Abstract
Plant endophytic fungi, which colonize plant tissues and form symbiotic relationships with their hosts, are known for their high diversity and wide distribution. These fungi often influence plant growth and development through the emission of volatile organic compounds (VOCs), whose effects can extend beyond host plants to non-host species. In this study, we isolated two endophytic fungi, Trametes hirsuta RR1 and Talaromyces pinophilus RR2 from healthy rice roots. The VOCs mixtures produced by strains RR1 and RR2 were both able to promote rice growth when these strains were co-cultured with rice seedlings. Specifically, strain RR1 and RR2 increased rice shoot fresh weight by 44.22% and 26.69%, root fresh weight by 58.24% and 41.76%, shoot length by 30.35% and 25.07%, and root length by 29.11% and 4.23%, respectively. They significantly enhanced the contents of chlorophyll a and carotenoids, which increased by 18.61% and 17.04%, and by 18.73% and 31.55%, respectively. Gas chromatography–mass spectrometry (GC-MS) was applied to analyze the VOCs emitted by the two strains. The analysis successfully identified a total of 13 major compounds. Among them, at appropriate concentrations, 1-pentanol, methyl DL-2-methylbutyrate, ethylbenzene, 2-ethyl-p-xylene, ethyl benzoate and dimethyl phthalate, can promote rice growth and alter the contents of photosynthetic pigments and hydrogen peroxide to varying degrees. This study provides an important basis for the in-depth research and development of biofumigants for promoting crop growth.
1. Introduction
Endophytic fungi are microorganisms that asymptomatically colonize plant tissues for all or part of their life cycle [1,2,3,4]. Widely distributed across diverse plant species, they establish mutualistic associations with their hosts [5,6,7]. By playing a pivotal role in maintaining ecosystem stability, these fungi act as key drivers of ecological processes. They employ multiple functional mechanisms to enhance host plant growth and improve resistance to abiotic and biotic stresses [8,9,10], primarily through phytohormone secretion, facilitation of mineral nutrient acquisition (e.g., nitrogen, phosphorus, and potassium), and suppression of pathogenic microbial invasion [11,12,13,14,15,16,17]. Beyond these mechanisms, volatile organic compounds (VOCs), as a class of organic chemicals, also play a pivotal role in mediating the interactions between endophytic fungi and their host plants [18,19,20].
VOCs are natural products that volatilize under ambient conditions. Characterized by low boiling points, low molecular weight, and high vapor pressure, they serve as key mediators in fungal-plant interactions [21,22,23]. Studies have shown that VOCs produced by various endophytic fungi promote plant growth and help plants adapt to environmental conditions by inducing systemic resistance, modulating hormone levels, and enhancing nutrient acquisition. For instance, VOCs emitted by Trichoderma spp. have been shown to strengthen plant defense, increase nutrient availability, and suppress plant pathogen invasion, thereby facilitating plant growth [24]. VOCs from Aureobasidium sp. contribute to nutrient acquisition and significantly promote the growth of Arabidopsis thaliana, as evidenced by increased seedling fresh weight, expanded leaf area, and enhanced lateral root formation [25]. VOCs produced by Streptomyces setonii promote plant growth through activation of auxin and ethylene signaling pathways in A. thaliana, while concurrently enhancing salt tolerance by inducing abscisic acid signaling and anthocyanin biosynthesis [26]. Moreover, VOCs released by Cladosporium sp., Geopyxis sp., Didymella sp., and Chalara sp. indirectly elevate terpenoid defense compound concentrations in white spruce, supporting the “plant partnership hypothesis” that highlights the pivotal role of fungal VOCs in strengthening tree resistance mechanisms [27].
Studies have indicated that VOCs produced by certain fungi can suppress plant growth and development. This inhibitory effect may be attributed to the ability of VOCs to diminish key enzyme activities or disrupt ion channels that are essential for normal plant physiological processes [28,29,30,31]. For example, VOCs from Cochliobolus sativus and Fusarium culmorum were found to reduce barley biomass, manifested as reduced root length and decreased surface area of aerial parts [29]. VOCs produced by Muscodor yucatanensis inhibited root elongation in tomato, amaranth, and barnyard grass, particularly during the first 15 days of fungal growth [30]. Furthermore, among 23 common VOCs tested, 1-octen-3-one, 2-ethylhexanal, 3-methylbutanal, and butanal were shown to completely prevent seedling formation in A. thaliana and significantly reduce plant fresh weight [31].
Trametes hirsuta is a fungal species belonging to the phylum Basidiomycota, class Agaricomycetes, order Polyporales, family Polyporaceae, and genus Trametes [32]. It demonstrates significant potential in several fields, particularly in biodegradation and soil remediation [33,34,35,36,37]. For instance, this fungus can assist in the phytoextraction of heavy metals from contaminated sites by enhancing their uptake in host plants [38]. Its ability to degrade lignin contributes to the improvement of soil quality and structure, thereby supporting plant growth and development [39]. Talaromyces pinophilus is a filamentous fungus classified within the phylum Ascomycota, class Eurotiomycetes, order Eurotiales, family Trichocomaceae, and genus Talaromyces [40]. Research has demonstrated that T. pinophilus can enhance growth metrics in wheat (Triticum aestivum), including the elevation of osmolytes (e.g., soluble sugars, proteins, and amino acids), photosynthetic pigments, and the activity of enzymatic antioxidants such as catalase, peroxidase, and superoxide dismutase [40]. It also improves the uptake of essential nutrients (e.g., calcium, potassium, and magnesium) while reducing heavy metal absorption in plants cultivated in sewage sludge-amended soil [41]. Its ability to secrete amylolytic and cellulolytic enzymes indicates a promising potential for biotechnological applications [42]. Furthermore, T. pinophilus exhibits notable mycoparasitic capabilities. For instance, it can colonize tissues of cucurbit plants including pumpkin and cucumber, establishing a symbiotic relationship with the host plants that confers potential protective benefits [43]. Antagonistic assays through dual culture with Botrytis cinerea demonstrated that the antifungal activity of T. pinophilus relies on this mycoparasitism, which was morphologically verified by the formation of mycelial overgrowth [44]. Although T. hirsuta and T. pinophilus have been extensively studied, the specific components of the VOCs they produce and the effects of these VOCs on plant growth and development are not yet known.
In this study, two endophytic fungi, T. hirsuta RR1 and T. pinophilus RR2, were isolated from healthy rice roots. The plant growth-promoting effects of the VOCs produced by these fungi were evaluated through non-contact co-cultivation of the fungal strains with rice. Furthermore, gas chromatography-mass spectrometry (GC-MS) was employed to analyze the specific composition of VOCs produced by both fungi and to assess the growth-regulating effects of individual compounds.
2. Materials and Methods
2.1. Strains and Plant Materials
The endophytic strains (RR1 and RR2) used in this study were isolated from the roots of healthy rice seedlings collected from experimental fields of Nanjing Agricultural University, Nanjing, China, in 2022. They were cultured on potato dextrose agar (PDA) medium (Haibo Biotechnology Co., Ltd., Qingdao, China) at 26 °C in the dark. Rice (Oryza Sativa spp. japonica) was used as plant material.
2.2. Molecular Identification of the Strains
Identification of the two strains was conducted based on the internal transcribed spacer (ITS) gene sequence [45,46]. The strains were cultured on PDA medium at 26 °C for 7 days. Mycelia were then harvested, and genomic DNA (gDNA) was extracted using the CTAB method [47]. The ITS region was amplified by PCR using a TIANGEN Golden Easy PCR kit (TIANGEN Biotech Co., Ltd., Beijing, China), with the gDNA as the template. The amplification reactions employed primer sets and conditions as described by Lu et al. [48]. The PCR products with evident bands, detected with 1% agarose gel electrophoresis, were sent to Tsingke Biotech Co., Ltd., Nanjing, China, for sequencing. The resulting sequences were validated using BLAST (Basic Local Alignment Search Tool) on the NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 21 October 2025). A phylogenetic tree was reconstructed with MEGA X (v10.2.6) [49] using the Maximum Likelihood (ML) method under the Tamura-Nei model, with 1000 bootstrap replicates.
2.3. Co-Culture of Strains with Rice
To avoid direct plant-fungal contact, rice seedlings were exposed to fungal VOCs via a double plate-within-a-plate system [50]. Briefly, an uncovered Petri dish (35 × 35 mm; Solarbio Science and Technology Co., Ltd., Beijing, China) was pre-positioned at the lower-right corner of a Petri dish (150 × 150 mm). Subsequently, molten 1/2 Murashige and Skoog (MS) solid medium (Solarbio Science and Technology Co., Ltd., Beijing, China) was then added to the Petri dish (150 × 150 mm). After solidification, half of the 1/2 MS solid medium was removed from the Petri dish (150 × 150 mm), and three rice seedlings were transplanted into the remaining medium. Molten PDA medium was transferred into the Petri dish (35 × 35 mm) using a micropipette (DLAB Scientific Co., Ltd., Beijing, China), followed by fungal inoculation after medium solidification.
Healthy and fully filled rice seeds were selected for the plant-fungal co-cultivation experiments. Rice seeds were surface-sterilized following the method of Jing et al. [51]. After sterilization, the seeds were thoroughly rinsed with sterile water and then placed in Petri dishes (100 × 100 mm) containing 1/2 MS solid medium [52]. The seeds were germinated at 26 °C in a constant temperature incubator (LISK instrument Equipment Co., Ltd., Nanjing, China) for 2–3 days. Then, they were transferred to a plant growth chamber (Kesheng Lab Instrument Co., Ltd., Ningbo, China) and cultivated under a 16 h light/8 h dark photoperiod at 26/24 °C with a relative humidity of 70% and light intensity of 10,000 lm/m2.
For fungal inoculation, mycelial plugs (5 mm diameter) taken from fungal cultures grown on PDA medium were transferred to Petri dishes (35 × 35 mm) containing fresh PDA medium. The PDA culture medium without strains was used as a control. Since the PDA medium inoculated with fungi is placed in separate Petri dishes (35 × 35 mm), it can form physical isolation from the 1/2 MS solid medium, thereby ensuring that the 1/2 MS medium is not contaminated by fungal mycelia or spores. After 7 days of rice cultivation, uniformly grown seedlings were selected and transferred to new Petri dishes (150 × 150 mm) containing 1/2 MS solid medium. Each treatment included six biological replicates. All co-culture samples were maintained in a plant growth chamber at 26/24 °C with a 16 h/8 h (light/dark) photoperiod.
Seven days after inoculation, rice samples were harvested for phenotypic and physiological analysis. The shoot length, root length, and fresh weight of rice seedlings were measured. Hydrogen peroxide (H2O2) content was measured according to the method of Li et al. [53]. Briefly, 0.2 g of fresh rice samples were added to 2 mL of 0.1% trichloroacetic acid (TCA), ground thoroughly on ice, and centrifuged at 1200× g for 15 min. Then, 0.5 mL of the supernatant was mixed with 0.5 mL of phosphate-buffered saline (PBS; 10 mmol/L, pH 7.0) and 1 mL of potassium iodide (KI) solution. The absorbance was measured at 390 nm, and the H2O2 content was calculated using the standard formula. The contents of chlorophyll a, chlorophyll b, and carotenoids were determined following the method of Lichtenhaler and Wellburn [54]. In brief, 0.2 g of rice samples was accurately weighed and thoroughly ground. Subsequently, 10 mL of an ethanol-acetone mixture (1:1, v/v) was added, and the extraction was performed in the dark overnight (not more than 12 h). Absorbance was measured at 663, 646, and 470 nm once the plant tissues had turned white. The contents of individual photosynthetic pigments were calculated using the standard formula. All reagents mentioned above were purchased from Shandong Lilkang Medical Technology Co., Ltd., Dezhou, China and Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
2.4. Identification of VOCs Produced by Two Strains
VOCs were analyzed using headspace solid-phase microextraction (HS-SPME; Agilent Technologies Co., Ltd., Santa Clara, CA, USA) coupled with GC-MS (Agilent Technologies Co., Ltd., Santa Clara, CA, USA). Briefly, 5 mL of molten PDA medium was aliquoted into a sterile 20 mL headspace vial (Zhejiang Sainz Scientific Instrument Co., Ltd., Ningbo, China) and solidified on a slanted surface. Strains were inoculated onto the medium and incubated at 26 °C for 7 days. A sterile PDA plug without fungal inoculation served as the control. The headspace was collected for 1 h and analyzed by GC-MS.
VOCs were extracted using an SPME fiber (50/30 μm Divinyl benzene/Carboxen/Polydimethylsiloxane, DVB/CAR/PDX; Anpel Laboratory Technologies Co., Ltd., Shanghai, China) assembly exposed to the vial headspace for 45 min at ambient temperature. GC-MS analysis was performed on an Agilent 7890N/5975C system equipped with an HP-5MS capillary column (0.25 mm × 30 m, 0.25 μm). The carrier gas was helium at a constant flow rate of 1.0 mL/min. The injector temperature was maintained at 240 °C, and samples were introduced in splitless mode with an injection volume of 1.0 μL. The oven temperature program was initialized at 30 °C (3 min hold), ramped at 5 °C/min to 220 °C, then raised to 270 °C (1 min hold). Mass spectrometric detection was conducted in electron impact (EI) mode at 70 eV ionization energy. The ion source temperature was set at 230 °C, with mass spectra acquired over a range of 20–450 m/z at a scanning rate of 3.35 scans per second. Compound identification was achieved by matching mass spectral data with the NIST/EPA/NIH Mass Spectral Library 2005 (NIST05) database (Agilent).
2.5. Effects of Different Concentrations of Pure VOCs on Rice Growth
The composition of VOCs in two strains was previously identified using GC-MS. We investigated the effects of six pure compounds, including 1-pentanol, methyl DL-2-methylbutyrate, ethylbenzene, 2-ethyl-p-xylene, ethyl benzoate, and dimethyl phthalate, on rice growth under in vitro conditions. The experimental procedure was consistent with that described in Section 2.3, except that 10 μL of pure VOC standard solutions (100) and their serial dilutions (10−1, 10−2, and 10−3) were pipetted onto sterile filter paper (10 × 10 mm) instead of applying a mycelial inoculum. The standard solutions included 1-pentanol (99.5%), methyl DL-2-methylbutyrate (98%), ethylbenzene (99.5%), 2-ethyl-p-xylene (98%), ethyl benzoate (99%), and dimethyl phthalate (99.7%), with vial concentrations of 0.018, 0.089, 0.019, 0.085, 0.013, and 0.026 mg/mL, respectively. All compounds were obtained from Macklin Biochemical Technology Co., Ltd., Shanghai, China. Filter paper without VOCs in a Petri dish (35 × 35 mm) was used as a control in the seedling Petri dish (150 × 150 mm). After 7 days of co-cultivation, rice growth parameters, H2O2 content, and pigment levels (including chlorophyll a, chlorophyll b, and carotenoids) were measured according to the method outlined in Section 2.3. There were six replications of each treatment.
3. Results
3.1. Characteristics of Two Strains
Strain RR1 formed grayish-white, flocculent colonies with dense aerial mycelia, whereas strain RR2 exhibited an orange center with white margins, along with dense aerial mycelia, flat colony morphology, and a smooth surface (Figure 1A). Phylogenetic analysis based on ITS sequences placed RR1 in a clade with T. hirsuta with 96% bootstrap support, while RR2 clustered with T. pinophilus supported by 93% bootstrap values (Figure 1B). These findings suggest that strain RR1 can be identified as T. hirsuta, while strain RR2 can be determined to be T. pinophilus. The ITS sequences of strains RR1 and RR2 are presented in Supplementary Materials.
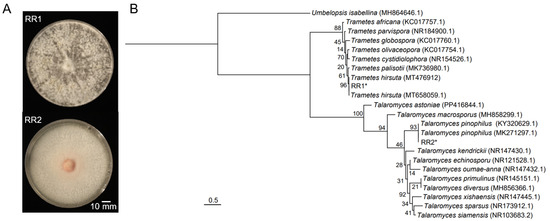
Figure 1.
Colony morphology and phylogenetic analysis of strains RR1 and RR2. (A) Colony morphologies of the two strains. (B) Maximum likelihood (ML) phylogenetic tree based on ITS sequences of the two strains and related species. The ML bootstrap values based on 1000 replications are indicated above the branches. The two strains from this study are marked with asterisks (*).
3.2. Effects of VOCs Mixtures Produced by Two Strains on the Growth and Development of Rice
To investigate the effects of VOCs produced by the two strains on rice growth, 7-day-old uniformly grown rice seedlings were co-cultured with the strains for 7 days to evaluate plant growth under VOC exposure (Figure 2A).
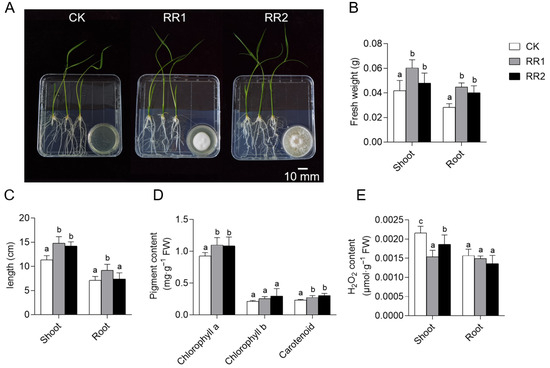
Figure 2.
Effects of VOCs produced by strains RR1 and RR2 on rice growth. (A) Promotion of rice growth by the two strains at 7 days post-inoculation. (B) Fresh weight of rice shoots and roots. (C) Shoot and root length of rice. (D) Pigment content in rice shoots. (E) H2O2 content in rice shoots and roots. Different lowercase letters above the bars indicate significant differences based on LSD multiple range test (p < 0.05).
The results showed that, compared to the control group, treatment with each strain resulted in varying degrees of increase in fresh weight, shoot length, and root length. Specifically, the VOCs produced by strains RR1 and RR2 increased rice shoot fresh weight by 44.22% and 26.69%, root fresh weight by 58.24% and 41.76%, and shoot length by 30.35% and 25.07%, respectively (Figure 2B,C). The VOCs released by both strains promoted root growth, and the effect was most pronounced with strain RR1 (Figure 2C).
To assess the impact of VOC exposure on the photosynthetic and antioxidant activity of rice, pigment and H2O2 contents were measured (Figure 2D,E). The results showed that VOCs from both strains significantly increased the contents of chlorophyll a and carotenoids. Specifically, chlorophyll a content increased by 18.61% and 17.04%, and carotenoid content increased by 18.73% and 31.55%, respectively (Figure 2D). A marked reduction in H2O2 content was also observed, with decreases of 28.67% and 13.74% in the shoots treated with RR1 and RR2, respectively. Although a decreasing trend was noted in the roots, the change was not significant (Figure 2E). These findings revealed that the VOCs produced by strains RR1 and RR2 exert substantial growth-promoting effects on rice.
3.3. Identification of VOC Mixture Produced by the Two Strains
Strains RR1 and RR2 were individually cultured in headspace vials containing PDA medium for 7 days, followed by GC-MS analysis within a 4 h period. The results revealed distinct differences in both the variety and quantity of VOCs produced by the two strains. A total of 13 VOCs were identified, including alcohols, esters, and alkenes (Table 1). Among these, strains RR1 and RR2 produced 8 and 7 VOCs, respectively. Two VOCs, 1,2,4,5-tetramethylbenzene and dimethyl phthalate, were common to both strains (Table 1).

Table 1.
VOCs identified from strains RR1 and RR2 by GC-MS.
3.4. Effects of Different Concentrations of Pure VOCs on the Growth and Development of Rice
Among the 13 detected VOCs, six pure compounds, namely 1-pentanol, methyl DL-2-methylbutyrate, ethylbenzene, 2-ethyl-p-xylene, ethyl benzoate, and dimethyl phthalate, were purchased. Subsequently, their impacts on rice growth were investigated (Figure 3 and Figure 4).
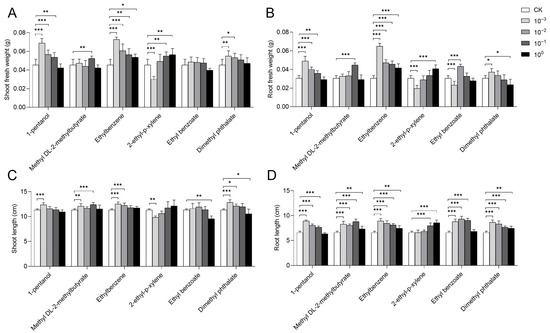
Figure 3.
Effects of pure compounds on rice growth. Changes in rice growth after 7 days of cultivation with six different concentrations of pure compounds are shown. The concentration gradients included a standard solution (100) along with solutions diluted 10-fold (10−1), 100-fold (10−2), and 1000-fold (10−3). (A) Fresh weight of rice shoots. (B) Fresh weight of rice roots. (C) Shoot length of rice. (D) Root length of rice. Error bars represent the mean ± standard error of the mean (SEM). The “*”, “**”, and “***” represent the result of LSD-t test, indicating the significant difference between control and treatment (p < 0.05, 0.01, and 0.001, respectively).
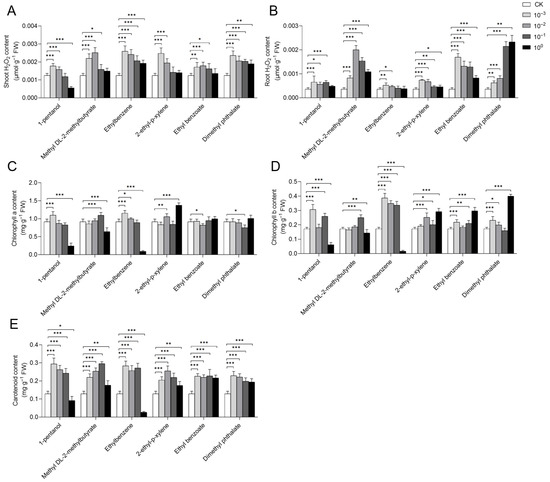
Figure 4.
Effects of pure compounds on hydrogen peroxide and pigment contents in rice. Changes in rice growth after 7 days of cultivation with six different concentrations of pure compounds are shown. The concentration gradients included a standard solution (100) along with solutions diluted 10-fold (10−1), 100-fold (10−2), and 1000-fold (10−3). (A) H2O2 content in rice shoots. (B) H2O2 content in rice roots. (C) Chlorophyll a content in rice shoots. (D) Chlorophyll b content in rice shoots. (E) Carotenoid content in rice shoots. Error bars represent the mean ± standard error of the mean (SEM). The “*”, “**”, and “***” represent the result of LSD-t test, indicating the significant difference between control and treatment (p < 0.05, 0.01, and 0.001, respectively).
Analysis of plant growth indicated that ethylbenzene promoted rice growth at all tested concentrations (Figure 3A–D). The standard solutions of most compounds, such as 1-pentanol, methyl DL-2-methylbutyrate, ethyl benzoate, and dimethyl phthalate, exerted weak or even inhibitory effects on rice growth, but these effects were gradually restored at reduced concentrations.
For 1-pentanol, all diluted solutions (10−1, 10−2, and 10−3) promoted rice growth, as evidenced by increases in shoot fresh weight, root fresh weight, shoot length, and root length, with the most pronounced effects observed at the 10−3 dilution (Figure 3A–D). For methyl DL-2-methylbutyrate, the 10−1 dilution demonstrated the most significant growth-promoting effect, increasing shoot fresh weight, root fresh weight, shoot length, and root length by 15.44%, 46.45%, 9.41%, and 33.42%, respectively, compared to the control (Figure 3A-D). All tested concentrations of methyl DL-2-methylbutyrate significantly enhanced root length (Figure 3D). With ethyl benzoate, the 10−2 dilution significantly enhanced both root fresh weight and root length, whereas its standard solution significantly reduced shoot length by 16.18% (Figure 3B–D). Regarding dimethyl phthalate, its 10−3 dilution significantly promoted shoot fresh weight, root fresh weight, shoot length, and root length, with increases of 20.96%, 21.86%, 13.09%, and 30.89%, respectively. The standard solution of dimethyl phthalate significantly improved root length but concurrently inhibited both root fresh weight and shoot length (Figure 3B–D).
Furthermore, the growth-promoting effect of 2-ethyl-p-xylene on rice was enhanced with increasing concentration. Specifically, its standard solution significantly increased shoot fresh weight, root fresh weight, and root length by 24.26%, 33.88%, and 30.13%, respectively, though no significant difference was observed in shoot length (Figure 3A–D). The 10−3 dilution of 2-ethyl-p-xylene significantly inhibited shoot fresh weight, root fresh weight, and shoot length.
The effects of pure compounds on H2O2 content in rice were also analyzed, which revealed that several treatments significantly enhanced H2O2 levels in both the shoots and roots. Specifically, this included diluted solutions (10−2 and 10−3) of 1-pentanol, ethylbenzene, and 2-ethyl-p-xylene, diluted solutions (10−1, 10−2, and 10−3) of methyl DL-2-methylbutyrate and ethyl benzoate, as well as all tested concentrations of dimethyl phthalate (Figure 4A,B). Notably, all concentrations of ethylbenzene significantly increased H2O2 content in the shoots, whereas ethyl benzoate primarily exerted its effect in the roots.
Pigment content analysis revealed that specific treatments significantly increased the levels of chlorophyll a, chlorophyll b, and carotenoids in rice. These treatments included a 10−3 dilution of 1-pentanol, a 10−1 dilution of methyl DL-2-methylbutyrate, 10−2 and 10−3 dilutions of ethylbenzene, and both the 10−2 dilution and the standard solution of 2-ethyl-p-xylene (Figure 4C–E). The standard solutions of 1-pentanol and ethylbenzene significantly inhibited the content of these photosynthetic pigments. We also found that none of the tested concentrations of ethyl benzoate or dimethyl phthalate significantly increased chlorophyll a content (Figure 4C). Furthermore, all diluted solutions (10−1, 10−2, and 10−3) of the six compounds significantly enhanced carotenoid content in rice (Figure 4E).
These results demonstrated that the growth-promoting effects varied depending on the compound and its concentration (Figure 3 and Figure 4). Specifically, treatments with the 10−3 dilutions of 1-pentanol, ethylbenzene, and dimethyl phthalate, the 10−1 dilution of methyl DL-2-methylbutyrate and ethyl benzoate, and the standard solution of 2-ethyl-p-xylene enhanced multiple physiological indices in rice. These improvements, which included growth traits, antioxidant capacity (H2O2 content), and photosynthetic pigment levels (including chlorophyll a, chlorophyll b, and carotenoids), contributed to promoted plant growth and improved environmental adaptability.
4. Discussion
Both plants [55] and microorganisms [56] have the ability to produce VOCs, which play a key role in mediating interactions between endophytic fungi and their host plants. For instance, VOCs from Trichoderma viride and Trichoderma pseudokoningii have been shown to enhance biomass in tomato plants and promote growth in A. thaliana, respectively [57]. Similarly, VOCs produced by Trichoderma asperellum and Trichoderma koningii exert growth-promoting effects on lettuce and A. thaliana, respectively [58,59]. In another study, VOCs released by the plant growth-promoting fungus (PGPF) Phoma sp. significantly improved tobacco growth under in vitro conditions [60]. In this study, the fungal-rice co-culture experiments demonstrated that VOCs from both strains RR1 and RR2 enhanced rice growth and significantly increased chlorophyll a and carotenoid contents. Notably, strain RR1 exhibited superior growth-promoting efficacy compared to RR2. GC-MS analysis revealed that the two strains produced 8 and 7 VOCs, respectively, with only two compounds common to both. These findings indicate that the VOCs produced by strain RR1 may possess stronger growth-promoting effects or that certain synergistic interactions among the emitted VOCs collectively contribute to enhanced plant growth and development.
Hydrogen peroxide (H2O2) is widely present in various plant tissues and represents one of the most abundant reactive oxygen species (ROS) in cells [61,62]. It plays an important role in plant growth and development, and its concentration is closely related to metabolic activity and stress resistance in plants [63,64,65,66,67]. For instance, the endophytic fungus Phomopsis liquidambaris enhances iron absorption in peanuts (Arachis hypogaea L.) by reducing hydrogen peroxide levels to alleviate oxidative stress [68]. Arbuscular mycorrhizal (AM) fungi enhance drought tolerance in Eucalyptus grandis seedlings by reducing the accumulation of reactive substances such as hydrogen peroxide and superoxide anions [69]. During Fusarium oxysporum infection of cucumber roots, Trichoderma harzianum enhances plant resistance by reducing the accumulation of reactive oxygen species (ROS; hydrogen peroxide and superoxide anion) and reactive nitrogen species (nitric oxide), thereby mitigating oxidative and nitrosative stress, respectively [70]. In this study, VOCs produced by strains RR1 and RR2 were found to reduce H2O2 levels in rice plants. This phenomenon may therefore be attributed to the ability of fungal VOCs to scavenge accumulated H2O2 in plant tissues, thereby alleviating oxidative stress and enhancing environmental adaptability.
However, different concentrations of VOCs have different effects on plant growth. For instance, 6-pentyl-α-pyrone, a VOC emitted by Trichoderma sp., promotes the growth of A. thaliana at a lower concentration (0.083 mg/mL), while reducing both fresh weight and root length by approximately 50% at a higher concentration (0.332 mg/mL) [71]. 1-octen-3-ol (mushroom alcohol) at concentrations of 10 and 100 mg/L significantly inhibits Arabidopsis seed germination and seedling growth [72]. Furthermore, 1-hexanol, a volatile compound commonly released by fungi and bacteria, exhibits a dual effect on plant growth, with promotion at low concentrations and inhibition at high ones [73]. These findings demonstrate that when present at elevated concentrations, certain VOCs exhibit phytotoxic effects on plants and inhibit their growth. When these high-concentration VOCs are properly diluted or maintained at inherently low levels, their toxicity is reduced or eliminated, thereby allowing their growth-promoting potential to be realized. This study demonstrated that, among all tested compounds at standard concentration (high concentration), only 2-ethyl-p-xylene concurrently increased rice biomass, H2O2 levels, and pigment content. The standard solutions of most other compounds exhibited weak promotive effects or even inhibitory effects. Furthermore, we found that although the standard solutions of methyl DL-2-methylbutyrate and ethylbenzene increased rice biomass to some extent, they concurrently reduced pigment content. These results suggest that the growth promotion induced by these compounds at standard concentration is not mediated through enhanced photosynthesis in the leaves. Future research should, therefore, focus on the synergistic mechanisms of VOCs to enhance our understanding of their roles in plant growth regulation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15232451/s1, The ITS sequences of strains RR1 and RR2.
Author Contributions
Conceptualization, C.Z. and C.C.; methodology, D.S., C.Z. and C.C.; software, D.S. and X.L.; validation, D.S., Q.X. and J.X.; investigation, D.S., Q.X., C.L. and L.L.; resources, C.Z. and C.C.; data curation, D.S. and Q.X.; writing—original draft preparation, D.S., C.Z. and C.C.; writing—review and editing, D.S., C.Z. and C.C.; visualization, D.S. and C.C.; supervision, Y.Z., C.Z. and C.C.; funding acquisition, C.Z. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Jiangsu Agricultural Science and Technology Innovation Foundation [CX(24)1016] and the National Natural Science Foundation of China (32470113).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. J. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, K.; Feng, Z.; Qin, Y.; Zhou, Y.; Feng, G.; Zhu, H.; Yao, Q. Tomato plant growth promotion and drought tolerance conferred by three arbuscular mycorrhizal fungi is mediated by lipid metabolism. Plant Physiol. Biochem. 2024, 208, 108478. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, X.; Li, C.; Wang, H.; Yu, Y.; Huang, B. Regulation mechanism of plant response to heavy metal stress mediated by endophytic fungi. Int. J. Phytorem. 2023, 25, 1596–1613. [Google Scholar] [CrossRef]
- Yamaji, K.; Watanabe, Y.; Masuya, H.; Shigeto, A.; Yui, H.; Haruma, T. Root fungal endophytes enhance heavy-metal stress tolerance of Clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS ONE 2016, 11, e0169089. [Google Scholar] [CrossRef]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2021, 13, 39–55. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, F.; Gupta, A.; Vasundhara, M. Endophytic fungi: A potential source of industrial enzyme producers. Biotech 2022, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.C.P.; Tavares, D.G.; Nery, E.M. Enzyme production by Induratia spp. isolated from coffee plants in Brazil. Braz. Arch. Biol. Technol. 2020, 63, e20180673. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Choi, D.H.; Kim, J.G.; Lee, I.S. Endophytic fungi of salt-tolerant plants: Diversity and ability to promote plant growth. J. Microbiol. Biotechnol. 2021, 31, 1526–1532. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Wang, B.; Li, Q.; Zu, J.; Yu, J.; Ding, Z.; Zhou, F. Screening of endophytic fungi from Cremastra appendiculata and their potential for plant growth promotion and biological control. Folia Microbiol. 2023, 68, 121–133. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Zabalgogeazcoa, I.; Vazquez de Aldana, B.R.; Boyom, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. South Afr. J. Bot. 2017, 109, 146–153. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Campos, V.P.; Pinho, R.S.C.; Freire, E.S. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Cienc. Agrotec. 2010, 34, 525–535. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Hussein, K.A. Biological control and plant growth promotion properties of volatile organic compound-producing antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef]
- Jiang, Z.; Peng, F.; Yu, J.; Li, Q. Plant growth-promoting effects and possible mechanisms of a plant endophytic fungus Aureobasidium sp. JRF1. Plant Physiol. Biochem. 2025, 222, 109724. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Gong, Y.; Kong, S.Y.; Wan, Z.Y.; Liu, J.Q.; Xing, K.; Qin, S. Aerial signaling by plant-associated Streptomyces setonii WY228 regulates plant growth and enhances salt stress tolerance. Microbiol. Res. 2024, 286, 127823. [Google Scholar] [CrossRef]
- Ullah, A.; Shah, A.; Chen, S.E.; Shah, A.; Rodriguez-Ramos, J.C.; Zaman, R.; Erbilgin, N. Alliance between conifer trees and endophytic fungi against insect defoliators. Plant Cell Environ. 2025, 48, 5236–5249. [Google Scholar] [CrossRef]
- Junker, R.R.; Tholl, D. Volatile organic compound mediated interactions at the plant-microbe interface. J. Chem. Ecol. 2013, 39, 810–825. [Google Scholar] [CrossRef]
- Fiers, M.; Lognay, G.; Fauconnier, M.L. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE 2013, 8, e66805. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Oropeza, F.; Duarte, G.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Anaya, A.L. Allelochemical effects of volatile compounds and organic extracts from Muscodor yucatanensis, a tropical endophytic fungus from Bursera simaruba. J. Chem. Ecol. 2010, 36, 1122–1131. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Rodriguez-Saona, C.; Bennett, J.W. Common gas phase molecules from fungi affect seed germination and plant health in Arabidopsis thaliana. AMB Express 2014, 4, 53. [Google Scholar] [CrossRef]
- Olou, B.A.; Krah, F.S.; Piepenbring, M.; Yorou, N.S.; Langer, E. Diversity of Trametes (Polyporales, Basidiomycota) in tropical Benin and description of new species Trametes parvispora. MycoKeys 2020, 65, 25–47. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, R.; Yin, D.; Wang, C.; Liu, S.; Kües, U.; Jia, R.; Xiao, Y.; Fang, Z.; Liu, J. ThIPK1 regulates lignocellulolytic enzyme expression during wood degradation in white-rot fungi. mBio 2025, 16, e01243. [Google Scholar] [CrossRef]
- Wang, C.; Jia, Y.; Luo, J.; Chen, B.; Pan, C. Characterization of thermostable recombinant laccase F from Trametes hirsuta and its application in delignification of rice straw. Bioresour. Technol. 2024, 395, 130382. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, K.; Zhu, R.; Wang, X.; Waigi, M.G.; Li, S. Biotransformation of bisphenol A in vivo and in vitro by laccase-producing Trametes hirsuta La-7: Kinetics, products, and mechanisms. Environ. Pollut. 2023, 321, 121155. [Google Scholar] [CrossRef]
- Wang, C.; Wu, K.; Pang, N.; Zhao, H.; Liu, S.; Zhang, X.; Xiao, Y.; Fang, Z.; Liu, J. Transcriptome analysis reveals the mechanism of tolerance to copper toxicity in the white rot fungus Trametes hirsuta AH28-2. Ecotoxicol. Environ. Saf. 2025, 296, 118194. [Google Scholar] [CrossRef] [PubMed]
- Rathankumar, A.K.; Saikia, K.; Cabana, H.; Kumar, V.V. Surfactant-aided mycoremediation of soil contaminated with polycyclic aromatic hydrocarbons. Environ. Res. 2022, 209, 112926. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Butt, T.A.; Naqvi, S.T.A.; Yousaf, S.; Qureshi, M.K.; Zafar, M.I.; Farooq, G.; Nawaz, I.; Iqbal, M. Lead tolerant endophyte Trametes hirsuta improved the growth and lead accumulation in the vegetative parts of Triticum aestivum L. Heliyon 2020, 6, e04188. [Google Scholar] [CrossRef]
- Yue, H.M. Degradation Characteristics and Mechanism of Alkali Lignin by Trametes hirsute X-13. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2022. [Google Scholar]
- Peterson, S.W.; Jurjević, Ž. The Talaromyces pinophilus species complex. Fungal Biol. 2019, 123, 745–762. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Tayeh, N.A.; Ali, O.M.; Abdel Latef, A.A.H.; Loutfy, N. The effect of endophytic Talaromyces pinophilus on growth, absorption and accumulation of heavy metals of Triticum aestivum grown on sandy soil amended by sewage sludge. Plants 2021, 10, 2659. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, L.S.; Li, C.X.; Liao, G.Y.; Lin, X.; Luo, X.M.; Zhao, S.; Feng, J.X. Identification of a novel transcription factor TP05746 involved in regulating the production of plant-biomass-degrading enzymes in Talaromyces pinophilus. Front. Microbiol. 2019, 10, 2875. [Google Scholar] [CrossRef]
- Su, L.; Niu, Y.C. Multilocus phylogenetic analysis of Talaromyces species isolated from cucurbit plants in China and description of two new species, T. cucurbitiradicus and T. endophyticus. Mycologia 2018, 110, 375–386. [Google Scholar] [CrossRef]
- Abdel-Rahim, I.R.; Abo-Elyousr, K.A.M. Talaromyces pinophilus strain AUN-1 as a novel mycoparasite of Botrytis cinerea, the pathogen of onion scape and umbel blights. Microbiol. Res. 2018, 212, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Lee, S.B.; Taylor, J.W. Isolation of DNA from fungal mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 282–287. [Google Scholar]
- Lu, Q.; Wang, Y.; Li, N.; Ni, D.; Yang, Y.; Wang, X. Differences in the characteristics and pathogenicity of Colletotrichum camelliae and C. fructicola isolated from the Tea plant [Camellia sinensis (L.) O. Kuntze]. Front. Microbiol. 2018, 9, 3060. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Lee, Y.C.; Johnson, J.M.; Chien, C.T.; Sun, C.; Cai, D.; Lou, B.; Oelmüller, R.; Yeh, K.W. Growth promotion of Chinese cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesized auxin. Mol. Plant Microbe Interact. 2010, 24, 421–431. [Google Scholar] [CrossRef]
- Jing, M.; Xu, X.; Peng, J. Comparative genomics of three Aspergillus strains reveals insights into endophytic lifestyle and endophyte-induced plant growth promotion. J. Fungi 2022, 8, 690. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Z.L. Guidelines for Plant Physiology Experiments, 5th ed.; Higher Education Press: Beijing, China, 2016; pp. 206–207. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Insam, H.; Seewald, S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Baiyee, B.; Ito, S.; Sunapapo, A. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol. Molec. Plant Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017, 29, 67–75. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kimura, M.; Miyazawa, M. Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8-3 for growth promotion effects on tobacco. Microbes Environ. 2013, 28, 42–49. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Li, P.X.; Schoelynck, J. Hydrogen peroxide variation patterns as abiotic stress responses of Egeria densa. Front. Plant Sci. 2022, 13, 855477. [Google Scholar] [CrossRef]
- Crawford, T.; Lehotai, N.; Strand, Å. The role of retrograde signals during plant stress responses. J. Exp. Bot. 2018, 69, 783–2795. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, X.; Tang, W.; Takahashi, K.; Pan, X.; Dai, J.; Ren, H.; Zhu, X.; Pan, S.; Zheng, H.; et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 2021, 599, 278–282. [Google Scholar] [CrossRef]
- Bushra, A.A.; Seleiman, M.F.; Harrison, M.T. Hydrogen peroxide mitigates cu stress in wheat. Agriculture 2023, 13, 862. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Qureshi, M.K.; Gawroński, P.; Munir, S.; Jindal, S.; Kerchev, P. Hydrogen peroxide-induced stress acclimation in plants. Cell Mol. Life Sci. 2022, 79, 129. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.C.; Kong, L.J.; Cao, L.S.; Zhang, W.; Zhu, Q.; Ma, C.Y.; Sun, K.; Dai, C.C. Endophytic fungus Phomopsis liquidambaris enhances Fe absorption in peanuts by reducing hydrogen peroxide. Front. Plant Sci. 2022, 13, 872242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, Y.; Han, L.; Nie, Y.; Zhang, S.; Xie, X.; Hu, W.; Chen, H.; Tang, M. Insights on the impact of arbuscular mycorrhizal symbiosis on Eucalyptus grandis tolerance to drought stress. Microbiol. Spectr. 2023, 11, e0438122. [Google Scholar] [CrossRef]
- Chen, S.C.; Ren, J.J.; Zhao, H.J.; Wang, X.L.; Wang, T.H.; Jin, S.D.; Wang, Z.H.; Li, C.Y.; Liu, A.R.; Lin, X.M.; et al. Trichoderma harzianum improves defense against Fusarium oxysporum by regulating ROS and RNS metabolism, redox balance, and energy flow in cucumber roots. Phytopathology 2019, 109, 972–982. [Google Scholar] [CrossRef]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. The effects of low concentrations of the enantiomers of mushroom alcohol (1-octen-3-ol) on Arabidopsis thaliana. Mycology 2014, 5, 73–80. [Google Scholar] [CrossRef]
- Blom, D.; Fabbri, C.; Connor, E.C. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 2011, 13, 3047–3058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).