Leaf to Root Morphological and Anatomical Indicators of Drought Resistance in Coffea canephora After Two Stress Cycles
Abstract
1. Introduction
2. Results
2.1. Pressure–Volume Curve Parameters After Two Sequential Water-Stress Cycles
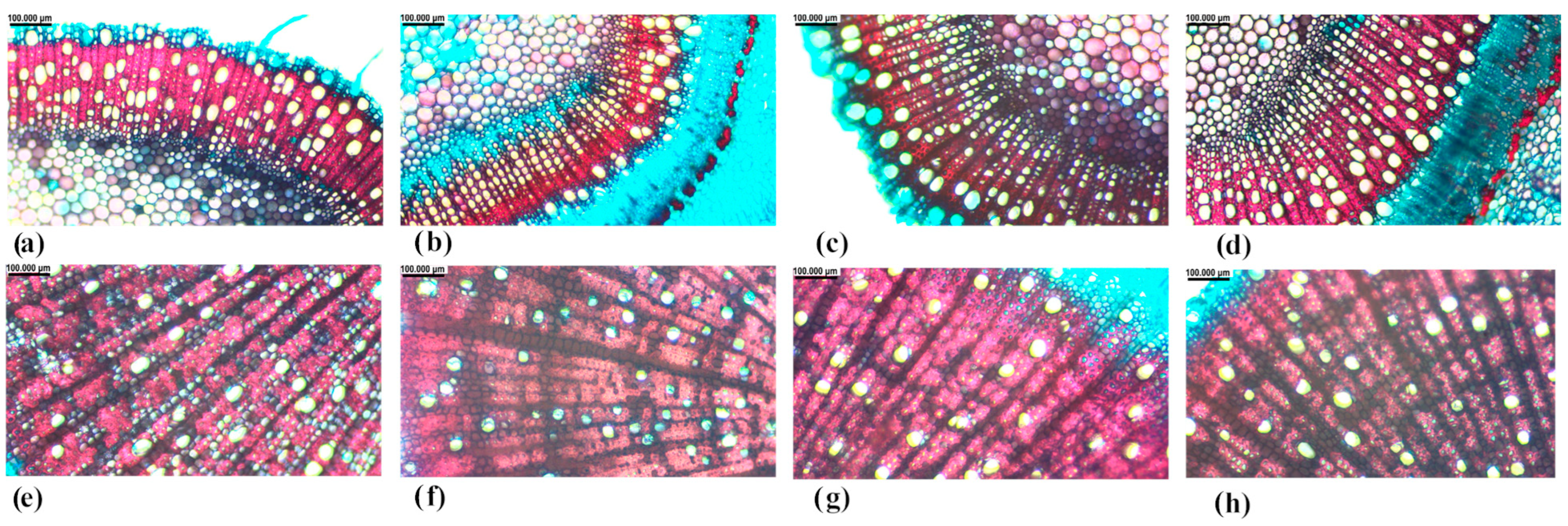
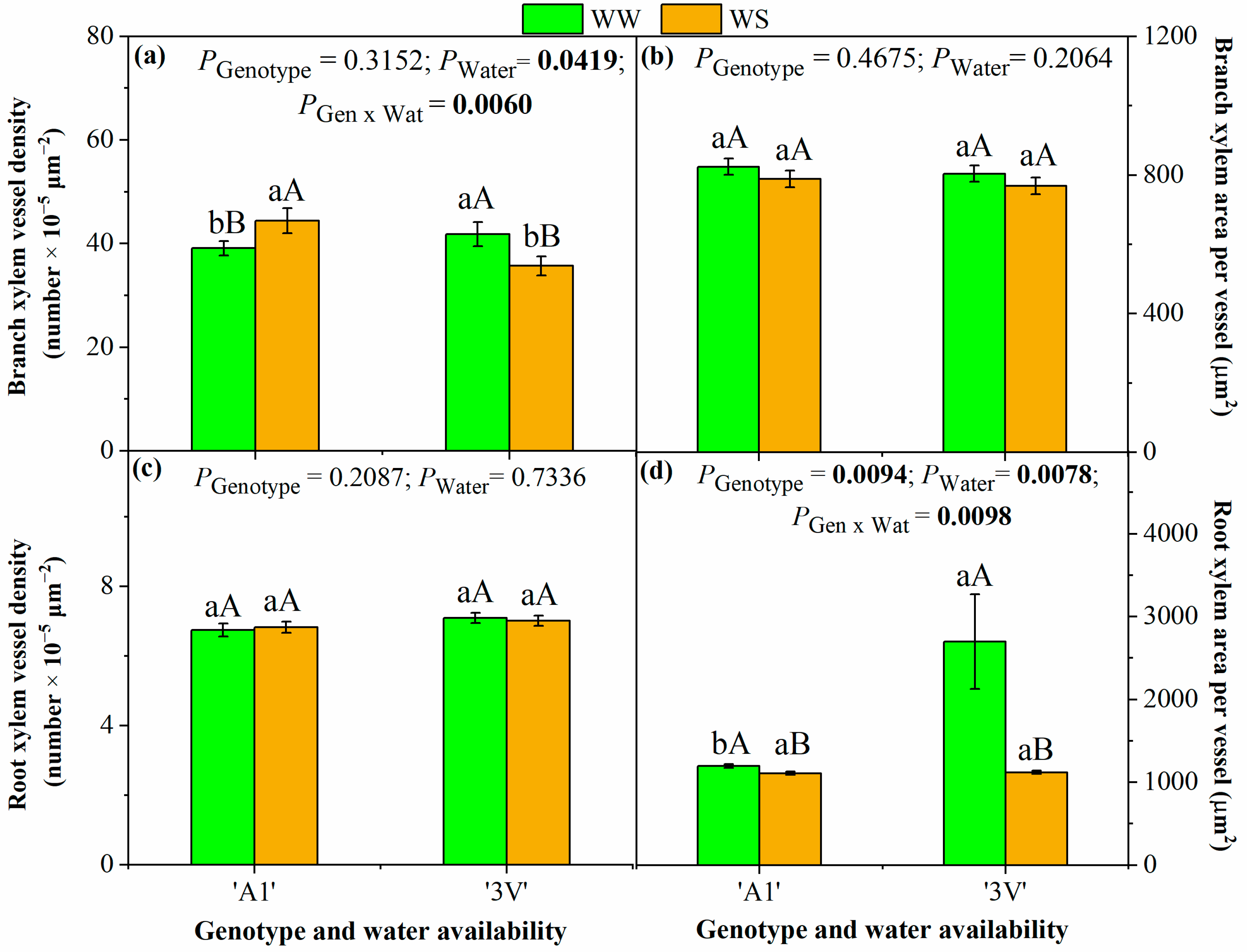
2.2. Root and Branch Xylem Anatomy Traits After Two Water-Stress Cycles
2.3. Growth Dynamics Along the Water-Stress Cycles
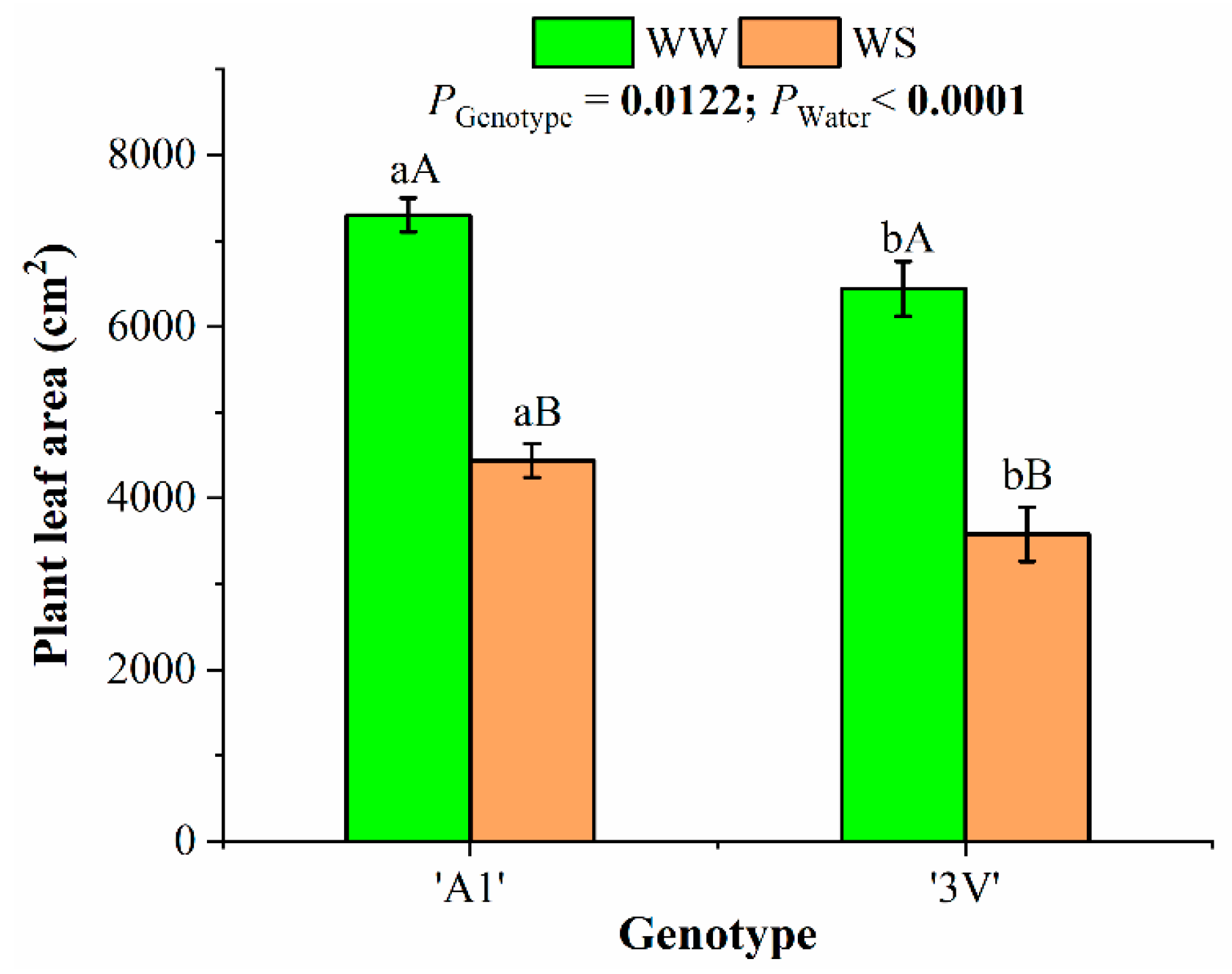
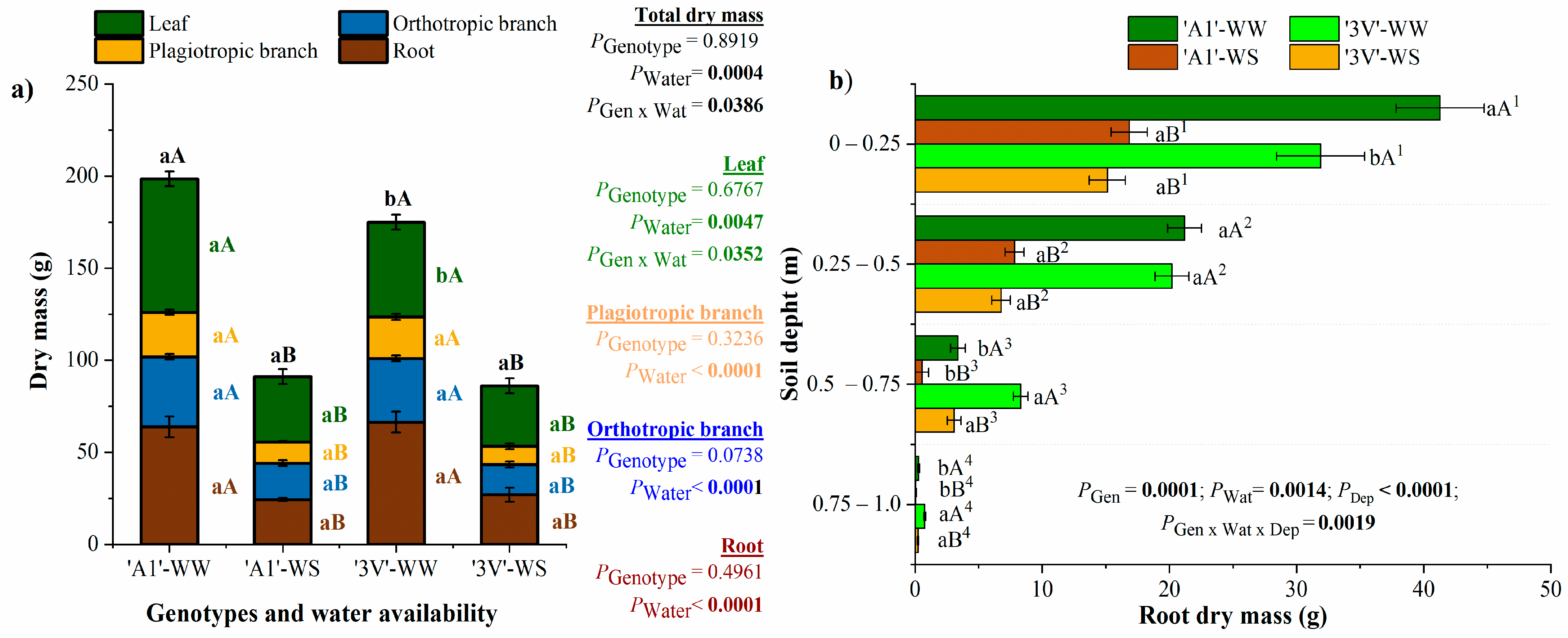
2.4. Plant Leaf Area, Plant Biomass Accumulation and Allocation in Plant Organs After Two Water-Stress Cycles
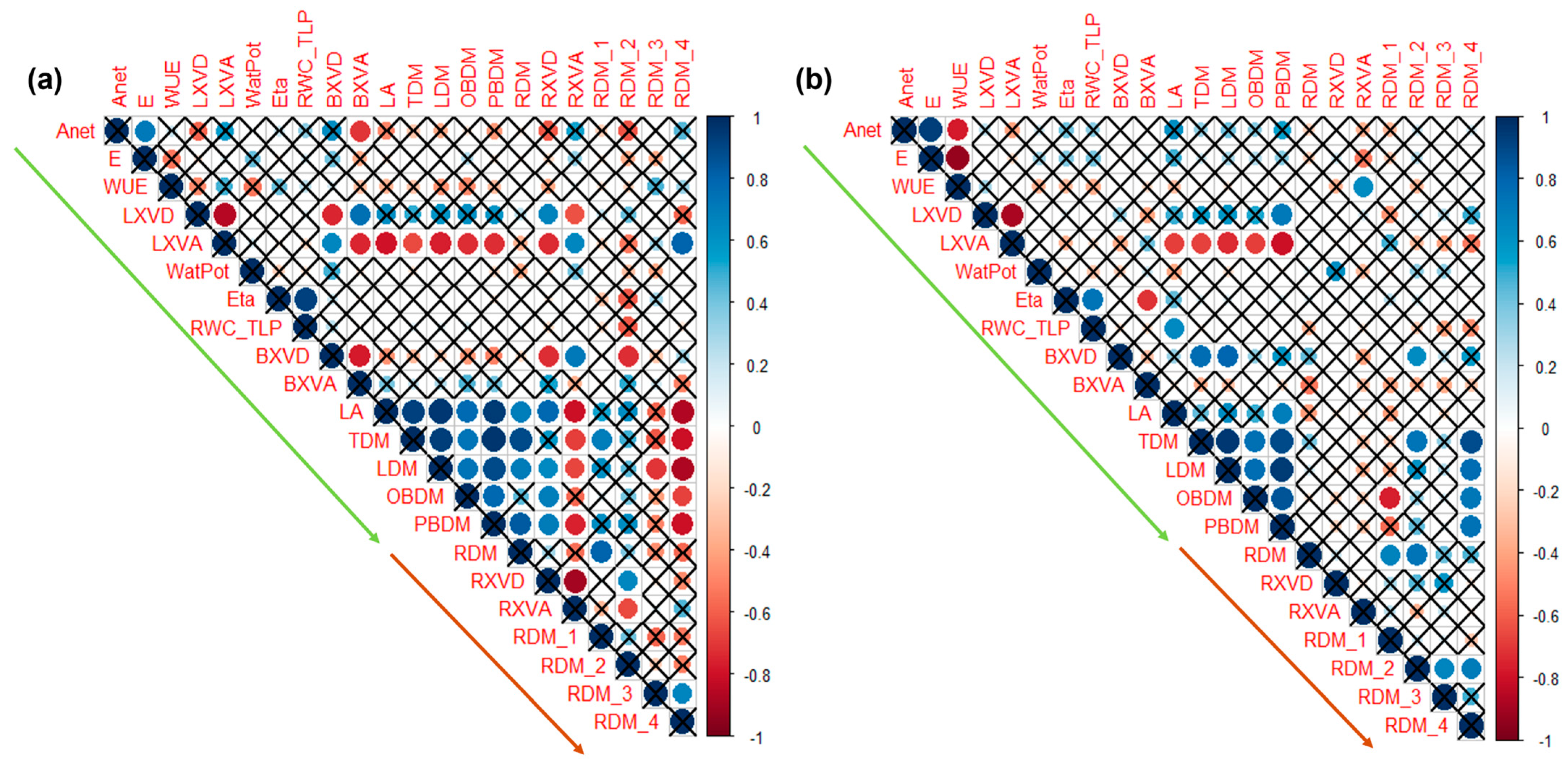
2.5. Correlations Among the Above-Ground and Below-Ground Traits After Two Water-Stress Cycles
3. Discussion
4. Conclusions
5. Material and Methods
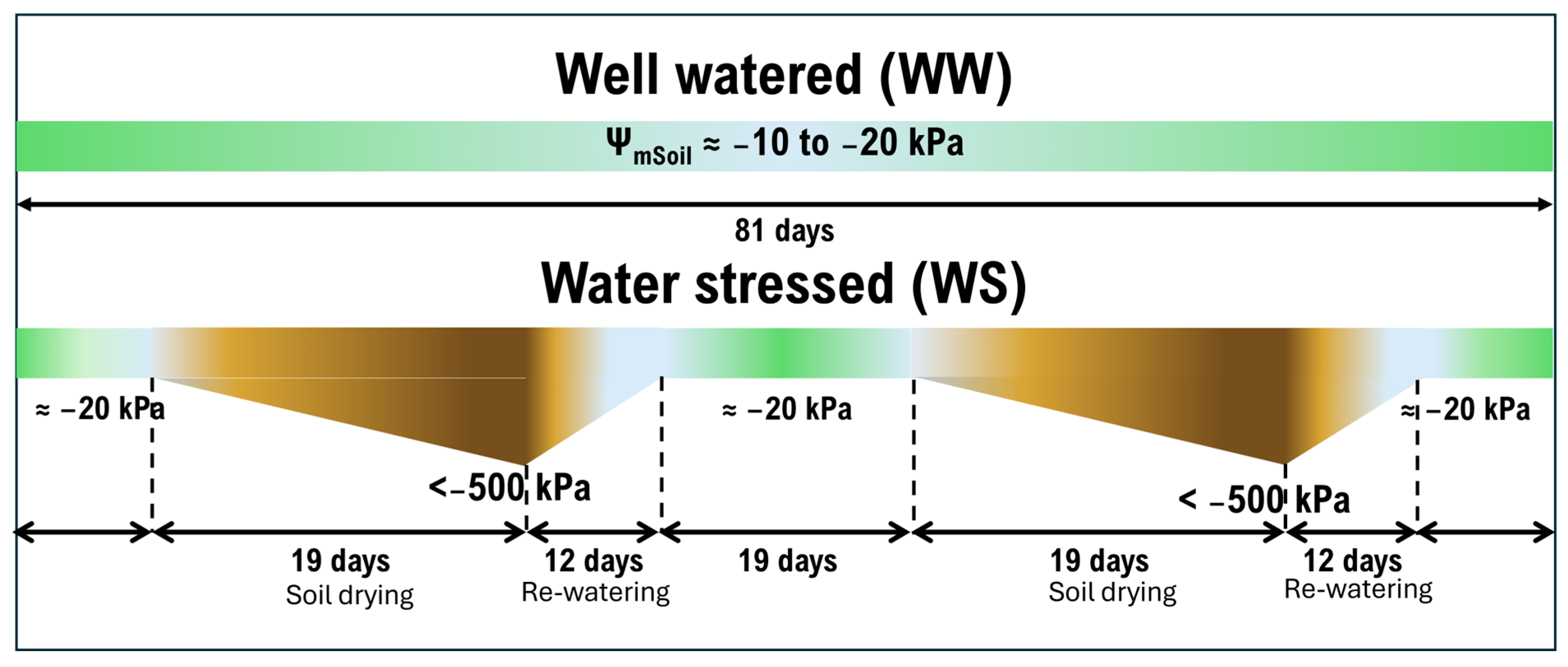
5.1. Plant Material, Experimental Conditions and Experimental Design
5.2. Pressure–Volume (P–V) Curve Measurements After Two Water-Stress Cycles
5.3. Measurmenents of Root and Branch Xylem Anatomy Traits After Two Water-Stress Cycles
5.4. Measurements of Growth Dynamics and Final Plant Leaf Area
5.5. Measurements of Biomass Allocation Traits After Two Water-Stress Cycles
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 2020, 201, 102953. [Google Scholar] [CrossRef]
- Crausbay, S.D.; Ramirez, A.R.; Carter, S.L.; Cross, M.S.; Hall, K.R.; Bathke, D.J.; Betancourt, J.L.; Colt, S.; Cravens, A.E.; Dalton, M.S.; et al. Defining ecological drought for the twenty-first century. B. Am. Meteor. Soc. 2017, 98, 2543–2550. [Google Scholar] [CrossRef]
- IPCC (The Intergovernmental Panel on Climate Change). 2022. Available online: https://www.ipcc.ch/report/ar6/wg2/chapter/chapter-4/ (accessed on 4 December 2024).
- Venancio, L.P.; Filgueiras, R.; Mantovani, E.C.; Amaral, C.H.D.; da Cunha, F.F.; dos Santos Silva, F.C.; Althoff, D.; Santos, R.A.; Cavatte, P.C. Impact of drought associated with high temperatures on Coffea canephora plantations: A case study in Espírito Santo State, Brazil. Sci. Rep. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Machado Filho, J.A.; Rodrigues, W.P.; Baroni, D.F.; Pireda, S.; Campbell, G.; de Souza, G.A.R.; Verdin Filho, A.C.; Arantes, S.D.; Arantes, L.O.; Cunha, M.; et al. Linking root and stem hydraulic traits to leaf physiological parameters in Coffea canephora clones with contrasting drought tolerance. J. Plant Physiol. 2021, 258, 153355. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Christou, A.; Kitta, E.; Katsoulas, N. Implementing sustainable irrigation in water-scarce regions under the impact of climate change. Agronomy 2020, 10, 1120. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and agronomic performance of the coffee crop in the context of climate change and global warming: A review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Pais, I.P.; Leitão, A.E.; Dubberstein, D.; Lidon, F.C.; Marques, I.; Semedo, J.N.; Rakočević, M.; Scotti-Campos, P.; Campostrini, E.; et al. Uncovering the wide protective responses in Coffea spp. leaves to single and superimposed exposure of warming and severe water deficit. Front. Plant Sci. 2024, 14, 1320552. [Google Scholar] [CrossRef]
- Rakočević, M. Coffee plant architecture. In ‘Coffee—A Glimpse into the Future’, ed. 114; DaMatta, F.M., Ramalho, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Martins, S.C.; Sanglard, M.L.; Morais, L.E.; Menezes-Silva, P.E.; Mauri, R.; Avila, R.T.; Vital, C.E.; Cardoso, A.A.; DaMatta, F.M. How do coffee trees deal with severe natural droughts? An analysis of hydraulic, diffusive and biochemical components at the leaf level. Trees 2019, 33, 1679–1693. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; Paulo, O.S.; Batista, D.; Lidon, F.C.; Rodrigues, A.P.; Partelli, F.L.; DaMatta, F.M.; Ribeiro-Barros, A.I.; Ramalho, J.C. Transcriptomic analyses reveal that Coffea arabica and Coffea canephora have more complex responses under combined heat and drought than under individual stressors. Int. J. Mol. Sci. 2024, 25, 7995. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.P.; Loh, R.K.; Arcuri, M.L.; Dezar, C.; Arge, L.W.; Falcão, T.; Romanel, E.; Morgante, C.V.; Cerqueira, J.V.A.; Ribeiro, T.P.; et al. Heterologous expression of coffee HB12 confers tolerance to water deficit in transgenic plants through an ABA-independent route. Environ. Exp. Bot. 2024, 228, 105983. [Google Scholar] [CrossRef]
- Costa, T.; Melo, J.; Carneiro, F.; Vieira, N.; Rêgo, E.; Lima, E.; Cotta, M.G.; Marraccini, P.; Andrade, A. Expression of candidates genes for drought tolerance related to ABA signaling in roots of C. canephora. In Congresso Brasileiro de Biotecnologia, 6. Brasília. [Resumos]; Sociedade Brasileira de Biotecnologia: Brasília, Brazil, 2015. [Google Scholar]
- Chekol, H.; Warkineh, B.; Shimber, T.; Mierek-Adamska, A.; Dąbrowska, G.B.; Degu, A. Drought Stress Responses in Arabica Coffee Genotypes: Physiological and Metabolic Insights. Plants. 2024, 13, 828. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. L. Sci. 2014, 72, 673–689. [Google Scholar] [CrossRef]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.-P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early Flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, Y.; Ma, N.; Zhang, X.; Tian, J.; Xu, Z.; Liu, Z. Drought-induced ecosystem resistance and recovery observed at 118 flux tower stations across the globe. Agric. For. Meteorol. 2024, 356, 110170. [Google Scholar] [CrossRef]
- Ramalho, J.; Chaves, M.M. Drought effects on plant water relations and carbon gain in two lines of Lupinus albus L. Eur. J. Agron. 1992, 1, 271–280. [Google Scholar] [CrossRef]
- Haddoudi, L.; Hdira, S.; Hanana, M.; Romero, I.; Haddoudi, I.; Mahjoub, A.; Jouira, H.B.; Djébali, N.; Ludidi, N.; Sanchez-Ballesta, M.T.; et al. Evaluation of the morpho-physiological, biochemical and molecular responses of contrasting Medicago truncatula Lines under water deficit stress. Plants 2021, 10, 2114. [Google Scholar] [CrossRef]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Pinheiro, H.P.; DaMatta, F.M.; Chaves, A.R.M.; Loureiro, M.E.; Ducatti, C. Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Ann. Bot. 2005, 96, 101–108. [Google Scholar] [CrossRef]
- Isaac, M.E.; Martin, A.R.; de Filho, E.M.V.; Rapidel, B.; Roupsard, O.; van den Meersche, K. Intraspecific trait variation and coordination: Root and leaf economics spectra in coffee across environmental gradients. Front. Plant Sci. 2017, 8, 1196. [Google Scholar] [CrossRef]
- DaMatta, F.M. Exploring drought tolerance in coffee: A physiological approach with some insights for plant breeding. Braz. J. Plant Physiol. 2004, 16, 1–6. [Google Scholar] [CrossRef]
- Erdiansyah, N.P.; Wachjar, A.; Sulistyono, E.; Supijatno, S. Growth response of seedlings of four robusta coffee (Coffea canephora Pierre. Ex. A. Froehner) clones to drought stress. Pelita Perkeb. 2019, 35, 1–11. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.Y.; Guo, L.L.; Liu, Y.; Wang, P.; Zhao, Y.D.; Cheng, Y.Q.; Lyu, Y.L.; Liu, L.Y. Influence of shrub roots on soil macropores using X-ray computed tomography in a shrub-encroached grassland in Northern China. J. Soils Sedim. 2019, 19, 1970–1980. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Lidon, F.C.; Marques, L.M.C.; Leitão, A.E.; Fortunato, A.S.; Pais, I.P.; Silva, M.J.; Scotti-Campos, P.; Lopes, A.; et al. Stress cross-response of the antioxidative system promoted by superimposed drought and cold conditions in Coffea spp. PLoS ONE 2018, 13, e0198694. [Google Scholar] [CrossRef]
- Dubberstein, D.; Lidon, F.C.; Rodrigues, A.P.; Semedo, J.N.; Marques, I.; Rodrigues, W.P.; Gouveia, D.; Armengaud, J.; Semedo, M.C.; Martins, S.; et al. Resilient and Sensitive Key Points of the Photosynthetic Machinery of Coffea spp. to the Single and Superimposed Exposure to Severe Drought and Heat Stresses. Front. Plant Sci. 2020, 11, 1049. [Google Scholar]
- Menezes-Silva, P.E.; Sanglard, L.M.; Ávila, R.T.; Morais, L.E.; Martins, S.C.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef]
- Baroni, D.F.; de Souza, G.A.R.; Bernado, W.d.P.; Santos, A.R.; Barcellos, L.C.d.S.; Barcelos, L.F.T.; Correia, L.Z.; de Almeida, C.M.; Verdin Filho, A.C.; Rodrigues, W.P.; et al. Stomatal and non-stomatal leaf responses during two sequential water stress cycles in young Coffea canephora plants. Stresses 2024, 4, 575–597. [Google Scholar] [CrossRef]
- Rakočević, M.; Matsunaga, F.T.; Pazianotto, R.A.A.; Ramalho, J.C.; Costes, E.; Ribeiro, R.V. Drought responses in Coffea arabica as affected by genotype and phenophase. I—Leaf distribution and branching. Exp. Agric. 2024, 60, e7. [Google Scholar] [CrossRef]
- Rakočević, M.; Costes, E.; Campostrini, E.; Ramalho, J.C.; Ribeiro, R.V. Drought responses in Coffea arabica as affected by genotype and phenophase. II—Photosynthesis at leaf and plant scales. Exp. Agric. 2024, 60, e22. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Lagiotis, G.; Madesis, P.; Stavridou, E. Echoes of a stressful past: Abiotic stress memory in crop plants towards enhanced adaptation. Agriculture 2023, 13, 2009. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J. Cumulative drought stress leads to a loss of growth resilience and explains higher mortality in planted than in naturally regenerated Pinus pinaster stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Zheng, R.; Zhang, M.; Zou, Z.; Heal, K.V.; Zhou, L. Xylem plasticity of root, stem, and branch in Cunninghamia lanceolata under drought stress: Implications for whole-plant hydraulic integrity. Front. Plant Sci. 2024, 15, 1308360. [Google Scholar] [CrossRef]
- Zhang, H.; McDowell, N.G.; Adams, H.D.; Wang, A.; Wu, J.; Jin, C.; Tian, J.; Zhu, K.; Li, W.; Zhang, Y.; et al. Divergences in hydraulic conductance and anatomical traits of stems and leaves in three temperate tree species coping with drought, N addition and their interactions. Tree Physiol. 2020, 40, 230–244. [Google Scholar] [CrossRef]
- Felouah, O.C.; Ammad, F.; Adda, A.; Bouzid, A.; Gharnaout, M.L.; Evon, P.; Merah, O. Morpho-anatomical modulation of seminal roots in response to water deficit in durum wheat (Triticum turgidum var. durum). Plants 2024, 13, 487. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Chaves, A.R.M.; Pinheiro, H.A.; Ducatti, C.; Loureiro, M.E. Drought tolerance of two field-grown clones of Coffea canephora. Plant Sci. 2003, 64, 111–117. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Maestri, M.; Barros, R.S.; Regazzi, A.J. Water relations of coffee leaves (Coffea arabica and C. canephora) in response to drought. J. Hortic. Sci. 1993, 68, 741–746. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Tien Le, D.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Schmidt, R.; Valani, G.P.; Ferreira, A.; Ribeiro-Barros, A.I.; Partelli, F.L. Root trait variability in Coffea canephora genotypes and its relation to plant height and crop yield. Agronomy 2020, 10, 1394. [Google Scholar] [CrossRef]
- Rakočević, M.; Baroni, D.F.; de Souza, G.A.R.; Bernado, W.P.; Almeida, C.M.; Matsunaga, F.T.; Rodrigues, W.P.; Ramalho, J.C.; Campostrini, E. Correlating Coffea canephora 3D architecture to plant photosynthesis at a daily scale and vegetative biomass allocation. Tree Physiol. 2023, 43, 556–574. [Google Scholar] [CrossRef]
- Silva, V.A.; Abrahão, J.C.d.R.; Reis, A.M.; Santos, M.d.O.; Pereira, A.A.; Botelho, C.E.; Carvalho, G.R.; de Castro, E.M.; Barbosa, J.P.R.A.D.; Botega, G.P.; et al. Strategy for selection of drought-tolerant arabica coffee genotypes in Brazil. Agronomy 2022, 12, 2167. [Google Scholar] [CrossRef]
- López, R.; Cano, F.J.; Martin-StPaul, N.K.; Cochard, H.; Choat, B. Coordination of stem and leaf traits define different strategies to regulate water loss and tolerance ranges to aridity. New Phytol. 2021, 230, 497–509. [Google Scholar] [CrossRef]
- Hecht, V.L.; Temperton, V.M.; Nagel, K.A.; Rascher, U.; Postma, J.A. Sowing density: A neglected factor fundamentally affecting root distribution and biomass allocation of field grown spring barley (Hordeum vulgare L.). Front. Plant Sci. 2016, 7, 944. [Google Scholar] [CrossRef]
- Park, S. Influences of Physical Soil Properties on Drought Severity in the Central Great Plains Based on Satellite Data and a Digital Soil Database. J. Geo. Soc. Korea. 2003, 38, 935–948. [Google Scholar]
- Fathi-Taperasht, A.; Shafizadeh-Moghadam, H.; Minaei, M.; Xu, T. Influence of drought duration and severity on drought recovery period for different land cover types: Evaluation using MODIS-based indices. Ecol. Indic. 2022, 141, 109146. [Google Scholar] [CrossRef]
- Sousa, J.S.; Neves, J.C.L.; Martinez, H.E.P.; Alvarez, V.H.V. Relationship between coffee leaf analysis and soil chemical analysis. Rev. Bras. Cienc. Solo 2018, 42, e0170109. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. Measurement of turgor pressure and water relations of plants by pressure bomb technique. J. Exp Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- Schölander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. SAP pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Dreyer, E.; Bousquet, F.; Ducrey, M. Use of pressure volume curves in water relation analysis on woody shoots: Influence of rehydration and comparison of four European oak species. Ann. For. Sci. 1990, 47, 285–297. [Google Scholar] [CrossRef]
- Ritchie, G.A.; Roden, J.R. Comparison between two methods of generating pressure-volume curves. Plant Cell Environ. 1985, 8, 49–53. [Google Scholar]
- Sack, L.; Pasquet-Kok, J. Leaf Pressure-Volume Curve Parameters. Prometheus Wiki. Available online: https://prometheusprotocols.net/ (accessed on 20 May 2024).
- Neto, B.C.; Silva, F.; Ferreira, T.; Crasque, J.; Arantes, L.; Filho, J.M.; de Souza, T.; Falqueto, A.; Dousseau-Arantes, S. Responses of wild piper species to drought and rehydration cycles considering stomatal closure as a marker of the alarm phase. Photosynthetica 2023, 61, 363–376. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. Available online: https://www.r-project.org// (accessed on 12 December 2024).
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R package for ANOVA and experimental designs. Appl. M. 2014, 5, 2952–2958. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, G.A.R.; Baroni, D.F.; Bernado, W.d.P.; Santos, A.R.; Barcellos, L.C.d.S.; Barcelos, L.F.T.; Correia, L.Z.; de Almeida, C.M.; Verdin Filho, A.C.; Rodrigues, W.P.; et al. Leaf to Root Morphological and Anatomical Indicators of Drought Resistance in Coffea canephora After Two Stress Cycles. Agriculture 2025, 15, 574. https://doi.org/10.3390/agriculture15060574
de Souza GAR, Baroni DF, Bernado WdP, Santos AR, Barcellos LCdS, Barcelos LFT, Correia LZ, de Almeida CM, Verdin Filho AC, Rodrigues WP, et al. Leaf to Root Morphological and Anatomical Indicators of Drought Resistance in Coffea canephora After Two Stress Cycles. Agriculture. 2025; 15(6):574. https://doi.org/10.3390/agriculture15060574
Chicago/Turabian Stylede Souza, Guilherme A. R., Danilo F. Baroni, Wallace de P. Bernado, Anne R. Santos, Larissa C. de S. Barcellos, Letícia F. T. Barcelos, Laísa Z. Correia, Claudio M. de Almeida, Abraão C. Verdin Filho, Weverton P. Rodrigues, and et al. 2025. "Leaf to Root Morphological and Anatomical Indicators of Drought Resistance in Coffea canephora After Two Stress Cycles" Agriculture 15, no. 6: 574. https://doi.org/10.3390/agriculture15060574
APA Stylede Souza, G. A. R., Baroni, D. F., Bernado, W. d. P., Santos, A. R., Barcellos, L. C. d. S., Barcelos, L. F. T., Correia, L. Z., de Almeida, C. M., Verdin Filho, A. C., Rodrigues, W. P., Ramalho, J. C., Rakočević, M., & Campostrini, E. (2025). Leaf to Root Morphological and Anatomical Indicators of Drought Resistance in Coffea canephora After Two Stress Cycles. Agriculture, 15(6), 574. https://doi.org/10.3390/agriculture15060574










