Abstract
Saccharomyces cerevisiae (SC) can be incorporated into ruminant diets as a postbiotic product. This study aimed to explore the effects of supplementing different levels of SC in the diets of mid-fattening Angus steers under heat stress conditions. A total of twenty-seven steers were randomly allocated into 3 groups: control, 30 g SC addition and 60 g SC addition groups. After a 7-day adaptation period followed by a 120-day experimental period, including respiratory rate, rectal temperature, growth performance, apparent digestibility of nutrients, rumen fermentation parameters, urine metabolites, serum biochemistry and antioxidant were measured. The results showed that the rectal temperature and respiratory rate of cattle decreased upon the addition of SC during heat stress. Meanwhile, the growth performance of cattle was improved in the 30 g SC addition group. The serum energy metabolism related indexes, such as non-esterified fatty acids, glucose, and β-hydroxybutyric acid, were altered. Additionally, the activity of catalase was significantly enhanced with the addition of SC. Overall, the addition of SC to the diets of mid-fattening Angus steer did not negatively affect rumen fermentation and nutrient apparent digestibility. Instead, it was capable of improving physiological performance under heat stress by modifying the energy metabolism and augmenting antioxidant capacity, which ultimately led to an improvement in growth performance. In conclusion, the most suitable level of SC to be added to the diet of mid-fattening Angus steers is 30 g/steer/d.
1. Introduction
In recent years, the production of ruminants has been substantially influenced by the phenomena of global warming. Prolonged periods of elevated temperatures, heat waves, and solar flares have imposed considerable stress on the beef industry [1]. During heat stress, beef cattle utilize their growth energy to cope with heat loads, which consequently leads to a decline in growth performance or even death [2]. Twenty years ago, the economic cost incurred by heat stress to the U.S. beef industry was estimated at $370 million [3]. Moreover, with the impacts of global warming, the economic losses caused by abnormally high temperatures has become immeasurable. Heat stress is able to induce cellular lipid peroxidation and oxidative stress by elevating the generation of reactive oxygen species [4]. Previous studies have shown that heat stress can affect production performance by affecting the antioxidant capacity and fermentation processes in both beef and dairy cattle [5,6]. The extent of heat stress endured by ruminants can be evaluated by the temperature-humidity index (THI), which integrates ambient temperature and humidity [7]. The currently acknowledged THI thresholds stand at 68 and 72 [8,9]. In order to alleviate the consequences of heat stress, feedlots typically implement strategies such as sprinkling, ventilation, and the provision of shade to reduce temperatures [10]. Dietary regulation of heat stress in beef cattle remains an important area of research. Research findings have demonstrated that the addition of essential oils, niacin, pyruvate creatine, and other additives to diets can improve the heat stress resistance capabilities of beef cattle [5,11,12].
Saccharomyces cerevisiae (SC), a postbiotic product, has been incorporated into ruminant diets to mitigate stress-related challenges. This study aimed to evaluate the effects of SC supplementation on mid-fattening Angus steers under heat stress conditions. SC represents a yeast cell and its derivatives, which are constituted by polysaccharides and glycoproteins. The chemical composition of these components is subject to variation in accordance with the specific strain and species of the yeast [13]. This yeast culture can supply rumen bacteria with essential vitamins, organic acids, amino acids, and other substrates that promote growth [14]. Saccharomyces cerevisiae provides a safeguard against oxidative stress and contributes to the modulation of immune responses [15]. Studies imply that SC can boost the proliferation of beneficial microorganisms in the rumen, attenuate the decline in rumen pH, and improve fiber digestibility [16]. Furthermore, the mano-oligosaccharides present in SC exhibit prebiotic attributes by serving as growth promoters that diminish inflammation and the susceptibility to infection, thereby aiding overall health [17]. Currently, dietary yeast and yeast-derived extracts are widely utilized as growth promoters, anti-stress agents, and methane-reducing additives in the dairy industry [16,18]. However, in the beef cattle industry, the efficacy of SC in alleviating heat stress and determining the optimal dosage remains a matter of contention. This study aimed to determine the optimal quantity of SC to be integrated into the diet of Angus steers during periods of heat stress, with particular emphasis on the impacts on growth performance and antioxidant capacity.
2. Materials and Methods
2.1. The Ethics of Animal Treatment
The Committee for Laboratory Animal Welfare and Ethical Inspection at China Agricultural University gave approval for all procedures involving the handling and treatment of animals (Permit No. AW 72303202-1-2). In line with the guidelines provided by ARRIVE, the methods utilized are elaborated in the manuscript.
2.2. Description of SC
The SC used in this experiment was provided by Diamond V (Original XPC, XPC; Diamond V, Cedar Rapids, IA, USA). The XPC postbiotic product line is amicrobial fermentate composed of hundreds of bioactive compounds. And its specific nutrient content test results are shown in Table 1.

Table 1.
Conventional nutritional composition of SC (DM basis).
2.3. Test Animal Feeding Management and Experimental Design
Twenty-seven Angus steers, aged 14 months and weighing 489 ± 24 kg (Mean ± SD), were housed in individual outdoor pens. Each pen in the cattle barn was equipped with a shaded roof at the top and a sand-based bedding material at the bottom. The total mixed ration (TMR) was provided to the bulls twice daily at 09:00 and 16:00 (GMT + 8). Fresh drinking water was available ad libitum throughout the experimental period. Prior to the initiation of the experiment, all animals were administered a broad-spectrum anthelmintic treatment to ensure parasite-free conditions. Additionally, the pens were thoroughly disinfected using a 2% glutaraldehyde solution to minimize microbial contamination. Fecal matter within the enclosures was removed daily throughout the experimental period to maintain hygienic conditions and minimize potential health risks to the animals. This experiment was carried out following a completely randomized design. Each treatment group included 9 cattle. Three dietary treatments were compared: a control group (no SC), 30 g SC per steer per day, and 60 g SC per steer per day. All group diets were formulated in accordance with the NASEM guidelines and are presented in Table 2 [19]. The experiment lasting for 127 days, with the initial 7 days designated as the adaptation period. During the experiment, ad libitum feeding and watering regimens were adopted. Immediately after the distribution of the total mixed ration (TMR), SC was sprinkled onto the surface to guarantee complete consumption by the animals.

Table 2.
Ingredients and nutrient compositions of the TMR basic diet (DM basis).
2.4. Data Collection, Sample Collection, and Testing Indicators
Before feeding each day, the orts were weighed to determine the actual feed intake. The body weight of the animals was measured before the morning feeding on the 0 d, 61 d, 91 d, and 121 d of the experiment, respectively. The average daily gain (ADG) was computed by dividing the body weight gain (BWG) by the number of experimental days. Meanwhile, the feed conversion ratio (FCR) was calculated as the dry matter intake (DMI) divided by the ADG. TMR and other feed samples were collected on 28 d, 29 d, 30 d, 88 d, 89 d, and 90 d of the experiment, respectively.
The rectal fecal samples and urine samples were collected from each steer on 29 d, 30 d, 31 d, 89 d, 90 d, and 91 d. Upon collection, fecal samples were combined and thoroughly mixed with tartaric acid (10%, v/v) at a ratio of 1:4 (g/mL) for nitrogen fixation. For urine samples, nitrogen fixation was carried out by mixing with 10 mL of diluted sulfuric acid (10%, v/v) per 60 mL of urine. The fecal and urine samples were stored at −20 °C for storage for subsequent analysis. The determination of neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid-insoluble ash in feed and fecal samples was performed in accordance with the method described by Van Soest [20]. The analyses for ash (AOAC Official Method 942.05), dry matter (DM) (AOAC Official Method 934.01), ether extract (EE) (AOAC Official Method 920.39), and crude protein (CP) (AOAC Official Method 984.13) were conducted following the procedures of the AOAC [21]. The apparent digestibility was calculated according to the method proposed by Keulen et al. [22]. Metabolism energy was calculated based on the guidelines provided by NASEM [19]. The urine metabolic indexes, including allantoin (ALN), uric acid (UA), inosine monophosphate (IMP), hippuric acid (HPA), and creatinine (CREA), were measured using kits purchased from Jiangsu Enzyme Immunity Industry Co.
At both the 30-day and 90-day marks of the experimental study, the ruminal fluid was harvested utilizing a naso-gastric tube, precisely three hours following the morning feeding session. To reduce the potential interference of saliva on the pH levels of the rumen fluid, the initial 200 mL of the rumen fluid sample was discarded. This step was crucial in ensuring that salivary contamination was minimized, allowing for more accurate assessments. Following this precautionary measure, approximately 200 mL of ruminal fluid was collected for analysis. Once the collection process was complete, the pH of the obtained fluid was promptly measured with the aid of a pH meter (testo 205, Lenzkirch, Germany). This measurement was vital for assessing the acidity of the ruminal environment. The collected sample was then subjected to a filtration process, which involved passing it through four layers of medical gauze to further ensure the purity of the sample. From the resulting filtrate, 10 mL was set aside for ammonia nitrogen determination, while another 10 mL was earmarked for the analysis of volatile fatty acids (VFA). To preserve the integrity of these samples, they were stored at −80 °C, having been pre-mixed with 2.5 mL of concentrated phosphoric acid (250 g/L) to prevent degradation over time. For the measurement of ammonia nitrogen, the established methodology detailed in Weatherburn was employed, facilitating reliable results [23]. Meanwhile, VFA was analyzed using gas chromatography (GC-2014 Shimadzu Corporation, Kyoto, Japan), with 2-ethylbutyric acid serving as the internal standard. This methodological framework was essential for obtaining precise and valid data regarding the ruminal contents.
Blood samples were collected from all steers via the tail vein prior to the morning feeding on days 31 and 91 of the experiment. Subsequently, these samples were centrifuged at 3000× g for 15 min to separate serum components. A fully automatic biochemical analyzer (Hitachi Co., Hitachi 7020, Tokyo, Japan) was employed to measure a variety of serum biochemical indicators, including aspartate aminotransferase (AST), total protein (TP), alkaline phosphatase (ALP), total cholesterol (CHO), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), urea nitrogen (UREA), non-esterified fatty acids (NEFA), glucose (GLU), β-hydroxybutyric acid (BHBA), creatinine (CREA), alanine aminotransferase (ALT), and albumin (ALB). The kits used for these determinations were procured from Beijing Jiuqiang Biotechnology Co., Ltd. (Beijing, China). As for the serum antioxidant and metabolic indexes, they were determined using the kits provided by Jiangsu Enzyme Immunity Industry Co., Ltd. (Yancheng, China). These indexes encompassed catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), reactive oxygen species (ROS), cortisol (COR), growth hormone (GH), heat shock protein 70 (HSP-70), and total antioxidant capacity (T-AOC). The oxidative stress index (OSI) was calculated based on the following equation: OSI = ROS/T-AOC.
2.5. Heat Stress Standards and Animal Behavior
This experiment was started on 21 June 2022 at Yongning County, Yinchuan City, Ningxia Hui Autonomous Region. The ambient temperature and humidity were automatically recorded at intervals of every 10 min by means of automatic temperature and humidity recorders (Shanghai Penghe Electronic Technology Co., Ltd., Shanghai, China). The temperature-humidity index (THI) was calculated according to the method proposed by Oliveira et al. [24]. Based on previous studies, THI ≥ 68 was designated as the threshold signifying the onset of heat stress [25,26].
Based on the weather forecast for the month of July, the hottest day of the week was selected. On that specific day, the respiration rates of the steers were recorded at 6:00 am and 2:00 pm using an ultra-high-definition zoomable camera. Additionally, the rectal temperatures of steers were determined using a thermometer on the hottest day within the month of July. The variations in temperature, humidity, and THI during the initial 9 weeks of the experiment are depicted in Figure 1 and Table 3.
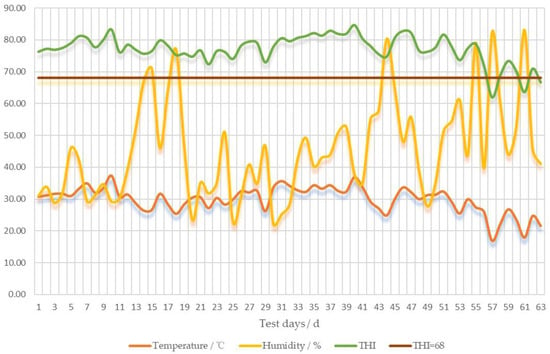
Figure 1.
Temperature, humidity and THI variations during the first 9 weeks of the test.

Table 3.
Temperature, humidity and THI variations during the first 9 weeks of the test.
2.6. Data Analysis
The experimental data were organized using Microsoft Excel 2019. Microsoft one-way ANOVA was performed in SPSS version 25.0 for statistical analysis. The model is specified as:
where: Y_ij: Observation (e.g., daily gain) of the jth cattle in the ith treatment group. μ: Overall mean. Treatment_i: Fixed effect of the ith treatment. ε_ij: Random error ~N (0, σ2).
Y_ij = μ + Treatment_i + ε_ij
Prior to conducting one-way ANOVA, we performed Levene’s test to assess the equality of variances across groups. The Least Significant Difference (LSD) method was adopted for multiple comparisons. The determination of significance was based on the p-value. A significance level was declared at p ≤ 0.05, and trends were identified when the p-value fell within the range of 0.05 ˂ p ≤ 0.10.
3. Results
3.1. Statistical Description of Results
3.1.1. Rectal Temperatures and Respiration Rate
Table 4 shows the impact of SC supplementation on the rectal temperature and respiratory rate of Angus steers. The rectal temperatures at 2:00 pm of the 60 g SC addition group was significantly lower than that of the control group (p = 0.027). The respiration rate at 6:00 am during the 7–14 d period of the 60 g SC addition group was significantly higher than that of the 30 g SC addition group (p = 0.039). The respiration rate at 2:00 pm during the 21–28 d period and at 2:00 pm during the 56–63 days period of both the 60 g SC addition group and the 30 g SC addition group were significantly lower than those of the control group (p < 0.05). With the supplementation of SC, a linear changing trend in the respiratory rate was observed for Angus steers at 6:00 am during the 21–28 d period, at 6:00 am during the 42–49 d period, and at 6:00 am during the 49–56 d period (0.05 < p ≤ 0.10). The rectal temperature and respiratory rate in other time periods were not affected by the addition of SC (p > 0.05). In conclusion, supplementation with SC reduced rectal temperature and respiratory rate, indicating improved heat stress resilience.

Table 4.
Effect of SC addition on rectal temperatures and respiration rate of Angus steers.
3.1.2. Growth Performance
The impacts of incorporating SC into the diets on the growth performance of Angus steers are presented in Table 5. The results indicated that the F/G in the 30 g SC addition group was significantly higher than that in both the control and the 60 g addition group (p = 0.013). Moreover, there was a tendency for ADG to increase during the periods of 0–90 days and 0–120 days in the 30 g SC addition group (0.05 < p ≤ 0.10).

Table 5.
Effect of SC addition on growth performance of Angus steers.
3.1.3. Apparent Nutrient Digestibility
Table 6 illustrates the impact of incorporating SC into the TMR on the apparent digestibility of Angus steers. With regard to the apparent digestibility of OM, CP, EE, NDF, and ADF, no significant differences were found among the groups (p > 0.05).

Table 6.
Effect of SC addition on apparent nutrient digestibility of Angus steers.
3.1.4. Ruminal Fermentation
The impacts of incorporating SC into the diet on ruminal fermentation in Angus steers are shown in Table 7. The acetate-to-propionate (A:P) ratio at 30 d was significantly lower in the 60 g SC addition group than that in the 30 g SC addition group (p = 0.034). No significant differences were found in other rumen fermentation indicators (p > 0.05).

Table 7.
Effect of SC addition on rumen fermentation of Angus steers.
3.1.5. Urine Metabolites
The impacts of incorporating SC addition into the diet on the urine metabolites of Angus steers are shown in Table 8. No significant differences were observed in the indexes among the different treatments (p > 0.05).

Table 8.
Effect of SC addition on urine metabolites of Angus steers.
3.1.6. Serum Biochemistry
Table 9 shows the impact of adding SC on serum biochemistry of Angus steers. The level of LDL-C at 90 d in the 60 g SC addition group was significantly lower than that in the 30 g SC addition group (p = 0.048). The NEFA level at 30 d in the 30 g SC addition group was significantly higher than those in both the 60 g SC addition group and the control group (p = 0.002). The BHBA level at 90 d in the 60 g SC addition group was significantly lower than those in both the 30 g SC addition group and the control group (p = 0.006). The ALP levels at 90 d exhibited a changing trend in relation to the amount of SC added (p = 0.088). As the amount of SC addition increases, the GLU level at 30 d shows a linear downward trend (p = 0.073). No significant differences were found in other serum biochemical indexes (p > 0.05).

Table 9.
Effect of SC addition on serum biochemistry of Angus steers.
3.1.7. Serum Oxidative Stress and Antioxidant Capacity
Table 10 illustrates the impact of adding SC on serum oxidative stress and the antioxidant capacity of Angus steers. The CAT activity at 30 d in the 60 g SC addition group was significantly higher than that in the control group (p = 0.049). The CAT activity at 90 d in both the 30 g SC addition group and the 60 g SC addition group was significantly higher than that in the control group (p = 0.005). No significant differences were detected in other serum oxidative stress and the antioxidant capacity indexes (p > 0.05).

Table 10.
Effect of SC addition on serum oxidative stress and antioxidant capacity of Angus steers.
4. Discussion
Cattle typically dissipate heat through evaporation and thermal exchange. This process diminishes fermentation heat and overall heat gain by reducing feed intakes, and as a result, it increases sweating, water intake, and respiration rate [27]. Heat stress has an adverse effect on protein synthesis and promotes muscle catabolism. Meanwhile, it also stimulating lipolysis and fat mobilization, ultimately resulting in physiological and behavioral anomalies in animals [27,28]. In addition, heat stress subjects animals to oxidative stress, resulting in dysfunction or inflammation [29]. In the present study, the average THI during the first 9 weeks was 77.1, which verified that the experimental animals were under heat stress. Under normal circumstances, the respiratory rate of cattle ranges from 20 to 60 cycles per minute. According to Mader et al., a respiratory rate exceeding 60 cycles per minute can be regarded as an indication of heat stress in cattle [30]. Besides, their rectal temperature will rise when cattle are under heat stress. A body temperature between 39.2 to 40 °C, accompanied by a decrease in feed intake, signals mild heat stress [25,31]. In this study, the addition of either 30 or 60 g of SC daily was capable of significantly reducing rectal temperature and respiratory rate in beef cattle during heat stress. This positive effect was more conspicuous at higher temperatures. Coincidentally, Lee et al. thought SC play a role in supporting thermoregulation during periods of heat load [32]. DU et al. found that adding SC to the diet of mid-lactation Holstein cows decreased the animals’ rectal temperature and respiratory rate, which was consistent with the results of the present study [33].
It has been reported that heat stress exerts negative impacts on DMI, ADG, and growth efficiency in beef cattle [33]. Angus steers in the 30 g SC addition group exhibited the best the best ADG and F/G. Improved growth performance is often associated with a more favorable physiological status, which is characterized by normal body temperature and respiratory rate [34]. This study has shown that supplementing with SC can improve the performance of beef cattle by positively influencing their physiological status. The effectiveness of yeast-based additives in enhancing the growth performance of beef cattle remains a subject of debate. In particular, it is crucial to note that the safety and efficacy of different sources of SC as feed additives for beef cattle need to be compared and verified [35,36]. Although some studies have indicated that the addition of SC does not significantly improve growth in fattening cattle [37,38], a meta-analysis has demonstrated that feeding SC can increase ADG by 6.5% and feed efficiency by 2.6%, which is in line with the results of this study [39]. Emma et al. have contended that incorporating SC can boost production performance by strengthening antioxidant capacity and optimizing the cattle’s immune systems [40]. These variations might be ascribed to differences in SC strains, the fermentation substrates utilized, the ingredients of beef cattle diets, and the specific application scenarios, all of which account for the observed differences in application effects.
The general consensus is that SC can augment the population of cellulolytic bacteria by protecting anaerobic rumen bacteria from O2 damage via respiratory activity [41]. Consequently, the majority of studies have reached the conclusion that the adding of SC to ruminant feeds, such as those for buffalo, beef cattle, and goats, would enhance fiber digestibility and alter the composition of rumen bacteria [42,43,44]. Nevertheless, different types of SC additions seem to yield different outcomes. Faccenda et al. and Takiya et al. respectively investigated the addition of SC and live or autolyzed yeast to cattle diets, yet neither of them discovered any impact of yeast cultures on nutrient digestibility [45,46]. Another study revealed that directly fed SC did not boost either the fermentation or the digestion process in sheep that were fed with sugar cane tops [47]. This study arrived at a similar conclusion, as adding SC did not significantly affect nutrient digestibility and rumen fermentation parameters. Interestingly, the 60 g SC addition group exhibited a numerically higher apparent digestibility compared to the other groups, which was not entirely consistent with the observed differences in growth performance. This discrepancy might imply that a daily intake of 60 g of SC could be excessive for beef cattle. As Emma et al. proposed that 12 g of SC per steer per day is appropriate for fattening beef cattle [40]. Furthermore, another study indicated that an intake of 12–18 g/day of SC in beef cattle diets is acceptable [48]. Additionally, the A:P at 30d ratio was significantly lower in the 60 g SC addition group than in the 30 g SC addition group, which might reflect a transient effect on rumen fermentation in beef cattle during the initial stages of feeding at this higher addition level. However, this effect did not seem to be sustainable and did not lead to any significant alterations in growth.
No significant differences were observed in any of the urine metabolite parameters of the beef cattle, which demonstrated that the addition of SC did not affect the hepatic and renal functions of the animals based solely on the urinary indicators. ALP levels in the blood can serve as an indicator of the health status of the cattle liver [49]. In this study, the serum ALP level exhibited a tendency of quadratic change with the increase of SC addition. This indicated that the high level of addition imposed a burden on the liver of the animals, which was in consistent with the previous speculation. LDL-C functions as a transport carrier for CHO, distributing it to organs that are unable to synthesize it independently. However, elevated levels of LDL-C can have adverse effects on organic health. The changes in LDL-C observed in this study suggest that the addition of SC may have a beneficial impact on lipid metabolism and cardiovascular health in fattening cattle. The levels of NEFA and GLU can be used as indexes to evaluate the energy metabolic status of beef cattle [50]. The observed change in NEFA content in the present study may be attributed to the increased energy requirements of the beef cattle in the 30 g SC addition group, which is associated with their accelerated growth rate. The reduction in GLU content may be explained by the presence of components such as polysaccharides and trace elements in SC, which can enhance insulin secretion and facilitate cellular uptake and utilization of glucose, thereby leading to a decrease in blood glucose levels [51]. Furthermore, it has been acknowledged that changes in the levels of NEFA and GLU under stress conditions are correlated with cortisol. It has been suggested that yeast products can improve the antioxidant capacity under stress conditions by reducing cortisol in cattle and thus improving production performance [52]. Although there was no significant difference in COR between groups in this study, the amelioration of NEFA and GLU content under heat stress was evident. Santinello et al. obtained similar results, demonstrating that supplementation with live yeast and selenium reduced blood NEFA and GLU levels in young bulls that had been transported over long distances [53].
Numerous studies have illustrated the impact of yeast additives on bolstering the antioxidant capacity of ruminants. Geng et al. put forward that the supplementation of active dry yeast can improve beef tenderness by mitigating oxidative stress [54]. Ma et al. indicated that the addition of active dry yeast can enhance growth performance by promoting ruminal fermentation and increasing the antioxidant capacity in weaned calves [55]. Additionally, Dai et al. proposed that feeding postbiotic products derived from SC can optimize the health and production performance of transition dairy cows [56]. Similar conclusions were drawn in the present study. CAT, which catalyzes the decomposition of H2O2, plays a crucial role in the antioxidant defense of aerobic cells [57]. In this study, it was found that the serum CAT levels of beef cattle fed with SC were significantly higher at both 30 d and 60 d. This indicated that SC was capable of enhancing the antioxidant capacity of the animals within a short time frame and that this improvement could be maintained throughout the heat stress period. This might be ascribed to the fact that SC is rich in amino acids, polysaccharides, and bioactive factors. These components can boost the immunity of beef cattle, activate the body’s antioxidant enzyme system, and curtail the production of free radicals, thereby reducing cellular damage [57].
5. Conclusions
The current study demonstrates that incorporating SC into the diets of mid-fattening Angus steer can promote growth performance through improving serum energy metabolism and anti-oxidative stress capacity. These findings suggest that dietary supplementation with 30 g SC per day is optimal for enhancing the growth performance and physiological resilience of mid-fattening Angus steers under heat stress conditions.
Author Contributions
Conceptualization, C.-X.S., S.-R.Y. and H.-W.S.; Data curation, S.-R.Y. and H.-W.S.; Funding acquisition, B.-H.C. and H.-W.S.; Investigation, S.-R.Y., H.-L.W., Y.-Q.L., S.-N.M., S.Z., H.-L.Z., Y.-W.L. and W.-X.Z.; Methodology, C.-X.S., S.-R.Y. and H.-W.S.; Project administration, B.-H.C. and H.-W.S.; Software, C.-X.S. and S.-R.Y.; Supervision, B.-H.C. and H.-W.S.; Validation, Y.H.; Writing—original draft, C.-X.S.; Writing—review & editing, Y.-Q.L., H.-L.W. and H.-W.S. All authors have read and agreed to the published version of the manuscript.
Funding
The funding sources for this research included the National Key R&D Program of China (2023YFD300904 & 2022YFD1602310), the 2115 Talent Development Program of China Agricultural University, and China Agriculture Research Systems of MOF and MARA (CARS-37).
Institutional Review Board Statement
The Committee for Laboratory Animal Welfare and Ethical Inspection at China Agricultural University gave approval for all procedures involving the handling and treatment of animals (Permit No. AW 72303202-1-2).
Data Availability Statement
The original contributions made in this study are incorporated within the article. For any further inquiries, please direct them to the corresponding author.
Conflicts of Interest
The authors state that there are no conflicts of interest.
References
- Wankar, A.K.; Rindhe, S.N.; Doijad, N.S. Heat stress in dairy animals and current milk production trends, economics, and future perspectives: The global scenario. Trop. Anim. Heal. Prod. 2021, 53, 70. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Zhang, F.J.; Weng, X.G.; Wang, J.F.; Zhou, D.; Zhang, W.; Zhai, C.C.; Hou, Y.X.; Zhu, Y.H. Effects of temperature–humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows1. J. Anim. Sci. 2014, 92, 3026–3034. [Google Scholar] [CrossRef]
- Chen, H.; Yang, M.; Shang, X.; Chen, H.; Li, Y.; Li, Y.; Li, L.; Qu, M.; Song, X. Pogostemon cablin essential oil as feed additive promotes the repair of the rumen epithelial barrier in heat-stressed beef cattle. Anim. Nutr. 2024, 18, 433–440. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Shen, Y.; Chen, P.; Li, Y.; Cao, Y.; Li, Q.; Xu, H.; Gao, Y.; Li, J. Effects of chromium propionate supplementation on lactation performance, nutrient digestibility, rumen fermentation patterns, and antioxidant status in Holstein cows under heat stress. Anim. Feed. Sci. Technol. 2023, 305, 115765. [Google Scholar] [CrossRef]
- Wang, X.; Bjerg, B.S.; Choi, C.Y.; Zong, C.; Zhang, G. A review and quantitative assessment of cattle-related thermal indices. J. Therm. Biol. 2018, 77, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zimbelman, R.B.; Collier, R.J. Feeding Strategies for High-Producing Dairy Cows During Periods of Elevated Heat and Humidity. In Proceedings of the Tri-State Dairy Nutrition Conference, Fort Wayne, IN, USA, 19–20 April 2011; pp. 111–126. [Google Scholar]
- Armstrong, D.V. Heat Stress Interaction with Shade and Cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.A.; Canozzi, M.E.A.; Rodhermel, J.C.B.; Schwegler, E.; La Manna, A.; Clariget, J.; Bianchi, I.; Moreira, F.; Olsson, D.C.; Peripolli, V. Strategies to alleviate heat stress on performance and physiological parameters in feedlot-finished cattle under heat stress conditions. A systematic review-meta-analysis. J. Therm. Biol. 2024, 119, 103798. [Google Scholar] [CrossRef]
- Zou, B.; Long, F.; Xue, F.; Chen, C.; Zhang, X.; Qu, M.; Xu, L. Protective Effects of Niacin on Rumen Epithelial Cell Barrier Integrity in Heat-Stressed Beef Cattle. Animals 2024, 14, 313. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Yu, H.; Xu, L.; Qu, M.; Li, Y. Improved antioxidant activity and rumen fermentation in beef cattle under heat stress by dietary supplementation with creatine pyruvate. Anim. Sci. J. 2020, 91, e13486. [Google Scholar] [CrossRef] [PubMed]
- Bzducha-Wróbel, A.; Błażejak, S.; Tkacz, K. Cell wall structure of selected yeast species as a factor of magnesium binding ability. Eur. Food Res. Technol. 2012, 235, 355–366. [Google Scholar] [CrossRef]
- Newbold, C.J.; Rode, L.M. Dietary additives to control methanogenesis in the rumen. Int. Congr. Ser. 2006, 1293, 138–147. [Google Scholar] [CrossRef]
- Sanchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Baker, L.M.; Kraft, J.; Karnezos, T.P.; Greenwood, S.L. Review: The effects of dietary yeast and yeast-derived extracts on rumen microbiota and their function. Anim. Feed. Sci. Technol. 2022, 294, 115476. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Connolly, A.; Kiers, A. A review of 733 published trials on Bio-Mos®, a mannan oligosaccharide, and Actigen®, a second generation mannose rich fraction, on farm and companion animals. J. Appl. Anim. Nutr. 2015, 3, e8. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Martin, C.; Morgavi, D.P. The use of direct-fed microbials for mitigation of ruminant methane emissions: A review. Animal 2014, 8, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrient Requirements of Beef Cattle. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016; p. 494. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Van Keulen, J.; Young, B.A. Evaluation of Acid-Insoluble Ash as a Natural Marker in Ruminant Digestibility Studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Esmay, M.L. Systems Model Analysis of Hot Weather Housing for Livestock. Trans. ASABE 1982, 25, 1355–1359. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef]
- Edwards-Callaway, L.N.; Cramer, M.C.; Cadaret, C.N.; Bigler, E.J.; Engle, T.E.; Wagner, J.J.; Clark, D.L. Impacts of shade on cattle well-being in the beef supply chain. J. Anim. Sci. 2020, 99, skaa375. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.D.; Rhoads, R.P.; Sanders, S.R.; Duff, G.C.; Baumgard, L.H. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 2010, 38, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Monteiro, A.P.; Thompson, I.M.; Hayen, M.J.; Dahl, G.E. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.L.; Gaughan, J.B.; Johnson, L.J.; Hahn, G.L. Tympanic temperature in confined beef cattle exposed to excessive heat load. Int. J. Biometeorol. 2010, 54, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Davison, T.M.; Jonsson, N.N.; Mayer, D.G.; Gaughan, J.B.; Ehrlich, W.K.; McGowan, M.R. Comparison of the impact of six heat-load management strategies on thermal responses and milk production of feed-pad and pasture fed dairy cows in a subtropical environment. Int. J. Biometeorol. 2016, 60, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Olm, J.C.W.; Lees, J.C.; Gaughan, J.B. Influence of feeding Saccharomyces cerevisiae on the heat load responses of lactating dairy cows during summer. Int. J. Biometeorol. 2022, 66, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Feng, L.; Chen, P.; Jiang, W.; Zhang, Y.; Liu, W.; Zhai, R.; Hu, Z. Effects of Saccharomyces Cerevisiae Cultures on Performance and Immune Performance of Dairy Cows During Heat Stress. Front. Vet. Sci. 2022, 9, 851184. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, K.; Song, X.; Lan, L.; Liu, S.; Hu, R.; Luo, J. The anti-heat stress effects of Chinese herbal medicine prescriptions and rumen-protected γ-aminobutyric acid on growth performance, apparent nutrient digestibility, and health status in beef cattle. Anim. Sci. J. 2020, 91, e13361. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of Levucell®SC (Saccharomyces cerevisiae CNCM I-1077) as a feed additive for dairy cows, cattle for fattening, minor ruminant species and camelids. EFSA J. 2017, 15, e04944. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of Actisaf® Sc47 (Saccharomyces cerevisiae CNCM I-4407) as a feed additive for cattle for fattening, dairy cows, weaned piglets and sows. EFSA J. 2019, 17, e05600. [Google Scholar] [CrossRef]
- Keyser, S.A.; McMeniman, J.P.; Smith, D.R.; MacDonald, J.C.; Galyean, M.L. Effects of Saccharomyces cerevisiae subspecies boulardii CNCM I-1079 on feed intake by healthy beef cattle treated with florfenicol and on health and performance of newly received beef heifers1. J. Anim. Sci. 2007, 85, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Deters, E.L.; Stokes, R.S.; Genther-Schroeder, O.N.; Hansen, S.L. Effects of a Saccharomyces cerevisiae fermentation product in receiving diets of newly weaned beef steers. I. Growth performance and antioxidant defense1. J. Anim. Sci. 2018, 96, 3897–3905. [Google Scholar] [CrossRef]
- Wagner, J.J.; Engle, T.E.; Belknap, C.R.; Dorton, K.L. Meta-analysis examining the effects of Saccharomyces cerevisiae fermentation products on feedlot performance and carcass traits Prof. Anim. Sci. 2016, 32, 172–182. [Google Scholar] [CrossRef]
- Rients, E.L.; Deters, E.L.; McGill, J.L.; Belknap, C.R.; Hansen, S.L. Effects of feeding a Saccharomyces cerevisiae fermentation product and ractopamine hydrochloride to finishing beef steers on growth performance, immune system, and muscle gene expression. J. Anim. Sci. 2023, 101, skac311. [Google Scholar] [CrossRef]
- Newbold, C.J.; Wallace, R.J.; McIntosh, F.M. Mode of action of the yeast Saccharomyces cerevisiae as a feed additive for ruminants. Br. J. Nutr. 1996, 76, 249–261. [Google Scholar] [CrossRef]
- Va, S.; Supapong, C.; Chanjula, P. Effects of yeast and dried kratom leaves (Mitragyna speciosa [Korth] Havil.) supplementation on digestibility, rumen fermentation, blood metabolites and nitrogen balance in goats. Anim. Biosci. 2024, 37, 228–239. [Google Scholar] [CrossRef]
- Treon, E.; Sidney, T.S.; Taiwo, G.A.; Idowu, M.; Leal, Y.; Ologunagba, D.; Ogunade, I.M. 123 Effects of a Saccharomyces cerevisiae based direct-fed microbial on performance, health, and rumen bacterial community of newly weaned beef steers during a 56-d receiving period. J. Anim. Sci. 2024, 102, 341–342. [Google Scholar] [CrossRef]
- Anjum, M.I.; Javaid, S.; Ansar, M.S.; Ghaffar, A. Effects of yeast (Saccharomyces cerevisiae) supplementation on intake, digestibility, rumen fermentation and milk yield in Nili-Ravi buffaloes. Iran J. Vet. Res. 2018, 19, 96–100. [Google Scholar]
- Takiya, C.S.; Chesini, R.G.; de Freitas, A.C.; Grigoletto, N.T.S.; Vieira, D.J.C.; Poletti, G.; Martins, N.P.; Sbaralho, O.P.; Roth, N.; Acedo, T.; et al. Dietary supplementation with live or autolyzed yeast: Effects on performance, nutrient digestibility, and ruminal fermentation in dairy cows. J. Dairy Sci. 2024, 107, 4495–4508. [Google Scholar] [CrossRef]
- Faccenda, A.; Zambom, M.A.; de Avila, A.S.; Garcias, J.; Eckstein, E.I.; Fornari, J.L.; de Almeida, K.V.; Santos, G.T. Nutrient digestibility and ruminal parameters of cattle fed dried brewers grains and Saccharomyces cerevisiae. Livest. Sci. 2019, 225, 109–115. [Google Scholar] [CrossRef]
- Arcos-García, J.L.; Castrejón, F.A.; Mendoza, G.D.; Pérez-Gavilán, E.P. Effect of two commercial yeast cultures with Saccharomyces cerevisiae on ruminal fermentation and digestion in sheep fed sugar cane tops. Livest. Prod. Sci. 2000, 63, 153–157. [Google Scholar] [CrossRef]
- Shen, Y.; Davedow, T.; Ran, T.; Saleem, A.M.; Yoon, I.; Narvaez, C.; Mcallister, T.A.; Yang, W. Ruminally protected and unprotected Saccharomyces cerevisiae fermentation products as alternatives to antibiotics in finishing beef steers1. J. Anim. Sci. 2019, 97, 4323–4333. [Google Scholar] [CrossRef] [PubMed]
- Sakowski, T.; Kuczyńska, B.; Puppel, K.; Metera, E.; Słoniewskia, K.; Barszczewskic, J. Relationships between physiological indicators in blood and yield as well as chemical composition of milk obtained from organic dairy cows. J. Sci. Food Agric. 2012, 92, 2905–2912. [Google Scholar] [CrossRef]
- Ran, T.; Shen, Y.Z.; Saleem, A.M.; AlZahal, O.; Beauchemin, K.A.; Yang, W.Z. Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: Growth performance, carcass traits, blood metabolites, and fecal Escherichia coli. J. Anim. Sci. 2018, 96, 4385–4397. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.-H.; Cheng, L.; Kang, K.; Tian, G.; Al-Mamun, M.; Xue, B.; Wang, L.-Z.; Zou, H.-W.; Gicheha, M.G.; Wang, Z.-S. Effects of yeast and yeast cell wall polysaccharides supplementation on beef cattle growth performance, rumen microbial populations and lipopolysaccharides production. J. Integr. Agric. 2020, 19, 810–819. [Google Scholar] [CrossRef]
- Batista, L.H.C.; Cidrini, I.A.; Prados, L.F.; Cruz, A.A.C.; Torrecilhas, J.A.; Siqueira, G.R.; Resende, F.D. A meta-analysis of yeast products for beef cattle under stress conditions: Performance, health and physiological parameters. Anim. Feed. Sci. Technol. 2022, 283, 115182. [Google Scholar] [CrossRef]
- Santinello, M.; Lora, I.; Villot, C.; Cozzi, G.; Penasa, M.; Chevaux, E.; Martin, B.; Guerra, A.; Simoni, M.; De Marchi, M. Impact of live yeast and selenium supplementation on blood metabolites and rumen pH of young bulls after long-transport to the fattening unit. Animal 2024, 18, 101375. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.Y.; Feng, X.; Luan, J.M.; Ji, S.; Jin, Y.H.; Zhang, M. Improved tenderness of beef from bulls supplemented with active dry yeast is related to matrix metalloproteinases and reduced oxidative stress. Animal 2022, 16, 100517. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Wang, Z.; Cao, G.; Hu, R.; Wang, X.; Zou, H.; Kang, K.; Peng, Q.; Xue, B.; et al. Active dry yeast supplementation improves the growth performance, rumen fermentation, and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Kong, F.; Han, H.; Shi, W.; Song, H.; Yoon, I.; Wang, S.; Liu, X.; Lu, N.; Wang, W.; et al. Effects of postbiotic products from Saccharomyces cerevisiae fermentation on lactation performance, antioxidant capacity, and blood immunity in transition dairy cows. J. Dairy Sci. 2024, 107, 10584–10598. [Google Scholar] [CrossRef] [PubMed]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef]
- Alugongo, G.M.; Xiao, J.X.; Chung, Y.H.; Dong, S.Z.; Li, S.L.; Yoon, I.; Wu, Z.H.; Cao, Z.J. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Performance and health. J. Dairy Sci. 2017, 100, 1189–1199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).