Abstract
The residues of the herbicides aminopyralid and iodosulfuron-methyl-sodium are phytotoxic to rotational crops. Their behaviour therefore needs to be studied under different agronomic practises and climatic conditions. The objective of this work was to use controlled laboratory conditions to study the effect of the following: (i) the application of green compost (GC) to agricultural soil, (ii) herbicide dose, (iii) soil moisture, and (iv) soil microbial activity on the degradation rate of aminopyralid and iodosulfuron-methyl-sodium. Moreover, the formation of two iodosulfuron-methyl-sodium metabolites (metsulfuron-methyl and 2-amino-4-methyl-4-methoxy methyl-triazine) and the dissipation mechanism of labelled 14C-iodosulfuron-methyl-sodium under the same conditions were also studied. Aminopyralid and iodosulfuron-methyl showed slower degradation and half-life values (DT50) that were up to 4.6 and 1.4 times higher, respectively, in soil amended with GC, as the higher organic carbon (OC) content of this soil increased herbicide adsorption. The DT50 values were up to 2.6 and 1.9 times higher for aminopyralid and iodosulfuron-methyl sodium, respectively, in soils treated with the double herbicide dose compared to soils treated with the agronomic dose. The DT50 values for aminopyralid were up to 2.3 times higher in soils with moisture equal to 25% (H25%) of their water-holding capacity (WHC) than in soils with H50%. However, the DT50 values for iodosulfuron-methyl-sodium were slightly lower in soils with H25% than in soils with H50%, due to the formation of bound residues. A biodegradation process significantly contributes to the dissipation of both herbicides. Higher amounts of metabolite metsulfuron-methyl were formed in the GC-amended soil in all cases. The percentages of 14C extractable in soils treated with both doses of herbicide under H25% were slightly higher than in soils under higher soil moisture (H50%) over time, due to the slower degradation of 14C-(iodosulfuron-methyl+metabolites). The higher persistence of the herbicides and their metabolites when the doses were applied at a high rate in soil amended with GC and under low moisture content may have negative consequences for the rotational crop. In the case of adverse conditions leading to the persistence of herbicides in the soil during the primary crop, the intervals for crop rotation should be increased.
1. Introduction
The use of herbicides is essential in agriculture, as weed abundance causes uncontrolled damage and crop losses [1]. However, residual herbicides in the soil system and on crops could cause a problem if the contamination enters the food chain [2]. This issue needs to be urgently addressed, with the use of non-chemical control methods for protecting crops from weeds and/or diseases now being promoted accordingly by the European Directive 2009/128/EC on the sustainable use of pesticides [3]. Furthermore, improved agronomic practises are now being put in place worldwide to decrease or even avoid the use of herbicides in general [4].
Rotation is a beneficial agronomic method for crop systems, as they complement each other through the use of nutrients and/or water, helping to break the cycle of diseases, pests, and weeds [5]. However, it is not sufficient to eliminate the total use of herbicides for maintaining adequate crop development, so the use of herbicides should not be discarded in an integrated weed management system [6]. The sustainable use of herbicides in a crop rotation system requires a thorough understanding of their behaviour. Certain factors, such as the carryover of herbicides on the subsequent crop, affect the stability of the rotational system if the harvest is affected by the herbicide residues in the soil from a previous crop [7,8].
Several studies have already indicated that the persistence of herbicide residues in the soil under specific conditions poses a serious hazard to the next crop, including plant damage and a reduced yield. There is also a threat to the surrounding environment due to soil and water contamination [9,10,11], hence, the importance of exploring herbicide management practises compatible with crop rotation.
Rotations of wheat or barley/sunflower or leguminous species are frequent under rainfed conditions, and the herbicides used on cereal crops are essential for their future persistence or carryover effect because certain phytotoxic effects have been reported when there is insufficient rainfall [12,13]. The potential for carryover injury to rotational crops is influenced by the amount of herbicide present in the soil, the susceptibility of the rotation crop, and the conditions after application, such as the chemical half-life (DT50), the herbicide rate applied, and the precipitation/irrigation regime. In addition, soil characteristics such as soil organic matter (OM) and clay content, soil microbial activity, and OM from the organic amendments applied modify herbicide persistence or its carryover potential. Herbicides can be adsorbed or bound by soil colloids, and potential injury to rotational crops may be caused by the release of herbicide residues if soil moisture is increased by rainfall/irrigation or by other environmental conditions [14]. Herbicides vary in their potential persistence and carryover, and those used in rotational systems belong to different chemical groups, with ones from the 6-chloropicolinate and sulfonylurea groups frequently applied [8,10,15].
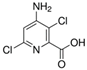
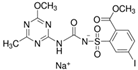
Aminopyralid (4-amino-3,6-dichloropyridine-2-carboxylic acid) belongs to the 6-chloropicolinate group that controls a broad spectrum of broad-leaved weeds in post-emergence wheat and barley. It acts by auxin mimicking during plant cell division and growth. It is a strong acid herbicide that is non-volatile and highly soluble in water. Based on its chemical properties, it is mobile with a high potential for leaching to groundwater. It is moderately persistent in soil systems but not so in surface water under normal conditions [16,17]. In turn, iodosulfuron-methyl-sodium (sodium ({[5-iodo-2-(methoxycarbonyl)phenyl] sulfonyl}carbamoyl)(4-methoxy-6-methyl- 1,3,5-triazin-2-yl)azanide) belongs to the sulfonylurea group. It is applied to post-emergence cereals, acting on the cellular metabolism of plants by inhibiting acetolactate synthase. It is a strong acid herbicide, highly soluble in water, and semi-volatile. It is not persistent in soil, but it is present in aquatic systems [16,18].
Trace amounts of 6-chloropicolinate and sulfonylurea herbicides in the soil lead to the phytotoxicity of current and subsequent crops, and environmental pollution and ecological toxicity have been reported [8,12,19,20]. Aminopyralid and iodosulfuron-methyl and its main metabolites have very high and very high-to-medium mobility in soil, respectively [17,18,21]. Their widespread use has the potential to contaminate surface and ground water [22,23,24]. Most 6-chloropicolinate and sulfonylurea herbicides are easily hydrolysed in an acidic environment, while some of them are very stable under alkaline and neutral conditions, meaning they degrade very slowly [17,18,19,20]. Therefore, the high persistence of these herbicides in soils after long-term application may damage subsequent sensitive crops, as previously reported [19,20,25,26].
Aminopyralid has soil residual properties [26]. Laboratory studies have reported DT50 values of 26.2–144.7 days in soils under aerobic degradation [17], while, in field trials, aminopyralid degraded rapidly in agricultural soils from Alaska and grasslands from China, with DT50 values of 8.2–23.0 days [20,26]. High soil OM content, microbial activity, temperature, and increased precipitation, which controls soil water content, accelerate herbicide degradation, decreasing DT50 values in agricultural soils and grasslands [26,27]. Precipitation events may also increase herbicide mobility, which contributes to its dissipation in the soil [20]. Furthermore, some studies have reported the adsorption of the herbicide by soils with high OM content, which reduced its bioavailability for degrading [20,28].
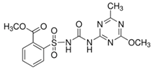
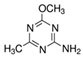
In laboratory studies, iodosulfuron-methyl-sodium records very low-to-moderate persistence in soil under aerobic conditions in the dark, with DT50 values of 0.8–23.1 days [18]. Changes in the persistence of iodosulfuron-methyl in agricultural soils of a combined wheat-poplar tree cultivation or winter wheat crops have been reported [29,30]. Moreover, the effects of environmental conditions on the dissipation of iodosulfuron-methyl-sodium under laboratory conditions have been observed, with its degradation rate positively related to temperature, moisture, and soil OM content, but inversely related to the herbicide dose applied [31]. The degradation of iodosulfuron-methyl-sodium under laboratory conditions gives rise to the formation of four major metabolites and three minor ones [16,18]. The formation of metsulfuron-methyl (methyl 2-(4-methoxy-6-methyl-1,3,5-triazin-2-yl carbamoylsulfamoyl)benzoate) and 2-amino-4-methyl-4-methoxymethyl-triazine (AMMT), as two of the major transformation products from iodosulfuron-methyl-sodium in different soils, has only been reported in a handful of studies [32,33,34]. Metsulfuron-methyl and AMMT record low-to-medium and medium-to-very high persistence, respectively [18]. Metsulfuron-methyl has been categorised as a potential leacher in soils, indicating a high risk of groundwater contamination [35] and may significantly damage rotation crops [36].
The effect that the application of organic amendments to soil has on the dissipation of aminopyralid or iodosulfuron-methyl-sodium has not been previously studied. Furthermore, there are no prior studies on the influence that combined agronomic practises and climatic conditions have on the dissipation, metabolite formation, and the persistence of aminopyralid and iodosulfuron-methyl-sodium in agricultural soils, which is essential for the production of rotational crops and agricultural sustainability. The hypothesis of this work is that the biodegradation process increases the dissipation of herbicides in agricultural soils.
Therefore, this novel work set out to apply controlled laboratory conditions (20 °C, in the dark) to study the effect of the following: (i) the use of GC as a soil amendment (2.5% w/w), (ii) herbicide rate (agronomic and double this rate), (iii) soil moisture content (25% and 50% of WHC), and (iv) microbial activity (non-sterile and sterile soils) on the degradation rate of the herbicides aminopyralid and iodosulfuron-methyl-sodium, the formation of iodosulfuron-methyl-sodium metabolites (metsulfuron-methyl and AMMT), and the dissipation mechanism of 14C-iodosulfuron-methyl-sodium (mineralized, extractable and non-extractable fractions). The ultimate aim was to evaluate herbicide and metabolite availability and soil residues in order to avoid carryover injury to rotational crops through their persistence in the treated soil. Understanding herbicide dissipation in agricultural soils and the effect that different management practises have on its behaviour would lead to appropriate weed control without crop injury.
2. Materials and Methods
2.1. Herbicides and Reagents
The commercial formulations used were Intensity® 10 (Corteva Agriscience, S.L.U., Seville, Spain), containing 30% w/w aminopyralid, and Hussar® Plus (Bayer AG, Lyon, France) containing 5% w/v iodosulfuron-methyl-sodium (purity 99%). The PESTANALTM analytical standards of the herbicides aminopyralid (purity ≥ 99.7%) and iodosulfuron-methyl-sodium and its metabolites metsulfuron-methyl (purity ≥ 98.0%) and AMMT (purity ≥ 97.0%) were supplied by Sigma-Aldrich, Merck (Taufkirchen, Germany). The labelled phenyl-U-14C-iodosulfuron-methyl-sodium (specific activity 2.87 MBq mg−1, purity 99.1%) was supplied by IZOTOP Co. Ltd. (Budapest, Hungary). The characteristics of the herbicides [16] and the adsorption Kd values [28] are included in Table 1. HPLC grade methanol and ammonium formate were supplied by VWR International Eurolab (Barcelona, Spain).

Table 1.
Characteristics of herbicides and metabolites [16,28].
2.2. Experimental Setup
An agricultural sandy loam soil (S) composed of 11.1% clay, 28.1% silt, and 60.8% sand was sampled (0–20 cm) at the Muñovela experimental farm (IRNASA-CSIC, Salamanca, Spain, 40°54′15″ N latitude and 5°46′26″ W longitude). This soil contains illite, kaolinite, and montmorillonite as clay minerals. According to the data registered in the information on phytosanitary treatments, there had been no application of aminopyralid or iodosulfuron-methyl-sodium in the field where soil was collected. The sample was sieved (<2 mm) and refrigerated at 4 °C until use.
The organic amendment used in this study was GC, produced by aerobic composting plant residues (grass, leaves and branches) from the pruning of plants and trees in parks and gardens for six months at a local green waste treatment plant (Viveros El Arca, Salamanca, Spain).
The soil was amended with GC (<2 mm) at a dose of 2.5% (w/w) (S+GC). The physicochemical characteristics of GC, S, and S+GC were determined in air-dried and sieved (<2 mm) samples by standard analytical methods [37] (Table 2) by the Analytical and Instrumentation Service at IRNASA-CSIC. Briefly, soil particle size distribution was measured using a Malvern Mastersizer 3000-Hydro LV laser diffractometer (Malvern Panalytical, Malvern, UK). Clay minerals (montmorillonite, illite and kaolinite) were qualitatively identified in the soil clay fraction using an X-ray Philips PW1710 diffractometer (Philips, Eindhoven, the Netherlands). The pH (1:2.5 w/v) was determined using a pH metre. The electrical conductivity (EC) (1:5 w/v) was determined using a WTW LF91 conductivity metre (WTW, Weilheim, Germany). Organic carbon (OC) and total nitrogen (N) content was measured with a LECO CHN628 elemental analyser (LECO Corporation, St Joseph, MI, USA). Dissolved organic carbon (DOC) content was determined in a soil/water (1/2 w/v) or GC/water (1/100 w/v ratio) suspension, after shaking (24 h, 20 °C), centrifuging (20 min at 10,000 rpm) and filtering (<0.45 μm), using a Shimadzu TOC-5050 (Shimadzu, Columbia, MD, USA) total OC analyser.

Table 2.
Physicochemical characteristics of the organic amendment (GC), unamended (S), and GC-amended (S+GC) soils.
Herbicide dissipation experiments were conducted in duplicate samples of S and S+GC. The soils were incubated at 20 °C for one week before the start of the dissipation experiments. The concentrations of the commercial formulations of aminopyralid and iodosulfuron-methyl sodium added to 500 g of S and S+GC corresponded to the agronomic dose (D1 = 1.5 mg kg−1) and double this dose (D2 = 3 mg kg−1). The soil WHC is the percentage of water that a given soil can hold per unit of mass without dripping, after being saturated. The soil moisture content was maintained at 25% (H25%) and 50% (H50%) of their maximum WHC throughout this study by weighing the soil flasks and adding sterile Milli-Q ultrapure water every 2–3 days. The soil moisture contents were 8.5% and 15.8% for S, and 8.9% and 16.6% for S+GC at H25% and H50%, respectively. The soils were incubated at 20 °C in the dark. In addition, sterilised soil samples were prepared by autoclaving 500 g of S and S+GC at 120 °C for 1 h over five consecutive days. The herbicides were applied to the sterilised samples at the D2 dose, as in the non-sterilised soils. These soil samples were handled in a sterile cabinet and were used as controls to verify the contribution that biological and chemical degradation make to herbicide dissipation [38].
2.3. Herbicide Extraction
Two soil subsamples (5 g) of each duplicate treatment were taken the day after herbicide application (day 0) for herbicide analysis and thereafter repeatedly at different time intervals up to 120 days. The herbicides were extracted with 10 mL of a methanol/water solution (50:50). The resulting mixture was shaken by sonication for 1 h in an ultrasonic bath at 20 °C and then kept under intermittent mechanical shaking in a thermostatic chamber at 20 °C for 24 h. The suspension was centrifuged at 3000 rpm for 15 min, and the supernatant, which corresponds to the soil extract, was filtered through 0.45 µm pore size nylon filters [38]. Finally, the extracts were transferred to glass vials for the quantitative determination of the herbicides.
2.4. Herbicide Analysis
Herbicides were analysed by Ultra-High-Performance Liquid Chromatography-Quadrupole Time-Of-Flight-Mass Spectrometry (UHPLC-QTOF-MS, Agilent Technologies, Avondale, AZ, USA), equipped with an HPLC Infinity II, a 6546A QTOF mass spectrometer, and Mass Hunter Qualitative and Quantitative Analysis Software version 10.1, as the data acquisition and processing system, using the method optimised by Douibi et al. [39].
A Zorbax® Eclipse Plus C18 (50 mm × 2.1 mm i.d., 1.8 μm particle size) column was used (Agilent Technologies, Avondale, AZ, USA) at 30 °C. The gradient profile was as follows: 0–0.25 min, 95% water with 0.1% formic acid (A) and 5% acetonitrile (B); 0.25–2.5 min, 55% A and 45% B; 2.5–3.5 min, 100% B; and 3.5–4 min, 95% A and 5% B. The flow rate was 0.4 mL min−1, and the sample injection volume was 4 μL. The QTOF mass spectrometer operated in positive electrospray ionisation mode (ESI) with an ion source with Jet Stream Technology (AJS) under multiple-reaction monitoring mode (MRM). Ultra-pure nitrogen (N2) was used as the nebuliser and sheath gas. Ultra-high-purity N2 was used as collision gas in product ion scanning experiments. The ESI parameters were set as follows: the capillary voltage was 3.5 kV; the temperature of the sheath gas and the flow rate were 350 °C and 11 L min−1, respectively; the source temperature was set at 225 °C and the flow rate of the drying gas was 12 L min−1; the nebuliser gas pressure was 30 psi; the fragmentor voltage was 110 V; the mass analyser scanned from 100 to 1050 (m/z); the QTOF acquisition rate was 1.5 Hz; the energies for collision-induced dissociation (CID) experiments were set at 10, 20, and 40 eV, respectively; and all MS data were acquired with reference masses at m/z 121.05 and 922.01 in the positive ESI mode to ensure mass accuracy and reproducibility.
Quantification involved monitoring the positive molecular ion [m/z] [M + H]+ 206.97 (aminopyralid); 507.98 (iodosulfuron-methyl-sodium), 382.08 (metsulfuron-methyl), and 141.08 (AMMT). The limit of detection (LOD) was 0.001 µg g−1, while the limit of quantitation (LOQ) was 0.005 µg g−1 for iodosulfuron-methyl-sodium, metsulfuron-methyl and AMMT. For amimopyralid, the LOD and LOQ were 0.01 and 0.05 µg g−1, respectively. Calibration curves were obtained by plotting peak areas versus concentration using standards prepared in blank soil (S or S+GC) extracts to avoid any under/overestimation of herbicide/metabolite concentrations determined in the soil extracts. The mean recovery values when performing the extraction and analysis for each analytical grade herbicide and metabolite spiked at 0.25 and 0.50 mg kg−1 in five blank soil samples (S and S+GC) were between 90% and 99% for aminopyralid, 76% and 92% for iodosulfuron-methyl-sodium, 91% and 98% for metsulfuron-methyl, and 85% and 93% for AMMT (Table S1). Chromatograms for the extracts of blank soils (without herbicides), extracts of soil samples spiked with 0.005 and 1 µg mL−1 of herbicide/metabolite standards, and extracts of soil samples treated with herbicides applied to D2, at time 0 days for aminopyralid and iodosulfuron-methyl-sodium, 70 days for metsulfuron-methyl and 120 days for AMMT are included in Tables S2–S5.
2.5. Dissipation Mechanism and Analysis of 14C Labelled Iodosulfuron-methyl-sodium
Duplicate samples of S and S+GC with 14C labelled iodosulfuron-methyl were incubated at the same time as unlabelled herbicide to study the herbicide dissipation mechanism considering the mineralized, extractable, and non-extractable fractions. A 10 mL volume of an aqueous solution of herbicide was labelled with 14C-iodosulfuron-methyl and added to 500 g of S and S+GC for concentrations of 1.5 (D1) and 3.0 (D2) mg kg−1 of dry soil and an activity of approximately 50 Bq g−1. The initial moisture content of both S and S+GC was adjusted to H25% and H50%, as in the dissipation experiments with unlabelled herbicide. In these samples, a 14CO2 trap (a vial containing 1 mL of NaOH 1 M) was attached to the lid with a stainless-steel clip [40].
Labelled iodosulfuron-methyl was extracted from soil samples: 2 × 5 g of each duplicate treatment was extracted with 10 mL of a methanol:water solution (50:50) by sonication for 1 h in an ultrasonic bath at 20 °C and then kept under intermittent mechanical shaking in a thermostatic chamber at 20 °C for 24 h. The quantitative determination of 14C-iodosulfuron-methyl was performed by liquid scintillation using a Beckman LS 6500 liquid scintillation counter (Beckman Instruments Inc., Fullerton, CA, USA). The 14C-activity of the solutions, associated with parent compound iodosulfuron-methyl and possible transformation compounds, was measured in disintegration per minute (dpm), determined in duplicate in 1 mL of extract to which 4 mL of scintillation cocktail was added (AquaLight+, HIDEX, Turku, Finland). The remaining 14C-activity in the soil was determined by combusting 1 g dried samples using a Biological Oxidiser (RJ. Harvey OX-500 Instrument Corporation, NJ, USA) under excess O2 at 900 °C. The 14CO2 generated was trapped in a mixture of ethanolamine (1 mL) and a scintillation cocktail (Oxysolve C-400, Zinsser Analytic, Berkshire, UK, 15 mL) and determined as previously indicated. 14CO2 from the mineralization of labelled iodosulfuron-methyl retained in 1 M NaOH (1 mL) in the scintillation vial was determined at different sampling times by mixing 4 mL of scintillation cocktail as previously indicated. The mass balance in 14C residues was calculated as a percentage of the total amount of 14C radioactivity obtained from the different fractions.
2.6. Data Analysis
The herbicide dissipation curves were fitted to a single first-order (SFO) kinetic model and a first-order multi-compartment (FOMC) one, using the Excel Solver add-in package [41] to calculate DT50 (the time elapsed for the dissipation of 50% of the herbicide), DT90 (the time elapsed for the dissipation of 90% of the herbicide), and the dissipation rates for the different soil treatments. Goodness of fit was determined by calculating the coefficient of determination (r2) and the chi-square test (ꭓ2).
Standard deviation (SD) was used to indicate the variability between replicates of the same treatment. One-way ANOVA was performed to determine significant differences in herbicide dissipation parameters and metabolite amounts across treatments. Means were compared by the Tukey post hoc test (p < 0.05). IBM SPSS Statistics v. 29.0 software (IBM Inc., Chicago, IL, USA) was used.
3. Results and Discussion
3.1. Degradation of Aminopyralid and Iodosulfuron-methyl-sodium in Unamended and Amended Soils
Figure 1 presents the degradation curves of aminopyralid and iodosulfuron-methyl-sodium applied at doses D1 and D2 in S and S+GC, incubated at moistures of H25% and H50%. These curves were fitted to a biphasic (FOMC) for aminopyralid and a single first-order (SFO) kinetic model for iodosulfuron-methyl-sodium [41], with goodness-of-fit parameters χ2 ≤ 8.3 and 7.1 and r2 ≥ 0.90 and 0.99, respectively (Table 3 and Table 4). Aminopyralid degraded rapidly between 0 and 28 d, and degradation then slowed through 120 days. The iodosulfuron-methyl degradation rate was constant through 120 days, since curves fitted the SFO kinetic model. The concentrations were in the ranges 32.3–49.4% (S) and 52.4–63.5% (S+GC) for aminopyralid, and 0.4–2.3% (S) and 0.8–5.0% (S+GC) for iodosulfuron-methyl-sodium at 120 d after application (Figure 1). On the contrary, the degradation curves of aminopyralid and iodosulfuron-methyl-sodium in unamended agricultural soils under laboratory conditions have been reported to fit the SFO kinetic model [17] and biphasic kinetic models [18,33,34], respectively.
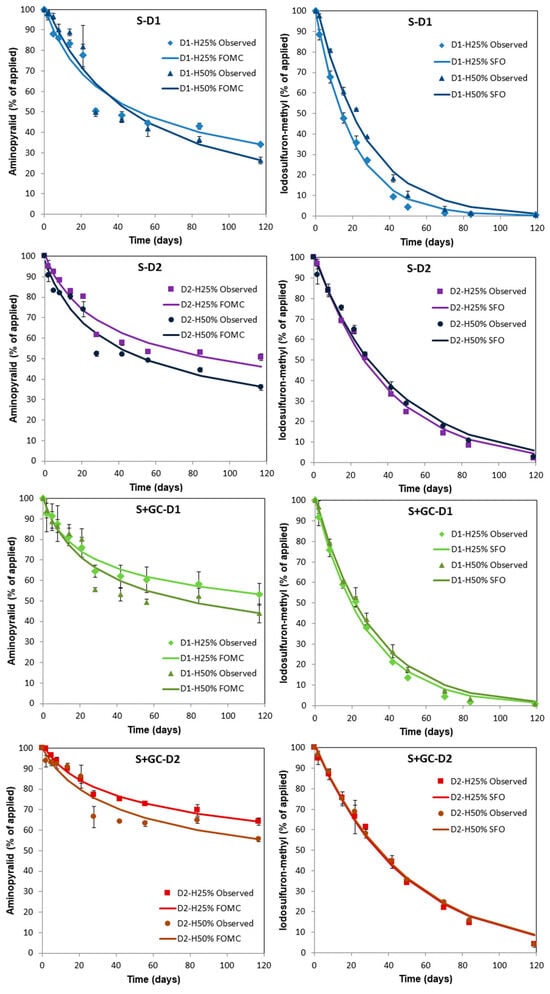
Figure 1.
Dissipation kinetics of aminopyralid (left) and iodosulfuron-methyl-sodium (right) applied to unamended (S) and GC-amended (S+GC) soils at two doses (D1 = agronomic and D2 = double agronomic) under two soil moisture regimes (H25% = 25% and H50% = 50% of WHC). Bars indicate the standard deviation of the mean (n = 2).

Table 3.
Dissipation parameters of aminopyralid applied to agronomic (D1) and double agronomic (D2) doses in a non-sterile and sterile unamended (S, SS) and GC-amended (S+GC, SS+GC) soils incubated at moisture levels of 25% (H25%) and 50% (H50%) of soil WHC.

Table 4.
Dissipation parameters of iodosulfuron-methyl-sodium applied to agronomic (D1) and double agronomic (D2) doses in non-sterile and sterile unamended (S, SS) and GC-amended (S+GC, SS+GC) soils incubated at moisture levels of 25% (H25%) and 50% (H50%) of soil WHC.
The values of the dissipation kinetic parameters, DT50 and DT90, are included in Table 3 and Table 4 for aminopyralid and iodosulfuron-methyl-sodium, respectively. The DT50 values calculated in S for aminopyralid (42.8–86.8 days) and iodosulfuron-methyl-sodium (14.0–29.2 days) fall within the range of values of 26.2–144.7 days (20 °C, pF2 soil moisture) [17] and 0.8–40.3 days (20 °C, 25–50% MWHC soil moisture) [18,33,34], respectively, as determined in previous laboratory studies. Iodosulfuron-methyl-sodium is more soluble in water than aminopyralid (Table 1), and this could explain its greater availability to be degraded faster.
The degradation DT50 values were up to 4.6 times higher for aminopyralid and 1.4 times higher (p < 0.05) for iodosulfuron-methyl-sodium in S+GC than in S (Table 3 and Table 4). S+GC has a higher OC content than S (Table 1), which increased the adsorption of the herbicides [28], decreasing their bioavailability for degradation [20].
The DT50 values were 1.3 to 2.6 times (aminopyralid), and 1.6 to 1.9 times (iodosulfuron-methyl-sodium) higher (p < 0.05) in soils treated with D2 compared to soils treated with D1 (Table 3 and Table 4), as microorganisms might take longer to degrade a larger amount of compound and there may be possible herbicide toxicity for soil microbial communities [42,43,44].
The DT50 values of aminopyralid were up to 2.3 times higher (p < 0.05) in soils with moisture H25% than in soils with H50%, indicating the slowest degradation rates, which favoured the formation of bound residues that increased herbicide retention, leading to a greater persistence of this herbicide in the soil. This increase was greater (p < 0.05) for S+GC due to higher herbicide retention and decreased microbial activity in soils with a lower moisture content [20,26,27,42]. This greater herbicide persistence in soils with lower moisture was corroborated by DT90 values, which were up to two orders of magnitude higher in soils with H25% (Table 3). By contrast, the DT50 values for iodosulfuron-methyl were up to 1.3 times lower (p < 0.05) in S with H25% than in S with H50%, due to the formation of bound residues over time and greater herbicide retention in the soil [42]. However, other studies have found that the degradation rate of iodosulfuron-methyl-sodium and metsulfuron-methyl is positively related to soil moisture content [31,34,45]. This factor’s effect on the iodosulfuron-methyl dissipation rate in S+GC was not significant (Table 4), which may be due to a higher adsorption of the herbicide by S+GC, irrespective of its soil moisture content.
Although aminopyralid degradation was very slow (DT50 > 1000 days) in all the sterile treatments, the slowest degradation was observed in sterile soils with moisture H25% (Table 3). At 120 days, the percentage of herbicide remaining in these soils exceeded 90%, indicating that aminopyralid degradation is due mainly to biotic processes (biodegradation by microorganisms), and both chemical degradation and photodegradation are minority processes [17]. Ahmed et al. [46] have reported the importance of microorganisms in the breakdown process of clopyralid, a picolinic acid herbicide from the same aminopyralid group.
The iodosulfuron-methyl-sodium degradation rate in sterile soils was up to 4.2 times slower than in non-sterile soils (Table 4). Iodosulfuron-methyl-sodium degraded more slowly in anaerobic soil incubations than under aerobic conditions [18]. The lower DT50 values of iodosulfuron-methyl in the non-sterile soils than in a sterile system indicate that biodegradation significantly contributed to herbicide dissipation, although both chemical and biological degradation contribute to its dissipation in the soil, as previously observed for iodosulfuron-methyl and other sulfonylurea herbicides [18,43,47,48].
3.2. Formation of Metsulfuron-methyl and AMMT Metabolites in Unamended and Amended Soils
Figure 2 shows the amounts of metsulfuron-methyl and AMMT formed over time during the degradation of iodosulfuron-methyl-sodium in S and S+GC for all the treatments studied. Metsulfuron-methyl is the main metabolite of iodosulfuron-methyl formed in the soil by the reductive elimination of iodine from the phenyl ring [18,34]. The maximum amount of metsulfuron methyl was detected between 42 and 70 days (32.3–45.6%) in S, and between 70 and 84 days (41.0–59.2%) in S+GC. The highest percentage of metsulfuron-methyl formed in S+GC was 1.3 times higher than in S, although it was reached at a later date (Table 5). These results might be due to the higher adsorption of the metabolite by the GC-amended soil [28] and its lower availability for further degradation. Metsulfuron-methyl is degraded mainly by soil microorganisms via O-demethylation, sulfonylurea bridge cleavage, and the triazine ring opening [47].
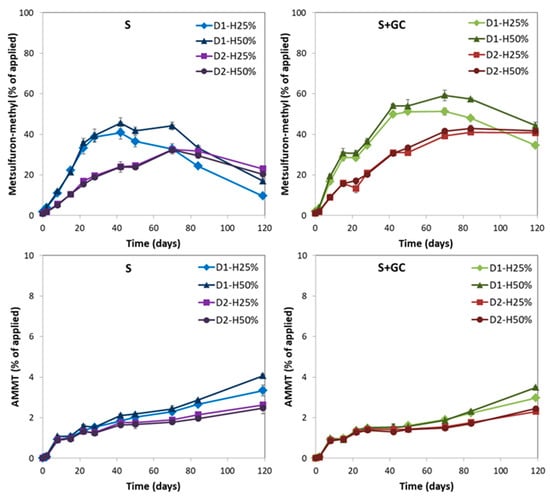
Figure 2.
Formation of metsulfuron-methyl and AMMT, expressed as percentages of iodosulfuron-methyl-sodium applied initially to unamended (S) and GC-amended (S+GC) soils at two doses (D1 = agronomic and D2 = double agronomic doses) under two soil moisture regimes (H25% = 25% and H50% = 50% of WHC). Bars indicate the standard deviation of the mean (n = 2).

Table 5.
The formation of metsulfuron-methyl and AMMT from the degradation of iodosulfuron-methyl-sodium applied to agronomic (D1) and double agronomic (D2) doses in unamended (S) and GC-amended (S+GC) soils incubated at moisture levels of 25% (H25%) and 50% (H50%) of soil WHC at the sampling time for maximum amounts determined and at the end of the incubation period.
Both iodosulfuron-methyl and metsulfuron-methyl are degraded to the triazine derivative AMMT by cleavage of the sulfonylurea bridge [32,49,50]. AMMT was detected at lower amounts than metsulfuron-methyl, and the highest amounts of AMMT were detected in S (2.5–4.1%) and S+GC (2.3–3.5%) at the end of the incubation period (Figure 2). Slightly lower amounts of AMMT were formed in S+GC than in S (Table 5), probably due to the slower degradation of iodosulfuron-methyl and metsulfuron-methyl from GC-amended soils and its higher adsorption by S+GC (Table 1). The formation of iodosulfuron-methyl metabolites in unamended soils has been reported in previous studies [32,33,34].
The formation of metsulfuron-methyl and AMMT in sterile soils is presented in Table S6, respectively. The peak amounts of metsulfuron-methyl were formed at 120 days and then decreased up to 16.3 times for all the soil treatments with respect to the non-sterile soils. The maximum amounts of AMMT formed in sterile S and S+GC decreased up to 3.0 times compared to non-sterile soils. These results indicate that the degradation of iodosulfuron-methyl-sodium is due mainly due to biodegradation, as indicated above [18,32].
3.3. Dissipation Mechanism of 14C-iodosulfuron-methyl-sodium in Unamended and Amended Soils
Figure 3 shows the total 14C-iodosulfuron-methyl mass balance (percentage of 14C applied initially; values shown in the graphs are adjusted to 100% over time), including mineralized, extractable, and non-extractable fractions, in both S and S+GC over 165 days, in order to determine the herbicide dissipation mechanism. The total mass balance was ≥100% at least up to 59 days after herbicide application at D1 in S and S+GC. The total 14C mass balance decreased to 76–88% at D1 but remained close to 100% at D2 at the end of the dissipation period. Previous studies have found high mass balances of 14C radioactivity considering mineralization, other volatiles, and non-extractable and extractable residues formed from 14C-iodosulfuron-methyl-sodium in four agricultural soils over 77 days [33,34]. The mass balance results here indicate that the extractable fractions account for the highest amounts through to the end of the dissipation period in all the soil treatments, indicating that herbicide residues and its potential metabolites were available over the incubation period. They did not mineralize or form non-extractable residues as the main dissipation processes.
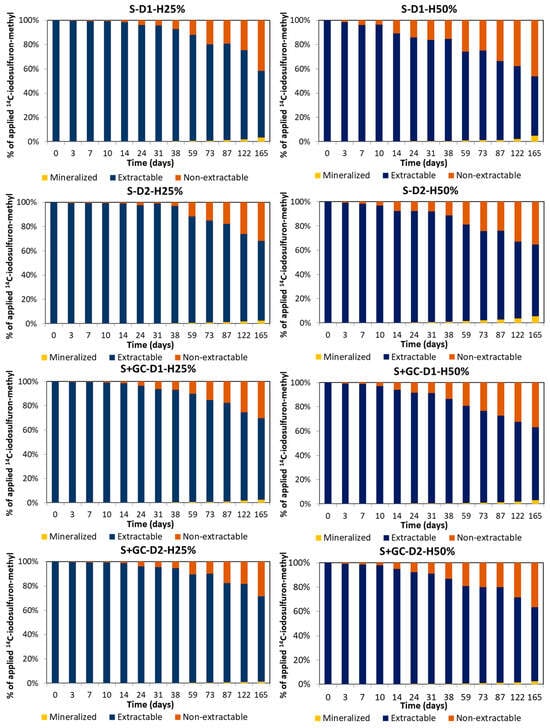
Figure 3.
Total mass balance of 14C-iodosulfuron-methyl-sodium (mineralized, extractable, and non-extractable fractions) applied at two doses (D1 = agronomic and D2 = double agronomic) to unamended (S) and GC-amended (S+GC) soils under two soil moisture regimes (H25% = 25% and H50% = 50% of WHC).
The 14C-herbicide mineralized fraction was low in all cases, but, over time, it was higher in S than in S+GC recording values of 2.6–5.2% and 1.4–2.6%, respectively (Figure 3). These results indicate the influence that OC added with GC to soil has on decreasing herbicide biodegradation. Similar results have been reported for other pesticides, revealing the decrease in mineralization in amended soils [42,51,52,53]. Iodosulfuron-methyl mineralization was low over incubation time because the herbicide is 14C-labelled in the phenyl group, with the aromatic ring less accessible for mineralization by microorganisms. However, according to the EFSA report [18], the mineralization of the phenyl ring of 14C-iodosulfuron-methyl to 14CO2 in unamended soils accounted for 38% of the applied radioactivity after 99 days, which is much higher than the values recorded in this study. The amount of 14CO2 evolved in S treated with D2 and H50% was higher than in S-D1-H50%. In all the other treatments, the percentages of 14CO2 evolved were higher in soils with D1 when compared to soils with D2. The percentages of 14CO2 evolved in soils under H50% were higher than in soils under H25%, indicating that herbicide mineralization was slower under low soil moisture content (Figure 3). Low iodosulfuron-methyl-sodium mineralization has been reported in agricultural soils with a moisture content equivalent to field capacity [33,34].
The 14C extractable fraction varied between 37% and 69% for all the treatments at the end of the incubation period. The amount of extractable 14C residues was up to 1.6 times higher in GC-amended soils than in unamended ones at 165 days (Figure 3), indicating an inverse relationship with the degree of adsorption (Table 1). The higher adsorption of the herbicide and its metabolites by amended soils may explain the increased persistence of these compounds in amended soils [28,54,55]. In all cases, the percentages of 14C extractable were up to 1.5 times higher in soils treated with herbicide at D2 when compared with soils treated with D1. The percentages of 14C extractable in S and S+GC treated with D1 or D2 under H25% were slightly higher than in soils under H50% over time (Figure 3). These results may be due to the slower degradation of iodosulfuron-methyl and its metabolites in soils with a lower moisture content, as observed in previous studies [31,34,43,56]. Seven metabolites were detected from the degradation of 14C-triazine-metsulfuron-methyl in a non-sterile soil after 60 days of incubation [44], indicating that high amounts of the transformation products from iodosulfuron-methyl and metsulfuron-methyl may be present in the extractable fraction throughout the soil incubation period, as they are more persistent than the parent compound (Tables S7 and S8) [16,18,57].
The 14C non-extractable fraction increased over time, recording values of 32–35% in S and 27–37% in S+GC (Figure 3). These percentages were similar to those reported for the formation of non-extractable residues in unamended soils for 14C-iodosulfuron-methyl, which accounted for 27–39% of the applied radioactivity after 99–120 days [18], albeit higher than those reported by Wijntjes et al. [34]. Furthermore, the formation of non-extractable residues of metsulfuron-methyl has been reported in seven soils, with this amount up to 18.2–34.4% of the radioactivity at 90 days [57]. In general, the formation of non-extractable 14C residues was higher in S than in S+GC. The percentages of non-extractable residues in S treated with D1 were slightly higher than in S with D2. However, the amounts of non-extractable residues were lower in S+GC with D1 than in S+GC with D2. The effect of soil moisture was also observed in the formation of non-extractable residues, with this fraction higher in all the soil treatments under H50%. This result may be due to a higher formation and adsorption of iodosulfuron-methyl metabolites by soils with a higher moisture content, while the degradation rate and the formation of metabolites were slower in soils with less moisture, as indicated above.
Overall, the extractable fraction of 14C-[herbicide+metabolites] was lower in S than in S+GC over time. More mineralized and bound fractions were recorded in soils incubated at H50% than in those incubated at H25% for both doses over time. The EFSA report [18] states that the persistence of 14C-iodosulfuron-methyl-sodium under laboratory and aerobic conditions in the dark ranged from very low to moderate. The labelled compound was degraded to at least seven metabolites (>5% of the radioactivity), with metsulfuron-methyl as the main metabolite (up to 88.5% of the radioactivity), and the amount of AMMT formed was up to 40.9% of the radioactivity.
4. Conclusions
This laboratory study has investigated the influence that different treatments of an agricultural soil (organic amendment application, herbicide rate, soil moisture, and microbial activity) have on aminopyralid and iodosulfuron-methyl-sodium dissipation and persistence. The Results and Discussion indicate that the dissipation of these herbicides in unamended and organically amended soils was a function of soil OC, moisture content, microbial activity, and the herbicide rate applied. The most favourable conditions contributing to the acceleration of herbicide dissipation were those when the herbicide was applied at a low dose on unamended soil under higher soil moisture. The greater persistence of herbicides applied at a high rate in soil amended with green compost and under 25% moisture content of WHC may negatively affect rotational crops. This study of herbicide degradation under controlled laboratory conditions has the limitation that the results cannot be fully extrapolated to real field conditions, where other factors, such as soil type, organic amendment characteristics, agronomic and climatic conditions, control the dissipation of these compounds. The applications of new agronomic practises for improving crop production, soil conservation, and climate change, with longer periods of drought, make it increasingly essential to study the fate of herbicides under these new practises in order to predict their persistence in today’s field scenarios. It would therefore be pertinent to study their dissipation under real field conditions, where other processes are involved in their persistence.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15050552/s1, Table S1: Validation parameters for determination of herbicides (aminopyralid and iodosulfuron-methyl-sodium) and metabolites (metsulfuron-methyl and AMMT) from blank soil (S) and soil amended with green compost (S+GC) by UHPLC-QTOF-MS analytical method; Table S2: Chromatograms for aminopyralid in extracts of soil (S) and soil+GC (S+GC); Table S3: Chromatograms for iodosulfuron-methyl-sodium in extracts of soil (S) and soil+GC (S+GC); Table S4: Chromatograms for metsulfuron-methyl in extracts of soil (S) and soil+GC (S+GC); Table S5: Chromatograms for AMMT in extracts of soil (S) and soil+GC (S+GC); Table S6: Formation of metsulfuron-methyl and AMMT from degradation of iodosulfuron-methyl-sodium applied to double agronomic (D2) dose in sterile unamended (SS) and GC-amended (SS+GC) soils incubated at moisture levels of 25% (H25%) and 50% (H50%) of soil WHC at the end of the incubation period; Table S7: Characteristics of iodosulfuron-methyl-sodium and its metabolites formed in soils; and Table S8: Characteristics of metsulfuron-methyl and its metabolites formed in soils. References [16,18,55] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.S.R.-C.; data curation, M.S.R.-C.; formal analysis, J.M.M.-B. and M.S.R.-C.; funding acquisition, M.S.R.-C.; investigation, J.M.M.-B., M.S.A. and M.S.R.-C.; Methodology, J.M.M.-B., M.S.A. and M.S.R.-C.; project administration, M.S.R.-C.; resources, J.M.M.-B., M.J.S.-M. and M.S.R.-C.; software, J.M.M.-B. and M.S.R.-C.; supervision, M.S.R.-C.; validation, J.M.M.-B. and M.S.R.-C.; visualisation, J.M.M.-B., M.S.A. and M.S.R.-C.; writing—original draft, M.J.S.-M. and M.S.R.-C.; writing—review and editing, J.M.M.-B., M.S.A., M.J.S.-M. and M.S.R.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Innovation (MICINN/AEI), grant number PID2020-113379RB-I00/AEI/10.13039/501100011033.
Data Availability Statement
The original contributions presented in this study are included in this article. Dataset is available upon request.
Acknowledgments
We thank Project CLU-2019-05—IRNASA/CSIC Unit of Excellence, funded by the regional government, the Junta of Castilla y León and co-financed by the European Union (ERDF “Europe drives our growth”). The authors thank J.M. Ordax for his technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Popp, J.; Petö, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. OJ L 2009, 309, 71–86. [Google Scholar]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and alternatives of herbicide-based weed management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Mesnage, R.; Székács, A.; Zaller, J.G. Herbicides: Brief history, agricultural use, and potential alternatives for weed control. In Emerging Issues in Analytical Chemistry, Herbicides; Mesnage, R., Zaller, J.G., Eds.; Elsevier in Cooperation with RTI Press: Amsterdam, The Netherlands, 2021; Volume 3, pp. 1–20. [Google Scholar]
- Riemens, M.; Sønderskov, M.; Moonen, A.-C.; Storkey, J.; Kudsk, P. An Integrated Weed Management framework: A pan-European perspective. Eur. J. Agron. 2022, 133, 126443. [Google Scholar] [CrossRef]
- Severo Silva, T.; Arneson, N.J.; Silva, D.V.; Werle, R. Evaluating cover crop tolerance to corn residual herbicides using field-treated soil in greenhouse bioassay. Weed Technol. 2023, 37, 500–511. [Google Scholar] [CrossRef]
- Mikkelson, J.R.; Lym, R.G. Aminopyralid soil residues affect crop rotation in North Dakota soils. Weed Technol. 2011, 25, 422–429. [Google Scholar] [CrossRef]
- Douglas, A. Residual Herbicides—Carryover and Behaviour in Dry Conditions, 7 May 2024. Available online: https://www.agric.wa.gov.au/grains-research-development/residual-herbicides-carryover-and-behaviour-dry-conditions (accessed on 18 November 2024).
- Fast, B.J.; Ferrell, J.A.; MacDonald, G.E.; Sellers, B.A.; MacRae, A.W.; Krutz, L.J.; Kline, W.N. Aminopyralid soil residues affect rotational vegetable crops in Florida. Pest Manag. Sci. 2011, 67, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Paporisch, A.; Laor, Y.; Rubin, B.; Eizenberg, H. Effect of repeated application of sulfonylurea herbicides on sulfosulfuron dissipation rate in soil. Agronomy 2020, 10, 1724. [Google Scholar] [CrossRef]
- Serim, A.T.; Maden, S. Effects of soil residues of sulfosulfuron and mesosulfuron methyl+ iodosulfuron methyl sodium on sunflower varieties. J. Agric. Sci. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Camacho, M.E.; Gannon, T.W.; Ahmed, K.A.; Mulvaney, M.J.; Heitman, J.L.; Amoozegar, A.; Leon, R.G. Evaluation of imazapic and flumioxazin carryover risk for Carinata (Brassica carinata) establishment. Weed Sci. 2022, 70, 503–513. [Google Scholar] [CrossRef]
- Adamson, D.M.; Sbatella, G.M.; Kniss, A.R.; Dayan, F.E. Reduced irrigation impact on soil-applied herbicide dissipation and rotational crop response. Weed Technol. 2024, 38, e11. [Google Scholar] [CrossRef]
- Sparangis, P.; Efthimiadou, A.; Katsenios, N.; Karkanis, A. Control of resistant false cleavers (Galium spurium L.) population to ALS-inhibiting herbicides and its impact on the growth and yield of durum wheat. Agronomy 2023, 13, 1087. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance aminopyralid. EFSA J. 2013, 11, 3352. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance iodosulfuron-methyl-sodium (approved as iodosulfuron). EFSA J. 2016, 14, 4453. [Google Scholar]
- Lei, Q.; Zhong, J.; Chen, S.-F.; Wu, S.; Huang, Y.; Guo, P.; Mishra, S.; Bhatt, K.; Chen, S. Microbial degradation as a powerful weapon in the removal of sulfonylurea herbicides. Environ. Res. 2023, 235, 116570. [Google Scholar] [CrossRef]
- Tomco, P.L.; Duddleston, K.N.; Schultz, E.J.; Hagedorn, B.; Stevenson, T.J.; Seefeldt, S.S. Field degradation of aminopyralid and clopyralid and microbial community response to application in Alaskan soils. Environ. Toxicol. Chem. 2016, 35, 485–493. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef]
- Rolando, C.A.; Scott, M.B.; Baillie, B.R.; Dean, F.; Todoroki, C.L.; Paul, T.S.H. Persistence of triclopyr, dicamba, and picloram in the environment following aerial spraying for control of dense pine invasion. Invasive Plant Sci. Manag. 2023, 16, 177–190. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, H.; Kaur, R.; Singh, K.; Bhullar, M.S. Groundwater monitoring and leaching of sulfonylurea herbicides and transformation products. Microchem. J. 2023, 194, 109273. [Google Scholar] [CrossRef]
- de Jesus, R.A.; Barros, G.P.; Bharagava, R.N.; Liu, J.; Mulla, S.I.; Azevedo, L.C.B.; Ferreira, L.F.R. Occurrence of pesticides in wastewater: Bioremediation approach for environmental safety and its toxicity. In Advances in Chemical Pollution, Environmental Management and Protection; Ferreira, L.F.R., Kumar, A., Bilal, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 9, pp. 17–33. [Google Scholar]
- Yu, C.Y.; Lian, J.L.; Gong, Q.; Ren, L.S.; Huang, Z.; Xu, A.X.; Dong, J.G. Sublethal application of various sulfonylurea and imidazolinone herbicides favors outcrossing and hybrid seed production in oilseed rape. BMC Plant Biol. 2020, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, J.; Dai, X.; Zhao, X.; Qiao, C.; Zhang, X.; Pu, E. Residue determination of triclopyr and aminopyralid in pastures and soil by gas chromatography-electron capture detector: Dissipation pattern under open field conditions. Ecotoxicol. Environ. Saf. 2018, 155, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bork, E.W.; Miller, A.J.; Hall, L.M. Legume re-establishment in northern temperate grasslands following application of aminocyclopyrachlor and aminopyralid. Crop Prot. 2023, 169, 106264. [Google Scholar] [CrossRef]
- García-Miro, A.; Ordax, J.M.; Sánchez-Martín, M.J.; Marín-Benito, J.M.; Rodríguez-Cruz, M.S. Adsorption of ionizable herbicides by agricultural soils without amendment and green compost-amended soils. Rev. Cienc. Agrar. 2022, 45, 614–617. [Google Scholar]
- Pavlidis, G.; Karasali, H.; Tsihrintzis, V.A. Dynamics of changes in the concentrations of herbicides and nutrients in the soils of a combined wheat-poplar tree cultivation: A field experimental model during the growing season. Agrofor. Syst. 2021, 95, 321–338. [Google Scholar] [CrossRef]
- Rouchaud, J.; Moulard, C.; Eelen, H.; Bulcke, R. Persistence of the sulfonylurea herbicide iodosulfuron-methyl in the soil of winter wheat crops. Toxicol. Environ. Chem. 2003, 85, 103–120. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Tang, M.-Z.; Yuan, M.; Xu, Z. Effects of environmental conditions and microbes on degradation of iodosulfuron-methyl-sodium in soil. J. Ecol. Rural Environ. 2006, 22, 76–79. [Google Scholar]
- Kaur, H.; Kaur, P.; Sharma, S.; Bhullar, M.S. Response of soil enzymatic and microbial activities to mixture formulation of mesosulfuron methyl and iodosulfuron methyl and its degradation in soil. Soil Sediment Contam. 2024, 33, 1–22. [Google Scholar] [CrossRef]
- Schaeffer, A.; Wijntjes, C. Changed degradation behavior of pesticides when present in mixtures. Eco-Environ. Health 2022, 1, 23–30. [Google Scholar] [CrossRef]
- Wijntjes, C.; Weber, Y.; Höger, S.; Nguyen, K.T.; Hollert, H.; Schäffer, A. Decelerated degradation of a sulfonylurea herbicide in four fungicide-treated soils. Environ. Sci. Adv. 2022, 1, 70–82. [Google Scholar] [CrossRef]
- Hall, K.E.; Ray, C.; Ki, S.J.; Spokas, K.A.; Koskinen, W.C. Pesticide sorption and leaching potential on three Hawaiian soils. J. Environ. Manag. 2015, 159, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sondhia, S. Persistence of metsulfuron in wheat crop and soil. Environ. Monit. Assess. 2008, 147, 463–469. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis. Part 3: Chemical Methods; SSSA Series; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- OECD. Test No. 307: Aerobic and Anaerobic Transformation in Soil, OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Douibi, M.; Krishtammagari, A.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Mulching vs. organic soil amendment: Effects on adsorption-desorption of herbicides. Sci. Total Environ. 2023, 892, 164749. [Google Scholar] [CrossRef]
- Reid, B.J.; Fervor, T.R.; Semple, K.T. Induction of PAH-catabolism in mushroom compost and its use in the biodegradation of soil-associated phenanthrene. Environ. Pollut. 2002, 118, 65–73. [Google Scholar] [CrossRef] [PubMed]
- FOCUS, Forum for the Co-Ordination of Pesticide Fate Models and Their Use. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration; Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005-v. 2.0; FOCUS, Forum for the Co-Ordination of Pesticide Fate Models and Their Use: 2006. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 1 June 2024).
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of organic residues on pesticide behavior in soils: A review of laboratory research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Barba, V.; Marín-Benito, J.M.; García-Delgado, C.; Sánchez-Martín, M.J.; Rodríguez–Cruz, M.S. Assessment of 14C–prosulfocarb dissipation mechanism in soil after amendment and its impact on the microbial community. Ecotoxicol. Environ. Saf. 2019, 182, 109395. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Igual, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Influence of herbicide triasulfuron on soil microbial community in an unamended soil and a soil amended with organic residues. Front. Microbiol. 2017, 8, 378. [Google Scholar] [CrossRef]
- Pons, N.; Barriuso, E. Fate of metsulfuron-methyl in soils in relation to pedo-climatic conditions. Pestic. Sci. 1998, 53, 311–323. [Google Scholar] [CrossRef]
- Ahmad, R.; James, T.K.; Rahman, A.; Holland, P.T. Dissipation of the herbicide clopyralid in an allophanic soil: Laboratory and field studies. J. Environ. Sci. Health B 2003, 38, 683–695. [Google Scholar] [CrossRef]
- Ismail, B.S.; Eng, O.K.; Tayeb, M.A. Degradation of triazine-2-14C metsulfuron-methyl in soil from an oil palm plantation. PLoS ONE 2015, 10, e138170. [Google Scholar]
- Singh, S.B.; Sharma, R.; Singh, N. Persistence of pyrazosulfuron in rice-field and laboratory soil under Indian tropical conditions. Pest Manag. Sci. 2012, 68, 828–833. [Google Scholar] [CrossRef]
- Gennari, M.; Abbate, C.; Baglieri, A.; Nègre, M. Fate and degradation of triasulfuron in soil and water under laboratory conditions. J. Environ. Sci. Health B 2008, 43, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; D’Agostina, A.; Arnoldi, A.; Marotta, E.; Zanardini, E.; Negri, M.; Valle, A.; Sorlini, C. Biodegradation of chlorsulfuron and metsulfuron-methyl by Aspergillus niger in laboratory conditions. J. Environ. Sci. Health B 2003, 38, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J. Agric. Food Chem. 2012, 60, 6936–6945. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Pose-Juan, E.; Rodríguez-Cruz, M.S. Effect of different rates of spent mushroom substrate on the dissipation and bioavailability of cymoxanil and tebuconazole in an agricultural soil. Sci. Total Environ. 2016, 550, 495–503. [Google Scholar] [CrossRef]
- Obregón Alvarez, D.; Mendes, K.F.; Tosi, M.; Fonseca de Souza, L.; Campos Cedano, J.C.; de Souza Falcão, N.P.; Dunfield, K.; Tsai, S.M.; Tornisielo, V.L. Sorption-desorption and biodegradation of sulfometuron-methyl and its effects on the bacterial communities in Amazonian soils amended with aged biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111222. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.B.; Raunaq; Das, T.K. Effect of fly ash on persistence, mobility and bio-efficacy of metribuzin and metsulfuron-methyl in crop fields. Ecotoxicol. Environ. Saf. 2013, 97, 236–241. [Google Scholar] [CrossRef]
- Ahmad, K.S. Adsorption evaluation of herbicide iodosulfuron followed by Cedrus deodora sawdust-derived activated carbon removal. Soil Sediment Contam. 2019, 28, 65–80. [Google Scholar] [CrossRef]
- Grey, T.L.; McCullough, P.E. Sulfonylurea herbicides’ fate in soil: Dissipation, mobility, and other processes. Weed Technol. 2012, 26, 579–581. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance metsulfuron-methyl. EFSA J. 2015, 13, 3936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).