Arsenic Accumulation in Pakchoi Influenced by Acidification-Driven Changes in Soil Properties and Arsenic Transformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pretreatment
2.2. Preparation of As-Contaminated Soil
2.3. Simulation of Acidification of Purple Soil
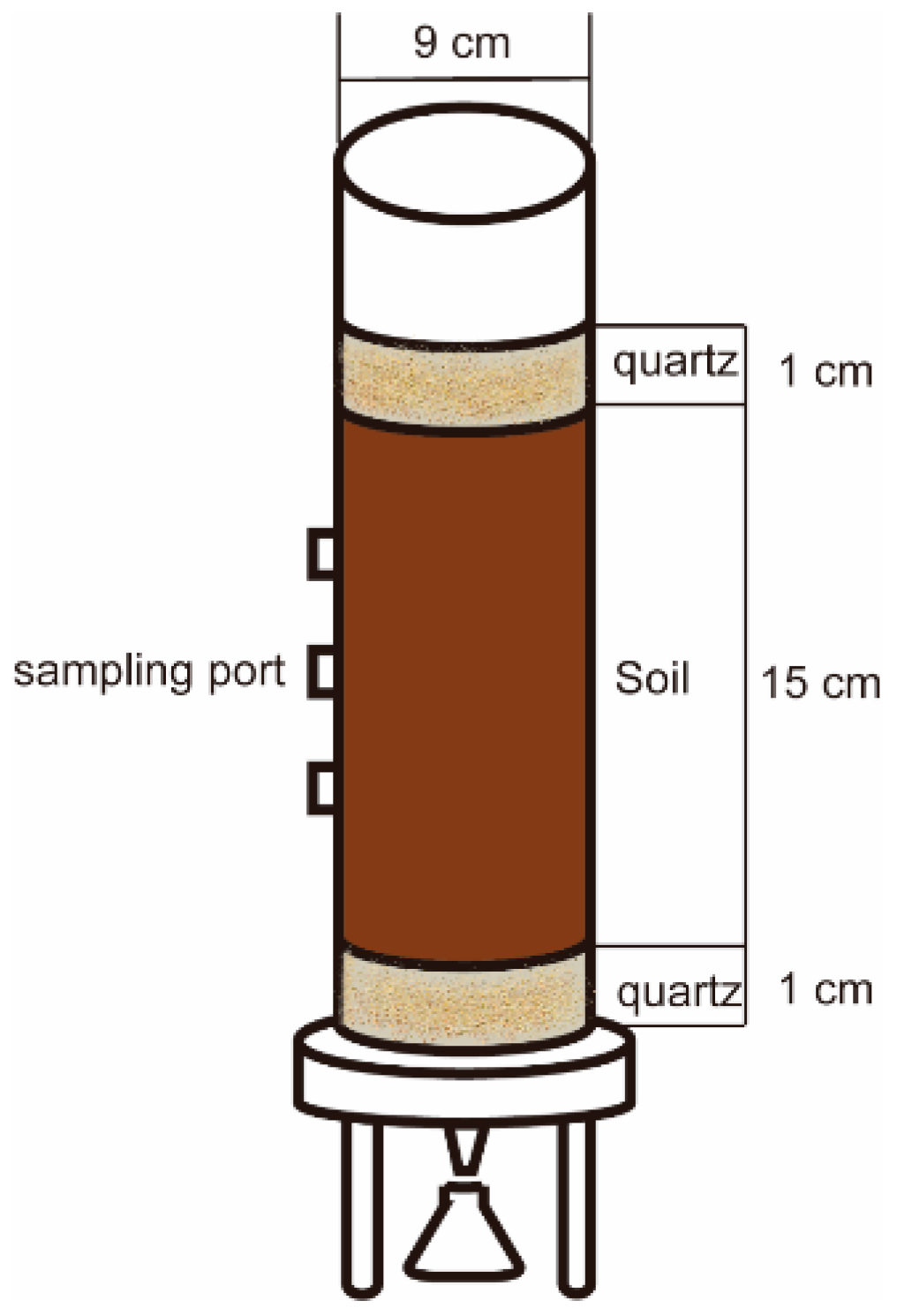
2.3.1. Simulation Test Method
2.3.2. Soil and Leachate Analyses and Measurements
2.4. Synergistic Responses of Crops to Acidification Combined with Arsenic Pollution
2.5. Statistical Analysis
3. Results and Discussion
3.1. Changes in Basic Soil Properties During Acidification
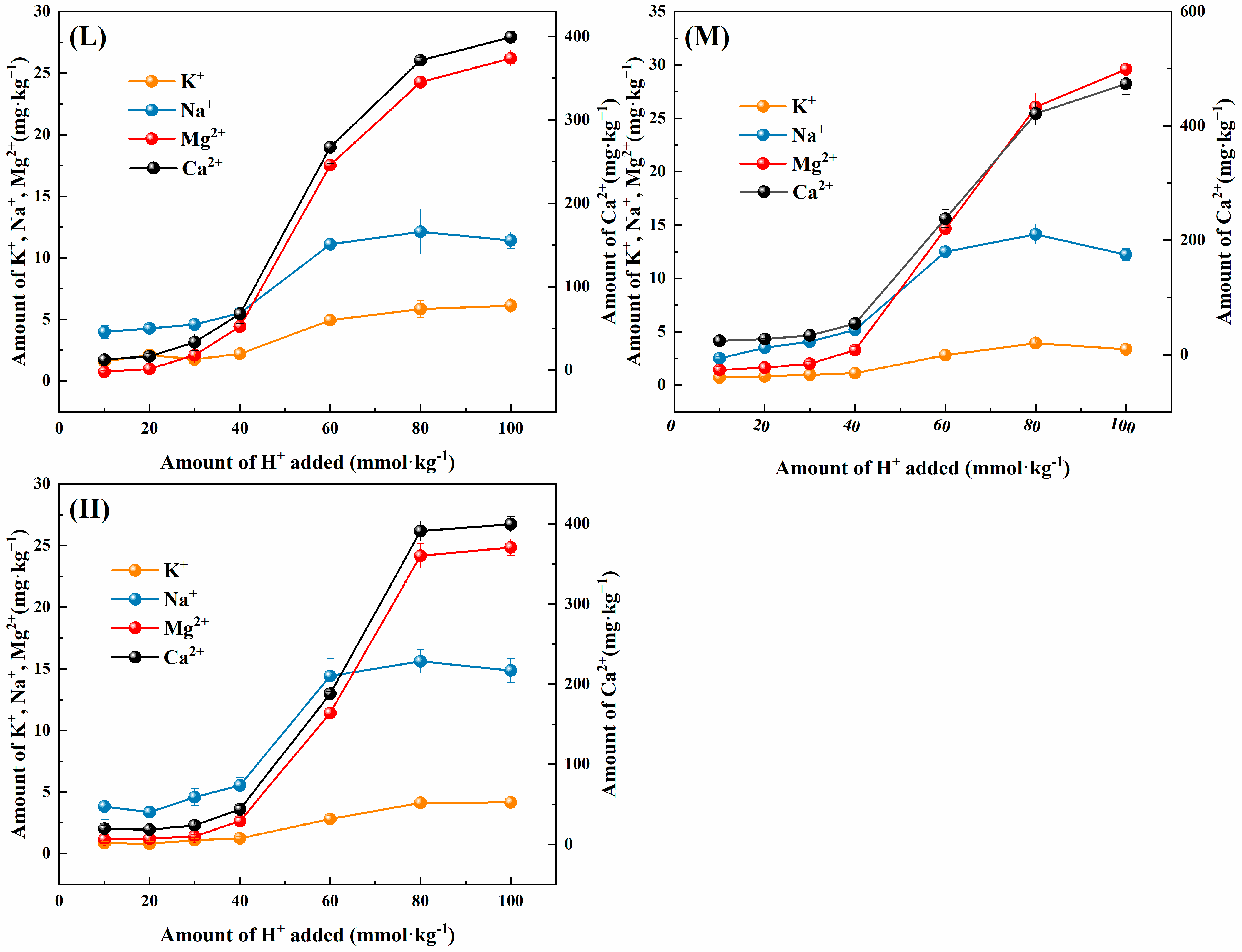
3.1.1. Changes in the Acid-Buffering Capacity
3.1.2. Changes in the Composition of Exchangeable Acids
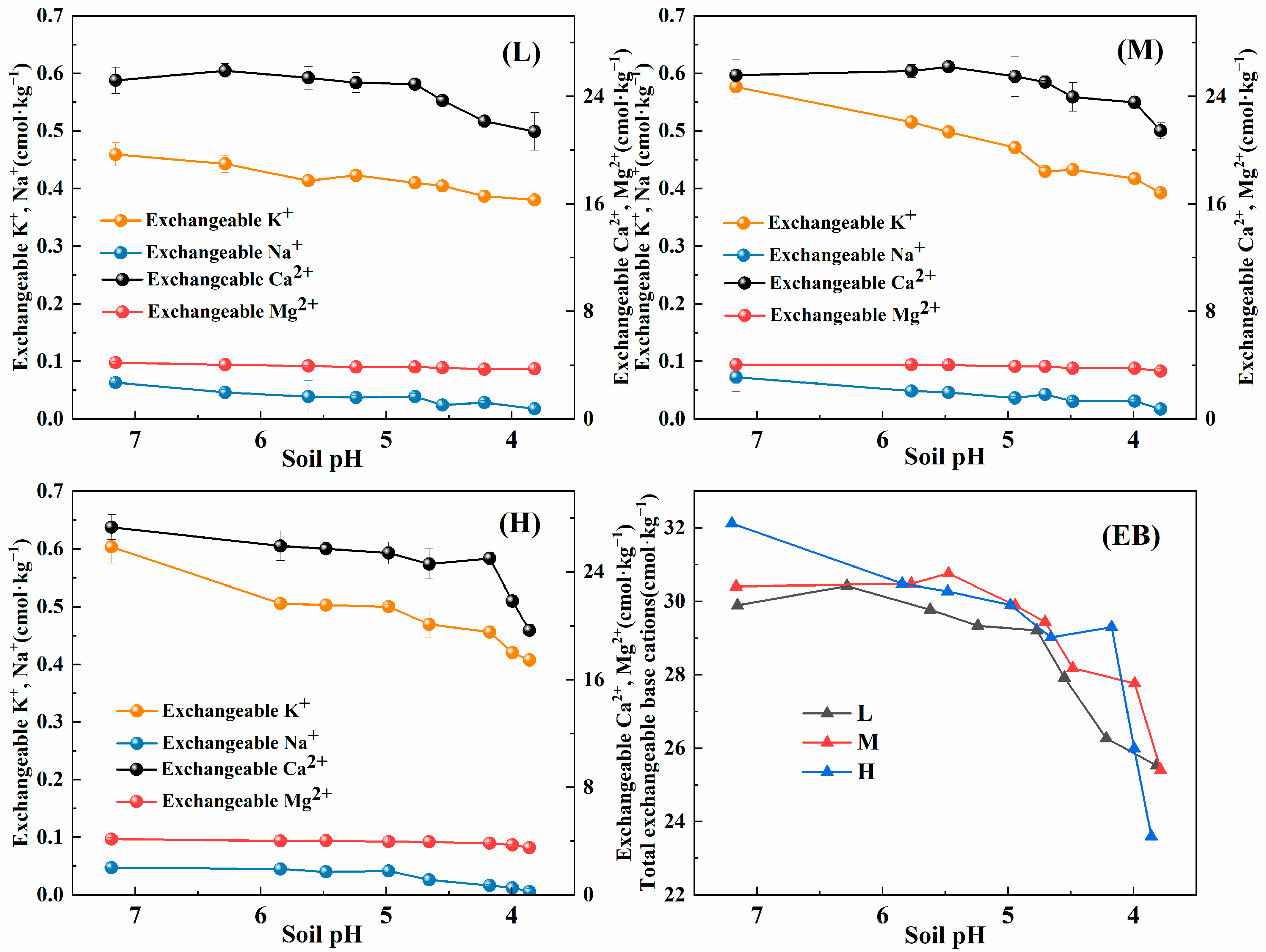
3.1.3. Changes in the Ionic Composition of Exchangeable Base Ions
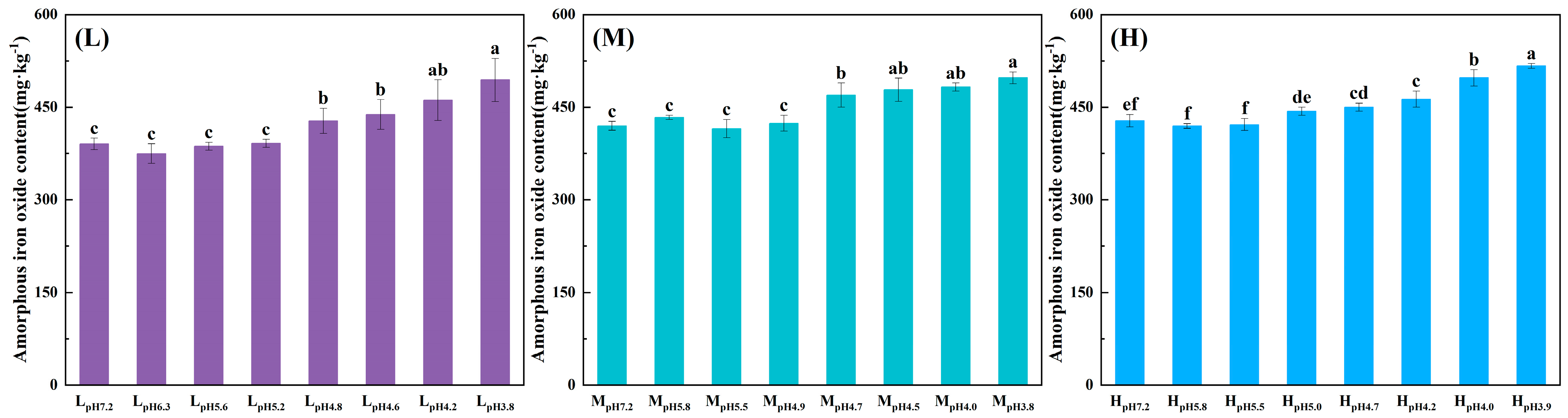
3.1.4. Changes in Soil Mineral Composition
3.2. Changes in Arsenic Forms During Soil Acidification
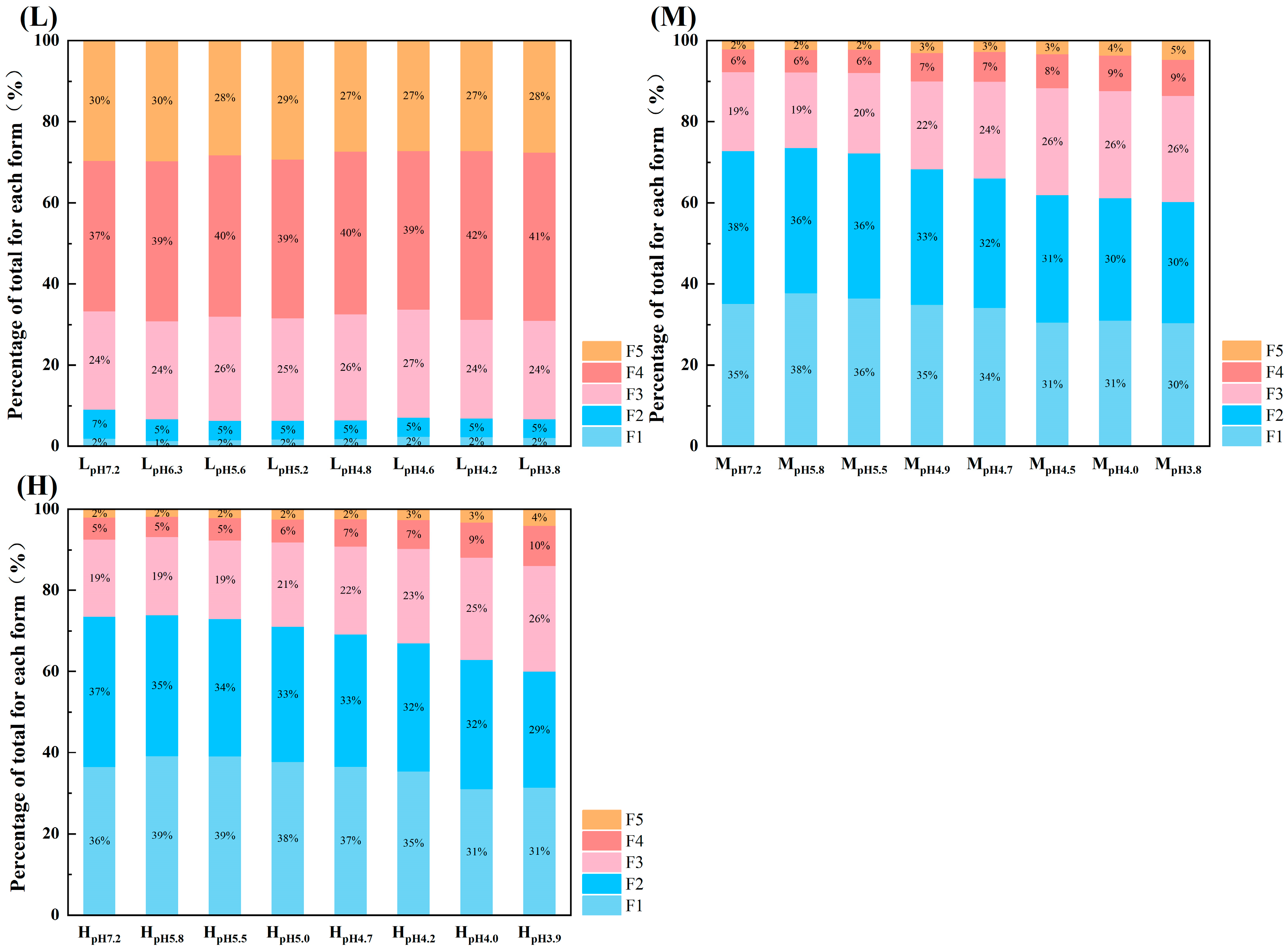
3.2.1. Morphological Fractionation of Arsenic
3.2.2. Changes in Migration Leaching and Availability of Arsenic
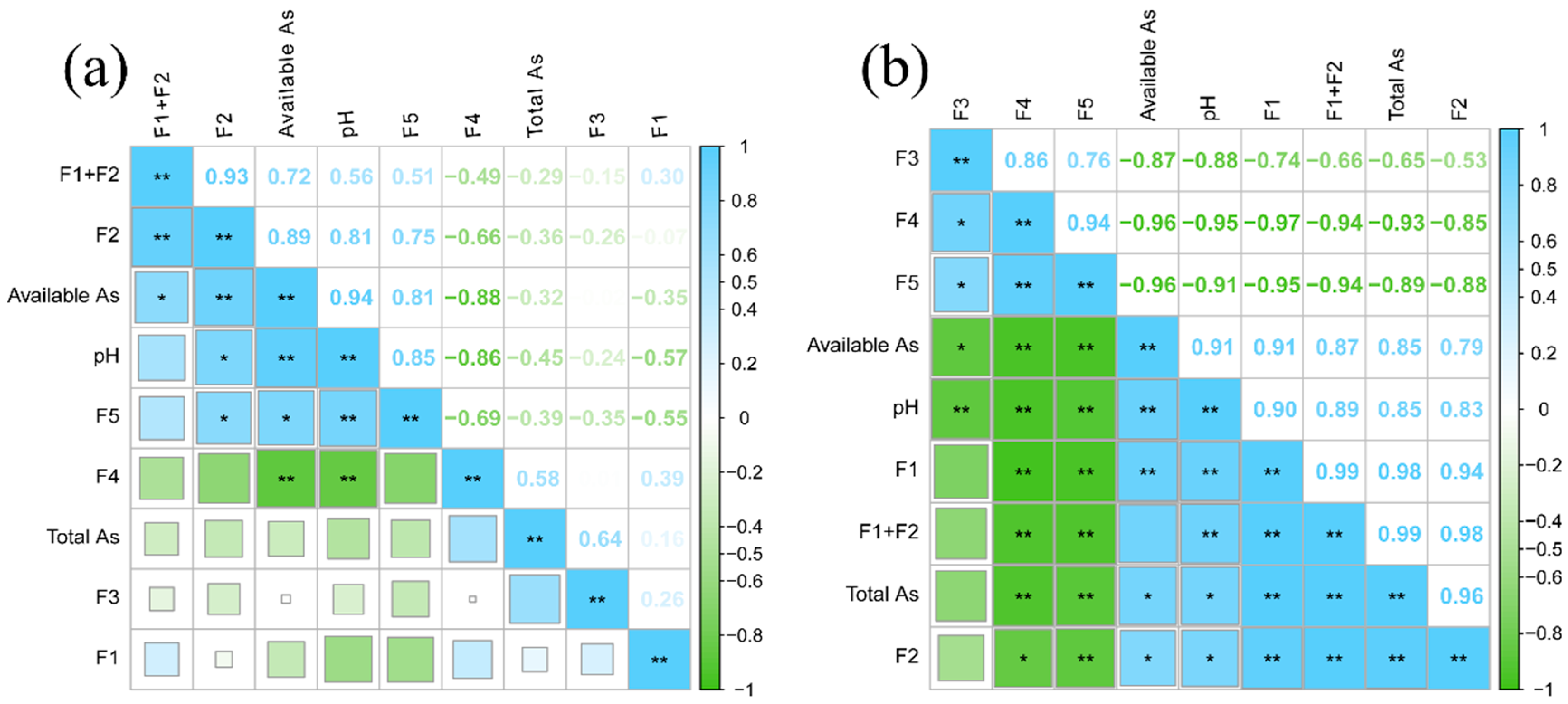
3.3. Arsenic Transformation in Relation to Soil Properties Driven by Acidification
3.4. Responses of Pakchoi to Soil Acidification Combined with Arsenic Contamination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Cai, J.P.; Luo, W.T.; Liu, H.Y.; Feng, X.; Zhang, Y.Y.; Wang, R.Z.; Xu, Z.W.; Zhang, Y.G.; Jiang, Y. Precipitation-mediated responses of soil acid buffering capacity to long-term nitrogen addition in a semi-arid grassland. Atmos. Environ. 2017, 170, 312–318. [Google Scholar] [CrossRef]
- Shi, Y.L.; Chen, W.Q.; Wu, S.L.; Zhu, Y.G. Anthropogenic Cycles of Arsenic in Mainland China: 1990–2010. Environ. Sci. Technol. 2017, 51, 1670–1678. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, H.M.; Yuan, H.Y.; Duan, G.L.; Jin, D.C.; Zhao, F.J.; Zhu, Y.G. Microbe mediated arsenic release from iron minerals and arsenic methylation in rhizosphere controls arsenic fate in soil-rice system after straw incorporation. Environ. Pollut. 2018, 236, 598–608. [Google Scholar] [CrossRef]
- Dai, Z.M.; Zhang, X.J.; Tang, C.; Muhammad, N.; Wu, J.J.; Brookes, P.C.; Xu, J.M. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Zhao, X.Q.; Shen, R.F. Molecular mechanisms of plant adaptation to acid soils: A review. Pedosphere 2023, 33, 14–22. [Google Scholar] [CrossRef]
- Ju, Y.W.; Luo, Z.W.; Bi, J.; Liu, C.Z.; Liu, X. Transfer of heavy metals from soil to tea and the potential human health risk in a regional high geochemical background area in southwest China. Sci. Total Environ. 2024, 908, 168122. [Google Scholar] [CrossRef]
- Li, Q.Q.; Luo, Y.L.; Wang, C.Q.; Li, B.; Zhang, X.; Yuan, D.G.; Gao, X.S.; Zhang, H. Spatiotemporal variations and factors affecting soil nitrogen in the purple hilly area of Southwest China during the 1980s and the 2010s. Sci. Total Environ. 2016, 547, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Bao, Y.H.; Wei, J.; He, X.B.; Tang, Q.; Wu, S.J.; Huang, P.; Ma, M.H.; Zhou, P.; Wang, M.F. Scaling properties of particle-size distributions of purple soils in a small agricultural watershed: A multifractal analysis. Catena 2022, 215, 106326. [Google Scholar] [CrossRef]
- Zhong, S.Q.; Han, Z.; Duo, J.; Ci, E.; Ni, J.P.; Xie, D.T.; Wei, C.F. Relationships between the lithology of purple rocks and the pedogenesis of purple soils in the Sichuan Basin, China. Sci. Rep. 2019, 9, 13272. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Wang, P.S.; Liu, L.; Zheng, Y.Y.; Xie, D.T. High negative surface charge increases the acidification risk of purple soil in China. Catena 2021, 196, 104819. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.B.; Müller, C.; Cai, Z.C. Temporal variations of crop residue effects on soil N transformation depend on soil properties as well as residue qualities. Biol. Fertil. Soils 2018, 54, 659–669. [Google Scholar] [CrossRef]
- Meng, H.Q.; Xu, M.G.; Lu, J.L.; He, X.H.; Li, J.W.; Shi, X.J.; Peng, C.; Wang, B.R.; Zhang, H.M. Soil pH Dynamics and Nitrogen Transformations Under Long-Term Chemical Fertilization in Four Typical Chinese Croplands. J. Integr. Agric. 2013, 12, 2092–2102. [Google Scholar] [CrossRef]
- Xie, J.; Wang, D.; Chen, Y.X.; Li, Z.Q.; Dai, W.C.; Huang, R.; Wang, Z.F.; Gao, M. Neutral purple soil acidification and mineralogical property changes due to long-term urea application in southwest China. Soil Tillage Res. 2024, 244, 106227. [Google Scholar] [CrossRef]
- Li, Q.Q.; Li, A.W.; Yu, X.L.; Dai, T.F.; Peng, Y.Y.; Yuan, D.; Zhao, B.; Tao, Q.; Wang, C.Q.; Li, B.; et al. Soil acidification of the soil profile across Chengdu Plain of China from the 1980s to 2010s. Sci. Total Environ. 2020, 698, 134320. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Meng, W.; Liu, N.; Wu, P. Accumulation and source apportionment of heavy metal(loid)s in agricultural soils based on GIS, SOM and PMF: A case study in superposition areas of geochemical anomalies and zinc smelting, Southwest China. Process Saf. Environ. Prot. 2022, 159, 964–977. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Tang, J.; Yang, J.; Guo, Z.; Xiao, Y.; Ge, Y.; Liu, T.; Hu, Q.; Ao, H.; et al. Cadmium absorption and translocation in rice plants are influenced by lower air temperatures during grain filling stage. Sci. Total Environ. 2024, 954, 176742. [Google Scholar] [CrossRef]
- Li, Y.; Tao, H.; Cao, H.; Wan, X.; Liao, X. Achieving synergistic benefits through integrated governance of cultivated cadmium contamination via multistakeholder collaboration. Nat. Commun. 2024, 15, 9817. [Google Scholar] [CrossRef]
- Zhao, K.L.; Liu, X.M.; Xu, J.M.; Selim, H.M. Heavy metal contaminations in a soil-rice system: Identification of spatial dependence in relation to soil properties of paddy fields. J. Hazard. Mater. 2010, 181, 778–787. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Simon, T.; Pechová, M. The availability of Cd, Pb and Zn and their relationships with soil pH and microbial biomass in soils amended by natural clinoptilolite. Plant Soil Environ. 2005, 51, 26–33. [Google Scholar] [CrossRef]
- Lu, M.; Huang, L.K.; Wang, Q.; Cao, X.R.; Lin, Q.; He, Z.L.; Feng, Y.; Yang, X.E. Soil properties drive the bacterial community to cadmium contamination in the rhizosphere of two contrasting wheat (Triticum aestivum L.) genotypes. J. Environ. Sci. 2023, 128, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Shi, Q.Y.; Li, Z.Y.; Deng, H.M.; Zhou, Q.; Li, L.H.; Zhao, L.Y.; Yuan, S.; Liu, Q.; Chen, Y.E. Rhodopseudomonas palustris shapes bacterial community, reduces Cd bioavailability in Cd contaminated flooding paddy soil, and improves rice performance. Sci. Total Environ. 2024, 926, 171824. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Lopipero, P.A.; Bates, M.N.; Steinmaus, C.M. Public health—Arsenic epidemiology and drinking water standards. Science 2002, 296, 2145–2146. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, B.O.; Orisakwe, O.E. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. J. Environ. Sci. Health Part C-Toxicol. Carcinog. 2020, 38, 204–244. [Google Scholar] [CrossRef]
- Qiao, J.T.; Liu, T.X.; Wang, X.Q.; Li, F.B.; Lv, Y.H.; Cui, J.H.; Zeng, X.D.; Yuan, Y.Z.; Liu, C.P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Zhao, F.J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.Y.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Feng, R.W.; Wang, L.Z.; Yang, J.G.; Zhao, P.P.; Zhu, Y.M.; Li, Y.P.; Yu, Y.S.; Liu, H.; Rensing, C.; Wu, Z.Y.; et al. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.Q.; Min, X.B.; Ke, Y.; Lin, Z.; Yang, Z.H.; Wang, S.; Peng, N.; Yan, X.; Luo, S.; Wu, J.H.; et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef] [PubMed]
- Standardization Administration of the People’s Republic of China. GB 15618-2018; Soil Environment Quality Risk Control Standard for Soil Contamination of Agriculture Land. China Environment Publishing Group: Beijing, China, 2018.
- Yang, J.K.; Barnett, M.O.; Jardine, P.M.; Basta, N.T.; Casteel, S.W. Adsorption, sequestration, and bioaccessibility of As(V) in soils. Environ. Sci. Technol. 2002, 36, 4562–4569. [Google Scholar] [CrossRef]
- Ministry of Agriculture of the People’s Republic of China. NY/T 1377-2007; Determination of Total Nitrogen, Total Phosphorus and Total Potassium in Soil—Kjeldahl Method. China Agriculture Press: Beijing, China, 2007.
- State Forestry Administration of the People’s Republic of China. LY/T 1246-1999; Determination of Exchangeable Potassium and Sodium in Forest Soil. China Standards Press: Beijing, China, 1999.
- State Forestry Administration of the People’s Republic of China. LY/T 1245-1999; Determination of Exchangeable Calcium and Magnesium in Forest Soil. China Standards Press: Beijing, China, 1999.
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- National Forestry Bureau of the People’s Republic of China. LY/T 1243–1999; Determination of Cation Exchange Capacity of Forest Soils. China Forestry Publishing House: Beijing, China, 1999.
- National Forestry Bureau of the People’s Republic of China. LY/T 1237–1999; Determination of Organic Matter in Forest Soils and Calculation of Carbon-Nitrogen Ratio. China Forestry Publishing House: Beijing, China, 1999.
- National Forestry Bureau of the People’s Republic of China. LY/T 1250-1999; Determination of Calcium Carbonate in Forest Soils. China Forestry Publishing House: Beijing, China, 1999.
- National Energy Administration of the People’s Republic of China. SY/T 5163-2018; Method for X-Ray Diffraction Analysis of Clay Minerals and Common Non-Clay Minerals in Sedimentary Rocks. China Petroleum Indus-try Press: Beijing, China, 2018.
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. (Eds.) Methods of Soil Analysis: Chemical Methods; SSSA Book Series; SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Chinese Standardization Administration. GB/T 22105.2-2008[S]; Soil Quality—Determination of Total Mercury, Total Arsenic and Total Lead by Atomic Fluorescence Spectrometry—Part 2: Determination of Total Arsenic In Soil. China Standard Press: Beijing, China, 2008.
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Woolson, E.A.; Kearney, P.C.; Axley, J.H. The Chemistry and Phytotoxicity of Arsenic in Soils: I. Contaminated Field Soils. Soil Sci. Soc. Am. Proc. 1971, 35, 938–943. [Google Scholar] [CrossRef]
- Wang, H.X.; Li, X.M.; Chen, Y.; Li, Z.B.; Hedding, D.W.; Nel, W.; Ji, J.F.; Chen, J. Geochemical behavior and potential health risk of heavy metals in basalt-derived agricultural soil and crops: A case study from Xuyi County, eastern China. Sci. Total Environ. 2020, 729, 139058. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Han, J.H.; Xue, W.X.; Wang, J.Y.; Wang, B.H.; Liu, L.J.; Zou, J.H. Overexpression of SmZIP plays important roles in Cd accumulation and translocation, subcellular distribution, and chemical forms in transgenic tobacco under Cd stress. Ecotoxicol. Environ. Saf. 2021, 214, 112097. [Google Scholar] [CrossRef]
- Kirkwood, D.E.; Nesbitt, H.W. Formation and evolution of soils from an acidified watershed: Plastic Lake, Ontario, Canada. Geochim. Cosmochim. Acta 1991, 55, 1295–1308. [Google Scholar] [CrossRef]
- Bartlett, R.J.; McIntosh, J.L. pH-Dependent Bonding of Potassium by a Spodosol. Soil Sci. Soc. Am. Proc. 1969, 33, 535–539. [Google Scholar] [CrossRef]
- Li, J.Y.; Xu, R.K. Inhibition of acidification of kaolinite and an Alfisol by aluminum oxides through electrical double-layer interaction and coating. Eur. J. Soil Sci. 2013, 64, 110–120. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C.X. Mobilization of soil-borne arsenic by three common organic acids: Dosage and time effects. Chemosphere 2016, 147, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, Y.L.; Xiang, H.M.; Zhang, J.E.; Li, S.F.; Yang, J.Y. Soil pH Responses to Simulated Acid Rain Leaching in Three Agricultural Soils. Sustainability 2020, 12, 280. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, S.Z.; Shan, X.Q.; Feng, M.H.; Zhu, Y.G.; McLaren, R.G. Adsorption of arsenate on soils. Part 2: Modeling the relationship between adsorption capacity and soil physiochemical properties using 16 Chinese soils. Environ. Pollut. 2005, 138, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Liu, Y.; Wang, D.J.; Wang, P.S.; Xu, R.K.; Xie, D.T. Characterizingsurface electrochemical properties of simulated bulk soil in situ by streaming potential measurements. Eur. J. Soil Sci. 2019, 70, 1063–1072. [Google Scholar] [CrossRef]
- Du, Y.; Tian, Z.; Zhao, Y.; Wang, X.; Ma, Z.; Yu, C. Exploring the accumulation capacity of dominant plants based on soil heavy metals forms and assessing heavy metals contamination characteristics near gold tailings ponds. J. Environ. Manag. 2023, 351, 119838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Li, Z.Y.; Lu, X.N.; Duan, Q.N.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.G.; Liu, F.C. Pollution in the urban soils of Lianyungang, China, evaluated using a pollution index, mobility of heavy metals, and enzymatic activities. Environ. Monit. Assess. 2017, 189, 34. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Zeng, X.B.; Lu, Y.H.; Bai, L.Y.; Su, S.M.; Wu, C.X. Dynamic arsenic aging processes and their mechanisms in nine types of Chinese soils. Chemosphere 2017, 187, 404–412. [Google Scholar] [CrossRef]
- Kim, E.J.; Yoo, J.C.; Baek, K. Arsenic speciation and bioaccessibility in arsenic-contaminated soils: Sequential extraction and mineralogical investigation. Environ. Pollut. 2014, 186, 29–35. [Google Scholar] [CrossRef]
- Shanmugapriya, S.; Subramanian, K. Developing a PLS path model to investigate the factors influencing safety performance improvement in construction organizations. KSCE J. Civ. Eng. 2016, 20, 1138–1150. [Google Scholar] [CrossRef]
- Tipper, E.T.; Stevenson, E.I.; Alcock, V.; Knight, A.C.G.; Baronas, J.J.; Hilton, R.G.; Bickle, M.J.; Larkin, C.S.; Feng, L.S.; Relph, K.E.; et al. Global silicate weathering flux overestimated because of sediment-water cation exchange. Proc. Natl. Acad. Sci. USA 2021, 118, e2016430118. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.P.; Yu, M.X.; Cao, N.N.; Yan, J.H. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]




| Soil Treatment | Fitted Equation (y = kx + b) | Acid-Buffering Capacity (mmol·kg−1) | |||
|---|---|---|---|---|---|
| k | b | R2 | |||
| L | A | −0.0582 | 6.9733 | 0.9722 | 17.18 |
| B | −0.0160 | 5.4560 | 0.9842 | 62.50 | |
| M | A | −0.0373 | 6.1550 | 0.9796 | 26.81 |
| B | −0.0163 | 5.3786 | 0.9712 | 61.35 | |
| H | A | −0.0406 | 6.2550 | 0.9936 | 24.63 |
| B | −0.0129 | 5.0730 | 0.9087 | 77.52 | |
| Treatment | Qtz | PI | MC | It | C | I/S | C/S | Hem |
|---|---|---|---|---|---|---|---|---|
| LpH7.2 | 35 | 22 | 6 | 5 | 6 | 8 | 15 | 3 |
| LpH5.6 | 34 | 20 | 9 | 7 | 5 | 6 | 16 | 3 |
| LpH4.6 | 36 | 19 | 8 | 6 | 3 | 6 | 18 | 4 |
| LpH3.8 | 38 | 18 | 5 | 6 | 4 | 7 | 19 | 3 |
| HpH7.2 | 37 | 20 | 8 | 6 | 7 | 7 | 12 | 3 |
| HpH3.9 | 39 | 16 | 7 | 6 | 4 | 9 | 20 | 3 |
| pH | Qtz | PI | MC | It | C | I/S | C/S | |
|---|---|---|---|---|---|---|---|---|
| pH | 1 | |||||||
| Qtz | −0.556 | 1 | ||||||
| PI | 0.856 * | −0.812 * | 1 | |||||
| MC | 0.247 | −0.472 | 0.122 | 1 | ||||
| It | −0.327 | −0.169 | −0.31 | 0.645 | 1 | |||
| C | 0.886 * | −0.254 | 0.61 | 0.108 | −0.215 | 1 | ||
| I/S | −0.065 | 0.594 | −0.349 | −0.484 | −0.541 | 0.136 | 1 | |
| C/S | −0.937 ** | 0.472 | −0.754 | −0.354 | 0.107 | −0.892 * | 0.252 | 1 |
| Soil Treatment | Biomass (g·pot−1, FW) | Plant Height (cm) |
|---|---|---|
| LpH6.9 | 16.23 ± 2.30 b | 17.1 ± 2.1 b |
| LpH6.1 | 21.13 ± 3.89 a | 21.6 ± 3.0 a |
| LpH5.4 | 11.73 ± 0.81 c | 12.3 ± 0.6 c |
| LpH4.6 | 6.78 ± 1.52 d | 8.10 ± 1.4 d |
| MpH6.9 | 6.80 ± 2.58 a | 7.6 ± 2.2 a |
| MpH6.1 | 5.04 ± 0.67 ab | 6.2 ± 0.6 ab |
| MpH5.4 | 2.34 ± 1.51 bc | 3.9 ± 1.0 b |
| MpH4.6 | 0 ± 0 d | - |
| HpH6.9 | 3.97 ± 1.73 a | 5.1 ± 0.7 a |
| HpH6.1 | 1.48 ± 0.68 b | 3.2 ± 0.3 b |
| HpH5.4 | 0.40 ± 0.26 b | 2.5 ± 0.3 b |
| HpH4.6 | 0 ± 0 b | - |
| Soil Treatment | Aboveground As Content (mg·kg−1) | BCF | TF |
|---|---|---|---|
| LpH6.9 | 0.37 ± 0.01 a | 0.11 ± 0.002 a | 0.367 ± 0.003 a |
| LpH6.1 | 0.32 ± 0.14 a | 0.09 ± 0.039 a | 0.193 ± 0.084 b |
| LpH5.4 | 0.30 ± 0.15 a | 0.08 ± 0.043 a | 0.139 ± 0.070 b |
| LpH4.6 | 0.33 ± 0.02 a | 0.12 ± 0.022 a | 0.223 ± 0.043 b |
| MpH6.9 | 14.98 ± 6.42 a | 0.19 ± 0.082 a | 0.008 ± 0.002 a |
| MpH6.1 | 18.81 ± 6.63 a | 0.24 ± 0.084 a | 0.007 ± 0.002 a |
| MpH5.4 | 18.27 ± 11.1 a | 0.23 ± 0.142 a | 0.011 ± 0.007 a |
| MpH4.6 | - | - | - |
| HpH6.9 | 25.91 ± 7.91 a | 0.21 ± 0.064 a | 0.009 ±0.003 ab |
| HpH6.1 | 24.87 ± 3.89 a | 0.20 ± 0.032 a | 0.007 ±0.001 b |
| HpH5.4 | 27.58 ± 0.29 a | 0.22 ± 0.002 a | 0.012 ±0.000 a |
| HpH4.6 | - | - | - |
| Parameters | Biomass | Aboveground As Content | BCF | TF | |
|---|---|---|---|---|---|
| Soil acidification | F | 178.57 | 47.968 | 0.172 | 6.665 |
| p-value | 0.00 ** | 0.00 ** | 0.914 | 0.003 ** | |
| As contamination | F | 34.10 | 19.871 | 10.460 | 108.10 |
| p-value | 0.00 ** | 0.00 ** | 0.001 ** | 0.000 ** | |
| Soil acidification × As contamination | F | 7.72 | 5.701 | 0.302 | 10.399 |
| p-value | 0.00 ** | 0.001 ** | 0.873 | 0.000 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Jiang, Z.; Wei, S. Arsenic Accumulation in Pakchoi Influenced by Acidification-Driven Changes in Soil Properties and Arsenic Transformation. Agriculture 2025, 15, 364. https://doi.org/10.3390/agriculture15040364
Chen J, Jiang Z, Wei S. Arsenic Accumulation in Pakchoi Influenced by Acidification-Driven Changes in Soil Properties and Arsenic Transformation. Agriculture. 2025; 15(4):364. https://doi.org/10.3390/agriculture15040364
Chicago/Turabian StyleChen, Jianjie, Zhenmao Jiang, and Shiqiang Wei. 2025. "Arsenic Accumulation in Pakchoi Influenced by Acidification-Driven Changes in Soil Properties and Arsenic Transformation" Agriculture 15, no. 4: 364. https://doi.org/10.3390/agriculture15040364
APA StyleChen, J., Jiang, Z., & Wei, S. (2025). Arsenic Accumulation in Pakchoi Influenced by Acidification-Driven Changes in Soil Properties and Arsenic Transformation. Agriculture, 15(4), 364. https://doi.org/10.3390/agriculture15040364






