Effects of Sewage Sludge Compost and Vermicompost on Wheat Yield and Vitality
Abstract
1. Introduction
2. Materials and Methods
2.1. Composts and Vermicomposts Used
2.2. Temperature and Rainfall
2.3. Sampling and Sample Preparation
2.4. Physical, Chemical, and Biological Analyses
2.5. Analysis of Physiological Parameters
2.6. Statistical Analyses
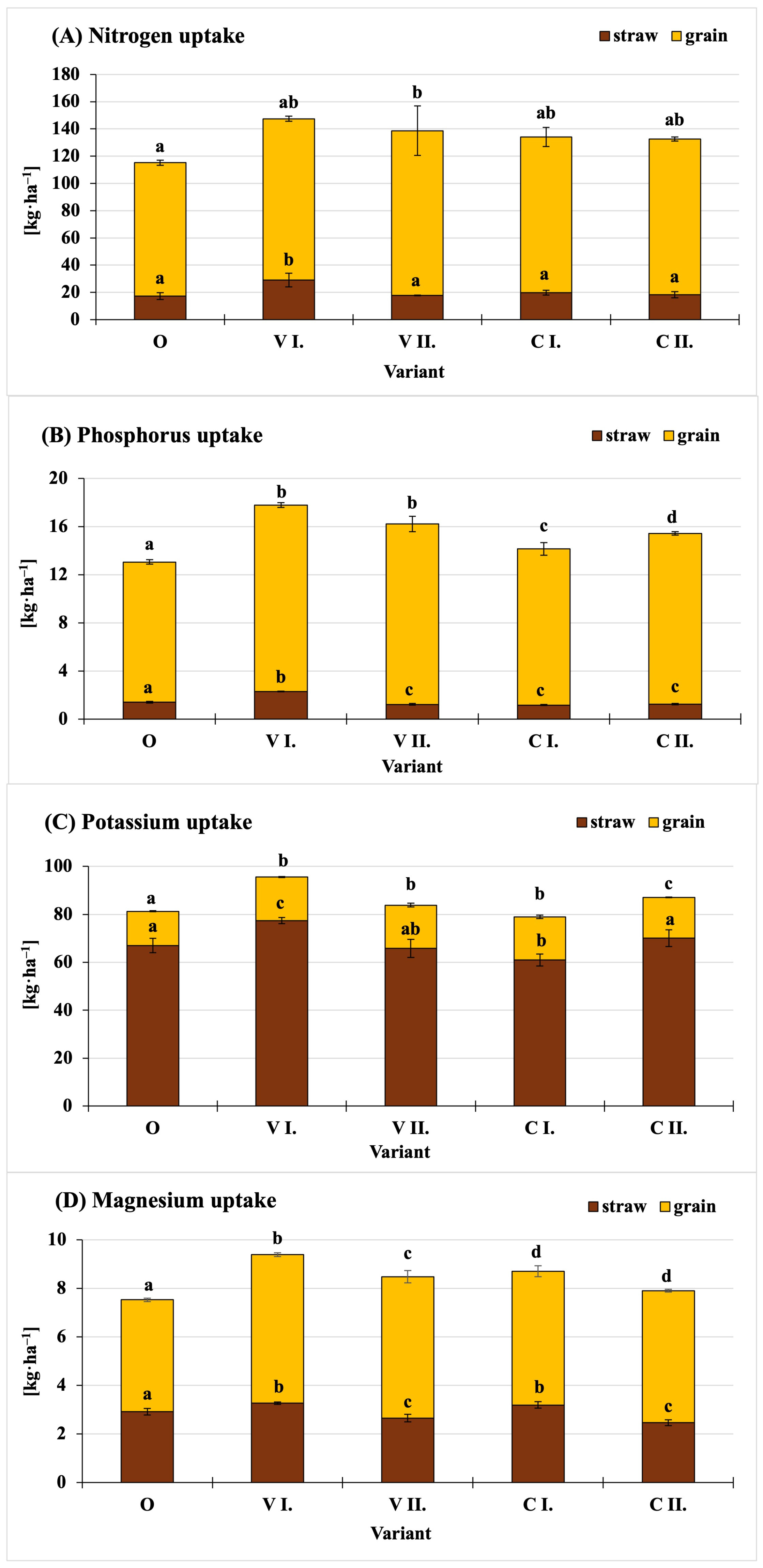
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPEJ | Soil Ecological Evaluation Units |
| WWTP | wastewater treatment plant |
| EC | electrical conductivity |
| NGE | number of grains per ear |
| TGW | Thousand-grain weight |
| WUEi | water use efficiency (internal) |
| ABA | abscisic acid |
| WP | water potential |
| SD | standard deviation |
| TCB | total coliform bacteria |
References
- Munroe, G. Manual of On-Farm Vermicomposting and Vermiculture; Organic Agriculture Centre of Canada: Truro, NS, Canada, 2007; pp. 39–40. [Google Scholar]
- Addison, K. Vermicomposting. Available online: http://journeytoforever.org/compost_worm.html (accessed on 27 August 2014).
- Edwards, C.A.; Arancon, N.Q.; Sherman, R.L. Vermiculture Technology: Earthworms, Organic Wastes, and Environmental Management; CRC Press: Boca Raton, FL, USA, 2010; 578p. [Google Scholar]
- Tu, J.C.; Zhao, Q.J.; Wei, L.L.; Yang, Q.Q. Heavy metal concentration and speciation of seven representative municipal sludges from wastewater treatment plants in Northeast China. Environ. Monit. Assess. 2012, 184, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Latare, A.M.; Kumar, O.; Singh, S.K.; Gupta, A. Direct and residual effect of sewage sludge on yield, heavy metals content and soil fertility under rice-wheat system. Ecol. Eng. 2014, 69, 17–24. [Google Scholar] [CrossRef]
- Bourioug, M.; Alaoui-Sossé, L.; Laffray, X.; Raouf, N.; Benbrahim, M.; Badot, P.M.; Alaoui-Sossé, B. Evaluation of sewage sludge effects on soil properties, plant growth, mineral nutrition state, and heavy metal distribution in European larch seedlings (Larix decidua). Arab. J. Sci. Eng. 2014, 39, 5325–5335. [Google Scholar] [CrossRef]
- Umaru, A.; Ehiomogue, P.; Ojedele, S.; Orji, F.; Okosa, I.; Ikechukwu-Edeh, C. Nutrient release patterns from compost, vermicomposting, and long-term effect on soil fertility status. Poljoprivredna Tehnika 2019, 44, 50–59. [Google Scholar] [CrossRef]
- Dume, B.; Hanč, A.; Švehla, P.; Michal, P.; Chane, A.D. Vermicomposting Technology as a Process Able to Reduce the Content of Potentially Toxic Elements in Sewage Sludge. Agronomy 2022, 12, 2049. [Google Scholar] [CrossRef]
- Didone, M.; Tosello, G. Moulded pulp products manufacturing with thermoforming. Packag. Technol. Sci. 2018, 32, 7–22. [Google Scholar] [CrossRef]
- Li, X.; Xing, M.; Yang, J.; Huang, Z. Compositional and functional features of humic acid-like fractions from vermicomposting of sewage sludge and cow dung. J. Hazard. Mater. 2011, 185, 740–748. [Google Scholar] [CrossRef]
- Adhikary, S. Vermicompost, the story of organic gold. A review. Agric. Sci. 2012, 13, 905–917. [Google Scholar] [CrossRef]
- El Jawaher, A. Research Article Vermicomposting of Organic Waste with Eisenia fetida Increases the Content of Exchangeable Nutrients in Soil. Pak. J. Biol. Sci. 2020, 23, 501–509. [Google Scholar] [CrossRef][Green Version]
- Peyvast, G.H.; Olfati, J.A.; Madeni, S.; Forghani, A. Effect of vermicompost on the growth and yield of spinach (Spinacia oleracea L.). J. Food Agric. Environ. 2008, 6, 110–113. [Google Scholar]
- Wang, D.; Shi, Q.; Wang, X.; Wei, M.; Hu, J.; Liu, J.; Yang, F. Influence of cow manure vermicompost on the growth, metabolite contents, and antioxidant activities of Chinese cabbage (Brassica campestris ssp. chinensis). Biol. Fertil. Soils 2010, 46, 689–696. [Google Scholar] [CrossRef]
- Lazcano, C.; Domínguez, J. The use of vermicompost in sustainable agriculture: Impact on plant growth and soil fertility. Soil Nutrients 2011, 10, 187. [Google Scholar]
- Rocha, J.F.; Kusdra, J.F.; Moreno, A.D.L.; Picazevicz, A.A.C. Growth and production of lettuce on substrates based on detritivorous earthworms drilocomposts. Comunicata Scientiae 2022, 13, e3646. [Google Scholar] [CrossRef]
- Isea-León, F.; Acosta-Balbás, V.; Rial-Betancoutd, L.B.; Medina-Gallardo, A.L.; Célestin, B.M. Evaluation of the Fatty Acid Composition of Earthworm Eisenia andrei Meal as an Alternative Lipid Source for Fish Feed. J. Food Nutr. Res. 2019, 7, 696–700. [Google Scholar] [CrossRef]
- Sosnecka, A.; Kacprzak, M.; Rorat, A. Vermicomposting as an alternative way of biodegradable waste management for small municipalities. J. Ecol. Eng. 2016, 17, 91–96. [Google Scholar] [CrossRef]
- Dvořák, J.; Mančíková, V.; Pižl, V.; Elhottová, D.; Šilerová, M.; Roubalová, R.; Škanta, F.; Procházková, P.; Bilej, M. Microbial environment affects innate immunity in two closely related earthworm species Eisenia andrei and Eisenia fetida. PLoS ONE 2013, 8, e79257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burg, P.; Zemánek, P.; Badalíková, B.; Masan, V.; Zatloukal, P.; Čížková, A.; Vašinka, M. Hloubková Aplikace Organické Hmoty u Vinic a Ověření Jejího Vlivu na Půdní a Růstové Podmínky; Mendel University Press: Brno, Czech Republic, 2021. [Google Scholar] [CrossRef]
- Kruk, J.; Dziurka, M.; Płytycz, B. Identification of new fluorophores in coelomic fluid of Eisenia andrei earthworms. PLoS ONE 2019, 14, e0214757. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Abbas, R.; Bellitürk, K.; Hussain, S.; Hussain, S.; Elshikh, M. Enhancing wheat crop resilience to drought stress through cellulolytic microbe-enriched cow dung vermicompost. ACS Omega 2024, 9, 2123–2133. [Google Scholar] [CrossRef]
- Nawaz, M.; Peigné, J.; Fouladidorhani, M.; Lamandé, M.; Arthur, E. Long-term conservation tillage in organic farming maintains sandy loam soil functioning despite increased penetration resistance. Soil Use Manag. 2024, 40, e13150. [Google Scholar] [CrossRef]
- Batista, A.; Pessoa, T.; Putti, F.; Andreote, F.; Libardi, P. Root influences rhizosphere hydraulic properties through soil organic carbon and microbial activity. Plants 2024, 13, 1981. [Google Scholar] [CrossRef]
- Kiran, S. Effects of vermicompost on some morphological, physiological, and biochemical parameters of lettuce (Lactuca sativa var. crispa) under drought stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 352–358. [Google Scholar] [CrossRef][Green Version]
- Ahmed, M.; Zarebanadkouki, M.; Ahmadi, K.; Kroener, E.; Kostka, S.; Kaestner, A.; Carminati, A. Engineering rhizosphere hydraulics: Pathways to improve plant adaptation to drought. Vadose Zone J. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Tammam, A.; El-Aggan, W.; Helaly, A.A.; Badr, G.; El-Dakak, R. Proteomics and photosynthetic apparatus response to vermicompost attenuation of salinity stress Vicia faba leaves. Acta Physiol. Plant. 2023, 45, 17. [Google Scholar] [CrossRef]
- Mahmoud, I.Y.; Mahmoud, E.K.; Ibrahim, D. Effects of Vermicompost and Water Treatment Residuals on Soil Physical Properties and Wheat Yield. Int. Agrophys. 2015, 29, 157–164. [Google Scholar] [CrossRef]
- Alamer, K.H.; Perveen, S.; Khaliq, A.; Haq, M.Z.U.; Ibrahim, M.; Ijaz, B. Mitigation of Salinity Stress in Maize Seedlings by the Application of Vermicompost and Sorghum Water Extracts. Plants 2022, 11, 2548. [Google Scholar] [CrossRef]
- Wu, D. Vermicompost Improves Tomato Yield and Quality by Promoting Carbohydrate Transport to Fruit under Salt Stress. Horticulturae 2023, 9, 1015. [Google Scholar] [CrossRef]
- Dalorima, T.; Zakaria, A.J.; Majrashi, A.; Mahmud, K.; Mohd, K.S.; Muhammad, H.; Khandaker, M.M. Impacts of vermicomposting rates on growth, yield and qualities of red seedless watermelon. Aust. J. Crop Sci. 2018, 12, 1765–1773. [Google Scholar] [CrossRef]
- Alharbi, K.; Hafez, E.M.; Omara, A.E.; Osman, H.S. Mitigating Osmotic Stress and Enhancing Developmental Productivity Processes in Cotton through Integrative Use of Vermicompost and Cyanobacteria. Plants 2023, 12, 1872. [Google Scholar] [CrossRef]
- Beyk-Khormizi, A.; Sarafraz-Ardakani, M.R.; Sarghein, S.H.; Moshtaghioun, S.M.; Kouhi, S.M.M.; Yazdi, M.E.T. Effect of Organic Fertilizer on the Growth and Physiological Parameters of a Traditional Medicinal Plant under Salinity Stress Conditions. Horticulturae 2023, 9, 701. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- Talaat, N.; Abdel-Salam, S. An innovative, sustainable, and environmentally friendly approach for wheat drought tolerance using vermicompost and effective microorganisms: Upregulating the antioxidant defense machinery, glyoxalase system, and osmotic regulatory substances. BMC Plant Biol. 2024, 24, 866. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Ali, A.; Rahman, M. Effects of copper and vermicompost on growth and yield of cowpea (Vigna unguiculata L. Walp) and nutrient accumulation in its fruits. J. Biodivers. Conserv. Bioresour. Manag. 2020, 5, 13–18. [Google Scholar] [CrossRef]
- Ayyobi, H.; Olfati, J.; Peyvast, G. The effects of cow manure vermicompost and municipal solid waste compost on peppermint (Mentha piperita L.) in Torbat-e-Jam and Rasht regions of Iran. Int. J. Recycl. Organ. Waste Agric. 2014, 3, 147–153. [Google Scholar] [CrossRef]
- Rashtbari, M.; Ali, A.; Ghorchiani, M. Effect of vermicompost and municipal solid waste compost on growth and yield of canola under drought stress conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 2215–2222. [Google Scholar] [CrossRef]
- Pandey, N.; Adhikhari, M.; Bhantana, B. Trichoderma and its prospects in agriculture of Nepal: An overview. Int. J. Appl. Sci. Biotechnol. 2019, 7, 309–316. [Google Scholar] [CrossRef]
- Mamun, M.; Li, J.; Cui, A.; Chowdhury, R.; Hossain, L. Integrating adaptive approaches in addressing climate-induced stresses: Evidence of a mixed-method study in coastal Bangladesh. In Proceedings of the EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024. [Google Scholar] [CrossRef]
- Zannat, M.; Hasan, M.; Sikder, S.; Islam, M.; Sharmin, S. Evaluation of organic amendments in alleviating the adverse effect of water deficit stress on soybean. JST 2024, 22, 1–12. [Google Scholar] [CrossRef]
- Law, C.Z. No. 156/1998 Coll. Zákon o hnojivech, pomocných půdních látkách, rostlinných biostimulantech a substrátech a o agrochemickém zkoušení zemědělských půd.
- Hrčka, M.; Hřebečková, T.; Hanč, A.; Grasserová, A.; Cajthaml, T. Changes in the content of emerging pollutants and potentially hazardous substances during vermi/composting of a mixture of sewage sludge and moulded pulp. Environ. Pollut. 2024, 348, 123736. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- BS EN 13651; Soil Improvers and Growing Media. Extraction of Calcium Chloride/DTPA (CAD) Soluble Elements. CEN (BSI): Brussels, Belgium, 2001; 16p.
- Hanč, A.; Částková, T.; Kužel, S.; Cajthaml, T. Dynamics of a vertical-flow windrow vermicomposting system. Waste Manag. Res. 2017, 11, 1121–1128. [Google Scholar] [CrossRef]
- ČSN 460610; Osivo a Sadba. Zkoušení Osiva. Český normalizační institut: Praha, Czech Republic, 1984; 14p.
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Aechra, S.; Meena, R.H.; Meena, S.C.; Jat, H.; Doodhwal, K.; Singh-Shekhawat, A.; Kumar-Verma, A.; Jat, L. Effect of biofertilizers and vermicompost on physico-chemical properties of soil under wheat (Triticum aestivum) crop. Indian J. Agric. Sci. 2022, 92, 991–995. [Google Scholar] [CrossRef]
- Azarmi, R.; Giglou, M.T.; Taleshmikail, R.D. Influence of vermicompost on soil chemical and physical properties in tomato (Lycopersicum esculentum) field. Afr. J. Biotechnol. 2008, 7, 2397–2401. [Google Scholar]
- Atiyeh, R.M.; Edwards, C.A.; Supler, S.; Metzger, J.D. Pig manure vermicompost as a component of a horticultural bedding plant medium: Effects on physicochemical properties and plant growth. Bioresour. Technol. 2001, 78, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Diviš, J.; Jůza, J.; Moudrý, J.; Vondrys, J.; Bárta, J.; Štěrba, Z. Pěstování Rostlin; Učební texty pro obor provozní podnikatel a pozemkové úpravy a převody nemovitostí; Jihočeská univerzita v Českých Budějovicích: České Budějovice, Czech Republic, 2010; 260p, ISBN 978-80-7394-216-8. [Google Scholar]
- Petr, J.; Lipavský, J. Tvorba Výnosu Jarní Pšenice; Jarní pšenice—Tematická příloha časopisu Úroda; Profi Press: Praha, Czech Republic, 2002; pp. 8–9. ISSN 0139-6013. [Google Scholar]
- UKZUZ. Výsledky Zkoušek Užitné Hodnoty Sklizně. Available online: https://mze.gov.cz/public/portal/-q417721---0gOCJhKv/psenice-jarni-sdo-1?_linka=a308823 (accessed on 12 December 2022).
- Johnston, A.E. Soil and Plant Phosphate; International Fertilizer Industry Association: Paris, France, 2000; 46p, ISBN 2950629954. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P. Influences of vermicomposts on field strawberries: Part 2. Effects on soil microbiological and chemical properties. Bioresour. Technol. 2006, 97, 831–840. [Google Scholar] [CrossRef]
- Nweke, I.A. Plant nutrient release composition in vermicompost as influenced by Eudrilus eugenae using different organic diets. J. Ecol. Natur. Environ. 2013, 5, 346–351. [Google Scholar]
- Mistry, J.; Mukhopadhyay, A.; Baur, G. Status of NPK in vermicompost prepared from two common weed and two medicinal plants. Int. J. Appl. Sci. Biotechnol. 2015, 3, 193–196. [Google Scholar] [CrossRef]
- Erdawati, E.; Pratiwi, Y.; Saefurahman, G. Vermicompost from spent coffee grounds as a nutrient-rich organic fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2024, 1354, 012011. [Google Scholar] [CrossRef]
- Pattnaik, S.; Reddy, M. Nutrient status of vermicompost of urban green waste processed by three earthworm species—Eisenia fetida, Eudrilus eugeniae, and Perionyx excavatus. Appl. Environ. Soil Sci. 2010, 2010, 967526. [Google Scholar] [CrossRef]
- Manivannan, S.; Balamurugan, M.; Parthasarathi, K.; Gunasekaran, G.; Ranganathan, L.S. Effect of vermicompost on soil fertility and crop productivity-beans (Phaseolus vulgaris). J. Environ. Biol. 2009, 30, 275–281. [Google Scholar]
- Špaldon, E. Rostlinná Výroba; Státní zemědělské nakladatelství: Praha, Czech Republic, 1986; 714p. [Google Scholar]
- Hřivna, L. Výživa a Hnojení Porostů Pšenice Ozimé a Kvalita Produkce. Družstvo vlastníků Odrůd. Available online: http://farmseed2.druvod.cz/files/aktuality/vyziva_a_hnojeni_porostu_psenice_ozime_a_kvalita_produkce.pdf (accessed on 27 August 2014).
- Černý, J.; Shejbalová, Š.; Kovářík, J.; Kulhánek, M. Předseťové a Podzimní Hnojení Pšenice Ozimé. Agromanual; Česká zemědělská univerzita: Praha, Czech Republic, 2014; Available online: https://www.agromanual.cz/cz/clanky/vyziva-a-stimulace/hnojeni/predsetove-a-podzimni-hnojeni-psenice-ozime (accessed on 27 August 2014).
- Ryant, P. Výživa Rostlin. Available online: http://web2.mendelu.cz/af_221_multitext/vyziva_rostlin/pdf/biogenni_prvky (accessed on 28 April 2008).
- Awasthi, M.K.; Pandey, V.P.; Khan, J.; Bundela, P.S.; Wong, J.W.C. Co-composting of organic fraction of municipal solid waste with biowaste for its nutrient enrichment. Bioresour. Technol. 2016, 220, 208–214. [Google Scholar] [CrossRef]
- Hanc, A.; Dreslova, M. Effect of composting and vermicomposting on properties of particle size fractions. Bioresour. Technol. 2016, 217, 186–189. [Google Scholar] [CrossRef]
- Suthar, S.; Singh, S. Vermicomposting of domestic waste by using two epigeic earthworms (Perionyx excavatus and Perionyx sansibaricus). Int. J. Environ. Waste Manag. 2008, 5, 99–106. [Google Scholar] [CrossRef]
- Jara-Samaniego, J.; Pérez-Murcia, M.D.; Bustamante, M.A.; Pérez-Espinosa, A.; López, M.; Paredes, C. Composting and vermicomposting as sustainable options for sewage sludge treatment: Effects on greenhouse gas emissions and plant growth. J. Clean. Prod. 2017, 151, 388–395. [Google Scholar] [CrossRef]
- Madani, A.; Makarem, A.; Vazin, F.; Joudi, M. The impact of post-anthesis nitrogen and water availability on yield formation of winter wheat. Plant Soil Environ. 2012, 58, 9–14. [Google Scholar] [CrossRef]
- Parmar, R. Influence of elevated CO2 and temperature on yield attributes of rice and wheat in central India. J. Exp. Agric. Int. 2024, 46, 374–395. [Google Scholar] [CrossRef]
- Oliveira, E.; Siddique, K.; Bramley, H.; Stefanova, K.; Palta, J. Response of wheat restricted-tillering and vigorous growth traits to variables of climate change. Glob. Change Biol. 2014, 21, 857–873. [Google Scholar] [CrossRef]
- Zhang, X.; Högy, P.; Wu, X.; Schmid, I.; Wang, X.; Schulze, W.; Jiang, D.; Fangmeier, A. Physiological and proteomic evidence for the interactive effects of post-anthesis heat stress and elevated CO2 on wheat. Proteomics 2018, 18, 1800262. [Google Scholar] [CrossRef] [PubMed]
- Awtaq, S.; Azeez, A.; Mahmood, Y.; Abdulkareem, N.; Jamal, K. Kalar1 and Kalar2, newly released wheat varieties for cultivation under rain-fed conditions. Kurd. J. Appl. Res. 2021, 6, 35–43. [Google Scholar] [CrossRef]
- Islam, M.; De, R.; Hossain, M.; Haque, M.; Uddin, N.; Fakir, M.; Kader, A.; Dessoky, E.S.; Attia, A.O.; El-Hallous, E.I.; et al. Evaluation of the tolerance ability of wheat genotypes to drought stress: Dissection through culm-reserves contribution and grain filling physiology. Agronomy 2021, 11, 1252. [Google Scholar] [CrossRef]
- Ma, S.; Hou, J.; Wang, Y.; Wang, M.; Zhang, W.; Fan, Y.; Huang, Z. Post-flowering soil waterlogging curtails grain yield formation by restricting assimilates supplies to developing grains. Front. Plant Sci. 2022, 13, 944308. [Google Scholar] [CrossRef]
- Morgun, V.; Stasik, O.; Kiriziy, D.; Sokolovska-Sergiienko, O. Effect of drought on photosynthetic apparatus, activity of antioxidant enzymes, and productivity of modern winter wheat varieties. Regul. Mech. Biosyst. 2019, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Munjonji, L.; Ayisi, K.; Vandewalle, B.; Dhau, I.; Boeckx, P.; Haesaert, G. Yield performance, carbon assimilation and spectral response of triticale to water stress. Exp. Agric. 2016, 53, 100–117. [Google Scholar] [CrossRef]
- Lugassi, N.; Kelly, G.; Fidel, L.; Yaniv, Y.; Attia, Z.; Levi, A.; Alchantis, V.; Moshelion, M.; Raveh, E.; Carmi, N.; et al. Expression of Arabidopsis hexokinase in citrus guard cells controls stomatal aperture and reduces transpiration. Front. Plant Sci. 2015, 6, 1114. [Google Scholar] [CrossRef]
- Shellakkutti, N.; Thangamani, P.; Suresh, K.; Baales, J.; Zeisler-Diehl, V.; Klaus, A.; Hochholdinger, F.; Schreiber, L.; Kreszies, T. Cuticular transpiration is not affected by enhanced wax and cutin amounts in response to osmotic stress in barley. Physiol. Plant. 2022, 174, e13735. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Milhiet, T.; Parent, B.; Meziane, A.; Tardieu, F.; Chaumont, F. The plasma membrane aquaporin ZmPIP2;5 enhances the sensitivity of stomatal closure to water deficit. Plant Cell Environ. 2022, 45, 1146–1156. [Google Scholar] [CrossRef]
- Rathnasamy, S.A.; Kambale, R.; Elangovan, A.; Mohanavel, W.; Priyanka, S.; Ramasamy, G.; Alagarsamy, S.; Marimuthu, R.; Rajagopalan, V.R.; Manickam, S.; et al. Altering stomatal density for manipulating transpiration and photosynthetic traits in rice through CRISPR/Cas9 mutagenesis. Curr. Issues Mol. Biol. 2023, 45, 3801–3814. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Zhang, H.; Fu, P.; Zhai, F. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Priya, J.; Vijayalakshmi, D.; Vinitha, A.; Raveendran, M.; Prasad, V. Physiological plasticity of green gram stomata to photosynthesis traits under interactive effects of elevated CO2, drought and heat stress. Int. J. Environ. Clim. Chang. 2021, 11, 109–119. [Google Scholar] [CrossRef]
- Cavichioli, J.; Lisboa, L.; Vitorino, R.; Contiero, L.; Figueiredo, P.; Rocha, E. Physiological parameters and development of passion fruit subjected to water stress and propagation methods. Braz. Arch. Biol. Technol. 2022, 65, e22210145. [Google Scholar] [CrossRef]
- Lisboa, L.; Cunha, M.; Nakayama, F.; Figueiredo, P. Physiological parameters of cotton cultivars in west paulista. Agric. Environ. Sci. 2021, 7, 9. [Google Scholar] [CrossRef]
- Pridgeon, A.; Hetherington, A. ABA signalling and metabolism are not essential for dark-induced stomatal closure but affect response speed. Sci. Rep. 2021, 11, 5751. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, L.M.J.; Sun, W.; Aalto, J.; Erkkilä, K.; Maseyk, K.; Seibt, U.; Vesala, T.; Mammarella, I.; Chen, H. Influences of light and humidity on carbonyl sulfide-based estimates of photosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J. Normal cyclic variation in CO2 concentration in indoor chambers decreases leaf gas exchange and plant growth. Plants 2020, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Gunnerås, S.; Petersson, S.; Tarkowski, P.; Graham, N.; May, S.; Ljung, K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2010, 22, 2956–2969. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Hussain, S.; Bibi, A.; Khaliq, A.; Javed, T.; Kumar, U. Rice straw vermicompost enriched with cellulolytic microbes ameliorates the negative effect of drought in wheat through modulating the morpho-physiological attributes. Front. Environ. Sci. 2022, 10, 902999. [Google Scholar] [CrossRef]
- Zając, M.; Skrajna, T. Effect of Composted Organic Waste on Miscanthus sinensis Andersson Yield, Morphological Characteristics and Chlorophyll Fluorescence and Content. Agronomy 2024, 14, 1672. [Google Scholar] [CrossRef]
- Ormeño, E.; Olivier, R.; Mévy, J.P.; Baldy, V.; Fernandez, C. Compost may affect volatile and semi-volatile plant emissions through nitrogen supply and chlorophyll fluorescence. Chemosphere 2009, 77, 94–104. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Arena, C.; Vitale, E.; Rahimi, A.; Mirzapour, M.; Nasar, J.; Kisaka, O.; Sow, S.; Ranjan, S.; Gitari, H. Impact of different fertilizer sources under supplemental irrigation and rainfed conditions on eco-physiological responses and yield characteristics of dragon’s head (Lallemantia iberica). Plants 2023, 12, 1693. [Google Scholar] [CrossRef]
- Belmeskine, H.; Ouameur, W.; Dilmi, N.; Ali, A. The vermicomposting for agricultural valorization of sludge from Algerian wastewater treatment plant: Impact on growth of snap bean Phaseolus vulgaris L. Heliyon 2020, 6, e04679. [Google Scholar] [CrossRef]
- Nikzad, S.; Maibody, S.; Ehtemam, M.; Golkar, P.; Mohammadi, S. Response of seed yield and biochemical traits of Eruca sativa Mill. to drought stress in a collection study. Sci. Rep. 2023, 13, 11157. [Google Scholar] [CrossRef]
- Golkar, P.; Hamzeh, E.; Maibody, S.; Taghizadeh, M. Safflower’s (Carthamus tinctorius L.) physio-biochemical mechanisms to improve its drought tolerance. Acta Physiol. Plant. 2021, 43, 82. [Google Scholar] [CrossRef]
- Korotkova, I.V.; Chaika, T.O.; Romashko, T.P.; Chetveryk, O.O.; Rybalchenko, A.M.; Barabolia, O.V. Emmer wheat productivity formation depending on pre-sowing seed treatment method in organic and traditional technology cultivation. Regul. Mech. Biosyst. 2023, 14, 41–47. [Google Scholar] [CrossRef]
- Aslam, Z.; Bashir, S.; Hassan, W.; Bellitürk, K.; Ahmad, N.; Niazi, N.; Maitah, M. Unveiling the efficiency of vermicompost derived from different biowastes on wheat (Triticum aestivum L.) plant growth and soil health. Agronomy 2019, 9, 791. [Google Scholar] [CrossRef]
- Ceritoğlu, M.; Erman, M.; Ceritoğlu, F.; Bektaş, H. The response of grain legumes to vermicompost at germination and seedling stages. Legume Res. Int. J. 2021, 44, 936–941. [Google Scholar] [CrossRef]
- Vaculová, K.; Balounová, M.; Cerkal, R.; Ehrenbergerová, J. The effect of location and year on mineral content in spring barley grain. Kvasný Prumysl 2010, 56, 60–68. [Google Scholar] [CrossRef][Green Version]
- Berhe, D.; Zergaw, Y.; Kebede, T. Organic amendments: Direct application and residual effects on vegetative and reproductive growth of hot pepper. Sci. World J. 2022, 2022, 2805004. [Google Scholar] [CrossRef]
- Nawrin, K.; Uddin, M.; Ali, A.; Rahman, M. Effects of boron and vermicompost on growth, yield and nutrient content of chilli (Capsicum annuum L.). J. Biodivers. Conserv. Bioresour. Manag. 2021, 6, 31–36. [Google Scholar] [CrossRef]
- El-Dakak, R.; El-Aggan, W.; Badr, G.; Helaly, A.; Tammam, A. Positive salt tolerance modulation via vermicompost regulation of SOS1 gene expression and antioxidant homeostasis in Vicia faba plant. Plants 2021, 10, 2477. [Google Scholar] [CrossRef]
- Usmani, Z.; Kumar, V.; Gupta, P.; Gupta, G.; Rani, R.; Chandra, A. Enhanced soil fertility, plant growth promotion and microbial enzymatic activities of vermicomposted fly ash. Sci. Rep. 2019, 9, 10455. [Google Scholar] [CrossRef]



| TCB [CFU·g−1 fw] | Escherichia coli [CFU·g−1 fw] | Enterococci [CFU·g−1 fw] | Salmonella spp. [-] | |
|---|---|---|---|---|
| VI | 899 | 711 | 723 × 102 | negative |
| VII | 388 | 439 | 136 × 10 | negative |
| CI | 83 | 128 | 751 × 102 | negative |
| CII | 160 | 283 | 175 × 10 | negative |
| pH (H2O) [-] | pH (CaCl2) [-] | EC [µS·cm−1] | Moisture [%] | |
|---|---|---|---|---|
| Soil | 7.8 ± 0.0 a | 7.1 ± 0.0 | 204.7 ± 9.6 a | 9.9 ± 0.2 a |
| Vermicompost I. | 7.0 ± 0.0 b | - | 1713.7 ± 78.7 b | 68.7 ± 0.3 b |
| Vermicompost II. | 7.0 ± 0.0 b | - | 1901.3 ± 44.8 c | 72.7 ± 0.3 c |
| Compost I. | 7.1 ± 0.0 c | - | 1466.3 ± 51.3 d | 65.6 ± 0.6 d |
| Compost II. | 7.2 ± 0.0 d | - | 1545.7 ± 83.5 d | 64.9 ± 0.8 d |
| O | 8.1 ± 0.0 a | 7.1 ± 0.1 a | 127.0 ± 7.3 a | 10.1 ± 0.9 a |
| V I. | 8.0 ± 0.1 a | 7.2 ± 0.0 a | 118.2 ± 2.6 a | 15.4 ± 0.7 b |
| V II. | 8.1 ± 0.1 a | 7.3 ± 0.0 b | 126.5 ± 8.8 ab | 17.8 ± 0.5 c |
| C I. | 8.1 ± 0.0 a | 7.3 ± 0.0 b | 125.8 ± 7.3 a | 14.9 ± 0.8 bd |
| C II. | 8.2 ± 0.1 a | 7.3 ± 0.1 ab | 148.5 ± 13.5 b | 13.7 ± 0.4 d |
| Harvest [kg·ha−1] | Straw Harvest [kg·ha−1] | Grain Harvest [kg·ha−1] | Ear Length [cm] | NGE [pcs] | TGW [g] | |
|---|---|---|---|---|---|---|
| O | 7996.7 ± 230.3 a | 3676.7 ± 165.0 a | 4320.0 ± 65.6 a | 7.4 ± 0.5 a | 27 ± 2 a | 41.8 ± 0.5 ab |
| V I. | 9320.0 ± 113.6 b | 4233.3 ± 70.2 b | 5086.7 ± 66.6 b | 7.6 ± 0.3 a | 27 ± 5 a | 42.4 ± 1.7 b |
| V II. | 9486.7 ± 470.4 b | 4293.3 ± 246.8 b | 5193.3 ± 223.7 b | 7.1 ± 0.6 a | 28 ± 4 a | 42.3 ± 0.7 b |
| C I. | 9220.0 ± 230.7 b | 4236.7 ± 176.2 b | 4983.3 ± 202.1 b | 7.8 ± 0.4 a | 29 ± 4 a | 41.3 ± 1.1 ab |
| C II. | 9150.0 ± 168.2 b | 4140.0 ± 208.1 b | 5010.0 ± 50.0 b | 7.7 ± 0.2 a | 30 ± 1 a | 42.0 ± 1.3 ab |
| P [mg·kg−1] | K [mg·kg−1] | Mg [mg·kg−1] | |
|---|---|---|---|
| Soil | 57.5 ± 9.1 a | 397.7 ± 82.3 a | 130.9 ± 19.7 a |
| Vermicompost I. | 94.2 ± 7.8 b | 1147.9 ± 61.6 b | 994.3 ± 111.5 b |
| Vermicompost II. | 87.2 ± 15.2 b | 1938.6 ± 21.8 c | 633.9 ± 7.9 c |
| Compost I. | 88.2 ± 11.4 b | 2054.3 ± 19.4 d | 895.5 ± 5.6 b |
| Compost II. | 59.3 ± 7.0 a | 2312.7 ± 162.5 e | 660.2 ± 29.1 c |
| O | 43.5 ± 7.8 a | 304.7 ± 21.8 a | 122.9 ± 13.1 a |
| V I. | 51.0 ± 7.8 ab | 279.4 ± 40.5 a | 120.2 ± 9.6 a |
| V II. | 52.4 ± 10.9 ab | 444.0 ± 105.0 b | 149.6 ± 19.2 a |
| C I. | 56.4 ± 12.3 ab | 354.0 ± 66.6 ab | 135.2 ± 10.7 a |
| C II. | 61.1 ± 10.1 b | 597.7 ± 395.9 ab | 151.6 ± 34.7 a |
| Cd [mg·kg−1] | Pb [mg·kg−1] | As [mg·kg−1] | Cr [mg·kg−1] | Cu [mg·kg−1] | Ni [mg·kg−1] | Zn [mg·kg−1] | |
|---|---|---|---|---|---|---|---|
| Soil | 0.1 ± 0.0 a | 13.2 ± 2.9 a | 2.1 ± 0.5 a | <DL a | 4.2 ± 0.8 a | 0.7 ± 0.2 a | 8.1 ± 1.6 a |
| Vermicompost I. | 0.7 ± 0.1 bc | 22.9 ± 1.1 b | 5.2 ± 0.7 b | 27.4 ± 2.1 bd | 184.3 ± 9.8 b | 16.5 ± 1.5 b | 581.0 ± 29.6 b |
| Vermicompost II. | 0.8 ± 0.1 c | 25.5 ± 1.5 b | 68.2 ± 5.4 c | 27.3 ± 1.6 b | 118.4 ± 12.4 c | 17.5 ± 1.3 b | 675.4 ± 41.5 c |
| Compost I. | 0.6 ± 0.0 b | 20.8 ± 0.8 c | 4.4 ± 0.6 b | 23.2 ± 1.1 c | 169.6 ± 4.9 b | 16.7 ± 1.3 b | 523.6 ± 12.8 d |
| Compost II. | 0.7 ± 0.1 bc | 22.6 ± 1.7 bc | 74.5 ± 7.0 c | 23.5 ± 2.0 cd | 111.8 ± 5.7 c | 19.3 ± 1.7 b | 615.0 ± 39.1 bc |
| O | 0.1 ± 0.0 a | 11.6 ± 1.7 a | 1.4 ± 0.5 a | <DL a | 3.8 ± 0.4 ab | 0.6 ± 0.0 a | 7.5 ± 1.1 a |
| V I. | 0.1 ± 0.0 a | 11.3 ± 1.8 a | 1.4 ± 0.4 a | <DL a | 3.7 ± 0.3 a | 0.5 ± 0.1 a | 7.8 ± 0.9 a |
| V II. | 0.2 ± 0.0 b | 14.5 ± 3.1 a | 1.5 ± 0.6 a | <DL a | 4.8 ± 0.7 b | 0.8 ± 0.1 b | 11.4 ± 1.2 b |
| C I. | 0.2 ± 0.0 b | 12.8 ± 1.3 a | 1.5 ± 0.3 a | <DL a | 4.3 ± 0.2 b | 0.7 ± 0.1 ab | 9.5 ± 0.3 c |
| C II. | 0.2 ± 0.0 b | 13.2 ± 1.8 a | 1.6 ± 0.4 a | <DL a | 4.4 ± 0.3 b | 0.6 ± 0.0 a | 10.6 ± 2.4 abc |
| Fungi [μg PLFA·g−1 dw] | Bacteria [μg PLFA·g−1 dw] | B/F Ratio | |
|---|---|---|---|
| Soil | 0.08 ± 0.05 a | 1.19 ± 0.65 a | 15.54 ± 2.60 a |
| Vermicompost I. | 2.49 ± 0.63 b | 58.92 ± 21.22 b | 23.28 ± 3.49 b |
| Vermicompost II. | 3.85 ± 2.01 bc | 84.35 ± 47.65 bc | 21.92 ± 2.95 b |
| Compost I. | 5.56 ± 0.44 c | 130.47 ± 29.03 c | 23.64 ± 4.54 b |
| Compost II. | 6.36 ± 1.52 c | 121.57 ± 21.02 c | 19.68 ± 4.44 ab |
| O | 0.05 ± 0.02 a | 1.07 ± 0.52 a | 21.87 ± 5.53 a |
| V I. | 0.08 ± 0.01 a | 1.71 ± 0.07 b | 22.70 ± 3.04 a |
| V II. | 0.11 ± 0.01 b | 2.39 ± 0.54 c | 22.52 ± 3.93 a |
| C I. | 0.08 ± 0.02 ab | 2.21 ± 1.41 abc | 25.53 ± 9.32 a |
| C II. | 0.22 ± 0.19 ab | 2.44 ± 0.32 c | 17.50 ± 11.14 a |
| A [µmol CO2·m−2·s−1] | E [mmol H2O·m−2·s−1] | gs [mol H2O·m−2·s−1] | WUEi [μmol CO2·mol−1 H2O] | Fv/Fm | WP [MPa] | |
|---|---|---|---|---|---|---|
| O | 9.78 ± 0.55 ab | 3.14 ± 0.11 a | 0.19 ± 0.01 a | 52.7 ± 17.2 c | 0.818 ± 0.001 a | −1.69 ± 0.06 c |
| V I. | 10.65 ± 0.22 a | 2.24 ± 0.03 bc | 0.10 ± 0 c | 102.7 ± 6.1 a | 0.818 ± 0.002 a | −1.97 ± 0.07 a |
| V II. | 10.75 ± 0.22 a | 2.20 ± 0.04 bc | 0.10 ± 0 c | 106.3 ± 8.2 a | 0.813 ± 0.006 a | −1.88 ± 0.09 ab |
| C I. | 9.38 ± 0.54 b | 2.43 ± 0.13 b | 0.13 ± 0.01 b | 76.7 ± 30.9 b | 0.810 ± 0.008 a | −1.83 ± 0.02 abc |
| C II. | 9.80 ± 0.09 c | 2.07 ± 0.04 c | 0.09 ± 0 c | 72.3 ± 6.4 b | 0.809 ± 0.003 a | −1.76 ± 0.04 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrčka, M.; Kraus, K.; Hřebečková, T.; Tunklová, B.; Kubeš, J.; Hanč, A. Effects of Sewage Sludge Compost and Vermicompost on Wheat Yield and Vitality. Agriculture 2025, 15, 551. https://doi.org/10.3390/agriculture15050551
Hrčka M, Kraus K, Hřebečková T, Tunklová B, Kubeš J, Hanč A. Effects of Sewage Sludge Compost and Vermicompost on Wheat Yield and Vitality. Agriculture. 2025; 15(5):551. https://doi.org/10.3390/agriculture15050551
Chicago/Turabian StyleHrčka, Milan, Kamil Kraus, Tereza Hřebečková, Barbora Tunklová, Jan Kubeš, and Aleš Hanč. 2025. "Effects of Sewage Sludge Compost and Vermicompost on Wheat Yield and Vitality" Agriculture 15, no. 5: 551. https://doi.org/10.3390/agriculture15050551
APA StyleHrčka, M., Kraus, K., Hřebečková, T., Tunklová, B., Kubeš, J., & Hanč, A. (2025). Effects of Sewage Sludge Compost and Vermicompost on Wheat Yield and Vitality. Agriculture, 15(5), 551. https://doi.org/10.3390/agriculture15050551





