Dissipation of Two Acidic Herbicides in Agricultural Soil: Impact of Green Compost Application, Herbicide Rate, and Soil Moisture
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbicides and Reagents
2.2. Experimental Setup
2.3. Herbicide Extraction
2.4. Herbicide Analysis
2.5. Dissipation Mechanism and Analysis of 14C Labelled Iodosulfuron-methyl-sodium
2.6. Data Analysis
3. Results and Discussion
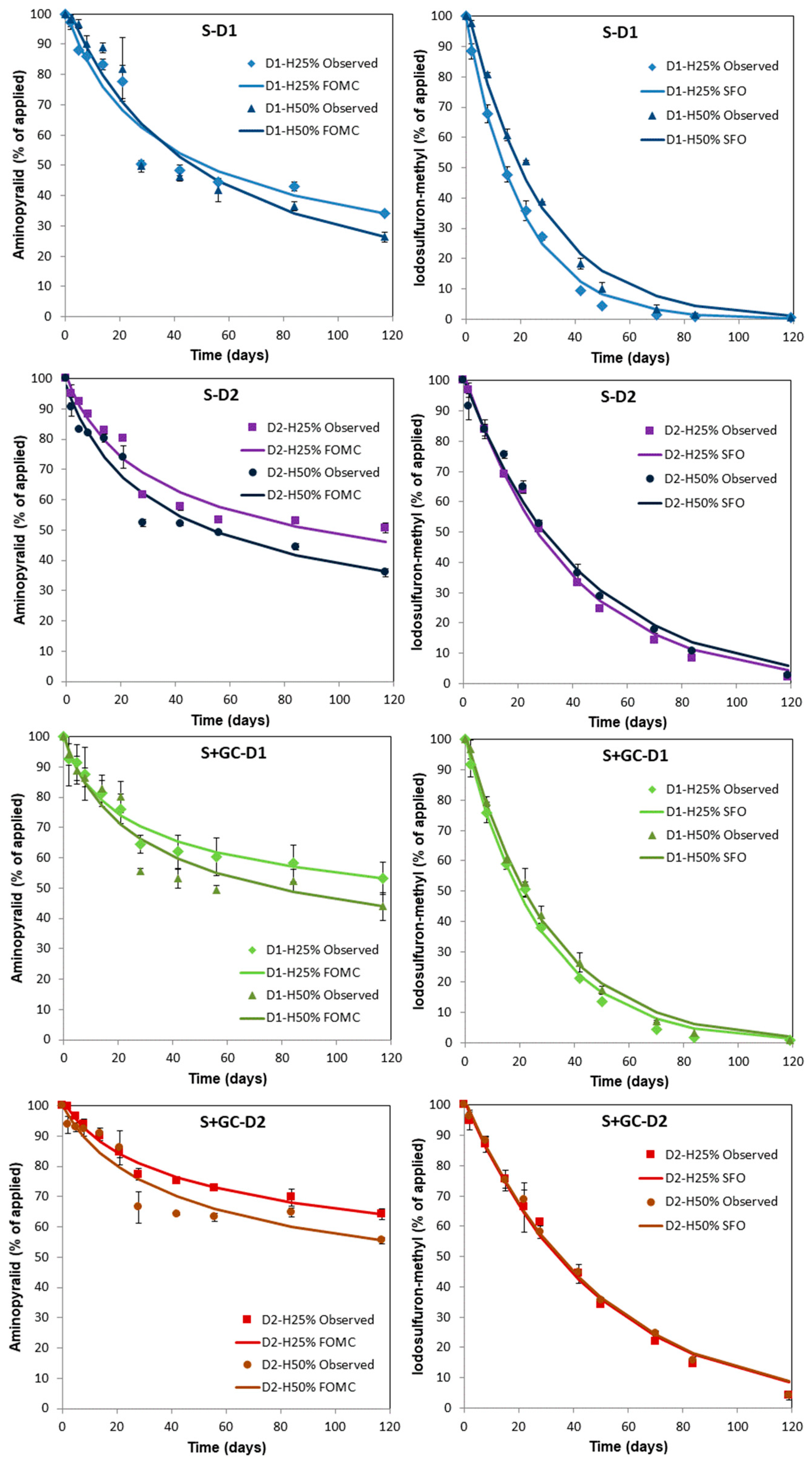
3.1. Degradation of Aminopyralid and Iodosulfuron-methyl-sodium in Unamended and Amended Soils
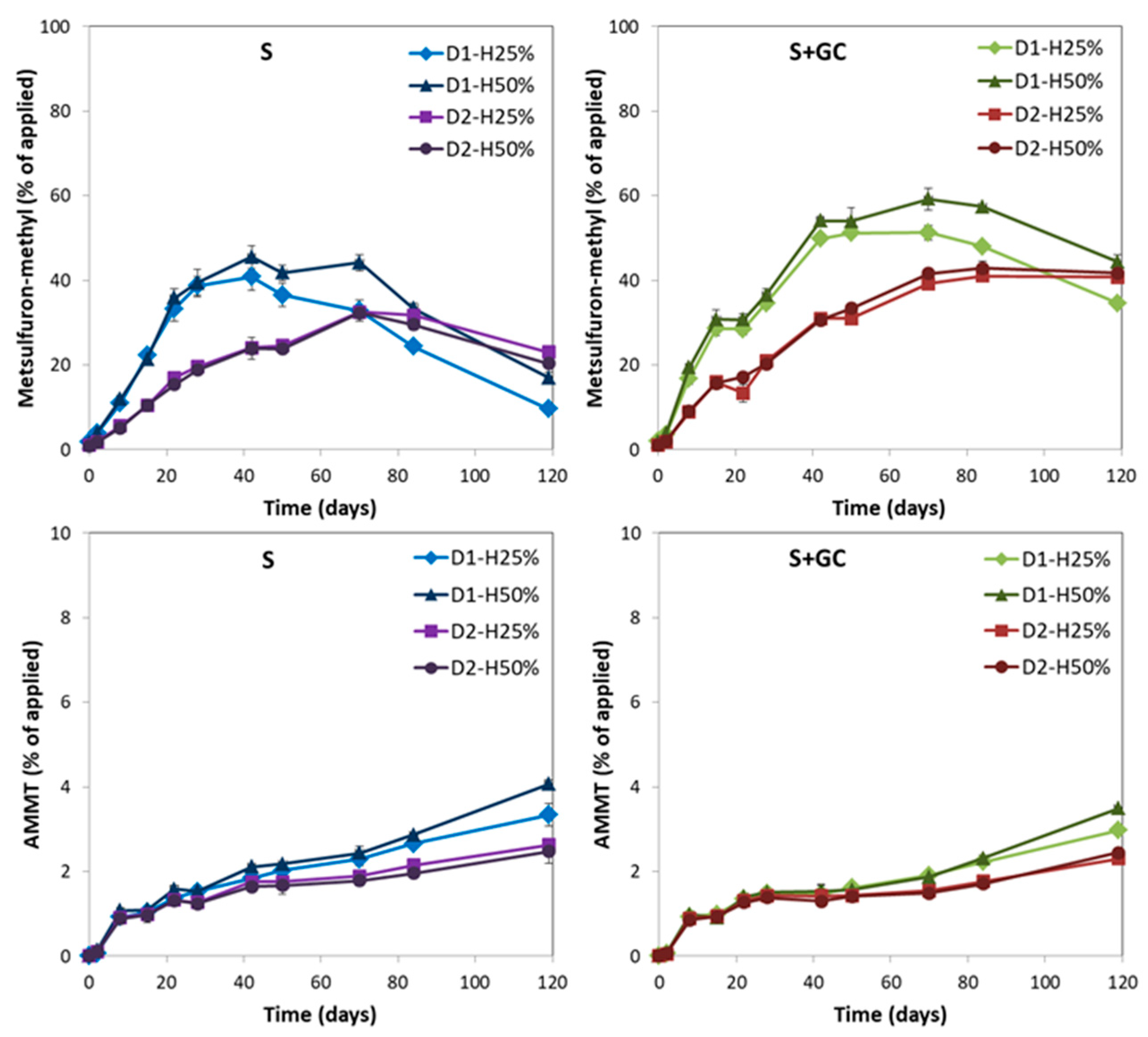
3.2. Formation of Metsulfuron-methyl and AMMT Metabolites in Unamended and Amended Soils
3.3. Dissipation Mechanism of 14C-iodosulfuron-methyl-sodium in Unamended and Amended Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popp, J.; Petö, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. OJ L 2009, 309, 71–86. [Google Scholar]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and alternatives of herbicide-based weed management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Mesnage, R.; Székács, A.; Zaller, J.G. Herbicides: Brief history, agricultural use, and potential alternatives for weed control. In Emerging Issues in Analytical Chemistry, Herbicides; Mesnage, R., Zaller, J.G., Eds.; Elsevier in Cooperation with RTI Press: Amsterdam, The Netherlands, 2021; Volume 3, pp. 1–20. [Google Scholar]
- Riemens, M.; Sønderskov, M.; Moonen, A.-C.; Storkey, J.; Kudsk, P. An Integrated Weed Management framework: A pan-European perspective. Eur. J. Agron. 2022, 133, 126443. [Google Scholar] [CrossRef]
- Severo Silva, T.; Arneson, N.J.; Silva, D.V.; Werle, R. Evaluating cover crop tolerance to corn residual herbicides using field-treated soil in greenhouse bioassay. Weed Technol. 2023, 37, 500–511. [Google Scholar] [CrossRef]
- Mikkelson, J.R.; Lym, R.G. Aminopyralid soil residues affect crop rotation in North Dakota soils. Weed Technol. 2011, 25, 422–429. [Google Scholar] [CrossRef]
- Douglas, A. Residual Herbicides—Carryover and Behaviour in Dry Conditions, 7 May 2024. Available online: https://www.agric.wa.gov.au/grains-research-development/residual-herbicides-carryover-and-behaviour-dry-conditions (accessed on 18 November 2024).
- Fast, B.J.; Ferrell, J.A.; MacDonald, G.E.; Sellers, B.A.; MacRae, A.W.; Krutz, L.J.; Kline, W.N. Aminopyralid soil residues affect rotational vegetable crops in Florida. Pest Manag. Sci. 2011, 67, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Paporisch, A.; Laor, Y.; Rubin, B.; Eizenberg, H. Effect of repeated application of sulfonylurea herbicides on sulfosulfuron dissipation rate in soil. Agronomy 2020, 10, 1724. [Google Scholar] [CrossRef]
- Serim, A.T.; Maden, S. Effects of soil residues of sulfosulfuron and mesosulfuron methyl+ iodosulfuron methyl sodium on sunflower varieties. J. Agric. Sci. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Camacho, M.E.; Gannon, T.W.; Ahmed, K.A.; Mulvaney, M.J.; Heitman, J.L.; Amoozegar, A.; Leon, R.G. Evaluation of imazapic and flumioxazin carryover risk for Carinata (Brassica carinata) establishment. Weed Sci. 2022, 70, 503–513. [Google Scholar] [CrossRef]
- Adamson, D.M.; Sbatella, G.M.; Kniss, A.R.; Dayan, F.E. Reduced irrigation impact on soil-applied herbicide dissipation and rotational crop response. Weed Technol. 2024, 38, e11. [Google Scholar] [CrossRef]
- Sparangis, P.; Efthimiadou, A.; Katsenios, N.; Karkanis, A. Control of resistant false cleavers (Galium spurium L.) population to ALS-inhibiting herbicides and its impact on the growth and yield of durum wheat. Agronomy 2023, 13, 1087. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance aminopyralid. EFSA J. 2013, 11, 3352. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance iodosulfuron-methyl-sodium (approved as iodosulfuron). EFSA J. 2016, 14, 4453. [Google Scholar]
- Lei, Q.; Zhong, J.; Chen, S.-F.; Wu, S.; Huang, Y.; Guo, P.; Mishra, S.; Bhatt, K.; Chen, S. Microbial degradation as a powerful weapon in the removal of sulfonylurea herbicides. Environ. Res. 2023, 235, 116570. [Google Scholar] [CrossRef]
- Tomco, P.L.; Duddleston, K.N.; Schultz, E.J.; Hagedorn, B.; Stevenson, T.J.; Seefeldt, S.S. Field degradation of aminopyralid and clopyralid and microbial community response to application in Alaskan soils. Environ. Toxicol. Chem. 2016, 35, 485–493. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef]
- Rolando, C.A.; Scott, M.B.; Baillie, B.R.; Dean, F.; Todoroki, C.L.; Paul, T.S.H. Persistence of triclopyr, dicamba, and picloram in the environment following aerial spraying for control of dense pine invasion. Invasive Plant Sci. Manag. 2023, 16, 177–190. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, H.; Kaur, R.; Singh, K.; Bhullar, M.S. Groundwater monitoring and leaching of sulfonylurea herbicides and transformation products. Microchem. J. 2023, 194, 109273. [Google Scholar] [CrossRef]
- de Jesus, R.A.; Barros, G.P.; Bharagava, R.N.; Liu, J.; Mulla, S.I.; Azevedo, L.C.B.; Ferreira, L.F.R. Occurrence of pesticides in wastewater: Bioremediation approach for environmental safety and its toxicity. In Advances in Chemical Pollution, Environmental Management and Protection; Ferreira, L.F.R., Kumar, A., Bilal, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 9, pp. 17–33. [Google Scholar]
- Yu, C.Y.; Lian, J.L.; Gong, Q.; Ren, L.S.; Huang, Z.; Xu, A.X.; Dong, J.G. Sublethal application of various sulfonylurea and imidazolinone herbicides favors outcrossing and hybrid seed production in oilseed rape. BMC Plant Biol. 2020, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, J.; Dai, X.; Zhao, X.; Qiao, C.; Zhang, X.; Pu, E. Residue determination of triclopyr and aminopyralid in pastures and soil by gas chromatography-electron capture detector: Dissipation pattern under open field conditions. Ecotoxicol. Environ. Saf. 2018, 155, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bork, E.W.; Miller, A.J.; Hall, L.M. Legume re-establishment in northern temperate grasslands following application of aminocyclopyrachlor and aminopyralid. Crop Prot. 2023, 169, 106264. [Google Scholar] [CrossRef]
- García-Miro, A.; Ordax, J.M.; Sánchez-Martín, M.J.; Marín-Benito, J.M.; Rodríguez-Cruz, M.S. Adsorption of ionizable herbicides by agricultural soils without amendment and green compost-amended soils. Rev. Cienc. Agrar. 2022, 45, 614–617. [Google Scholar]
- Pavlidis, G.; Karasali, H.; Tsihrintzis, V.A. Dynamics of changes in the concentrations of herbicides and nutrients in the soils of a combined wheat-poplar tree cultivation: A field experimental model during the growing season. Agrofor. Syst. 2021, 95, 321–338. [Google Scholar] [CrossRef]
- Rouchaud, J.; Moulard, C.; Eelen, H.; Bulcke, R. Persistence of the sulfonylurea herbicide iodosulfuron-methyl in the soil of winter wheat crops. Toxicol. Environ. Chem. 2003, 85, 103–120. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Tang, M.-Z.; Yuan, M.; Xu, Z. Effects of environmental conditions and microbes on degradation of iodosulfuron-methyl-sodium in soil. J. Ecol. Rural Environ. 2006, 22, 76–79. [Google Scholar]
- Kaur, H.; Kaur, P.; Sharma, S.; Bhullar, M.S. Response of soil enzymatic and microbial activities to mixture formulation of mesosulfuron methyl and iodosulfuron methyl and its degradation in soil. Soil Sediment Contam. 2024, 33, 1–22. [Google Scholar] [CrossRef]
- Schaeffer, A.; Wijntjes, C. Changed degradation behavior of pesticides when present in mixtures. Eco-Environ. Health 2022, 1, 23–30. [Google Scholar] [CrossRef]
- Wijntjes, C.; Weber, Y.; Höger, S.; Nguyen, K.T.; Hollert, H.; Schäffer, A. Decelerated degradation of a sulfonylurea herbicide in four fungicide-treated soils. Environ. Sci. Adv. 2022, 1, 70–82. [Google Scholar] [CrossRef]
- Hall, K.E.; Ray, C.; Ki, S.J.; Spokas, K.A.; Koskinen, W.C. Pesticide sorption and leaching potential on three Hawaiian soils. J. Environ. Manag. 2015, 159, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sondhia, S. Persistence of metsulfuron in wheat crop and soil. Environ. Monit. Assess. 2008, 147, 463–469. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis. Part 3: Chemical Methods; SSSA Series; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- OECD. Test No. 307: Aerobic and Anaerobic Transformation in Soil, OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Douibi, M.; Krishtammagari, A.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Mulching vs. organic soil amendment: Effects on adsorption-desorption of herbicides. Sci. Total Environ. 2023, 892, 164749. [Google Scholar] [CrossRef]
- Reid, B.J.; Fervor, T.R.; Semple, K.T. Induction of PAH-catabolism in mushroom compost and its use in the biodegradation of soil-associated phenanthrene. Environ. Pollut. 2002, 118, 65–73. [Google Scholar] [CrossRef] [PubMed]
- FOCUS, Forum for the Co-Ordination of Pesticide Fate Models and Their Use. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration; Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005-v. 2.0; FOCUS, Forum for the Co-Ordination of Pesticide Fate Models and Their Use: 2006. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 1 June 2024).
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of organic residues on pesticide behavior in soils: A review of laboratory research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Barba, V.; Marín-Benito, J.M.; García-Delgado, C.; Sánchez-Martín, M.J.; Rodríguez–Cruz, M.S. Assessment of 14C–prosulfocarb dissipation mechanism in soil after amendment and its impact on the microbial community. Ecotoxicol. Environ. Saf. 2019, 182, 109395. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Igual, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Influence of herbicide triasulfuron on soil microbial community in an unamended soil and a soil amended with organic residues. Front. Microbiol. 2017, 8, 378. [Google Scholar] [CrossRef]
- Pons, N.; Barriuso, E. Fate of metsulfuron-methyl in soils in relation to pedo-climatic conditions. Pestic. Sci. 1998, 53, 311–323. [Google Scholar] [CrossRef]
- Ahmad, R.; James, T.K.; Rahman, A.; Holland, P.T. Dissipation of the herbicide clopyralid in an allophanic soil: Laboratory and field studies. J. Environ. Sci. Health B 2003, 38, 683–695. [Google Scholar] [CrossRef]
- Ismail, B.S.; Eng, O.K.; Tayeb, M.A. Degradation of triazine-2-14C metsulfuron-methyl in soil from an oil palm plantation. PLoS ONE 2015, 10, e138170. [Google Scholar]
- Singh, S.B.; Sharma, R.; Singh, N. Persistence of pyrazosulfuron in rice-field and laboratory soil under Indian tropical conditions. Pest Manag. Sci. 2012, 68, 828–833. [Google Scholar] [CrossRef]
- Gennari, M.; Abbate, C.; Baglieri, A.; Nègre, M. Fate and degradation of triasulfuron in soil and water under laboratory conditions. J. Environ. Sci. Health B 2008, 43, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; D’Agostina, A.; Arnoldi, A.; Marotta, E.; Zanardini, E.; Negri, M.; Valle, A.; Sorlini, C. Biodegradation of chlorsulfuron and metsulfuron-methyl by Aspergillus niger in laboratory conditions. J. Environ. Sci. Health B 2003, 38, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J. Agric. Food Chem. 2012, 60, 6936–6945. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Pose-Juan, E.; Rodríguez-Cruz, M.S. Effect of different rates of spent mushroom substrate on the dissipation and bioavailability of cymoxanil and tebuconazole in an agricultural soil. Sci. Total Environ. 2016, 550, 495–503. [Google Scholar] [CrossRef]
- Obregón Alvarez, D.; Mendes, K.F.; Tosi, M.; Fonseca de Souza, L.; Campos Cedano, J.C.; de Souza Falcão, N.P.; Dunfield, K.; Tsai, S.M.; Tornisielo, V.L. Sorption-desorption and biodegradation of sulfometuron-methyl and its effects on the bacterial communities in Amazonian soils amended with aged biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111222. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.B.; Raunaq; Das, T.K. Effect of fly ash on persistence, mobility and bio-efficacy of metribuzin and metsulfuron-methyl in crop fields. Ecotoxicol. Environ. Saf. 2013, 97, 236–241. [Google Scholar] [CrossRef]
- Ahmad, K.S. Adsorption evaluation of herbicide iodosulfuron followed by Cedrus deodora sawdust-derived activated carbon removal. Soil Sediment Contam. 2019, 28, 65–80. [Google Scholar] [CrossRef]
- Grey, T.L.; McCullough, P.E. Sulfonylurea herbicides’ fate in soil: Dissipation, mobility, and other processes. Weed Technol. 2012, 26, 579–581. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance metsulfuron-methyl. EFSA J. 2015, 13, 3936. [Google Scholar] [CrossRef]

| Aminopyralid | Iodosulfuron- methyl-sodium | Metsulfuron-methyl (Metabolite) | AMMT (Metabolite) | |
|---|---|---|---|---|
| Chemical structure |  | 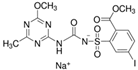 | 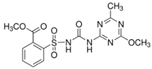 | 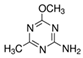 |
| Molecular mass | 207.03 | 529.24 | 381.36 | 140.15 |
| Dissociation constant (pKa), 25 °C | 2.56 | 3.22 | 3.75 | - |
| Solubility—In water, pH 7, 20 °C (mg L−1) | 2480 | 25,000 | 2800 | - |
| Octanol–water partition coefficient (log Kow), pH 7, 20 °C | −2.7 | −0.7 | −1.9 | 0.52 |
| DT50 in lab, 20 °C (days) | 55.5 | 2.7 | 23.2 | 144 |
| DT50 in field (days) | 12.1 | 3.2 | 13.3 | - |
| Vapour pressure, 20 °C (mPa) | 2.59 × 10−5 | 2.6 × 10−6 | 1.0 × 10−6 | - |
| GUS leaching potential index 1 | 3.34 | 1.19 | 3.28 | 5.04 |
| Kd (mL g−1) | 11.2 (GC) 0.72 (S) 3.43 (S+GC) | 3.39 (GC) 0.19 (S) 0.27 (S+GC) | 93.8 (GC) 0.12 (S) 1.09 (S+GC) | 7.11 (GC) 0.21 (S) 0.80 (S+GC) |
| pH | EC 1 (dS/m) | OC 2 (%) | DOC 3 (%) | N (%) | C/N | |
|---|---|---|---|---|---|---|
| GC | 7.8 | 0.33 | 19.1 | 0.703 | 2.100 | 11.4 |
| S | 6.2 | 0.10 | 1.12 | 0.003 | 0.126 | 8.9 |
| S+GC | 6.3 | 0.29 | 1.37 | 0.007 | 0.162 | 8.5 |
| M0 (%) | α/β k (d−1) | DT50 (d) | DT90 (d) | ꭓ2 | r2 | Model | |
|---|---|---|---|---|---|---|---|
| S-D1-H25% | 101.94 | 0.583/21.3 | 48.6 ± 0.6 de | 1086 | 7.1 | 0.931 | FOMC |
| S-D1-H50% | 104.50 | 1.229/56.6 | 42.8 ± 0.1 e | 312 | 8.3 | 0.924 | FOMC |
| S-D2-H25% | 101.13 | 0.356/14.5 | 86.8 ± 0.6 c | 9267 | 4.6 | 0.948 | FOMC |
| S-D2-H50% | 97.70 | 0.507/19.2 | 56.3 ± 0.3 de | 1790 | 5.5 | 0.950 | FOMC |
| S+GC-D1-H25% | 100.32 | 0.225/7.3 | 153 ± 5.4 b | 2 × 105 | 3.2 | 0.897 | FOMC |
| S+GC-D1-H50% | 100.79 | 0.353/12.3 | 75.3 ± 1.6 cd | 8308 | 6.6 | 0.896 | FOMC |
| S+GC-D2-H25% | 101.81 | 0.204/13.6 | 395 ± 18 a | 1 × 106 | 1.6 | 0.976 | FOMC |
| S+GC-D2-H50% | 100.16 | 0.286/17.0 | 175 ± 3.1 b | 5 × 104 | 5.2 | 0.882 | FOMC |
| SS-D2-H25% | 96.09 | 9.0 × 10−5 | 8044 ± 184 | 3 × 104 | 2.7 | 0.092 | SFO |
| SS-D2-H50% | 97.47 | 4.0 × 10−4 | 1781 ± 97.1 | 5918 | 2.5 | 0.416 | SFO |
| SS+GC-D2-H25% | 100.01 | 0.020/0.009 | 4 × 1011 ± 1 × 103 | 3 × 106 | 3.5 | 0.817 | FOMC |
| SS+GC-D2-H50% | 97.59 | 4.0 × 10−5 | 2 × 104 ± 200 | 6 × 104 | 3.1 | 0.037 | SFO |
| M0 (%) | k (d−1) | DT50 (d) | DT90 (d) | ꭓ2 | r2 | Model | |
|---|---|---|---|---|---|---|---|
| S-D1-H25% | 99.63 | 0.050 | 14.0 ± 0.6 e | 46.5 | 4.5 | 0.996 | SFO |
| S-D1-H50% | 104.83 | 0.038 | 18.5 ± 0.7 d | 61.4 | 7.1 | 0.990 | SFO |
| S-D2-H25% | 102.80 | 0.026 | 26.2 ± 0.1 c | 87.0 | 4.5 | 0.993 | SFO |
| S-D2-H50% | 100.83 | 0.024 | 29.2 ± 1.6 bc | 97.0 | 4.8 | 0.990 | SFO |
| S+GC-D1-H25% | 100.6 | 0.036 | 19.2 ± 0.8 d | 63.8 | 4.7 | 0.994 | SFO |
| S+GC-D1-H50% | 102.34 | 0.033 | 20.9 ± 1.6 d | 69.5 | 3.9 | 0.995 | SFO |
| S+GC-D2-H25% | 101.64 | 0.021 | 33.2 ± 1.6 ab | 110 | 3.9 | 0.989 | SFO |
| S+GC-D2-H50% | 102.08 | 0.021 | 33.8 ± 0.5 a | 112 | 3.4 | 0.993 | SFO |
| SS-D2-H25% | 102.54 | 0.007 | 95.9 ± 3.1 | 319 | 8.0 | 0.854 | SFO |
| SS-D2-H50% | 103.86 | 0.011 | 64.7 ± 2.0 | 215 | 6.8 | 0.945 | SFO |
| SS+GC-D2-H25% | 104.34 | 0.005 | 127 ± 4.8 | 423 | 8.9 | 0.742 | SFO |
| SS+GC-D2-H50% | 107.50 | 0.007 | 99.8 ± 2.7 | 332 | 9.4 | 0.803 | SFO |
| Time (d) | % Metabolite Max. | Time (d) | % Metabolite | |
|---|---|---|---|---|
| Metsulfuron-methyl | ||||
| S-D1-H25% | 42 | 40.8 ± 3.3 c | 120 | 9.6 ± 0.5 e |
| S-D1-H50% | 42 | 45.6 ± 2.7 c | 120 | 17.0 ± 0.3 d |
| S-D2-H25% | 70 | 32.5 ± 1.0 d | 120 | 23.0 ± 1.4 c |
| S-D2-H50% | 70 | 32.3 ± 0.4 d | 120 | 20.3 ± 2.0 c |
| S+GC-D1-H25% | 70 | 51.2 ± 1.2 b | 120 | 34.7 ± 0.4 b |
| S+GC-D1-H50% | 70 | 59.2 ± 2.6 a | 120 | 44.4 ± 1.8 a |
| S+GC-D2-H25% | 84 | 41.0 ± 0.4 c | 120 | 40.8 ± 0.4 a |
| S+GC-D2-H50% | 84 | 42.8 ± 1.6 c | 120 | 41.7 ± 1.8 a |
| AMMT | ||||
| S-D1-H25% | 120 | 3.3 ± 0.3 c | 120 | 3.3 ± 0.3 c |
| S-D1-H50% | 120 | 4.1 ± 0.1 a | 120 | 4.1 ± 0.1 a |
| S-D2-H25% | 120 | 2.6 ± 0.1 d | 120 | 2.6 ± 0.1 d |
| S-D2-H50% | 120 | 2.5 ± 0.3 d | 120 | 2.5 ± 0.3 d |
| S+GC-D1-H25% | 120 | 3.0 ± 0.0 c | 120 | 3.0 ± 0.0 c |
| S+GC-D1-H50% | 120 | 3.5 ± 0.0 b | 120 | 3.5 ± 0.0 b |
| S+GC-D2-H25% | 120 | 2.3 ± 0.0 d | 120 | 2.3 ± 0.0 d |
| S+GC-D2-H50% | 120 | 2.4 ± 0.1 d | 120 | 2.4 ± 0.1 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of Two Acidic Herbicides in Agricultural Soil: Impact of Green Compost Application, Herbicide Rate, and Soil Moisture. Agriculture 2025, 15, 552. https://doi.org/10.3390/agriculture15050552
Marín-Benito JM, Andrades MS, Sánchez-Martín MJ, Rodríguez-Cruz MS. Dissipation of Two Acidic Herbicides in Agricultural Soil: Impact of Green Compost Application, Herbicide Rate, and Soil Moisture. Agriculture. 2025; 15(5):552. https://doi.org/10.3390/agriculture15050552
Chicago/Turabian StyleMarín-Benito, Jesús M., María Soledad Andrades, María J. Sánchez-Martín, and María Sonia Rodríguez-Cruz. 2025. "Dissipation of Two Acidic Herbicides in Agricultural Soil: Impact of Green Compost Application, Herbicide Rate, and Soil Moisture" Agriculture 15, no. 5: 552. https://doi.org/10.3390/agriculture15050552
APA StyleMarín-Benito, J. M., Andrades, M. S., Sánchez-Martín, M. J., & Rodríguez-Cruz, M. S. (2025). Dissipation of Two Acidic Herbicides in Agricultural Soil: Impact of Green Compost Application, Herbicide Rate, and Soil Moisture. Agriculture, 15(5), 552. https://doi.org/10.3390/agriculture15050552








