Effect of Short-Term Storage in Modified Atmosphere Packaging (MAP) and Controlled Atmosphere (CA) on Total Polyphenol Content and Antioxidant Activity in Juices from Haskap Berry (Lonicera caerulea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Harvest Timing
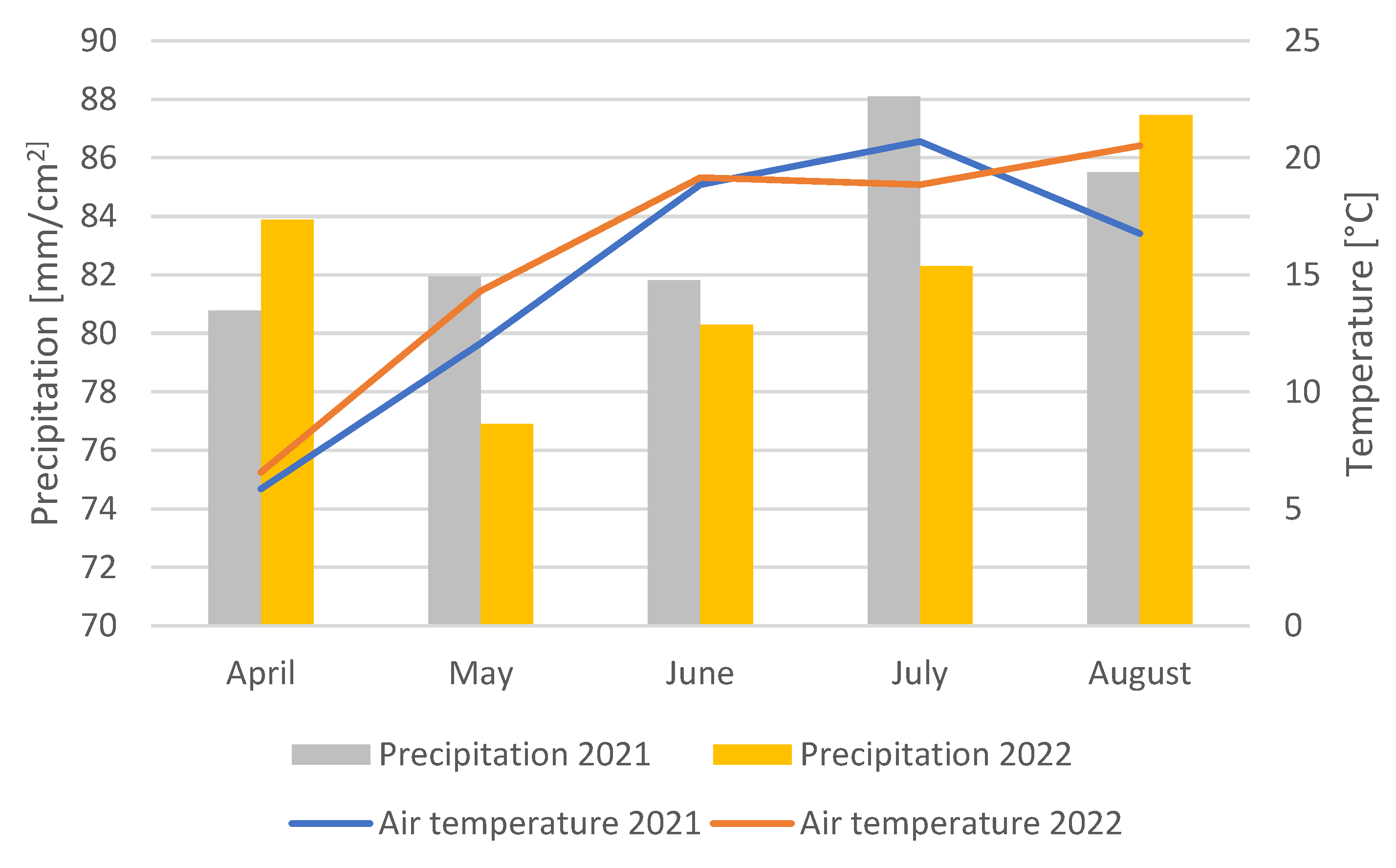
2.1.2. Weather Conditions
2.2. Storage Treatments
- Normal atmosphere (NA): Stored in a cold room (temperature 2 °C, relative humidity 90–92%).
- Controlled atmosphere (CA): 20% CO2 and 5% O2 (temperature 2 °C, relative humidity 90–92%).
- Modified atmosphere packaging (MAP): Xtend packaging (StePac LA Ltd., Johnson Matthey, Tefen, Israel/USA (Chicago, IL 60181)).
2.3. Juice Preparation
2.4. The Content of Bioactive Compounds and Antioxidant Activity
2.4.1. Total Polyphenols Content (TPC)
2.4.2. Radical Scavenging Capacity (RSC)—A DPPH Assay
2.5. Fruit Firmness
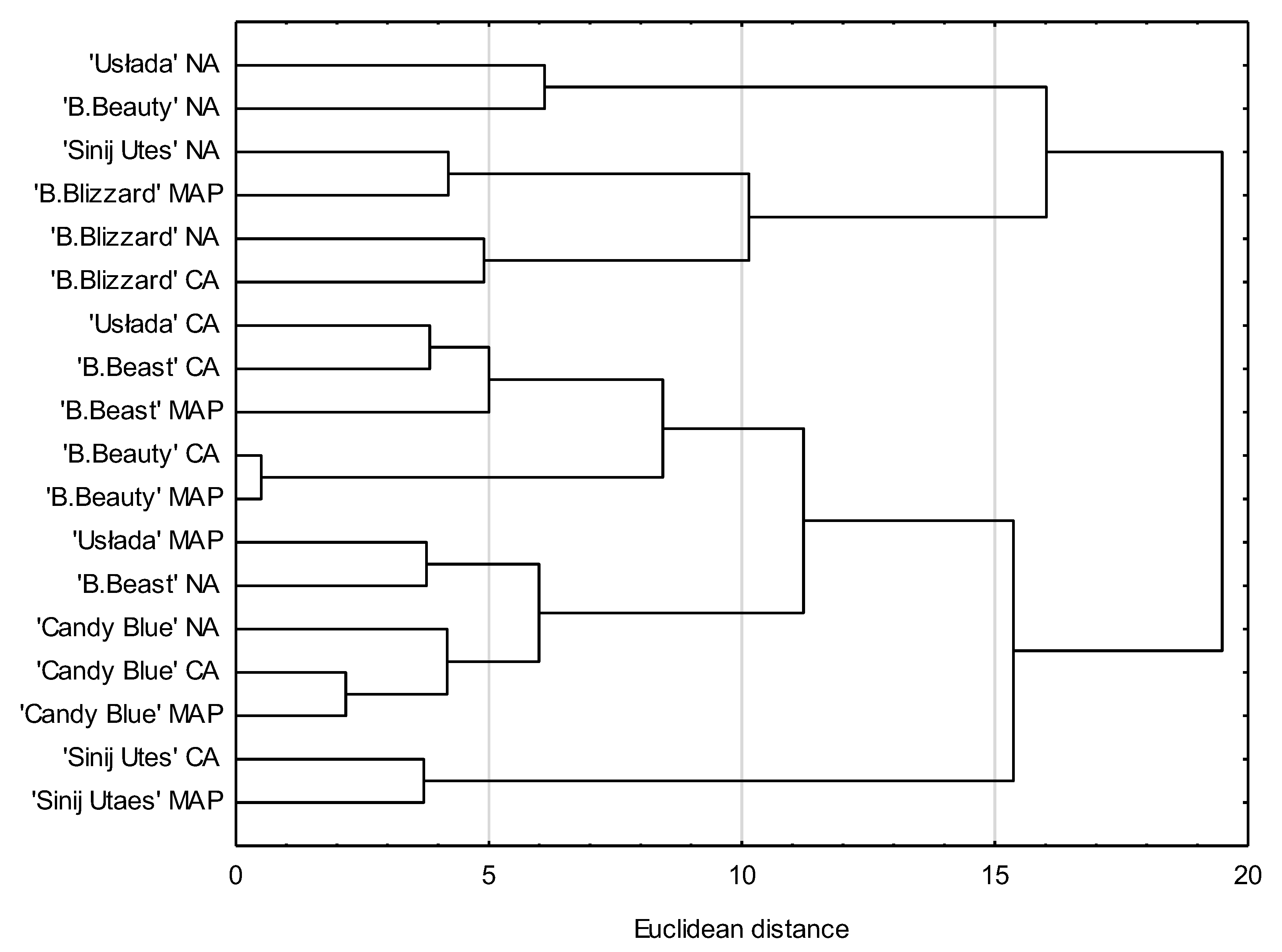
2.6. Statistical Analysis
3. Results and Discussion
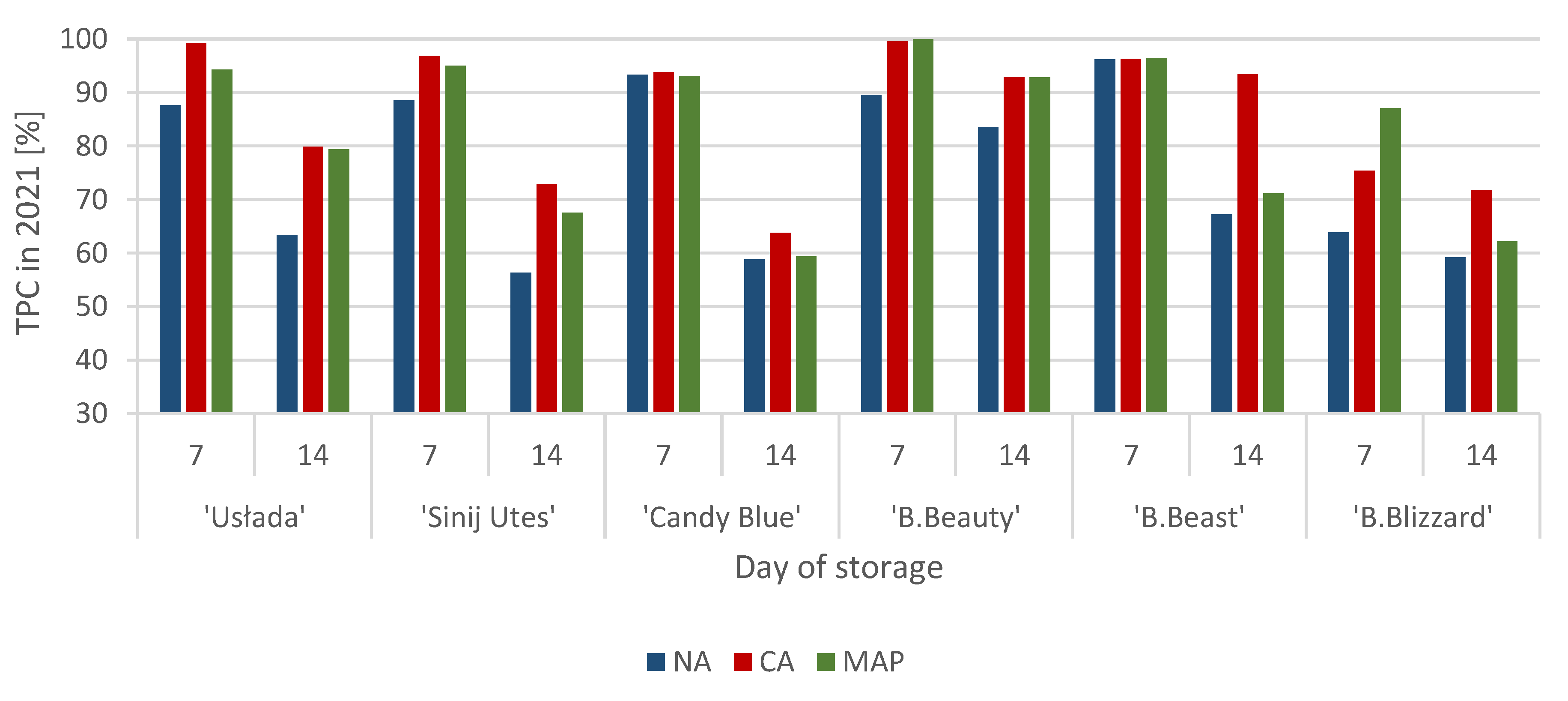
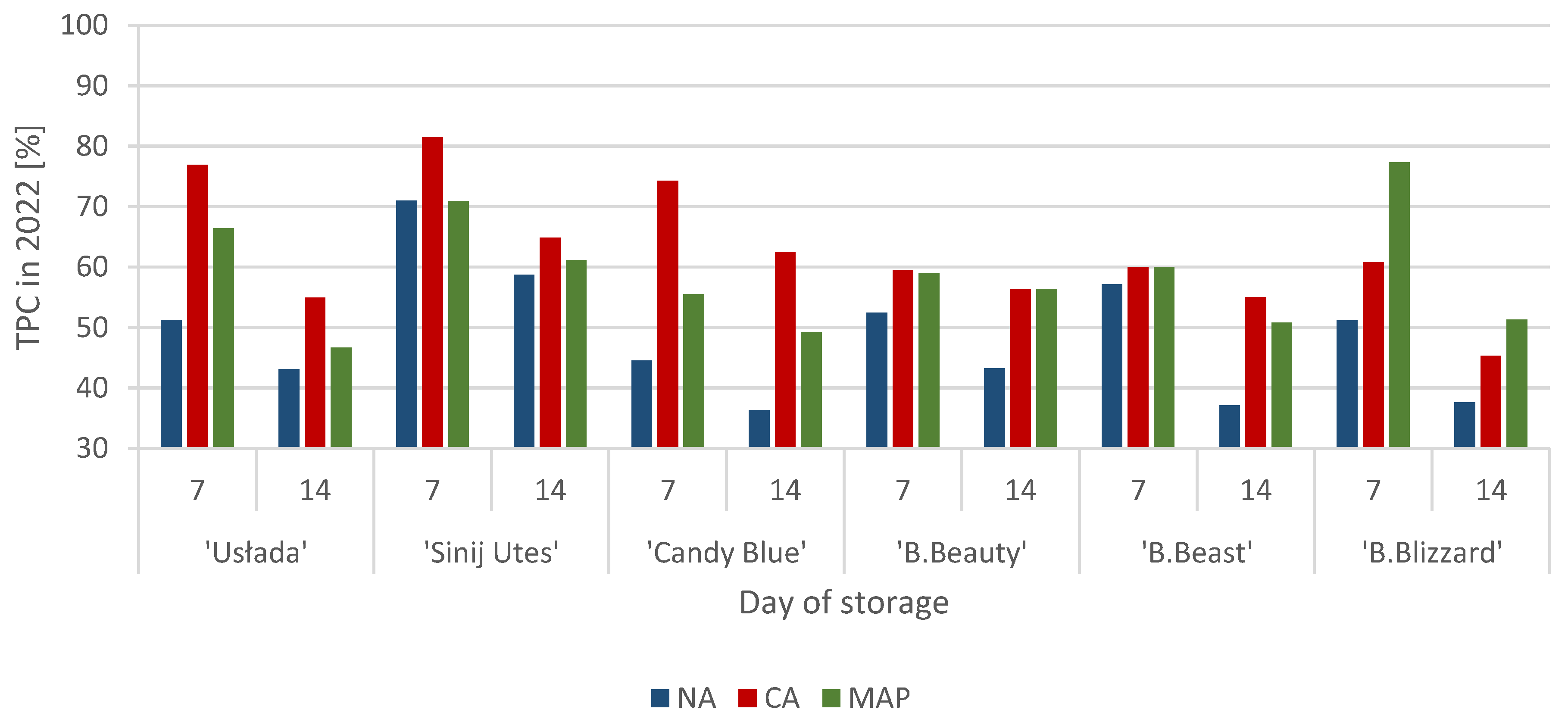
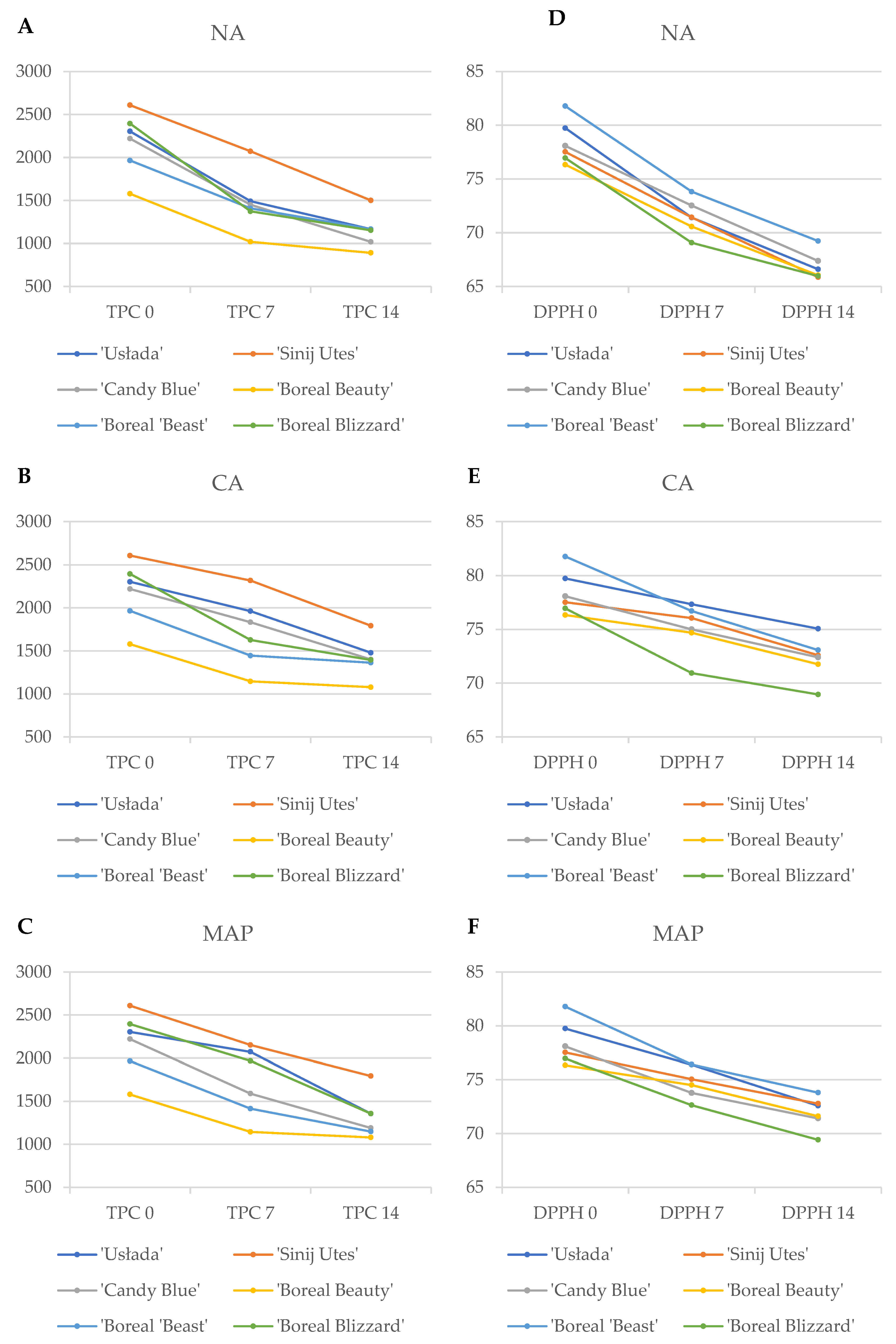
3.1. Polyphenol Content
3.2. Antioxidant Activity (DPPH)
3.3. Qualitative Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grobelna, A.; Kalisz, S.; Kieliszek, M. The Effect of the Addition of Blue Honeysuckle Berry Juice to Apple Juice on the Selected Quality Characteristics, Anthocyanin Stability, and Antioxidant Properties. Biomolecules 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Česonienė, L.; Labokas, J.; Jasutienė, I.; Šarkinas, A.; Kaškonienė, V.; Kaškonas, P.; Kazernavičiūtė, R.; Pažereckaitė, A.; Daubaras, R. Bioactive Compounds, Antioxidant, and Antibacterial Properties of Lonicera caerulea Berries: Evaluation of 11 Cultivars. Plants 2021, 10, 624. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of Modified (MAP) and Controlled Atmosphere (CA) Storage on the Quality and Bioactive Compounds of Blue Honeysuckle Fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Liu, X.; Fan, L. Simultaneous Optimization of the Acidified Water Extraction for Total Anthocyanin Content, Total Phenolic Content, and Antioxidant Activity of Blue Honeysuckle Berries (Lonicera caerulea L.) Using Response Surface Methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Fan, Z.; Yang, X.; Bao, Y.H.; Liang, M.; Guo, Y. Anthocyanins and Antioxidant Activity of Lonicera caerulea Berry Wine during Different Processes. Food Sci. Technol. 2021, 42, e25121. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Piekarska, J.; Szczypka, M.; Gorczykowski, M.; Sokół-łętowska, A.; Kucharska, A.Z. Evaluation of Immunotropic Activity of Iridoid-Anthocyanin Extract of Honeysuckle Berries (Lonicera caerulea L.) in the Course of Experimental Trichinellosis in Mice. Molecules 2022, 27, 1949. [Google Scholar] [CrossRef]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kucharska, A.Z. Effect of Pre-Treatment of Blue Honeysuckle Berries on Bioactive Iridoid Content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.J. Lonicera caerulea: An Updated Account of Its Phytoconstituents and Health-Promoting Activities. Trends Food Sci. Technol. 2021, 107, 130–149. [Google Scholar] [CrossRef]
- Auzanneau, N.; Weber, P.; Kosińska-Cagnazzo, A.; Andlauer, W. Bioactive Compounds and Antioxidant Capacity of Lonicera caerulea Berries: Comparison of Seven Cultivars over Three Harvesting Years. J. Food Compos. Anal. 2018, 66, 81–89. [Google Scholar] [CrossRef]
- Shevchuk, L.; Tereshchenko, Y.; Vintskovska, Y.; Levchuk, L.; Babenko, S.; Hrynyk, R. Yield and Content of Biologically Active Substances in Blue Honeysuckle Fruit (Lonicera caerulea L.) Grown in the Forest Steppe of Ukraine. Agron. Res. 2022, 20, 814–826. [Google Scholar] [CrossRef]
- Becker, R.; Szakiel, A. Phytochemical Characteristics and Potential Therapeutic Properties of Blue Honeysuckle Lonicera caerulea L. (Caprifoliaceae). J. Herb. Med. 2019, 16, 100237. [Google Scholar] [CrossRef]
- Bors, B. Breeding the Boreal Series of Haskap (Lonicera Caerulea). 2017, p. 2017. Available online: https://research-groups.usask.ca/fruit/documents/haskap/cshs-Poster-2017.pdf (accessed on 4 June 2024).
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap Berries (Lonicera caerulea L.)—A Critical Review of Antioxidant Capacity and Health-Related Studies for Potential Value-Added Products. Food Bioprocess Technol. 2014, 7, 1541–1554. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Skupień, K. Field Performance, fruit chemical composition and firmness under cold storage and simulated “shelf-life” conditions of three blue honey suckle cultigens (Lonicera caerulea). J. Fruit Ornam. Plant Res. 2008, 16, 83–91. Available online: https://www.inhort.pl/files/journal_pdf/journal_2008/full9%202008.pdf (accessed on 18 July 2024).
- Levon, V.; Grygorieva, O.; Antoniewska-Krzeska, A.; Šramková, K.F.; Zhurba, M. Variation of Fruits Morphometric Parameters and Iridoid Content of Lonicera caerulea L. Germplasm Collection. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 6, 262–271. [Google Scholar] [CrossRef]
- Khattab, R.; Brooks, M.S.-L.; Ghanem, A. Phenolic Analyses of Haskap Berries (Lonicera caerulea L.): Spectrophotometry Versus High Performance Liquid Chromatography. Int. J. Food Prop. 2016, 19, 1708–1725. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The Potential Health Benefits of Haskap (Lonicera caerulea L.): Role of Cyanidin-3-O-Glucoside. J. Funct. Foods. 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Gerbrandt, E.M.; Bors, R.H.; Meyer, D.; Wilen, R.; Chibbar, R.N. Fruit Quality of Japanese, Kuril and Russian Blue Honeysuckle (Lonicera caerulea L.) Germplasm Compared to Blueberry, Raspberry and Strawberry. Euphytica 2020, 216, 59. [Google Scholar] [CrossRef]
- Gazdik, Z.; Reznicek, V.; Adam, V.; Zitka, O.; Jurikova, T.; Krska, B.; Matuskovic, J.; Plsek, J.; Saloun, J.; Horna, A.; et al. Use of Liquid Chromatography with Electrochemical Detection for the Determination of Antioxidants in Less Common Fruits. Molecules 2008, 13, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Polak, N.; Jodłowska, M. Wpływ Odmiany Surowca Na Wybrane Wyróżniki Jakościowe Soków z Jagody Kamczackiej. Przemysł Spożywczy 2022, 76, 26–32. [Google Scholar] [CrossRef]
- Małodobry, M.; Bieniasz, M.; Dziedzic, E. Evaluation of the Yield and Some Components in the Fruit of Blue Honeysuckle (Lonicera caerulea Var. Edulis Turcz. Freyn.). Folia Hortic. 2010, 22, 45–50. [Google Scholar] [CrossRef]
- Koort, A.; Moor, U.; Põldma, P.; Kaiser, C.; Starast, M. Comparison of Regular Atmospheric Storage versus Modified Atmospheric Packaging on Postharvest Quality of Organically Grown Lowbush and Half-Highbush Blueberries. Sustainability 2018, 10, 3916. [Google Scholar] [CrossRef]
- Yan, H.; Mi, Y.; Li, Y.; Zang, H.; Guo, L.; Huo, J.; Man, Z.; Chen, Z.; Zhang, B.; Sang, M.; et al. First Report of Postharvest Fruit Rot Caused by Botrytis Cinerea on Blue Honeysuckle (Lonicera caerulea) Fruit in China. Plant Dis. 2024, 108, 527. [Google Scholar] [CrossRef]
- Jurikova, T.; Ercişli, S.; Rop, O.; Mlcek, J.; Balla, S.; Zitny, R.; Sochor, J.; Hegedusova, A.; Benedikova, D.; Ďurišová, L. The Evaluation of Anthocyanin Content of Honeyberry (Lonicera kamtschatica) Clones during Freezing in Relation to Antioxidant Activity and Parameters of Nutritional Value. Zemdirb. Agric. 2014, 101, 215–220. [Google Scholar] [CrossRef]
- Khattab, R.; Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Effect of Frozen Storage on Polyphenol Content and Antioxidant Activity of Haskap Berries (Lonicera caerulea L.). J. Berry Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Leisso, R.; Jarrett, B.; Richter, R.; Miller, Z. Fresh Haskap Berry Postharvest Quality Characteristics and Storage Life. Can. J. Plant Sci. 2021, 101, 1051–1063. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Błaszczyk, J.; Bieniasz, M.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Bogdał, S. The Effect of Harvest Date and Storage Conditions on the Quality of Remontant Strawberry Cultivars Grown in a Gutter System under Covers. Agriculture 2022, 12, 1193. [Google Scholar] [CrossRef]
- Krupa, T.; Klimek, K.; Zaraś-Januszkiewicz, E. Nutritional Values of Minikiwi Fruit (Actinidia arguta) after Storage: Comparison between DCA New Technology and ULO and CA. Molecules 2022, 27, 4313. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D. Controlled Atmosphere Storage. In Cold and Chilled Storage Technology; Springer: Boston, MA, USA, 1997; pp. 53–92. [Google Scholar] [CrossRef]
- Fang, Y.; Wakisaka, M. A Review on the Modified Atmosphere Preservation of Fruits and Vegetables with Cutting-Edge Technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Bors, B.; Sawchuk, E.; Dawson, J.; Sawatzky, R. Breeding and Selecting Haskap for Nutraceutical and Agronomic Suitability; Final Report on Saskatchewan Ministry of Agriculture: Saskatoon, SK, Canada, 2015; pp. 1–84. Available online: https://research-groups.usask.ca/fruit/documents/haskap/20080042.pdf (accessed on 26 July 2024).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wang, Z.; Svyantek, A.; Miller, Z.; Jarrett, B.; Kapus, A. Haskap Juicing Method Effects on Haskap Juice Quality. Appl. Sci. 2023, 13, 10784. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue Honeysuckle (Lonicera caerulea Subsp. Edulis (Turcz. Ex Herder) Hultén.) Berries and Changes in Their Ingredients across Different Locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef]
- Piasek, A.; Kusznierewicz, B.; Grzybowska, I.; Malinowska-Pańczyk, E.; Piekarska, A.; Azqueta, A.; Collins, A.R.; Namieśnik, J.; Bartoszek, A. The Influence of Sterilization with EnbioJet® Microwave Flow Pasteurizer on Composition and Bioactivity of Aronia and Blue-Berried Honeysuckle Juices. J. Food Compos. Anal. 2011, 24, 880–888. [Google Scholar] [CrossRef]
- Szajdek, A.; Dąbkowska, E.; Borowska, E.J. Wpływ obróbki enzymatycznej miazgi owoców jagodowych na zawartość polifenoli i aktywność przeciwutleniającą soku. Żywność 2006, 4, 59–67. Available online: https://wydawnictwo.pttz.org/magazine-archive/agnieszka-szajdek-ewa-dabkowska-eulalia-j-borowska-wplyw-obrobki-enzymatycznej-miazgi-owocow-jagodowych-na-zawartosc-polifenoli-i-aktywnosc-przeciwutleniajaca-soku/ (accessed on 18 July 2024).
- Kobori, R.; Yakami, S.; Kawasaki, T.; Saito, A.; Popovi´c, J.; Djordjevi´c, P.-D.; Fernando, L.; De Oliveira, C. Changes in the Polyphenol Content of Red Raspberry Fruits during Ripening. Horticulturae 2021, 7, 569. [Google Scholar] [CrossRef]
- Łysiak, G. The Sum of Active Temperatures as a Method of Determining the Optimum Harvest Date of “Šampion” and “Ligol” Apple Cultivars Impact of Transport Conditions on Storage and Shelf Life of Fruit View Project Impact of Climatic Conditions on the Quality of Frui. Acta Sci. Pol. Hortorum Cultus. 2012, 11, 3–13. Available online: https://bibliotekanauki.pl/articles/11542008.pdf (accessed on 24 March 2022).
- Zhang, Y.; Truzzi, F.; D’amen, E.; Dinelli, G. Effect of Storage Conditions and Time on the Polyphenol Content of Wheat Flours. Processes 2021, 9, 248. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Li, X.; Zhang, F.; Fan, Y.; Liu, Q.; Wan, X.; Lin, T. Postharvest UV-B Radiation Increases Enzyme Activity, Polysaccharide and Secondary Metabolites in Honeysuckle (Lonicera japonica Thunb.). Ind. Crops Prod. 2021, 171, 113907. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Application of Pulsed Electric Fields for Improving the Maceration Process during Vinification of Red Wine: Influence of Grape Variety. Eur. Food Res. Technol. 2008, 227, 1099–1107. [Google Scholar] [CrossRef]
- Kalisz, S.; Polak, N.; Cacak-Pietrzak, G.; Cendrowski, A.; Kruszewski, B. Impact of Production Methods and Storage Time on the Bioactive Compounds and Antioxidant Activity of Confitures Made from Blue Honeysuckle Berry (Lonicera caerulea L.). Appl. Sci. 2023, 13, 12999. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, I.J.; Kim, J.W.; Lee, S.K.; Ku, S.K.; Lee, H.J. Evaluation of in Vitro Anti-Oxidant and Anti-Inflammatory Activities of Korean and Chinese Lonicera Caerulea. Nutr. Res. Pract. 2018, 12, 486–493. [Google Scholar] [CrossRef]
- Zehfus, L.R.; Eskiw, C.; Low, N.H. Phenolic Profiles and Antioxidant Activities of Saskatchewan (Canada) Bred Haskap (Lonicera caerulea) Berries. BioRxiv. 2021. [Google Scholar] [CrossRef]
- Khattab, R.; Ghanem, A.; Brooks, M.S.-L. Stability of Haskap Berry (Lonicera caerulea L.) Anthocyanins at Different Storage and Processing Conditions. J. Field Robot. 2016, 5, 67. [Google Scholar] [CrossRef]


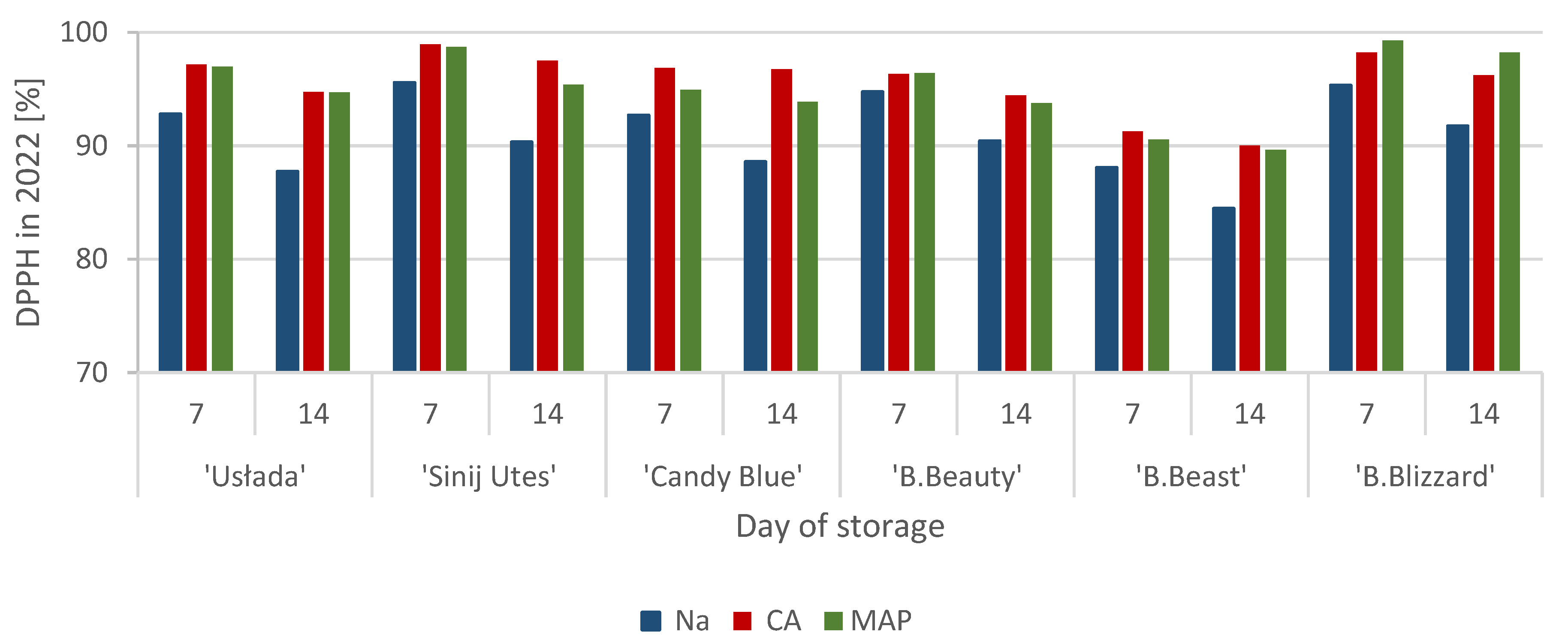


| June | July | August | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| ‘Usłada’ |  | |||||||||||
| ‘Sinij Utes’ |  | |||||||||||
| ‘Candy Blue’ |  | |||||||||||
| ‘Boreal Beauty’ |  | |||||||||||
| ‘Boreal Beast’ |  | |||||||||||
| ‘Boreal Blizzard’ |  | |||||||||||
| Total Polyphenols [mg GAE/L] | Antioxidant Activity [%] | |
|---|---|---|
| ‘Usłada’ | 1708.66 ± 6.47 d | 79.93 ± 0.49 b |
| ‘Sinij Utes’ | 2506.57 ± 2.36 a | 77.52 ± 0.07 cd |
| ‘Candy Blue’ | 1895.16 ± 5.84 c | 78.15 ± 0.04 c |
| ‘Boreal Beauty’ | 1036.20 ± 2.84 f | 76.43 ± 0.25 e |
| ‘Boreal Beast’ | 1463.77 ± 7.79 e | 81.77 ± 0.54 a |
| ‘Boreal Blizzard’ | 2340.74 ± 31.84 b | 76.95 ± 0.13 de |
| Cultivars | Storage Conditions | Total Polyphenols [mg GAE/L] | Antioxidant Activity [%] | Total Polyphenols [mg GAE/100 mL] | Antioxidant Activity [%] |
|---|---|---|---|---|---|
| 7 days | 7 days | 14 days | 14 days | ||
| ‘Usłada’ | Na | 1498.13 ± 1.03 i | 68.81 ± 0.04 f | 1082.61 ± 1.52 h | 63.24 ± 0.04 g |
| Ca | 1693.56 ± 2.19 g | 77.21 ± 0.07 b | 1364.72 ± 0.82 e | 74.58 ± 0.06 a | |
| MAP | 1610.66 ± 1.55 h | 75.46 ± 0.14 c | 1356.66 ± 6.23 e | 69.66 ± 0.05 d | |
| ‘Sinij Utes’ | Na | 2219.28 ± 5.31 c | 68.78 ± 0.05 f | 1411.02 ± 4.25 d | 61.68 ± 0.11 h |
| Ca | 2426.57 ± 2.14 a | 75.42 ± 0.10 c | 1853.21 ± 3.07 a | 69.60 ± 0.09 d | |
| MAP | 2380.02 ± 2.37 b | 73.57 ± 0.03 d | 1659.06 ± 3.86 b | 71.60 ± 0.19 c | |
| ‘Candy Blue’ | Na | 1767.37 ± 3.24 f | 72.73 ± 0.15 e | 999.5 ± 3.65 j | 65.64 ± 0.21 e |
| Ca | 1776.62 ± 5.04 e | 73.44 ± 0.23 d | 1209.11 ± 3.94 f | 69.33 ± 0.08 d | |
| MAP | 1764.09 ± 2.23 f | 73.48 ± 0.11 d | 1124.78 ± 1.59 g | 69.55 ± 0.02 d | |
| ‘Boreal Beauty’ | Na | 927.76 ± 2.25 l | 68.90 ± 0.15 f | 865.58 ± 1.68 l | 63.15 ± 0.11 g |
| Ca | 1031.40 ± 2.94 k | 75.95 ± 0.24 c | 961.67 ± 5.19 k | 71.49 ± 0.03 c | |
| MAP | 1035.28 ± 4.41 k | 75.51 ± 0.12 c | 962.08 ± 0.64 k | 71.73 ± 0.07 c | |
| ‘Boreal Beast’ | Na | 1407.35 ± 1.02 j | 75.61 ± 0.22 c | 1040.87 ± 1.59 i | 69.37 ± 0.09 d |
| Ca | 1409.35 ± 1.61 j | 78.78 ± 0.20 a | 1367.23 ± 5.26 e | 72.54 ± 0.07 b | |
| MAP | 1410.85 ± 1.91 j | 78.78 ± 0.14 a | 984.49 ± 4.90 jk | 74.28 ± 0.07 a | |
| ‘Boreal Blizzard’ | Na | 1495.29 ± 2.05 i | 64.78 ± 0.09 h | 1385.24 ± 1.24 de | 61.36 ± 0.05 h |
| Ca | 1764.92 ± 4.93 f | 66.30 ± 0.21 g | 1673.07 ± 5.11 b | 63.85 ± 0.10 f | |
| MAP | 2039.25 ± 2.95 d | 68.87 ± 0.23 f | 1455.62 ± 4.73 c | 63.24 ± 0.03 g |
| Total Polyphenols [mg GAE/L] | Antioxidant Activity [%] | |
|---|---|---|
| ‘Usłada’ | 2898.76 ± 3.63 a | 79.74 ± 0.13 b |
| ‘Sinij Utes’ | 2710.15 ± 2.91 b | 77.53 ± 0.13 c |
| ‘Candy Blue’ | 2544.46 ± 2.84 c | 78.02 ± 0.32 c |
| ‘Boreal Beauty’ | 2120.75 ± 9.35 f | 76.24 ± 0.28 e |
| ‘Boreal Beast’ | 2467.37 ± 8.40 d | 81.78 ± 0.14 a |
| ‘Boreal Blizzard’ | 2448.64 ± 1.54 e | 76.96 ± 0.05 d |
| Cultivars | Storage Conditions | Total Polyphenols [mg GAE/L] | Antioxidant Activity [%] | Total Polyphenols [mg GAE/L] | Antioxidant Activity [%] |
|---|---|---|---|---|---|
| 7 days | 7 days | 14 days | 14 days | ||
| ‘Usłada’ | Na | 1485.86 ± 2.29 e | 74.01 ± 0.16 b | 1250.60 ± 4.32 e | 69.98 ± 0.06 e |
| Ca | 2230.19 ± 4.01 c | 77.46 ± 0.08 a | 1592.26 ± 4.95 c | 75.55 ± 0.14 a | |
| MAP | 1925.93 ± 2.51 c | 77.31 ± 0.17 a | 1353.04 ± 3.52 d | 75.50 ± 0.34 a | |
| ‘Sinij Utes’ | Na | 1924.21 ± 1.74 c | 74.09 ± 0.05 b | 1591.35 ± 2.38 c | 70.04 ± 0.01 e |
| Ca | 2208.92 ± 3.96 b | 76.69 ± 0.16 b | 1757.58 ± 1.92 a | 75.59 ± 0.28 a | |
| MAP | 1922.67 ± 3.80 c | 76.51± 0.12 b | 1653.99 ± 4.12 b | 73.95 ± 0.16 b | |
| ‘Candy Blue’ | Na | 1133.70 ± 2.99 h | 72.32 ± 0.23 g | 924.64 ± 1.43 h | 69.12 ± 0.17 e |
| Ca | 1889.79 ± 6.04 d | 75.56 ± 0.09 c | 1351.92 ± 1.53 d | 75.47 ± 0.10 a | |
| MAP | 1413.54 ± 4.84 f | 74.06 ± 0.01 e | 1252.52 ± 2.03 e | 73.25 ± 0.11 b | |
| ‘Boreal Beauty’ | Na | 1112.99 ± 5.33 i | 72.25 ± 0.04 g | 916.86 ± 3.25 h | 68.95 ± 0.12 e |
| Ca | 1260.68 ± 5.48 g | 73.43 ± 0.07 f | 1194.44 ± 3.29 f | 72.02 ± 0.01 c | |
| MAP | 1250.73 ± 2.74 g | 73.50 ± 0.09 f | 1196.23 ± 2.72 f | 71.48 ± 0.06 c | |
| ‘Boreal Beast’ | Na | 1410.06 ± 0.45 f | 72.03 ± 0.02 g | 1350.58 ± 1.89 d | 69.09 ± 0.14 e |
| Ca | 1480.46 ± 5.99 e | 74.64 ± 0.08 d | 1357.90 ± 3.84 d | 73.61 ± 0.28 b | |
| MAP | 1415.36 ± 7.01 f | 74.04 ± 0.01 e | 1253.43 ± 8.66 e | 73.32 ± 0.21 b | |
| ‘Boreal Blizzard’ | Na | 1252.60 ± 3.68 g | 73.36 ± 0.14 f | 920.81 ± 6.07 h | 70.59 ± 0.08 e |
| Ca | 1488.64 ± 7.27 d | 75.57 ± 0.13 c | 1109.42 ± 1.11 g | 74.05 ± 0.14 b | |
| MAP | 1894.66 ± 3.68 d | 76.41 ± 0.09 b | 1255.87 ± 2.33 e | 75.59 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, B.A.; Bieniasz, M.; Błaszczyk, J. Effect of Short-Term Storage in Modified Atmosphere Packaging (MAP) and Controlled Atmosphere (CA) on Total Polyphenol Content and Antioxidant Activity in Juices from Haskap Berry (Lonicera caerulea L.). Agriculture 2025, 15, 253. https://doi.org/10.3390/agriculture15030253
Kowalczyk BA, Bieniasz M, Błaszczyk J. Effect of Short-Term Storage in Modified Atmosphere Packaging (MAP) and Controlled Atmosphere (CA) on Total Polyphenol Content and Antioxidant Activity in Juices from Haskap Berry (Lonicera caerulea L.). Agriculture. 2025; 15(3):253. https://doi.org/10.3390/agriculture15030253
Chicago/Turabian StyleKowalczyk, Barbara Anna, Monika Bieniasz, and Jan Błaszczyk. 2025. "Effect of Short-Term Storage in Modified Atmosphere Packaging (MAP) and Controlled Atmosphere (CA) on Total Polyphenol Content and Antioxidant Activity in Juices from Haskap Berry (Lonicera caerulea L.)" Agriculture 15, no. 3: 253. https://doi.org/10.3390/agriculture15030253
APA StyleKowalczyk, B. A., Bieniasz, M., & Błaszczyk, J. (2025). Effect of Short-Term Storage in Modified Atmosphere Packaging (MAP) and Controlled Atmosphere (CA) on Total Polyphenol Content and Antioxidant Activity in Juices from Haskap Berry (Lonicera caerulea L.). Agriculture, 15(3), 253. https://doi.org/10.3390/agriculture15030253







