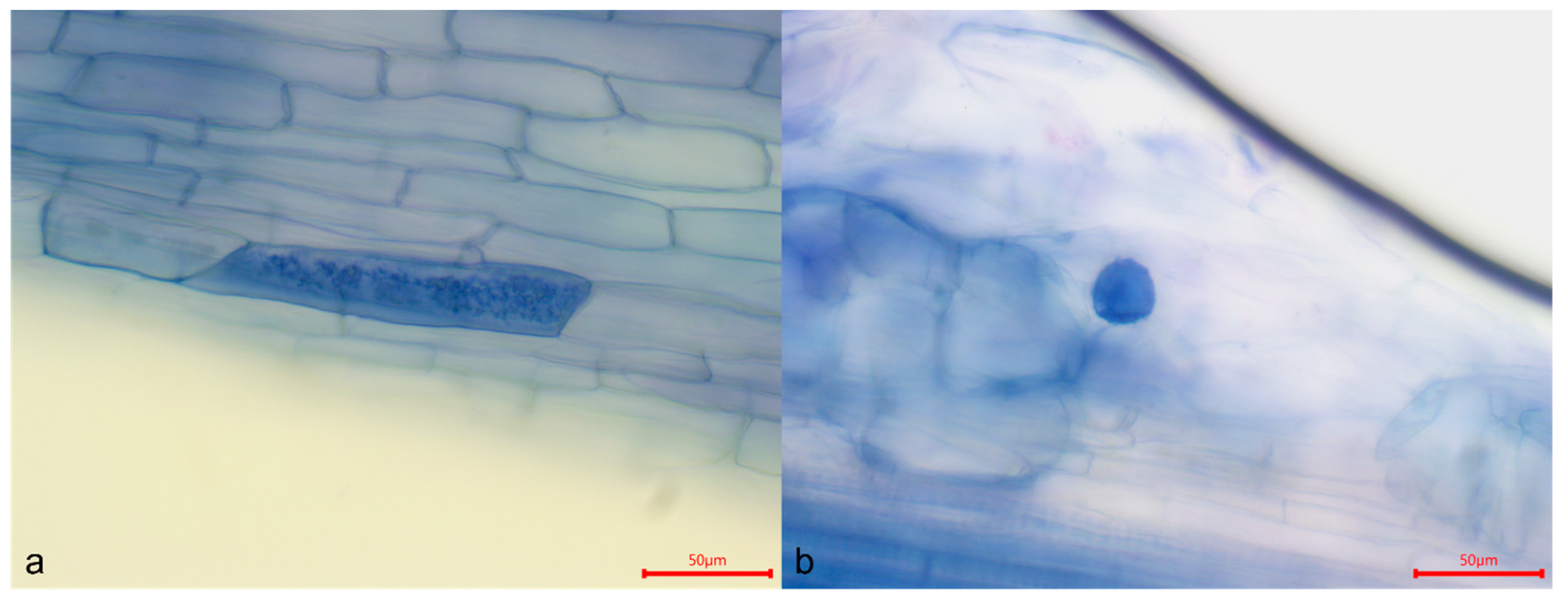
| Treatment | Stem Length (cm) | Root Length (cm) | Number of Leaves | Number of Roots | % Colonization |
|---|---|---|---|---|---|
| Control (uninoculated) | 12.4 ± 0.4 | 14.7 ± 3.0 | 2 ± 0.0 | 46 ± 4.0 | 0.0 |
| Glomus sp. colonized root | 13.6 ± 0.2 | 16.3 ± 2.5 | 3 ± 0.0 | 42 ± 7 | 30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Ceciliano, M.; Andrade-Torres, A.; Artavia-Salazar, E.; Solís-Ramos, L.Y. Establishing Monoxenic Culture of Arbuscular Mycorrhizal Fungus Glomus sp. Through In Vitro Root Organ Culture and Swietenia macrophylla King In Vitro Cultures. Agriculture 2025, 15, 673. https://doi.org/10.3390/agriculture15070673
Romero-Ceciliano M, Andrade-Torres A, Artavia-Salazar E, Solís-Ramos LY. Establishing Monoxenic Culture of Arbuscular Mycorrhizal Fungus Glomus sp. Through In Vitro Root Organ Culture and Swietenia macrophylla King In Vitro Cultures. Agriculture. 2025; 15(7):673. https://doi.org/10.3390/agriculture15070673
Chicago/Turabian StyleRomero-Ceciliano, Marysol, Antonio Andrade-Torres, Evelyn Artavia-Salazar, and Laura Yesenia Solís-Ramos. 2025. "Establishing Monoxenic Culture of Arbuscular Mycorrhizal Fungus Glomus sp. Through In Vitro Root Organ Culture and Swietenia macrophylla King In Vitro Cultures" Agriculture 15, no. 7: 673. https://doi.org/10.3390/agriculture15070673
APA StyleRomero-Ceciliano, M., Andrade-Torres, A., Artavia-Salazar, E., & Solís-Ramos, L. Y. (2025). Establishing Monoxenic Culture of Arbuscular Mycorrhizal Fungus Glomus sp. Through In Vitro Root Organ Culture and Swietenia macrophylla King In Vitro Cultures. Agriculture, 15(7), 673. https://doi.org/10.3390/agriculture15070673







