Assessing Seed Germination and Plant Growth of Eruca vesicaria (L.) Cav. Cultivated in Biochar-Enriched Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Substrate Characterization
2.2. Germination Assessment
2.3. Plant Growth and Biomass Analysis
2.4. Chlorophyll and Flavonols Assessment
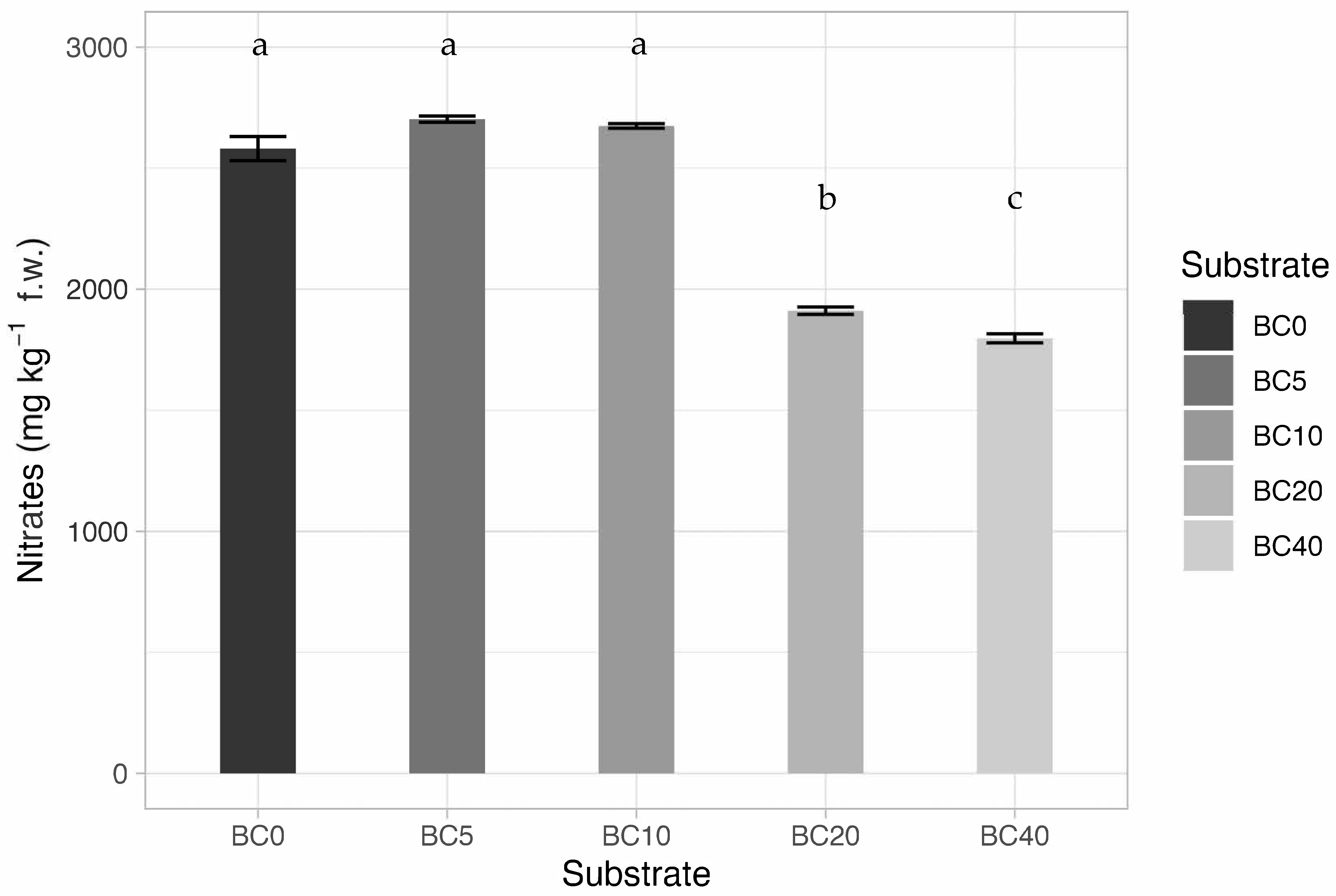
2.5. Nitrates
2.6. Mineral Content
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Substrates
3.2. Germination Assessment
3.3. Rocket Yield
3.4. Mineral and Heavy Metal Contents
3.5. Chlorophyll, Flavonols, Nitrate Content, and Leaf Color
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripodi, P.; Francese, G.; Mennella, G. Rocket Salad: Crop Description, Bioactive Compounds and Breeding Perspectives. Adv. Hortic. Sci. 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-Leaf and Multi-Leaf of Green and Red Lettuces Are Suitable Raw Materials for the Fresh-Cut Industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Hetta, M.H.; Owis, A.I.; Haddad, P.S.; Eid, H.M. The Fatty Acid-Rich Fraction of Eruca sativa (Rocket Salad) Leaf Extract Exerts Antidiabetic Effects in Cultured Skeletal Muscle, Adipocytes and Liver Cells. Pharm. Biol. 2017, 55, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, Z. Reducing the Nitrate Content in Vegetables Through Joint Regulation of Short-Distance Distribution and Long-Distance Transport. Front. Plant Sci. 2020, 11, 1079. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in Vegetables: Toxicity, Content, Intake and EC Regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Statement on Possible Public Health Risks for Infants and Young Children from the Presence of Nitrates in Leafy Vegetables. EFSA J. 2010, 8, 1935. [Google Scholar]
- The European Commission. Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs (Text with EEA Relevance). Available online: https://Faolex.Fao.Org/Docs/Pdf/Eur108181.Pdf (accessed on 6 September 2024).
- Fontana, E.; Nicola, S. Traditional and Soilless Culture Systems to Produce Corn Salad (Valerianella olitoria L.) and Rocket (Eruca sativa Mill.) with Low Nitrate Content. J. Food Agric. Environ. 2009, 7, 405–410. [Google Scholar]
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques—Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gasco, G. The Effect of Sewage Sludge Biochar on Peat-Based Growing Media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Lin, X.; Gonzalez Neira, A.C.; Tabay Zambon, F.; Hu, H.; Wang, X.; Huang, J.-H.; Fan, G. Substrate pH Influences the Nutrient Absorption and Rhizosphere Microbiome of Huanglongbing-Affected Grapefruit Plants. Front. Plant Sci. 2022, 13, 856937. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Bozdar, B.; Tu, P. Micronutrients and Their Effects on Horticultural Crop Quality, Productivity and Sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Mall, M.; Kumar, R.; Akhtar, M.Q. Horticultural Crops and Abiotic Stress Challenges. In Stress Tolerance in Horticultural Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–19. [Google Scholar]
- Schmilewski, G. The Role of Peat in Assuring the Quality of Growing Media. Mires Peat 2008, 3, 2. [Google Scholar]
- Tietjen, S.; Graubner, I.; Sradnick, A. Reducing Peat in Substrate Mixture Formulations for Press Pots Using the Taguchi Method. Sci. Hortic. 2022, 295, 110838. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; Van Winkel, A. Growing Media for Food and Quality of Life in the Period 2020–2050. Acta Hortic. 2019, 1305, 341–356. [Google Scholar] [CrossRef]
- Clarke, D.; Rieley, J. Strategy for Responsible Peatland Management; International Peatland Society: Jyväskylä, Finland, 2010; ISBN 9529940122. [Google Scholar]
- Loisel, J.; Gallego-Sala, A. Ecological Resilience of Restored Peatlands to Climate Change. Commun. Earth Environ. 2022, 3, 208. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Tzortzakis, N. Wood-Based Biochar Ratio Used for Partial Peat Replacement in Growing Media for Antirrhinum Majus Pot Production. Agriculture 2024, 14, 1860. [Google Scholar] [CrossRef]
- Del Bubba, M.; Anichini, B.; Bakari, Z.; Bruzzoniti, M.C.; Camisa, R.; Caprini, C.; Checchini, L.; Fibbi, D.; El Ghadraoui, A.; Liguori, F. Physicochemical Properties and Sorption Capacities of Sawdust-Based Biochars and Commercial Activated Carbons Towards Ethoxylated Alkylphenols and Their Phenolic Metabolites in Effluent Wastewater from a Textile District. Sci. Total Environ. 2020, 708, 135217. [Google Scholar] [CrossRef]
- Wood, R.; Mašek, O.; Erastova, V. Developing a Molecular-Level Understanding of Biochar Materials Using Public Characterization Data. Cell Rep. Phys. Sci. 2024, 5, 102036. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K. Development and Evaluation of Biochar-Based Secondary and Micronutrient Enriched Slow Release Nano-Fertilizer for Reduced Nutrient Losses. Biomass Convers. Biorefin 2023, 13, 12193–12204. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef] [PubMed]
- Linam, F.; Limmer, M.A.; Ebling, A.M.; Seyfferth, A.L. Rice Husk and Husk Biochar Soil Amendments Store Soil Carbon While Water Management Controls Dissolved Organic Matter Chemistry in Well-Weathered Soil. J. Environ. Manag. 2023, 339, 117936. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, R.; Sánchez, E.; Fabani, P.; Mazza, G.; Rodriguez, R. Almond Shell Biochar: Characterization and Application in Soilless Cultivation of Eruca sativa. Biomass Convers. Biorefin 2024, 14, 18183–18200. [Google Scholar] [CrossRef]
- Sánchez, E.; Zabaleta, R.; Fabani, M.P.; Rodriguez, R.; Mazza, G. Effects of the Amendment with Almond Shell, Bio-Waste and Almond Shell-Based Biochar on the Quality of Saline-Alkali Soils. J. Environ. Manag. 2022, 318, 115604. [Google Scholar] [CrossRef]
- Massa, D.; Bonetti, A.; Cacini, S.; Faraloni, C.; Prisa, D.; Tuccio, L.; Petruccelli, R. Soilless Tomato Grown Under Nutritional Stress Increases Green Biomass but Not Yield or Quality in Presence of Biochar as Growing Medium. Hortic. Environ. Biotechnol. 2019, 60, 871–881. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of Biochar Application on Nutritional Quality of Tomato (Lycopersicon esculentum). Crop Pasture Sci. 2015, 66, 747–755. [Google Scholar] [CrossRef]
- Simiele, M.; Argentino, O.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Terzaghi, M.; Montagnoli, A. Biochar Enhances Plant Growth, Fruit Yield, and Antioxidant Content of Cherry Tomato (Solanum lycopersicum L.) in a Soilless Substrate. Agriculture 2022, 12, 1135. [Google Scholar] [CrossRef]
- Yu, P.; Huang, L.; Li, Q.; Lima, I.M.; White, P.M.; Gu, M. Effects of Mixed Hardwood and Sugarcane Biochar as Bark-Based Substrate Substitutes on Container Plants Production and Nutrient Leaching. Agronomy 2020, 10, 156. [Google Scholar] [CrossRef]
- Awad, Y.M.; Lee, S.-E.; Ahmed, M.B.M.; Vu, N.T.; Farooq, M.; Kim, I.S.; Kim, H.S.; Vithanage, M.; Usman, A.R.A.; Al-Wabel, M. Biochar, a Potential Hydroponic Growth Substrate, Enhances the Nutritional Status and Growth of Leafy Vegetables. J. Clean. Prod. 2017, 156, 581–588. [Google Scholar] [CrossRef]
- Parlavecchia, M.; Carnimeo, C.; Loffredo, E. Soil Amendment with Biochar, Hydrochar and Compost Mitigates the Accumulation of Emerging Pollutants in Rocket Salad Plants. Water Air Soil. Pollut. 2020, 231, 554. [Google Scholar] [CrossRef]
- Milone, J.; Casas, C.; Vega, A.S. Guadua chacoensis Bamboo Biochar (Poaceae, Bambuseae) Affected Horticultural Species in a Saline-Alkaline Soil. Cienc. Suelo 2023, 41, 273–284. [Google Scholar]
- Batta, N.; Heuchan, S.M.; Stokes-Rees, J.; Moreira, C.; Berruti, F. Production of Biochar for Treatment of Retting Effluents and Utilization of Spent Biochar as Potential Germination Medium for Leafy Greens. Biomass Bioenergy 2024, 186, 107266. [Google Scholar] [CrossRef]
- Sun, R.; Wang, J.; Peng, Y.; Wang, H.; Chen, Q. Mitigation of Arsenic Accumulation in Arugula (Eruca sativa Mill.) Using. Fe/Al/Zn Impregnated Biochar Composites. Environ. Sci. Pollut. Res. 2021, 28, 4136–4146. [Google Scholar]
- Bini, L.; Renai, L.; Fichera, M.; Petrucci, W.A.; Lenzi, A.; Biricolti, S.; Giordani, E.; Rivoira, L.; Bruzzoniti, M.C.; Piesik, D. Assessing the Impact of Sustainable Biochar-Enriched Substrates on Safety and Quality of Tomato (Solanum lycopersicum L.) as Relevant Model Crop. ACS Agric. Sci. Technol. 2024, 4, 681–689. [Google Scholar]
- El-Kassaby, Y.A.; Moss, I.; Kolotelo, D.; Stoehr, M. Seed Germination: Mathematical Representation and Parameters Extraction. For. Sci. 2008, 54, 220–227. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved Equations for the Prediction of Seed Longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar]
- Wardle, D.A.; Ahmed, M.; Nicholson, K.S. Allelopathic Influence of Nodding Thistle (Carduus nutans L.) Seeds on Germination and Radicle Growth of Pasture Plants. N. Z. J. Agric. Res. 1991, 34, 185–191. [Google Scholar]
- Melville, A.H.; Galletta, G.J.; Draper, A.D.; Ng, T.J. Seed Germination and Early Seedling Vigor in Progenies of Inbred Strawberry Selections. HortScience 1981, 15, 749–750. [Google Scholar]
- Aravind, J.; Vimala, D.; Radharani, J.; Jacob, S.R.; Srinivasa, K. The Germinationmetrics Package: A Brief Introduction; ICAR-National Bureau of Plant Genetic Resources: New Delhi, India, 2019; pp. 1–62. [Google Scholar]
- Rodríguez, G.R.; Moyseenko, J.B.; Robbins, M.D.; Morejón, N.H.; Francis, D.M.; van der Knaap, E. Tomato Analyzer: A Useful Software Application to Collect Accurate and Detailed Morphological and Colorimetric Data from Two-Dimensional Objects. J. Vis. Exp. 2010, 37, 1856. [Google Scholar]
- Cataldo, D.A.; Schrader, L.E.; Youngs, V.L. Analysis by Digestion and Colorimetric Assay of Total Nitrogen in Plant Tissues High in Nitrate 1. Crop Sci. 1974, 14, 854–856. [Google Scholar]
- DIN ISO 11466:1995-03; Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. International Organization for Standardization: Geneva, Switzerland, 1995.
- Dinno, A. Package ‘Dunn. Test’. CRAN 2017. [Google Scholar] [CrossRef]
- Kassambara, A. Pipe-Friendly Framework for Basic Statistical Tests [R Package Rstatix Version 0.7.0]; Free Software Foundation Inc.: Boston, MA, USA, 2021. [Google Scholar]
- Wei, T.; Simko, V.R. Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.88). Available online: https://github.com/taiyun/corrplot (accessed on 20 September 2024).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Kassambara, A. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. CRAN 2016. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and Quality of Basil, Swiss Chard, and Rocket Microgreens Grown in a Hydroponic System. N. Z. J. Crop Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Nicola, S.; Pignata, G.; Casale, M.; Lo Turco, P.E.; Gaino, W. Overview of a Lab-Scale Pilot Plant for Studying Baby Leaf Vegetables Grown in Soilless Culture. Hortic. J. 2016, 85, 97–104. [Google Scholar]
- Teodoro, M.; Trakal, L.; Gallagher, B.N.; Šimek, P.; Soudek, P.; Pohořelý, M.; Beesley, L.; Jačka, L.; Kovář, M.; Seyedsadr, S.; et al. Application of Co-Composted Biochar Significantly Improved Plant-Growth Relevant Physical/Chemical Properties of a Metal Contaminated Soil. Chemosphere 2020, 242, 125255. [Google Scholar] [CrossRef]
- Carril, P.; Ghorbani, M.; Loppi, S.; Celletti, S. Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops. Plants 2023, 12, 2235. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N. Advances and Future Directions of Biochar Characterization Methods and Applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar]
- Mumme, J.; Getz, J.; Prasad, M.; Lüder, U.; Kern, J.; Mašek, O.; Buss, W. Toxicity Screening of Biochar-Mineral Composites Using Germination Tests. Chemosphere 2018, 207, 91–100. [Google Scholar]
- Prapagdee, S.; Tawinteung, N. Effects of Biochar on Enhanced Nutrient Use Efficiency of Green Bean, Vigna radiata L. Environ. Sci. Pollut. Res. 2017, 24, 9460–9467. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, S.; Thian, B.W.Y.; Fong, S.L.; Ee, A.W.L.; Arora, S.; Ghosh, S.; Li, S.F.Y.; Tan, H.T.W.; Dai, Y.; et al. Impacts of Biochar Concentration on the Growth Performance of a Leafy Vegetable in a Tropical City and Its Global Warming Potential. J. Clean. Prod. 2020, 264, 121678. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit Quality, Antioxidant Capacity, and Flavonoid Content of Organically and Conventionally Grown Blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.M.; Sareen, P.; Sengar, R.S.; Kumar, A. Plant Ionomics: A Newer Approach to Study Mineral Transport and Its Regulation. Acta Physiol. Plant 2013, 35, 2641–2653. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; AlJuhaimi, F.; Özcan, M.M.; Uslu, N.; Karrar, E. Effect of Heating Processes on Bioactive Properties, Phenolic Components and Mineral Amounts of Rocket (Eruca sativa Mill.) Leaves. Int. J. Food Sci. Technol. 2024, 59, 4755–4764. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar Additions Alter Phosphorus and Nitrogen Availability in Agricultural Ecosystems: A Meta-Analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- Buss, W.; Assavavittayanon, K.; Shepherd, J.G.; Heal, K.V.; Sohi, S. Biochar Phosphorus Release Is Limited by High PH and Excess Calcium. J. Environ. Qual. 2018, 47, 1298–1303. [Google Scholar] [CrossRef]
- Buss, W.; Bogush, A.; Ignatyev, K.; Masek, O. Unlocking the Fertilizer Potential of Waste-Derived Biochar. ACS Sustain. Chem. Eng. 2020, 8, 12295–12303. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Improving Electrochemical Characteristics of Plant Roots by Biochar Is an Efficient Mechanism in Increasing Cations Uptake by Plants. Chemosphere 2023, 313, 137365. [Google Scholar] [CrossRef]
- Gunarathne, V.; Mayakaduwa, S.; Vithanage, M. Biochar’s Influence as a Soil Amendment for Essential Plant Nutrient Uptake. In Essential Plant Nutrients: Uptake, Use Efficiency, and Management; Springer: Berlin/Heidelberg, Germany, 2017; pp. 47–67. ISBN 9783319588414. [Google Scholar]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of Cow Manure Biochar on Maize Productivity under Sandy Soil Condition. Soil. Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Fontes, P.C.R.; Pereira, P.R.G.; Conde, R.M. Critical Chlorophyll, Total Nitrogen, and Nitrate-Nitrogen in Leaves Associated to Maximum Lettuce Yield. J. Plant Nutr. 1997, 20, 1061–1068. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and Nitrite Content of Vegetables, Fruits, Grains, Legumes, Dairy Products, Meats and Processed Meats. J. Food Compos. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Signore, A.; Amoruso, F.; Gallegos-Cedillo, V.M.; Gómez, P.A.; Ochoa, J.; Egea-Gilabert, C.; Costa-Pérez, A.; Domínguez-Perles, R.; Moreno, D.A.; Pascual, J.A.; et al. Agro-Industrial Compost in Soilless Cultivation Modulates the Vitamin C Content and Phytochemical Markers of Plant Stress in Rocket Salad (Diplotaxis tenuifolia (L.) DC.). Agronomy 2023, 13, 544. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated Applications of Organic Amendments Promote Beneficial Microbiota, Improve Soil Fertility and Increase Crop Yield. Appl. Soil. Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Saffeullah, P.; Nabi, N.; Zaman, M.B.; Liaqat, S.; Siddiqi, T.O.; Umar, S. Efficacy of Characterized Prosopis Wood Biochar Amendments in Improving Growth, Nitrogen Use Efficiency, Nitrate Accumulation, and Mineral Content in Cabbage Genotypes. J. Soil. Sci. Plant Nutr. 2021, 21, 690–708. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Shen, Q.; Hedley, M.; Camps Arbestain, M.; Kirschbaum, M.U.F. Can Biochar Increase the Bioavailability of Phosphorus? J. Soil. Sci. Plant Nutr. 2016, 16, 268–286. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar Reduced Nitrate Leaching and Improved Soil Moisture Content Without Yield Improvements in a Four-Year Field Study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]

| BC0 | BC5 | BC10 | BC20 | BC40 | BC70 | |
|---|---|---|---|---|---|---|
| GP (%) | 84.33 (7.65) a | 85.17 (2.95) a | 88.33 (3.98) a | 86.33 (3.33) a | 86.00 (2.83) a | 29.17 (6.34) b |
| LastGermTime (d) | 8.25 (1.5) a | 7.50 (1) a | 7.25 (1.71) a | 7.00 (1.63) a | 9.00 (0.82) a | 9.50 (1.29) a |
| S (n d−1) | 35.67 (9.11) a | 35.43 (7.81) a | 40.13 (5.31) a | 35.26 (6.30) a | 34.06 (5.91) a | 7.21 (1.75) b |
| Dlag50 (d) | 3.20 (1) b | 3.27 (0.96) b | 2.85 (0.34) b | 2.98 (0.57) b | 3.50 (1) ab | 5.89 (0.41) a |
| TimeSpreadGerm (d) | 4.75 (0.96) a | 4.00 (1.63) a | 4.25 (1.71) a | 4.00 (1.63) a | 6.00 (0.82) a | 5.50 (1.29) a |
| GI (d) | 8.15 (0.81) a | 8.09 (0.88) a | 8.45 (0.47) a | 8.26 (0.6) a | 7.7 (0.98) ab | 5.47 (0.35) b |
| t50total (d) | 3.26 (0.99) a | 3.35 (0.96) a | 2.96 (0.51) a | 3.13 (0.72) a | 3.75 (1.12) a | 0 (0) b |
| MGT (d) | 3.88 (0.91) ab | 3.82 (0.95) ab | 3.42 (0.46) b | 3.56 (0.66) b | 4.11 (1.01) ab | 6.93 (1.28) a |
| BC0 | BC5 | BC10 | BC20 | BC40 | |
|---|---|---|---|---|---|
| Fresh Weight (g plant−1) | 0.59 (0.09) a | 0.49 (0.1) ab | 0.54 (0.07) ab | 0.68 (0.12) a | 0.36 (0.03) b |
| Dry Weight (g plant−1) | 0.033 (0.005) a | 0.025 (0.013) a | 0.025 (0.006) a | 0.035 (0.006) a | 0.023 (0.005) a |
| Number of Leaves (n plant−1) | 5.25 (0.48) a | 4.63 (0.53) a | 4.97 (0.54) a | 5.3 (0.18) a | 4.7 (0.57) a |
| Blade Lenght (cm) | 4.96 (1.01) a | 4.28 (0.81) a | 4.48 (0.56) a | 5.23 (1.26) a | 3.87 (0.42) a |
| Petiole Lenght (cm) | 12.19 (2.05) a | 10.55 (0.86) a | 10.99 (1.30) a | 12.38 (1.20) a | 9.79 (0.88) a |
| Leaf Area (cm2 plant−1) | 21.45 (6.08) ab | 15.88 (3.73) bc | 16.52 (1.32) bc | 24.58 (2.51) a | 12.43 (2.87) c |
| Specific Leaf Area (cm2 g−1) | 689.92 (69.75) ab | 645.24 (129.92) bc | 657.89 (110.92) bc | 711.76 (86.90) a | 588.69 (112.11) c |
| BC0 | BC5 | BC10 | BC20 | BC40 | |
|---|---|---|---|---|---|
| Elements (g kg−1 d.w.) | |||||
| Ca | 21.47 (0.66) b | 23.82 (0.89) ab | 25.98 (0.30) a | 22.06 (0.52) b | 16.37 (0.71) c |
| K | 55.79 (0.99) ab | 51.48 (0.81) b | 55.35 (1.10) ab | 53.94 (1.38) ab | 59.93 (2.5) a |
| Mg | 3.67 (0.40) b | 5.69 (0.04) a | 5.41 (0.05) a | 5.03 (0.19) ab | 5.28 (0.20) a |
| Na | 8.24 (0.26) b | 8.94 (0.03) ab | 11.36 (0.74) ab | 12.15 (0.43) a | 11.95 (0.06) a |
| P | 7.25 (0.11) a | 7.11 (0.15) a | 6.50 (0.08) b | 6.17 (0.19) b | 6.50 (0.10) b |
| Heavy metals (mg kg−1 d.w.) | |||||
| Fe | 420.67 (8.71) a | 415.60 (21.50) a | 456.15 (47.30) a | 418.20 (26.03) a | 396.56 (88.06) a |
| Cu | 4.82 (1.1) a | 2.73 (0.43) a | 4.24 (0.47) a | 2.82 (0.27) a | 3.91 (0.92) a |
| Mn | 173.58 (7.12) a | 174.56 (10.07) a | 192.98 (12.60) a | 150.11 (26.58) a | 165.25 (37.76) a |
| Ni | 2.07 (0.62) a | 1.34 (0.40) a | 1.99 (0.32) a | 1.88 (0.83) a | 1.46 (0.59) a |
| Zn | 16.98 (2.33) b | 18.67 (2.6) ab | 19.73 (1.66) ab | 18.02 (1.50) ab | 24.35 (2.20) a |
| BC0 | BC5 | BC10 | BC20 | BC40 | |
|---|---|---|---|---|---|
| Chl (µg cm−2) | 14.03 (1.13) a | 13.5 (0.88) a | 14.7 (0.99) a | 13.78 (0.97) a | 13.08 (1.13) a |
| Flav (µg cm−2) | 0.17 (0.03) a | 0.19 (0.04) a | 0.2 (0.03) a | 0.22 (0.03) a | 0.23 (0.01) a |
| L* | 50.31 (1.45) ab | 51.28 (0.64) ab | 50.51 (0.81) b | 51.87 (0.92) ab | 52.47 (0.77) a |
| a* | −5.08 (0.85) a | −3.78 (1.35) a | −4.47 (1) a | −3.77 (0.96) a | −4.59 (0.6) a |
| b* | 34.06 (0.66) a | 33.89 (0.49) a | 33.53 (0.48) a | 32.75 (0.81) a | 33.16 (1.06) a |
| PC1 | PC2 | |
|---|---|---|
| NL | 0.190 | 0.001 |
| FW | 0.257 * | 0.124 |
| DW | 0.168 | 0.002 |
| Flav | −0.250 * | 0.279 * |
| L* | −0.303 * | 0.165 |
| a* | −0.011 | 0.365 * |
| Ca | 0.294 * | 0.155 |
| Cu | 0.062 | −0.318 * |
| Fe | 0.263 * | 0.117 |
| K | −0.221 | −0.152 |
| Mg | −0.117 | 0.317 * |
| Mn | 0.139 | −0.099 |
| Na | −0.159 | 0.311 * |
| Ni | 0.232 * | −0.061 |
| P | 0.104 | −0.345 * |
| Zn | −0.304 * | 0.022 |
| Nitrates | 0.255 * | −0.115 |
| GP | 0.028 | 0.296 * |
| TimeSpreadGerm | −0.271 * | −0.214 |
| GI | 0.307 * | 0.170 |
| MGT | −0.244 * | −0.282 * |
| Variance percentage | 40.85% | 24.40% |
| Cumulative variance percentage | 40.85% | 65.25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bini, L.; Biricolti, S.; Lenzi, A.; Del Bubba, M.; Petrucci, W.A.; Giordani, E. Assessing Seed Germination and Plant Growth of Eruca vesicaria (L.) Cav. Cultivated in Biochar-Enriched Substrates. Agriculture 2025, 15, 302. https://doi.org/10.3390/agriculture15030302
Bini L, Biricolti S, Lenzi A, Del Bubba M, Petrucci WA, Giordani E. Assessing Seed Germination and Plant Growth of Eruca vesicaria (L.) Cav. Cultivated in Biochar-Enriched Substrates. Agriculture. 2025; 15(3):302. https://doi.org/10.3390/agriculture15030302
Chicago/Turabian StyleBini, Lorenzo, Stefano Biricolti, Anna Lenzi, Massimo Del Bubba, William Antonio Petrucci, and Edgardo Giordani. 2025. "Assessing Seed Germination and Plant Growth of Eruca vesicaria (L.) Cav. Cultivated in Biochar-Enriched Substrates" Agriculture 15, no. 3: 302. https://doi.org/10.3390/agriculture15030302
APA StyleBini, L., Biricolti, S., Lenzi, A., Del Bubba, M., Petrucci, W. A., & Giordani, E. (2025). Assessing Seed Germination and Plant Growth of Eruca vesicaria (L.) Cav. Cultivated in Biochar-Enriched Substrates. Agriculture, 15(3), 302. https://doi.org/10.3390/agriculture15030302







