Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans
Abstract
1. Introduction
2. Melatonin Synthesis and Secretion
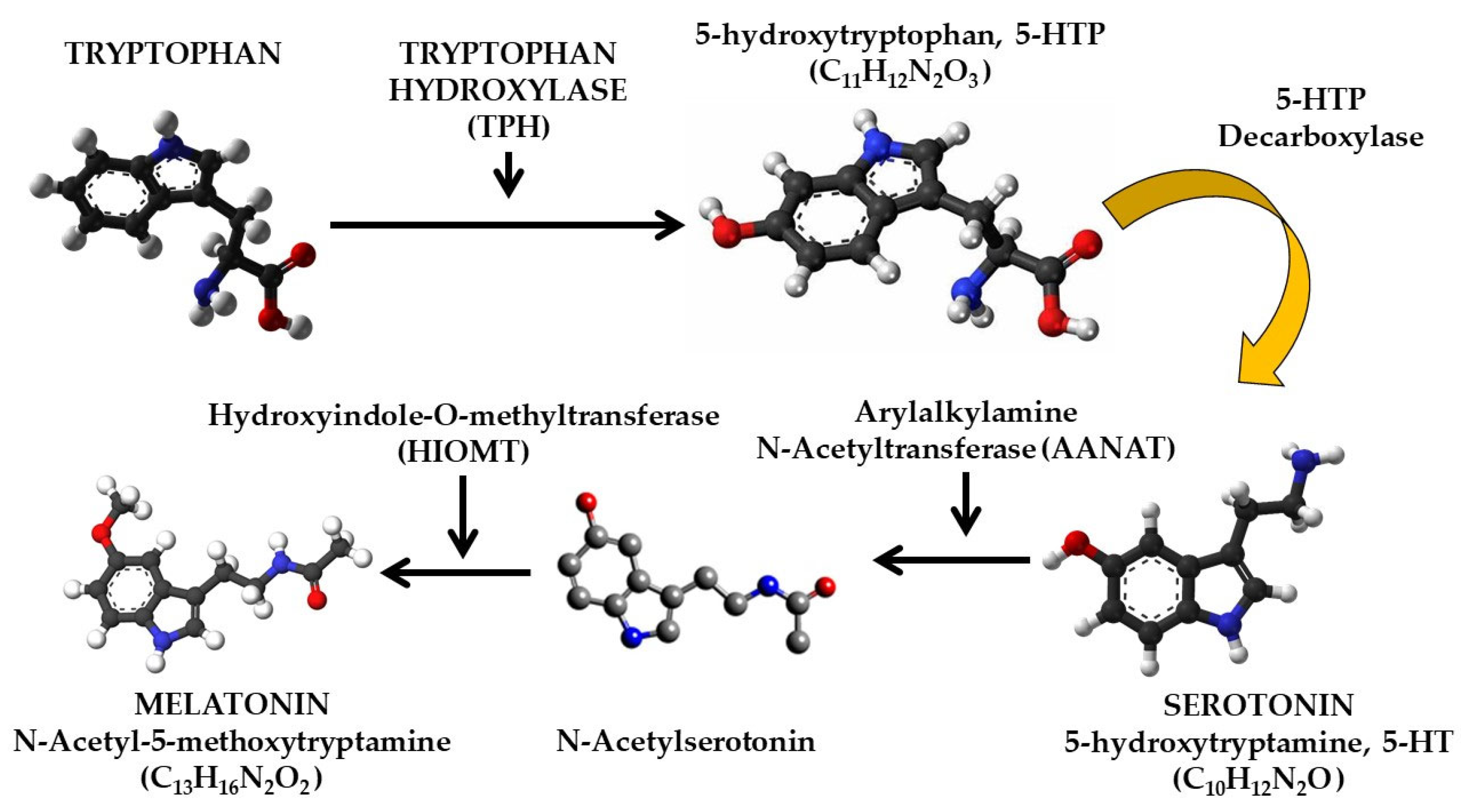
2.1. Pineal-Originated Melatonin
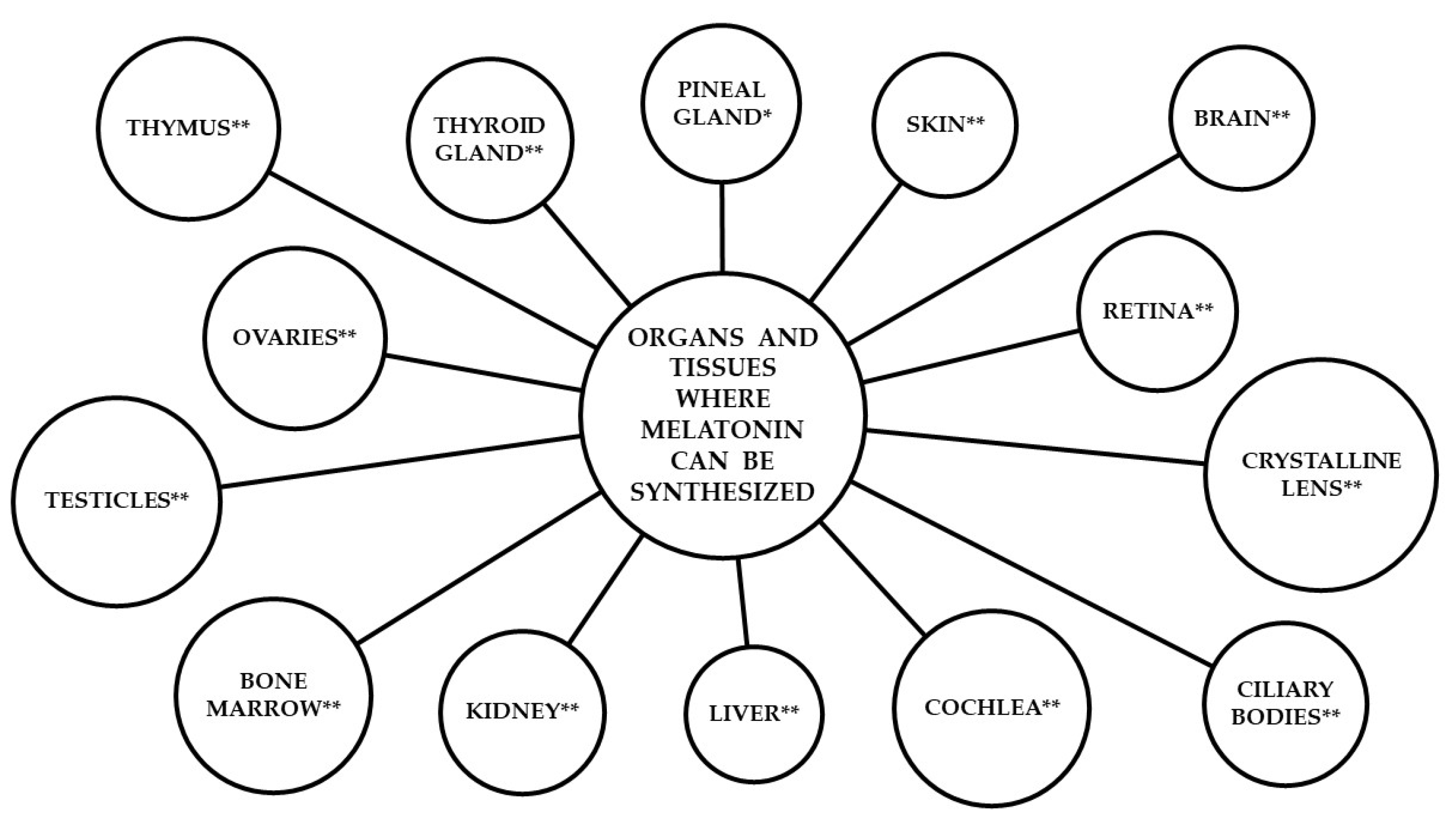
2.2. Extra-Pineal-Originated Melatonin
3. The Role of Melatonin
3.1. The Role of Melatonin in the Animal Body
3.2. The Role of Melatonin in the Human Body
4. Melatonin in Milk
4.1. Farming Technology Factors
4.1.1. Species and Breed
4.1.2. Environmental Conditions
4.1.3. Animals’ Productivity
4.1.4. Frequency of Milking
4.1.5. Lighting Conditions
4.1.6. Type and Intensity of Artificial Lighting
4.2. Nutritional Factors
4.2.1. Feeding Melatonin Rich Feedstuffs
4.2.2. Feeding Ruminally Protected L-Tryptophan
5. Sources of Melatonin in Human Nutrition
- The use in the kefir production process of raw milk with a very low, almost non-existent, melatonin hormonal content, which would explain the absence of melatonin in the finished product;
- The type of double fermentation (lactic + alcoholic) characteristic to kefir could be a factor in the depreciation of the melatonin content in the finished product.
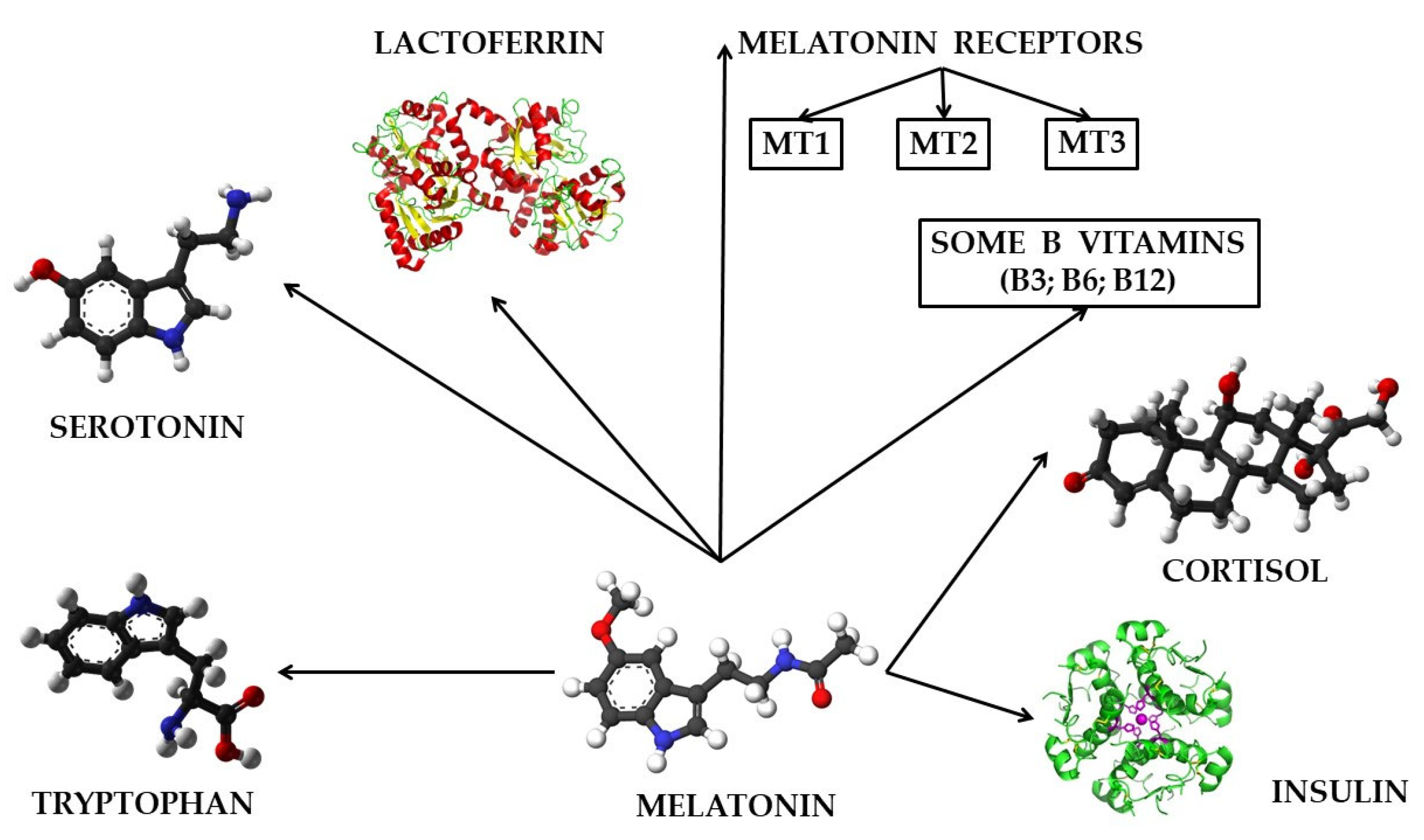
6. The Role of Melatonin in Relation to Other Bioactive Molecules on the Animal and Human Body
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reiter, R.J.; Sharma, R.; Tan, D.-X.; Chuffa, L.G.d.A.; da Silva, D.G.H.; Slominski, A.T.; Steinbrink, K.; Kleszczynski, K. Dual sources of melatonin and evidence for different primary functions. Front. Endocrinol. 2024, 15, 1414463. [Google Scholar] [CrossRef]
- Chlubek, D.; Sikora, M. Fluoride and Pineal Gland. Appl. Sci. 2020, 10, 2885. [Google Scholar] [CrossRef]
- Tan, D.-X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Reiter, R.J.; Zimmerman, S.; Hardeland, R. Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light. Biology 2023, 12, 89. [Google Scholar] [CrossRef]
- Andrani, M.; Dall’Olio, E.; De Rensis, F.; Tummaruk, P.; Saleri, R. Bioactive Peptides in Dairy Milk: Highlighting the Role of Melatonin. Biomolecules 2024, 14, 934. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Masters, A.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.L.; McFarlane, S.I. Melatonin, the Hormone of Darkness: From Sleep Promotion to Ebola Treatment. Brain Disord. Ther. 2014, 4, 1000151. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, W.D.; Pandi-Perumal, S.R.; Zakharia, R.; Bhatnagar, K.P.; Brzezinski, A. Melatonin and human reproduction: Shedding light on the darkness hormone. Gynecol. Endocrinol. 2009, 25, 779–785. [Google Scholar] [CrossRef]
- Patel, S.; Rahmani, B.; Gandhi, J.; Seyam, O.; Joshi, G.; Reid, I.; Smith, N.L.; Waltzer, W.C.; Khan, S.A. Revisiting the pineal gland: A review of calcification, masses, precocious puberty, and melatonin functions. Int. J. Neurosci. 2020, 130, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Horodincu, L.; Solcan, C. Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals 2023, 13, 2095. [Google Scholar] [CrossRef] [PubMed]
- Polakovičová, S.; Líška, J.; Varga, I.; Gálfiová, P. Morphology of the Human Pineal Gland Studied by Freeze-Fracturing in Scanning Electron Microscopy. Life 2024, 14, 1617. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2014, 35, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.Á.; Cuesta, A.; Chaves-Pozo, E.; Meseguer, J. Influence of Melatonin on the Immune System of Fish: A Review. Int. J. Mol. Sci. 2013, 14, 7979–7999. [Google Scholar] [CrossRef]
- Bisquert, R.; Planells-Cárcel, A.; Alonso-del-Real, J.; Muñiz-Calvo, S.; Guillamón, J.M. The Role of the PAA1 Gene on Melatonin Biosynthesis in Saccharomyces cerevisiae: A Search of New Arylalkylamine N-Acetyltransferases. Microorganisms 2023, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, K.; Zhou, Y.; Zhao, J.; Wang, J.; Lu, W. Role of Melatonin in Bovine Reproductive Biotechnology (Review). Molecules 2023, 28, 4940. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Mendes, L.; Queiroz, M.; Sena, C.M. Melatonin and Vascular Function. Antioxidants 2024, 13, 747. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.H.; Moloudizargari, M.; Ghobadi, E.; Fallah, M.; Abdollahi, M. Melatonin as a Multifunctional Anti-Cancer Molecule: Implications in Gastric Cancer. Life Sci. 2017, 185, 38–45. [Google Scholar] [CrossRef]
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin Anticancer Effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef]
- Fernández, A.; Ordóñez, R.; Reiter, R.J.; González-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, Y.M.; Song, K.-H.; Noh, H.; Lee, S.H. Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging Cell 2020, 19, e13111. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Bellaoui, M.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Markus, R.P.; Sousa, K.S.; da Silveira Cruz-Machado, S.; Fernandes, P.A.; Ferreira, Z.S. Possible Role of Pineal and Extra-Pineal Melatonin in Surveillance, Immunity, and First-Line Defense. Int. J. Mol. Sci. 2021, 22, 12143. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, S.C.; Clarkson, A.N.; Cakmak, Y.O. Neuromodulation of the Pineal Gland via Electrical Stimulation of Its Sympathetic Innervation Pathway. Front. Neurosci. 2020, 14, 264. [Google Scholar] [CrossRef]
- Lee, K.; Back, K. Functional Characterization of the Ciliate Stylonychia lemnae Serotonin N-Acetyltransferase, a Pivotal Enzyme in Melatonin Biosynthesis and Its Overexpression Leads to Peroxidizing Herbicide Tolerance in Rice. Antioxidants 2024, 13, 1177. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ding, D.; Bai, D.; Zhu, Y.; Sun, W.; Sun, Y.; Zhang, D. Melatonin biosynthesis pathways in nature and its production in engineered microorganisms. Synth. Syst. Biotechnol. 2022, 7, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.J.; Back, K. Functional Characterization of Arylalkylamine N-Acetyltransferase, a Pivotal Gene in Antioxidant Melatonin Biosynthesis from Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Choi, W.S. Use of Melatonin in Cancer Treatment: Where Are We? Int. J. Mol. Sci. 2022, 23, 3779. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Riestra, A.; Estrada-Reyes, R.; Torres-Sanchez, E.D.; Carreño-García, S.; Ortiz, G.G.; Benítez-King, G. Melatonin: A Neurotrophic Factor? Molecules 2022, 27, 7742. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. TEM 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Felder-Schmittbuhl, M.P.; Hicks, D.; Ribelayga, C.P.; Tosini, G. Melatonin in the mammalian retina: Synthesis, mechanisms of action and neuroprotection. J. Pineal Res. 2024, 76, e12951. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Bakalov, D.; Iliev, P.; Tafradjiiska-Hadjiolova, R. The Vital Role of Melatonin and Its Metabolites in the Neuroprotection and Retardation of Brain Aging. Int. J. Mol. Sci. 2024, 25, 5122. [Google Scholar] [CrossRef] [PubMed]
- Alkozi, H.A.; Pintor, J. TRPV4 activation triggers the release of melatonin from human non-pigmented ciliary epithelial cells. Exp. Eye Res. 2015, 136, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, M.C.; Guerrero, J.M.; Rubio, A.; Lardone, P.J.; Carrillo-Vico, A.; Carrascosa-Salmoral, M.P.; Jiménez-Jorge, S.; Arellano, M.V.; Leal-Noval, S.R.; Leal, M.; et al. Melatonin biosynthesis in the thymus of humans and rats. Cell Mol. Life Sci. 2007, 64, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Bonmatí-Carrión, M.-Á.; Tomas-Loba, A. Melatonin and Cancer: A Polyhedral Network Where the Source Matters. Antioxidants 2021, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Boga, J.A.; Tan, D.-X.; Davis, J.M.; Konturek, P.C.; Konturek, S.J.; Brzozowski, T. The photoperiod, circadian regulation and chronodisruption: The requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin. J. Physiol. Pharmacol. 2011, 62, 269–274. [Google Scholar]
- Bonmatí-Carrión, M.-Á.; Rol, M.-A. Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease. Antioxidants 2024, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, C.E.; Warmus, D.; Bonowicz, K.; Gagat, M.; Linowiecka, K.; Wolnicka-Glubisz, A.; Reiter, R.J.; Böhm, M.; Slominski, A.T.; Steinbrink, K.; et al. Ultraviolet Radiation-Induced Mitochondrial Disturbances Are Attenuated by Metabolites of Melatonin in Human Epidermal Keratinocytes. Metabolites 2023, 13, 861. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and Its Metabolites vs Oxidative Stress: From Individual Actions to Collective Protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Bae, S.M.; Jeong, J.; Jeon, H.J.; Bang, Y.R.; Yoon, I.Y. Effects of melatonin-rich milk on mild insomnia symptoms. Sleep. Med. Res. 2016, 7, 60–67. [Google Scholar] [CrossRef]
- Brzezinski, A.; Rai, S.; Purohit, A.; Pandi-Perumal, S.R. Melatonin, Clock Genes, and Mammalian Reproduction: What Is the Link? Int. J. Mol. Sci. 2021, 22, 13240. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, Y.; Chen, Y.; Li, B.; Wang, H.; Liu, S.; Adeniran, S.O.; Zheng, P. Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway. Animals 2024, 14, 2771. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Atayik, M.C.; Sirinzade, H.; Entezari, B.; Gurer-Orhan, H.; Cakatay, U. Melatonin and redox homeostasis. Melatonin Res. 2022, 5, 304–324. [Google Scholar] [CrossRef]
- Rzepka-Migut, B.; Paprocka, J. Melatonin-Measurement Methods and the Factors Modifying the Results. A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 1916. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H.C. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow–Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Slakey, L.M.; Xiang, S.; Yuan, L.; Dauchy, E.M.; Shan, B.; Brainard, G.C.; Hanifin, J.P.; et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3β. Mol. Endocrinol. 2012, 26, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, S.; Zhang, Y.; Zhang, Q. Melatonin Receptors: A Key Mediator in Animal Reproduction. Vet. Sci. 2022, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin Receptor Genes in Vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef]
- Peña-Delgado, V.; Carvajal-Serna, M.; Fondevila, M.; Martín-Cabrejas, M.A.; Aguilera, Y.; Álvarez-Rivera, G.; Abecia, J.A.; Casao, A.; Pérez-Pe, R. Improvement of the Seminal Characteristics in Rams Using Agri-Food By-Products Rich in Phytomelatonin. Animals 2023, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2020, 85, 5–13. [Google Scholar] [CrossRef]
- Karolczak, K.; Watala, C. Melatonin as a Reducer of Neuro- and Vasculotoxic Oxidative Stress Induced by Homocysteine. Antioxidants 2021, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef] [PubMed]
- Gombert, M.; Codoñer-Franch, P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. Int. J. Mol. Sci. 2021, 22, 6809. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Mirhosseini, N.; Reiter, R.J.; Azami, A.; Asemi, Z. Melatonin, a calpain inhibitor in the central nervous system: Current status and future perspectives. J. Cell Physiol. 2018, 234, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Vorster, A.P.A.; van Someren, E.J.W.; Pack, A.I.; Huber, R.; Schmidt, M.H.; Bassetti, C.L.A. Sleep Health. Clin. Transl. Neurosci. 2024, 8, 8. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, E1. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- Paditz, E. Postnatal Development of the Circadian Rhythmicity of Human Pineal Melatonin Synthesis and Secretion (Systematic Review). Children 2024, 11, 1197. [Google Scholar] [CrossRef]
- Paditz, E. Melatonin in infants-Physiology, pathophysiology and intervention options. Somnologie 2024, 28, 103–109. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Hara, C.C.P.; Ormonde, J.V.S.; Nunes, G.T.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef]
- Italianer, M.F.; Naninck, E.F.G.; Roelants, J.A.; van der Horst, G.T.J.; Reiss, I.K.M.; Goudoever, J.B.v.; Joosten, K.F.M.; Chaves, I.; Vermeulen, M.J. Circadian Variation in Human Milk Composition, a Systematic Review. Nutrients 2020, 12, 2328. [Google Scholar] [CrossRef]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Biggio, G.; Biggio, F.; Talani, G.; Mostallino, M.C.; Aguglia, A.; Aguglia, E.; Palagini, L. Melatonin: From Neurobiology to Treatment. Brain Sci. 2021, 11, 1121. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Milagres, M.P.; Minim, V.P.R.; A Minim, L.; A Simiqueli, A.; Moraes, L.E.S.; Martino, H.S.D. Night milking adds value to cow’s milk. J. Sci. Food Agric. 2013, 94, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Usturoi, M.G. The Technology of Milk and Dairy Products; Alfa Publishing House: Iasi, Romania, 2007. [Google Scholar]
- Rațu, R.N.; Cârlescu, P.M.; Usturoi, M.G.; Lipșa, F.D.; Veleșcu, I.D.; Arsenoaia, V.N.; Florea, A.M.; Ciobanu, M.M.; Radu-Rusu, R.-M.; Postolache, A.N.; et al. Effects of Dairy Cows Management Systems on the Physicochemical and Nutritional Quality of Milk and Yogurt, in a North-Eastern Romanian Farm. Agriculture 2023, 13, 1295. [Google Scholar] [CrossRef]
- Matei, M.; Zaharia, R.; Petrescu, S.-I.; Radu-Rusu, C.G.; Simeanu, D.; Mierliță, D.; Pop, I.M. Persistent Organic Pollutants (POPs): A Review Focused on Occurrence and Incidence in Animal Feed and Cow Milk. Agriculture 2023, 13, 873. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and metabolic properties of bovine milk oligosaccharides and their potential in the development of novel therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Berg, E.; de Campos Zani, S.C.; Chan, C.B. Cow’s Milk Bioactive Molecules in the Regulation of Glucose Homeostasis in Human and Animal Studies. Foods 2024, 13, 2837. [Google Scholar] [CrossRef]
- Usturoi, A.; Usturoi, M.-G.; Avarvarei, B.-V.; Pânzaru, C.; Simeanu, C.; Usturoi, M.-I.; Spătaru, M.; Radu-Rusu, R.-M.; Doliş, M.-G.; Simeanu, D. Research Regarding Correlation between the Assured Health State for Laying Hens and Their Productivity. Agriculture 2023, 13, 86. [Google Scholar] [CrossRef]
- Helmreich, S.; Wechsler, B.; Hauser, R.; Gygax, L. Effects of milking frequency in automatic milking systems on salivary cortisol, immunoglobulin A, somatic cell count and melatonin. Schweiz Arch Tierheilkd 2016, 158, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, P.; Kennedy, A.D.; Berry, R.J. Plasma melatonin and insulin-like growth factor-1 responses to dim light at night in dairy heifers. J. Pineal Res. 2006, 40, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Bal, M.A.; Penner, G.B.; Oba, M.; Kennedy, A.D. Effects of dim light at night on milk yield, milk composition and endocrine profile of lactating dairy cows. Can. J. Anim. Sci. 2008, 88, 609–612. [Google Scholar] [CrossRef]
- Boztepe, S.; Keskin, I.; Semacan, A.; Akyürek, F.; Aytekin, I.; Sahin, Ö. Melatonin Differences Between Day and Night Milk in Primiparous Holstein Friesian and Jersey Dairy Cattle. Selcuk. J. Agric. Food Sci. 2022, 36, 27–30. [Google Scholar] [CrossRef]
- Keskin, M.; Gül, S.; Karaaslan, İ.; Yakan, A. Controlling the photoperiod to raise the melatonin content of sheep milk. Photoperiod control and milk melatonin content. J. Hell. Vet. Med. Soc. 2023, 74, 6641–6648. [Google Scholar] [CrossRef]
- Şahin, Ö.; Akyürek, F.; Boztepe, S.; Aytekin, İ.; Keskin, İ. Determination of Melatonin Differences between Day and Night Milk in Dairy Cattle. J. Agric. Sci. Bilim. Derg. 2021, 27, 449–453. [Google Scholar] [CrossRef]
- Romanini, E.B.; Marchi Volpato, A.; Dos Santos, J.S.; De Santana, E.H.W.; De Souza, C.H.B.; Ludovico, A. Melatonin concentration in cow’s milk and sources of its variation. J. Appl. Anim. Res. 2019, 47, 140–145. [Google Scholar] [CrossRef]
- Asher, A.; Shabtay, A.; Brosh, A.; Eitam, H.; Agmon, R.; Cohen-Zinder, M.; Zubidat, A.E.; Haim, A. “Chrono-functional milk”: The difference between melatonin concentrations in night-milk versus day-milk under different night illumination conditions. Chronobiol. Int. 2015, 32, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.W.; Yang, G.Q.; Wang, I.F.; Fu, T.; Lian, H.X.; Sun, Y.; Han, L.Q.; Zhang, L.Y.; Gao, T.Y. Effects of the Circadian Rhythm on Milk Composition in Dairy Cows: Does Day Milk Differ from Night Milk? J. Dairy Sci. 2021, 104, 8301–8313. [Google Scholar] [CrossRef]
- Jo, J.-H.; Jalil, G.N.; Kim, W.-S.; Moon, J.-O.; Lee, S.-D.; Kwon, C.-H.; Lee, H.-G. Effects of Rumen-Protected L-Tryptophan Supplementation on Productivity, Physiological Indicators, Blood Profiles, and Heat Shock Protein Gene Expression in Lactating Holstein Cows under Heat Stress Conditions. Int. J. Mol. Sci. 2024, 25, 1217. [Google Scholar] [CrossRef]
- Stelwagen, K.; Phyn, C.V.; Davis, S.R.; Guinard-Flament, J.; Pomiès, D.; Roche, J.R.; Kay, J.K. Invited review: Reduced milking frequency: Milk production and management implications. J. Dairy. Sci. 2013, 96, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.H.; Bond, J.P.; McFadden, T.B. Milk yield responses to changes in milking frequency during early lactation are associated with coordinated and persistent changes in mammary gene expression. BMC Genom. 2013, 14, 296. [Google Scholar] [CrossRef]
- Asher, A.; Fialko, M.; Fares, F.; Moallem, U.; Yaacoby, S.; Gutman, R. The Effect of Short-Wavelength White LED Illumination throughout the Night on the Milk Fatty Acid Profile of High-Yielding Dairy Cows. Biology 2022, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.A.; Herlihy, M.M.; Nolan, M.B.; O’Brien, C.; Furlong, J.G.; Butler, S.T. Identification of the blue light intensity administered to one eye required to suppress bovine plasma melatonin and investigation into effects on milk production in grazing dairy cows. J. Dairy. Sci. 2021, 104, 12127–12138. [Google Scholar] [CrossRef]
- Adamczyk, K.; Herbut, P.; Godyń, D.; Angrecka, S.; Kupczyński, R.; Corrêa Vieira, F.M. Effect of light on dairy cattle in farm conditions—A review. Ann. Anim. Sci. 2024, 24, 1139–1151. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mon, M.; Takao, Y.; Shinoda, A.; Watanabe, T.; Kushibiki, S.; Obitsu, T.; Sugino, T. Exposure to blue LED light before the onset of darkness under a long-day photoperiod alters melatonin secretion, feeding behaviour and growth in female dairy calves. Jpn. Soc. Anim. Sci. 2020, 91, e13353. [Google Scholar] [CrossRef] [PubMed]
- Simeanu, D. Nutrition and Feeding of Animals; “Ion Ionescu de la Brad” Publishing House: Iasi, Romania, 2018. [Google Scholar]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyzetryptophan to melatonin in the cytoplasm or chloroplasts (Review article). J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Ding, Z.; Zeng, J.; Yan, Z.; Duan, H.; Lv, J.; Zhang, Y.; Zhang, L.; Hu, J. Melatonin Sources in Sheep Rumen and Its Role in Reproductive Physiology. Animals 2024, 14, 3451. [Google Scholar] [CrossRef]
- Holzmann, V.M.M.; Trentin, M.; De Almeida Rego, F.C.; Coelho Cunha Filho, L.F.; Ludovico, A. Melatonin concentration in the milk of cows supplemented with vitamins and milked twice daily. Semin. Agrar. 2019, 40, 2017–2026. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. The Uniqueness of Tryptophan in Biology: Properties, Metabolism, Interactions and Localization in Proteins. Int. J. Mol. Sci. 2020, 21, 8776. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Kim, C.-H.; Yang, W.-S. Physiologically Active Molecules and Functional Properties of Soybeans in Human Health-A Current Perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, D.; Lu, N.; Li, H.; Liu, G.; Cao, Z.; Yang, H.; Li, S.; Yu, X.; Shao, W.; et al. Analysis of Chemical Composition, Amino Acid Content, and Rumen Degradation Characteristics of Six Organic Feeds. Animals 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-L.; Lee, S.-H.; Son, G.-H.; Shin, J.-S.; Kim, M.-J.; Park, B.-K. Effect of Rumen-Protected L-Tryptophan or L-Ascorbic Acid on Plasma Metabolites and Milk Production Characteristics of Lactating Holstein Cows during Summer Conditions. Animals 2024, 14, 1820. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, S.; Liu, Y.; Han, H.; Wang, W.; Yi, Q.; Yan, L.; Ji, P.; Zhang, L.; Liu, G. Effects of Prepartum L-Tryptophan Supplementation on the Postpartum Performance of Holstein Cows. Animals 2024, 14, 1278. [Google Scholar] [CrossRef]
- Kollmann, M.T.; Locher, M.; Hirche, F.; Eder, K.; Meyer, H.H.; Bruckmaier, R.M. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Domest. Anim. Endocrinol. 2008, 34, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, D.; Radhakrishna, A.; Sivaraman, K.P.; Biju, V.M. Quantitative determination of melatonin in milk by LC-MS/MS. J. Food Sci. Technol. 2014, 51, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Kocadağı, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sturtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Kanova, M.; Kohout, P. Serotonin—Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep. Med. 2007, 8 (Suppl. S3), 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Tamura, H.; Reiter, R.J. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3melatonin membrane receptor: Hypothesis and significance. J. Pineal Res. 2007, 43, 317–320. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Flis, Z.; Molik, E. Importance of Bioactive Substances in Sheep’s Milk in Human Health. Int. J. Mol. Sci. 2021, 22, 4364. [Google Scholar] [CrossRef] [PubMed]
- Niaz, B.; Saeed, F.; Ahmad, A.; Imran, M.; Maan, A.; Khan, M.; Tufail, T.; Anjum, F.; Hussain, S.; Suleria, H. Lactoferrin (LF): A Natural Antimicrobial Protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Redwan, E.; Uversky, V.; El-Fakharany, E.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2015, 337, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent advances and prospects in purification and heterologous expression of lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Bablon, J.C.; Da Silva, C. A combination of melatonin, vitamin B6 and medicinal plants in the treatment of mild-to-moderate insomnia: A prospective pilot study. Complement. Ther. Med. 2019, 45, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Peukhuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.-G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Xia, A.-Y.; Zhu, H.; Zhao, Z.-J.; Liu, H.-Y.; Wang, P.-H.; Ji, L.-D.; Xu, J. Molecular Mechanisms of the Melatonin Receptor Pathway Linking Circadian Rhythm to Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1406. [Google Scholar] [CrossRef]
- Peschke, E.; Bähr, I.; Mühlbauer, E. Melatonin and Pancreatic Islets: Interrelationships between Melatonin, Insulin and Glucagon. Int. J. Mol. Sci. 2013, 14, 6981–7015. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E. Melatonin, endocrine pancreas and diabetes. J. Pineal Res. 2008, 44, 26–40. [Google Scholar] [CrossRef]
- Mulder, H.; Nagorny, C.L.; Lyssenko, V.; Groop, L. Melatonin receptors in pancreatic islets: Good morning to a novel type 2 diabetes gene. Diabetologia 2009, 52, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
| Species | Breed | Melatonin Content (pg/mL) | References | ||
|---|---|---|---|---|---|
| Daytime Milk | Nighttime Milk | Difference | |||
| Cattle | Jersey | 2.924 ± 0.216 a | 6.954 ± 0.567 a | 4.03 | [99] |
| Holstein Friesian | 2.912 ± 0.266 a | 11.314 ± 1.1 a | 8.402 | ||
| Sheep | Awassi | 6.12 ± 4.55 b | 11.06 ± 7.24 b | 4.94 | [100] |
| Melatonin Content (pg/mL) | Study Period | Duration of Photoperiod | N * | Geographical Area | References | ||
|---|---|---|---|---|---|---|---|
| Daytime Milk (Milking Time) | Nighttime Milk (Milking Time) | Difference | |||||
| 2.912 ± 0.266 (15:00–17:00) | 11.314 ± 1.1 (03:00–05:00) | 8.402 | January | 11 h of light 13 h of darkness | 27 | Konya, Turkey | [99] |
| 103.7 ± 6.61 a (07:00–16:00) | 163.13 ± 8.96 a (01:00) | 59.43 | Unspecified | Unspecified | 40 | Konya, Turkey | [101] |
| 4.03 a,b (15:00) | 39.43 a,b (02:00) | 35.4 | 2–16 June | 15 h of light 9 h of darkness | 10 | Viçosa, Brazil | [89] |
| 6.98 ± 3.05 (N.S. **) | 14.87 ± 7.69 (N.S. **) | 7.89 | S. *** | S. *** | 30 | Castro, Brazil | [102] |
| 5.36 ± 0.33 (12:30) | 30.7 ± 1.79 (04:30) | 25.34 | 1–15 November | 10.4 h of light 13.6 h of darkness | 28 | Israel | [103] |
| 3.3 ± 0.18 (12:30) | 17.81 ± 0.33 (04:30) | 14.51 | |||||
| 90.21 ± 7.21 c (15:00) | 120.07 ± 7.21 c (05:00) | 29.86 | August | 13 h of light 11 h of darkness | 10 | China | [104] |
| Feedstuff | Melatonin Content | Samples | Assessment Method | References |
|---|---|---|---|---|
| Corn (whole, yellow) | 1.3 ± 0.28 ng/g | 5 | HPLC ** | [29] |
| Corn, germs floor | 1.0 ± 0.1 ng/g | |||
| Wheat (Triticum aestivum L.) | 124.7 ± 14.9 ng/g FW * | 3 | HPLC-ECD *** | [118] |
| Barley (Hordeum vulgare L.) | 82.3 ± 6.0 ng/g FW * | |||
| Oat (Avena sativa L.) | 90.6 ± 7.7 ng/g FW * |
| Feedstuff | Protein Content (g per 100 g) | Tryptophan (g per 100 g) | References |
|---|---|---|---|
| Soybean | 36.49 | 0.59 | [120] |
| Soybean meal | 47.46 | 0.53 | [121] |
| Alfalfa hay | 19.61 | 0.24 | |
| Oat hay | 8.88 | 0.08 | |
| Wheat bran | 20.15 | 0.26 |
| Product Name | Melatonin Content | Number of Samples Performed | Assessment Method | References |
|---|---|---|---|---|
| Fresh/processed milk | ||||
| Whole cow milk | 14.45 ± 0.12 pg/mL−1 | 6 | LC-MS/MS * | [125] |
| Skimmed cow milk | 18.41 ± 0.62 pg/mL−1 | |||
| UHT milk | 4.16 pg/mL | 16 | ELISA (RE54041; IBL) ** | [102] |
| Other dairy products | ||||
| Colostrum, fresh | 0.06 ng/g | 5 | HPLC *** | [29] |
| Colostrum, powder | 0.6 ± 0.06 ng/g | |||
| Yogurt | 0.13 ± 0.01 ng/g | 5 | LC-MS/MS * | [29] |
| Probiotic yogurt | 126.7 ± 9 pg/mL | 5 | LC-MS/MS * | [126] |
| Kefir | Not detected | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronachi, V.-C.; Simeanu, C.; Matei, M.; Radu-Rusu, R.-M.; Simeanu, D. Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans. Agriculture 2025, 15, 273. https://doi.org/10.3390/agriculture15030273
Andronachi V-C, Simeanu C, Matei M, Radu-Rusu R-M, Simeanu D. Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans. Agriculture. 2025; 15(3):273. https://doi.org/10.3390/agriculture15030273
Chicago/Turabian StyleAndronachi, Vasile-Cosmin, Cristina Simeanu, Mădălina Matei, Răzvan-Mihail Radu-Rusu, and Daniel Simeanu. 2025. "Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans" Agriculture 15, no. 3: 273. https://doi.org/10.3390/agriculture15030273
APA StyleAndronachi, V.-C., Simeanu, C., Matei, M., Radu-Rusu, R.-M., & Simeanu, D. (2025). Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans. Agriculture, 15(3), 273. https://doi.org/10.3390/agriculture15030273










