Seawater Tolerance of the Beach Bean Vigna marina (Burm.) Merrill in Comparison with Mung Bean (Vigna radiata) and Adzuki Bean (Vigna angularis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
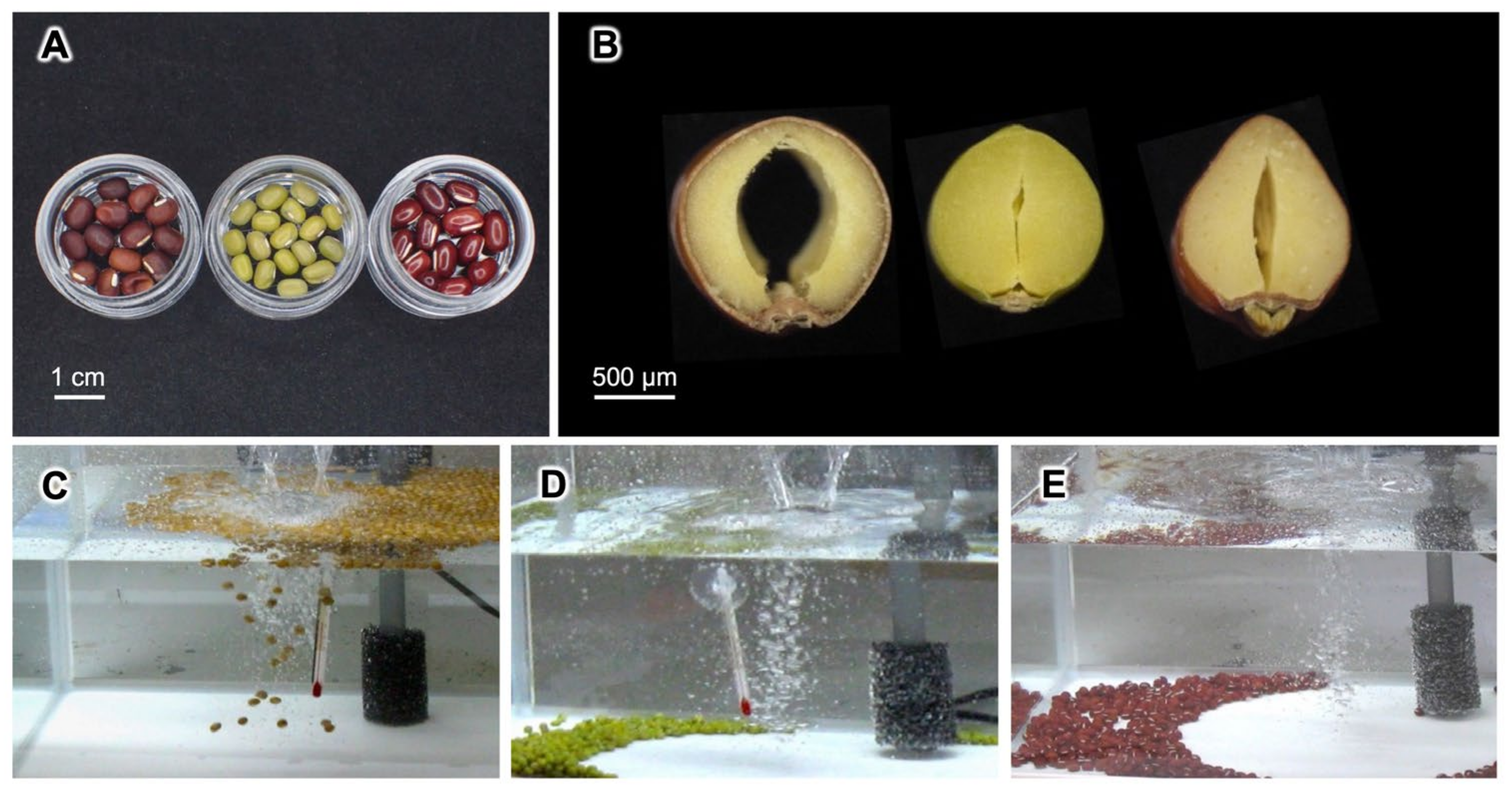
2.2. Seed Floating Experiments
2.3. Seed Germination Experiments
2.4. Seedling Growth Experiments
2.5. Chlorophyll a Fluorescence Measurement
3. Results
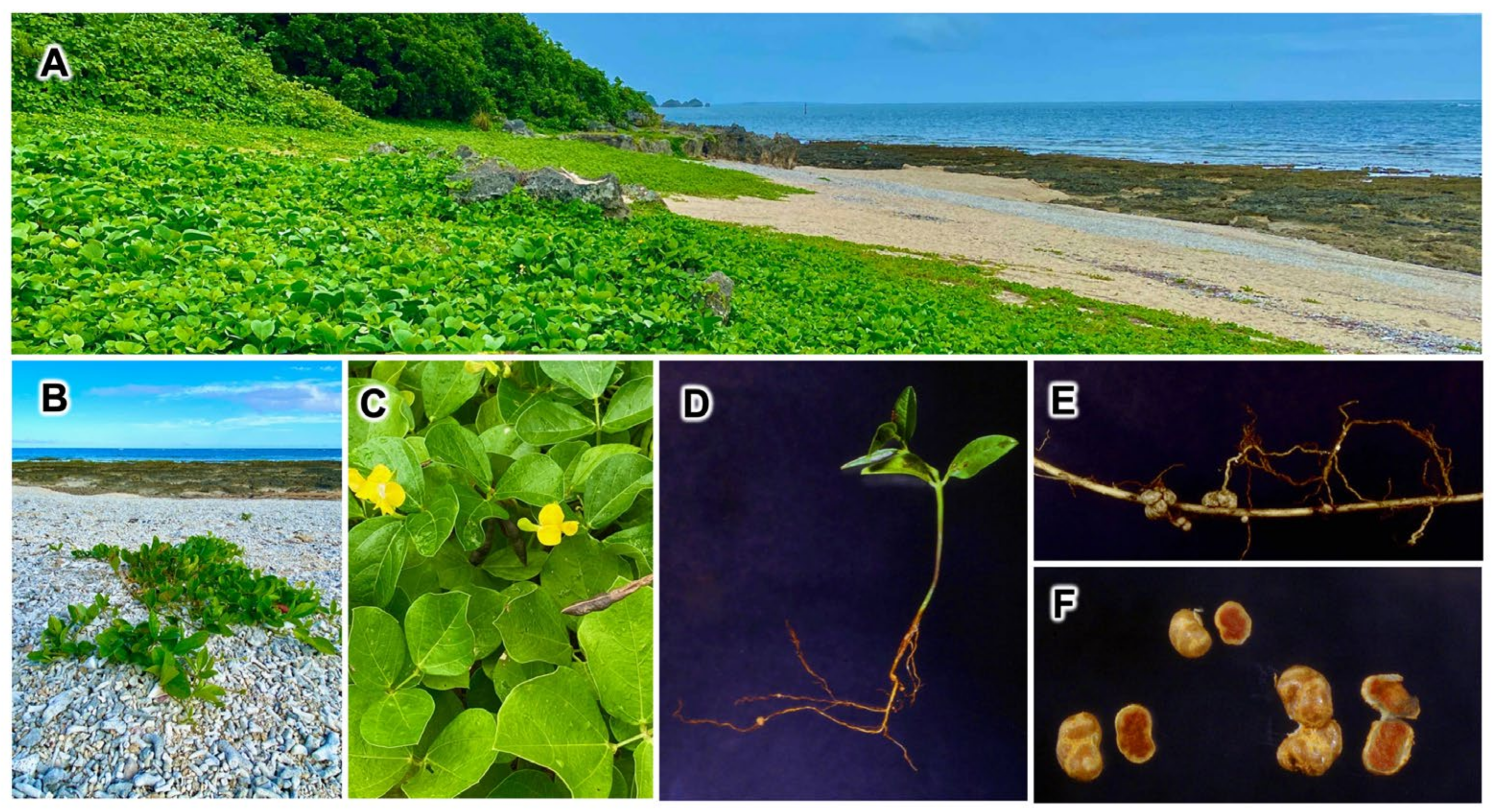
3.1. Natural Habitat of Vigna marina
3.2. Seawater Tolerance of Seeds
3.3. Seawater Tolerance of Seedlings
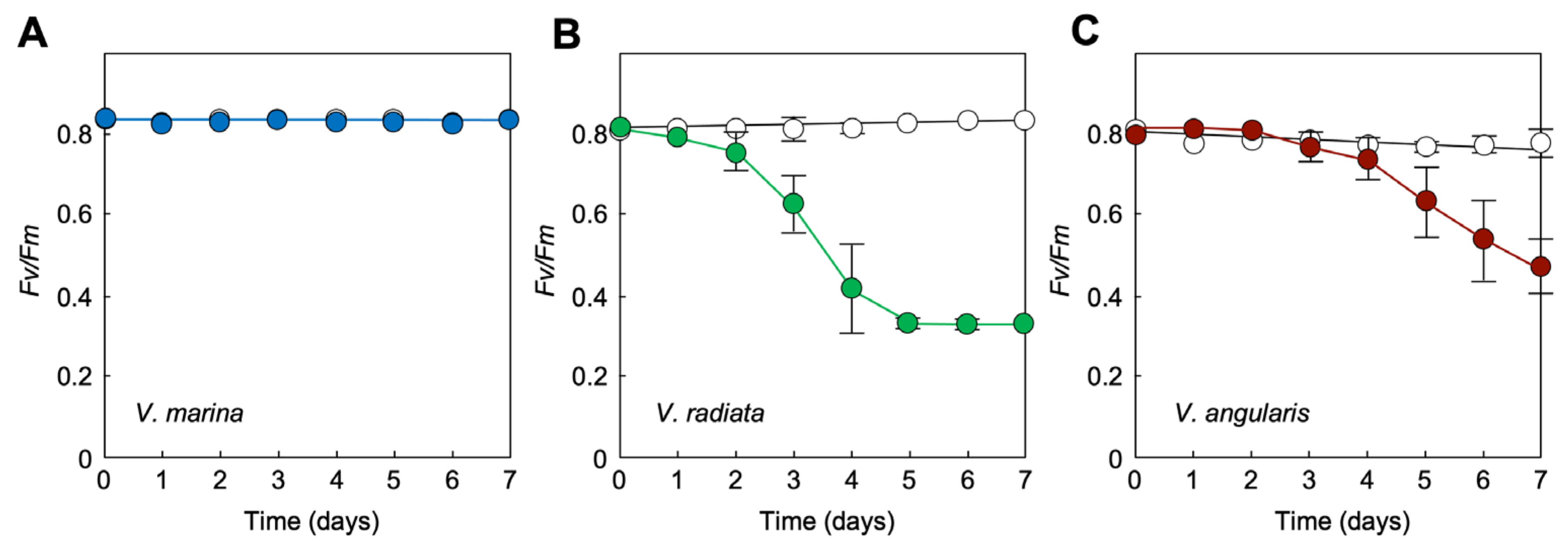
3.4. Seawater Tolerance of Photosynthetic Activity
4. Discussion
4.1. V. marina as a Seawater-Tolerant Legume Plant
4.2. Difference Between NaCl and Seawater Tolerance
4.3. V. marina as a Strong Candidate for Addressing Crop Production Challenges
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLeod, M.K.; Slavich, P.G.; Irhas, Y.; Moore, N.; Rachman, A.; Ali, N.; Iskandar, T.; Hunt, C.; Caniago, C. Soil salinity in Aceh after the December 2004 Indian Ocean tsunami. Agric. Water Manag. 2010, 97, 605–613. [Google Scholar] [CrossRef]
- Roy, K.; Sasada, K.; Kohno, E. Salinity status of the 2011 Tohoku-oki tsunami affected agricultural lands in northeast Japan. Int. Soil Water Conserv. Res. 2014, 2, 40–50. [Google Scholar] [CrossRef]
- Tchouaffe Tchiadje, N.F. Strategies to reduce the impact of salt on crops (rice, cotton and chili) production: A case study of the tsunami-affected area of India. Desalination 2007, 206, 524–530. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil. Sci. 2020, 72, 842–862. [Google Scholar] [CrossRef]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil salinization in agriculture: Mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Zorb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21 (Suppl. S1), 31–38. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Dasgupta, S.; Hossain, M.M.; Huq, M.; Wheeler, D. Climate change and soil salinity: The case of coastal Bangladesh. Ambio 2015, 44, 815–826. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Harouna, D.V.; Venkataramana, P.B.; Ndakidemi, P.A.; Matemu, A.O. Under-exploited wild Vigna species potentials in human and animal nutrition: A review. Glob. Food Secur. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Gerrano, A.S.; Mbuma, N.W.; Labuschagne, M.T. Breeding of vegetable cowpea for nutrition and climate resilience in sub-Saharan Africa: Progress, opportunities, and challenges. Plants 2022, 11, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.; Hall, A. Cowpea (Vigna unguiculata L. walp.). Field Crops Res. 1997, 53, 187–204. [Google Scholar] [CrossRef]
- Ben Gaied, R.; Brígido, C.; Sbissi, I.; Tarhouni, M. Sustainable strategy to boost legumes growth under salinity and drought stress in semi-arid and arid regions. Soil. Syst. 2024, 8, 84. [Google Scholar] [CrossRef]
- Breria, C.M.; Hsieh, C.H.; Yen, T.B.; Yen, J.Y.; Noble, T.J.; Schafleitner, R. A SNP-based genome-wide association study to mine genetic loci associated to salinity tolerance in mungbean (Vigna radiata L.). Genes 2020, 11, 759. [Google Scholar] [CrossRef]
- Singh, V.; Bell, M. Genotypic variability in achitectural development of mungbean (Vigna radiata L.) root systems and physiological relationships with shoot growth dynamics. Front. Plant Sci. 2021, 12, 725915. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Sharma, A.K.; Kumar, V.; Bhat, K.V. Salt stress and mungbean [Vigna radiata (L.) Wilczek]: Effects, physiological perspective and management practices for alleviating salinity. Arch. Agron. Soil Sci. 2019, 65, 1287–1301. [Google Scholar] [CrossRef]
- Patel, M.; Gupta, D.; Saini, A.; Kumari, A.; Priya, R.; Panda, S.K. Physiological phenotyping and biochemical characterization of mung bean (Vigna radiata L.) genotypes for salt and drought stress. Agriculture 2024, 14, 1337. [Google Scholar] [CrossRef]
- Podder, S.; Ray, J.; Das, D.; Sarker, B.C. Effect of salinity (NaCl) on germination and seedling growth of mungbean (Vigna radiata L.). J. Biosci. Agric. Res. 2020, 24, 2012–2019. [Google Scholar] [CrossRef]
- Dilipan, E.; Nisha, A.J. Assessing salinity tolerance and genetic variation in mung bean (Vigna radiata) through CAAT box and SCoT marker analysis. Ecol. Genet. Genom. 2024, 32, 100266. [Google Scholar] [CrossRef]
- Deeroum, A.; Thepphomwong, K.; Laosatit, K.; Somta, P. Inheritance of salt tolerance in wild mungbean (Vigna radiata var. sublobata). Agric. Nat. Resour. 2024, 58, 469–476. [Google Scholar]
- Mohamad Yunus, A.T.; Bin Chiu, S.; Ghazali, A.H. Vigna marina as a potential leguminous cover crop for high salinity soils. Pertanika J. Trop. Agric. Sci. 2024, 47, 481–494. [Google Scholar] [CrossRef]
- Chankaew, S.; Isemura, T.; Naito, K.; Ogiso-Tanaka, E.; Tomooka, N.; Somta, P.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL mapping for salt tolerance and domestication-related traits in Vigna marina subsp. oblonga, a halophytic species. Theor. Appl. Genet. 2014, 127, 691–702. [Google Scholar] [CrossRef]
- Lawn, R.; Cottrell, A. Seeds of Vigna marina (burm.) Merrill survive up to 25 years flotation in salt water. Qld. Nat. 2016, 54, 3–13. [Google Scholar]
- Maxted, N.; Mabuza-Diamini, P.; Moss, H.; Padulosi, S.; Jarvis, A.; Guarino, L. An Ecogeographic Study African Vigna. 2004. Available online: https://hdl.handle.net/10568/105017 (accessed on 5 January 2025).
- Hossain, K.K.; Nakamura, T.; Yamasaki, H. Effect of nitric oxide on leaf non-photochemical quenching of fluorescence under heat-stress conditions. Russ. J. Plant Physiol. 2011, 58, 629–633. [Google Scholar] [CrossRef]
- Lawn, R.; Watkinson, A. Habitats, morphological diversity, and distribution of the genus Vigna Savi in Australia. Aust. J. Agric. Res. 2002, 53, 1305–1316. [Google Scholar] [CrossRef]
- Singh, A.K.; Velmurugan, A.; Gupta, D.S.; Kumar, J.; Kesari, R.; Konda, A.; Singh, N.P.; Roy, S.D.; Biswas, U.; Kumar, R.R.; et al. Draft genome sequence of a less-known wild Vigna: Beach pea (V. marina cv. ANBp-14-03). Crop J. 2019, 7, 660–666. [Google Scholar] [CrossRef]
- Septiana, A.; Analuddin. Potential uses of marine bean (Vigna marina Burm.) as salt tolerant kegume in coastal salty land, Southeast Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 260, 012142. [Google Scholar] [CrossRef]
- Nakanishi, H. Dispersal ecology of the maritime plants in the Ryukyu Islands, Japan. Ecol. Res. 1988, 3, 163–173. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tsuda, Y.; Takayama, K.; Nagashima, R.; Tateishi, Y.; Kajita, T. The presence of a cryptic barrier in the West Pacific Ocean suggests the effect of glacial climate changes on a widespread sea-dispersed plant, Vigna marina (Fabaceae). Ecol. Evol. 2019, 9, 8429–8440. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhian, R.; Rajalakshmi, S.; Jayakumar, V. Salt tolerance characteristics of rhizobium species associated with Vigna marina. Indian J. Agric. Sci. 2009, 79, 980–985. [Google Scholar]
- Noda, Y.; Sugita, R.; Hirose, A.; Kawachi, N.; Tanoi, K.; Furukawa, J.; Naito, K. Diversity of Na+ allocation in salt-tolerant species of the genus Vigna. Breed. Sci. 2022, 72, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Iki, Y.; Tanoi, K.; Naito, K. Phenotypic responses in the root of salt-tolerant accessions of Vigna marina and Vigna luteola under salt stress. Genet. Resour. Crop Evol. 2023, 71, 2631–2640. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Takahashi, S.; Tamashiro, A.; Sakihama, Y.; Yamamoto, Y.; Kawamitsu, Y.; Yamasaki, H. High-susceptibility of photosynthesis to photoinhibition in the tropical plant Ficus microcarpa L. f. cv. Golden Leaves. BMC Plant Biol. 2002, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.; Machado, E.; Silva, J.; Lagôa, A.; Silveira, J. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Yang, X.; Lu, C. Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol. Plant. 2005, 124, 343–352. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil. Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quesada, V.; Garcia-Martinez, S.; Piqueras, P.; Ponce, M.R.; Micol, J.L. Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiol. 2002, 130, 951–963. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Sadhukhan, S. Impact of salinity on growth and development of plants with the central focus on Glycophytes: An overview. Bull. Environ. Pharmacol. Life Sci. 2023, 12, 235–266. [Google Scholar]
- Kester, D.R.; Pytkowicx, R.M. Sodium, magnesium, and calcim sulfate ion-pairs in seawater at 25 °C. Limnol. Oceanogr. 1969, 14, 686–692. [Google Scholar] [CrossRef]
- Dickson, A.G.; Goyet, C. Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Sea Water. Version 2; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 1994; p. 198. Available online: https://www.osti.gov/biblio/10107773 (accessed on 5 January 2025).
- Yamasaki, H.; Cohen, M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide 2016, 55–56, 91–100. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.; Zhou, M. Structure and function of an Arabidopsis thaliana sulfate transporter. Nat. Commun. 2021, 12, 4455. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal Behav. 2023, 18, 2030082. [Google Scholar] [CrossRef]
- Guo, J.; Shan, C.; Zhang, Y.; Wang, X.; Tian, H.; Han, G.; Zhang, Y.; Wang, B. Mechanisms of salt tolerance and molecular breeding of salt-tolerant ornamental plants. Front. Plant Sci. 2022, 13, 854116. [Google Scholar] [CrossRef]
- Shelar, P.V.; Mankar, G.D.; Sontakke, O.P.; Bhosale, K.S.; Nikalje, G.C.; Ahire, M.L.; Nikam, U.D.; Barmukh, R.B. A Review of the physio-biochemical and molecular mechanisms of salt tolerance in crop. Curr. Agric. Res. J. 2024, 12, 545–563. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Yamasaki, H.; Itoh, R.D.; Mizumoto, K.B.; Yoshida, Y.S.; Otaki, J.M.; Cohen, M.F. Spatiotemporal characteristics determining the multifaceted nature of reactive oxygen, nitrogen, and sulfur species in relation to poton homeostasis. Antioxid. Redox Signal 2024. [Google Scholar] [CrossRef] [PubMed]
- Padulosi, S.; Ng, N.Q. A useful and enexploited herb, Vigna marina (Lgeguminosae-Papliionoidease) and the taxonomic revision of its genetic diversity. Bulll. Nat. Plantention Belg. 1993, 62, 119–126. [Google Scholar] [CrossRef]
- Takahashi, Y.; Muto, C.; Iseki, K.; Naito, K.; Somta, P.; Pandiyan, M.; Senthil, N.; Tomooka, N. A new taxonomic treatment for some wild relatives of mungbean (Vigna radiata (L.) Wilcz.) based on their molecular phylogenetic relationships and morphological variations. Genet. Resour. Crop Evol. 2017, 65, 1109–1121. [Google Scholar] [CrossRef]
- Takahashi, Y.; Somta, P.; Muto, C.; Iseki, K.; Naito, K.; Pandiyan, M.; Natesan, S.; Tomooka, N. Novel genetic resources in the genus Vigna unveiled from gene bank accessions. PLoS ONE 2016, 11, e0147568. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Septiana, A.; Nakamura, S.P.; Naomasa, R.F.; Yamasaki, H. Seawater Tolerance of the Beach Bean Vigna marina (Burm.) Merrill in Comparison with Mung Bean (Vigna radiata) and Adzuki Bean (Vigna angularis). Agriculture 2025, 15, 228. https://doi.org/10.3390/agriculture15030228
Septiana A, Nakamura SP, Naomasa RF, Yamasaki H. Seawater Tolerance of the Beach Bean Vigna marina (Burm.) Merrill in Comparison with Mung Bean (Vigna radiata) and Adzuki Bean (Vigna angularis). Agriculture. 2025; 15(3):228. https://doi.org/10.3390/agriculture15030228
Chicago/Turabian StyleSeptiana, Andi, Shiori P. Nakamura, Riko F. Naomasa, and Hideo Yamasaki. 2025. "Seawater Tolerance of the Beach Bean Vigna marina (Burm.) Merrill in Comparison with Mung Bean (Vigna radiata) and Adzuki Bean (Vigna angularis)" Agriculture 15, no. 3: 228. https://doi.org/10.3390/agriculture15030228
APA StyleSeptiana, A., Nakamura, S. P., Naomasa, R. F., & Yamasaki, H. (2025). Seawater Tolerance of the Beach Bean Vigna marina (Burm.) Merrill in Comparison with Mung Bean (Vigna radiata) and Adzuki Bean (Vigna angularis). Agriculture, 15(3), 228. https://doi.org/10.3390/agriculture15030228




