A Review on the Mechanism of Soil Flame Disinfection and the Precise Control Technology of the Device
Abstract
1. Introduction
2. Soil Disinfection
3. Soil Flame Disinfection Technology
3.1. Research on the Impact of Flame on Soil Nutrients
3.2. Research on Flame Weed Control
3.3. Research on the Effects of Flame (Temperature) on Pests
| Type | Target Organism | Lethal Temperature Threshold | Critical Exposure Time | Source |
|---|---|---|---|---|
| Soil pests | Delia antigua (Meigen) | >40 °C | 1.3–1.4 h | [76,77] |
| Grubs | >50 °C | Egg and larval mortality within 10 min | [78] | |
| Agrotis ipsilon | >35 °C | 5 min | [79,80] |
3.3.1. Delia antigua (Meigen)
3.3.2. Grub
3.3.3. Agrotis ipsilon
3.4. Research on the Effects of Flame Disinfection on Pathogens
3.5. Research on the Effects of Flame on Soil Microbial Communities
3.6. Risks of Long-Term Continuous Use and the Necessity of Method Rotation
4. Research on Flame Disinfection Equipment and Precise Control Technology
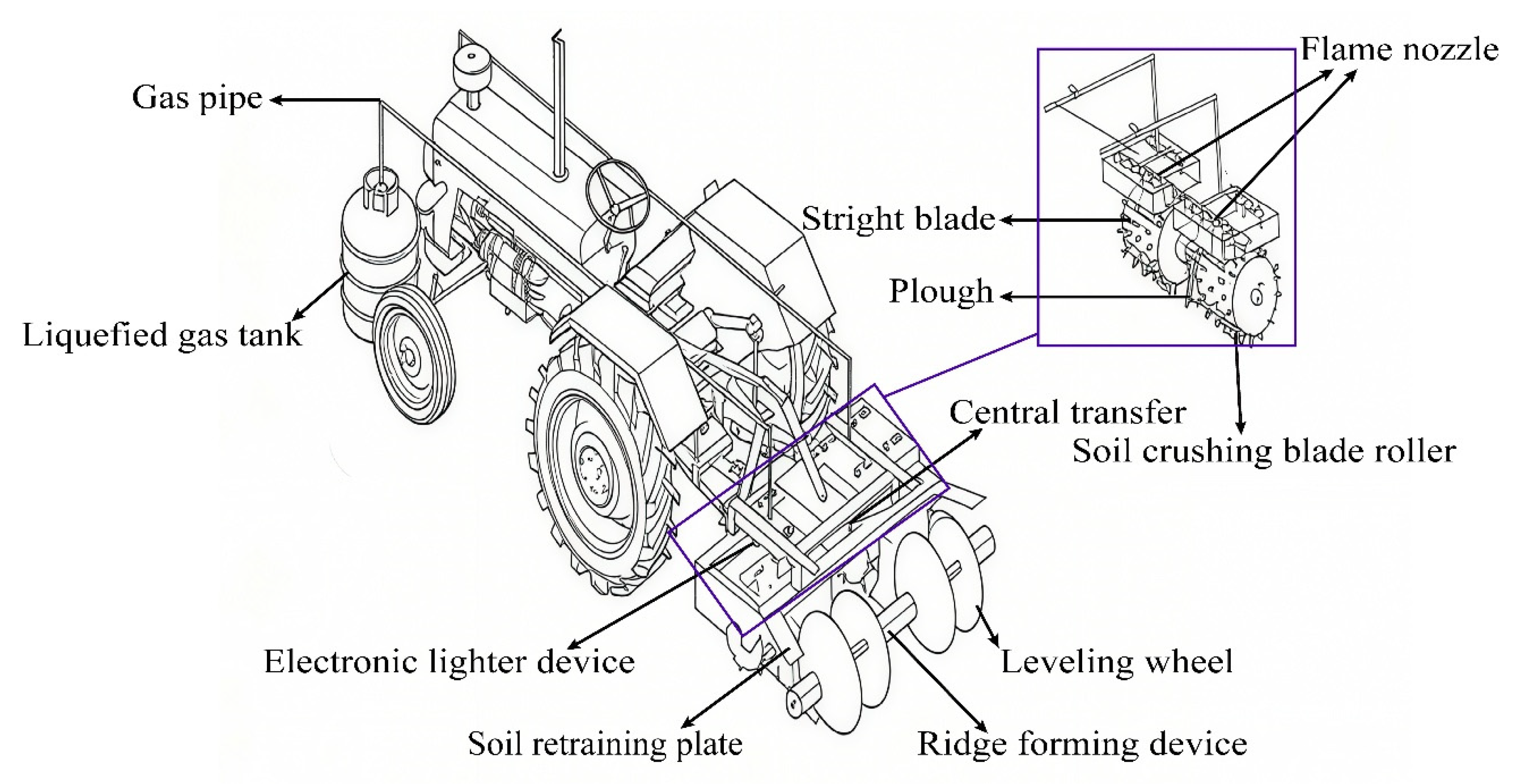
4.1. Development of Flame Disinfection Devices
4.2. Research on Precise Control Technology
5. Future Development Prospects
- (1)
- Technological Innovation and Precision Control
- (2)
- Advanced Modeling and Real-Time Monitoring for Precision Control
- (3)
- Standardization of Soil Flame Disinfection Technology
- (4)
- Specific Research Gaps
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, R.J. Research on the Current Situation and Countermeasures of Soil-Borne Diseases of Agricultural, Forestry and Horticultural Crops in China. New Agric. 2021, 12, 40. [Google Scholar]
- Tao, X.; Ye, W.; Vetukuri, R.R.; De Vries, S.; Kong, L.; Zhang, M. Editorial: Plant resistance to soil-borne diseases. Front. Plant Sci. 2024, 15, 3. [Google Scholar] [CrossRef]
- Xu, G.H.; Zhao, Q.L.; Gao, Y. Research and Experiment on Preventing Soil Pests by High Temperature Flaming Sterilization Technology. Agric. Eng. 2014, 4, 52–54. [Google Scholar]
- Cao, A.C.; Guo, M.X.; Wang, Q.X.; Li, Y.; Yan, D.D. Progress in Soil Disinfection Technology Worldwide. China Veg. 2010, 21, 17–22. [Google Scholar]
- Katan, J. Physical and cultural methods for the management of soil-borne pathogens. Crop Prot. 2000, 19, 725–731. [Google Scholar] [CrossRef]
- Zhang, H.R.; Yang, N.; Wen, D.; Wang, X.Y.; Yang, Y.J.; Sun, K.N.; Chen, N. Research Progress of Application of Soil Disinfection Technology in Production of Protected Vegetables. Shandong Agric. Sci. 2020, 52, 149–156. [Google Scholar]
- Zhang, J.X. Study on the Present Prevention and Control Situation of Soil Borne Diseases in Agriculture, Forestry and Gardening Crops and Strategies. Times Agric. Mach. 2016, 43, 146–147. [Google Scholar]
- Jin, N.; Chen, Y.P.; Liu, Q.; Jian, H. Research progresses in occurrence, diagnoses, pathogenic mechanisms and integrated management of vegetable root-knot nematodes in China. J. Plant Prot. 2022, 49, 424–438. [Google Scholar]
- Liu, S.D.; Miao, Z.Q.; Gao, W.D. Challenges, Opportunities and Obligations in Management of Soilborne Plant Diseases in China. Chin. J. Biol. Control 2011, 27, 433–440. [Google Scholar]
- Giannakakos, I.O.; Anastasiadis, I. Evaluation of chemical strategies as alternatives to methyl bromide for the control of root-knot nematodes in greenhouse cultivated crops. Crop Prot. 2005, 24, 499–506. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the roles of soil microbes in ecosystem succession. Nat. Rev. Microbiol. 2017, 15, 469–479. [Google Scholar] [CrossRef]
- Graham, E.B.; Camargo, A.P.; Wu, R.; Neches, R.Y.; Nolan, M.; Paez-Espino, D.; Kyrpides, N.C.; Jansson, J.K.; McDermott, J.E.; Hofmockel, K.S. A global atlas of soil viruses reveals unexplored biodiversity and potential biogeochemical impacts. Nat. Microbiol. 2024, 9, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ah-mad, W.; Andriuzzi, W.S.; et al. Soil Nematode Abundance and Functional Group Composition at a Global Scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Tian, Y.; Li, Y.; Du, X.; Liu, H.; Steinberger, Y.; Liang, W. Microfauna Community Assembly and Cas-cading Relationship with Microflora in Cropland Ecosystems along a Latitudinal Gradient. Agric. Ecosyst. Environ. 2023, 357, 108678. [Google Scholar] [CrossRef]
- Hu, H.T.; Zhu, Z.G.; Jiao, Z.J.; Min, Y.; Cao, C.X.; Yan, D.D.; Cao, A.C. Effects of soil disinfection by dazomet on control efficacy of cabbage clubroot disease and soil fungal communities in high mountain area. J. Huazhong Agric. Univ. 2019, 38, 25–31. [Google Scholar]
- Li, H.; Wei, X.L.; Wang, M.T. Residual level of chlorine disinfectant, the formation of disinfection by-products, and its impact on soil enzyme activity. Environ. Geochem. Health 2025, 47, 18. [Google Scholar]
- Brain, R.K. An Assessment of Progress toward Microbial Control of Plant-Parasitic Nematodes. J. Nematol. 1990, 22, 621–631. [Google Scholar]
- Ioannou, N. Soil Solarization as a Substitute for Methyl Bromide Fumigation in Greenhouse Tomato Production in Cyprus. Phytoparasitica 2000, 28, 248–256. [Google Scholar] [CrossRef]
- Nouri, M.; Homaee, M. Contribution of soil moisture variations to high temperatures over different climatic regimes. Soil Tillage Res. 2021, 213, 105115. [Google Scholar] [CrossRef]
- Purnama, I.; Malhat, F.M.; Mutamima, A.; Rusdiarso, B.; Noegrohati, S. Enhanced dissipation of azoxystrobin in loam soil under direct sunlight exposure. Int. J. Environ. Sci. Technol. 2025, 22, 4521–4534. [Google Scholar] [CrossRef]
- Wang, X.; Cao, A.; Yan, D.; Wang, Q.; Huang, B.; Zhu, J.; Wang, Q.; Li, Y.; Ouyang, C.; Guo, M. Evaluation of soil flame disinfestation (SFD) for controlling weeds, nematodes and fungi. J. Integr. Agric. 2020, 19, 164–172. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, H.; Zhao, Q.; Guo, D.; Gao, Q.; Guan, C. Research Progress of Soil Physical Disinfection Equipment. Agric. Eng. 2015, 5, 43–48. [Google Scholar]
- Mcavoy, T.; Reeman, J.H.; Reiter, M. Soil Persistence of Dimethyl Disulfide Fumigant due to Application Rate, Chemical Formulation, and Plastic Mulch Type. HortScience 2010, 45, 502–503. [Google Scholar]
- Lu, Z.J.; Chen, M.Y.; Huang, J.; Zhu, Q.Y.; Hu, X.J.; Wang, Y.H. The Application of Soil Chemical Fumigants in the Control of Root-Knot Nematodes. China Plant Prot. 2016, 36, 59–64. [Google Scholar]
- Wang, Q.X.; Yan, D.D.; Fang, W.S.; Xu, J.; Li, Y.; Cao, A.C. Research progress on the novel soil fumigant dimethyl disulfide. J. Plant Prot. 2023, 50, 32–39. [Google Scholar]
- Yates, S.R.; Gan, J.; Papiernik, S.K. Environmental Fate of Methyl Bromide as a Soil Fumigant. Rev. Environ. Contam. Toxicol. 2003, 177, 45–122. [Google Scholar]
- Nelson, S.D.; Locascio, S.J.; Allen, L.H.; Dickson, D.W.; Mitchell, D.J. Soil Flooding and Fumigant Alternatives to Methyl Bromide in Tomato and Eggplant Production. HortScience 2002, 37, 1057–1060. [Google Scholar] [CrossRef]
- Bell, C.H. Fumigation in the 21st century. Crop Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Gemmill, A.; Gunier, R.B.; Bradman, A.; Eskenazi, B.; Harley, K.G. Residential Proximity to Methyl Bromide Use and Birth Outcomes in an Agricultural Population in California. Environ. Health Perspect. 2013, 121, 737–743. [Google Scholar] [CrossRef]
- Minuto, A.; Gullino, M.L.; Lamberti, F.; D’Addabbo, T.; Tescari, E.; Ajwa, H.; Garibaldi, A. Application of an emulsifiable mixture of 1,3-dichloropropene and chloropicrin against root knot nematodes and soilborne fungi for greenhouse tomatoes in Italy. Crop Prot. 2006, 25, 1244–1252. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Yan, D.-D.; Mao, L.-G.; Ma, T.-T.; Liu, P.-F.; Wu, Z.-F.; Li, Y.; Guo, M.-X.; Cao, A.-C. Efficacy of 1,3-dichloropropene gelatin capsule formulation for the control of soilborne pests. Crop Prot. 2013, 48, 24–28. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Koike, S.; Baird, G.; Fennimore, S.; Samtani, J.; Bolda, M.; Dara, S.; Daugovish, O.; Lazarovits, G.; et al. Anaerobic soil disinfestation is an alternative to soil fumigation for control of some soilborne pathogens in strawberry production. Plant Pathol. 2018, 67, 51–66. [Google Scholar] [CrossRef]
- Minuto, A.; Gilardi, G.; Pome, A.; Garibaldi, A. Chemical and physical alternatives to methyl bromide for soil disinfestation: Results against soilborne diseases of protected vegetable crops. J. Plant Pathol. 2000, 82, 179–186. [Google Scholar]
- Cao, A.C.; Zhang, W.J.; Liu, J.H. Progress in the alternatives to methyl bromide in soil disinfestation. Plant Prot. 2007, 33, 15–20. [Google Scholar]
- Cao, A.; Guo, M.; Yan, D.; Mao, L.; Wang, Q.; Li, Y.; Duan, X.; Wang, P. Evaluation of Sulfuryl Fluoride as a Soil Fumigant in China. Pest Management Science 2014, 70, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.P.; Helmkamp, G.K.; Ervin, J.O. Effect of Bromide from a Soil Fumigant and from CaBr2 on Growth and Chemical Composition of Citrus Plants. Soil Sci. Soc. Am. J. 1956, 20, 209–212. [Google Scholar] [CrossRef]
- Cao, A.C.; Zhang, D.Q.; Fang, W.S.; Song, Z.X.; Ren, L.R.; Li, Q.J.; Li, W.J.; Wang, Q.X.; Yan, D.D.; Li, Y.; et al. Progress and challenges in the management of soil-borne diseases. Plant Prot. 2023, 49, 260–269. [Google Scholar]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Fawcett, L.; Anthony, S.G.; Young, C. A model for Sclerotinia sclerotiorum infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS ONE 2014, 9, e94049. [Google Scholar] [CrossRef]
- Su, H.; Zhang, R.P.; Wu, S.X.; Yao, H.Y.; Li, Y.Y. Mechanism and prevention status of continuous cropping obstacles. Soils 2024, 56, 2. [Google Scholar]
- Wallenhammar, A.-C.; Omer, Z.S.; Edin, E.; Jonsson, A. Influence of soil-borne inoculum of Plasmodiophora brassicae measured by qPCR on disease severity of clubroot-resistant cultivars of winter oilseed rape (Brassica napus L.). Pathogens 2021, 10, 433. [Google Scholar] [CrossRef]
- Leonard, S.; Merson, R.L.; Marsh, G.L.; York, G.K.; Wolcott, T. Flame sterilization of canned foods: An overview. J. Food Sci. 2010, 40, 246–249. [Google Scholar] [CrossRef]
- Stapleton, J.J. Soil solarization in various agricultural production systems. Crop Prot. 2000, 19, 837–841. [Google Scholar] [CrossRef]
- Tanaka, H.; Takaura, Y.; Ichino, Y.; Sakaguchi, R.; Negoro, J.; Asano, E.; Shibao, M. Effects of burning soil by a flame thrower on rise in temperature underground and on survial of Liriomyza trifolii (BURGESS) pupae. Ann. Rep. Kansai Plant Prot. Soc. 1995, 37, 27–28. [Google Scholar] [CrossRef]
- Liu, T.Y.; Zhu, H. Green, environmentally friendly and highly efficient soil disinfection technology Flame high-temperature disinfection. J. Changjiang Veg. 2017, 23, 58–59. [Google Scholar]
- Wszelaki, A.L.; Doohan, D.J.; Alexandrou, A. Weed control and crop quality in cabbage (Brassica oleracea (capitata group)) and tomato (Lycopersicon lycopersicum) using a propane flamer. Crop Prot. 2007, 26, 134–144. [Google Scholar] [CrossRef]
- Bond, W.; Grundy, A.C. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Song, Q.L.; Dong, X.B.; Li, Y.; Li, J.N. Impacts of Burning and Logging Disturbance on Soil Chemical Properties of Forest in Daxing’anling Mountain Region. For. Eng. 2010, 26, 4–7. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Liu, X.D.; Zhang, S.Y.; Li, C.Y. The Effects of Burning with Different Intensities on Soil Property in Chinese Fir Plantations. J. Northwest For. Univ. 2016, 31, 1–6+22. [Google Scholar] [CrossRef]
- Zhao, L.P.; Wei, N.; Tan, S.T.; He, Q.B.; Wang, Z.B.; Fan, W.N.; Sun, P. Effects of Winter Fire on Vegetation Community Characteristics and Soil Properties of A Typical Steppe on the Loess Plateau. Acta Agrestia Sin. 2018, 26, 576–583. [Google Scholar]
- Liu, G.; Li, B.; Gong, D.; Li, W.; Liu, X. Effects of forest fire on soil chemical properties of Pinus tabuliformis forest in Pinggu District of Beijing. J. Beijing For. Univ. 2019, 41, 29–40. [Google Scholar]
- Yang, J.J. Effects of Simulated Fire and Warming on Soil Properties and Greenhouse Gas Flux of Leymus chinensis Grassland. Master’s Thesis, Northwest A&F University, Shanxi, China, 2020. [Google Scholar]
- Zhou, S.H.; Zhang, Y.; Cui, X.Y. Temporal and spatial dynamics of soil available potassium in a post-fire Larix gmelinii forest. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 141–147. [Google Scholar]
- Li, Y.; Cheng, J.M.; Wei, L.; Chen, F. Changes of soil chemical properties after different burning years in typical steppe of Yunwun Mountains. Acta Ecol. Sin. 2013, 33, 2131–2138. [Google Scholar] [CrossRef]
- Guo, W.L.; Feng, L.; Tian, X.S. Effect of Flame Weeding in Orchards. J. Weeds 2019, 37, 35–39. [Google Scholar]
- Ascard, J. Effects of flame weeding on weed species at different developmental stages. Weed Res. 2006, 35, 397–411. [Google Scholar] [CrossRef]
- Ulloa, S.M.; Datta, A.; Knezevic, S.Z. Growth Stage-Influenced Differential Response of Foxtail and Pigweed Species to Broadcast Flaming. Weed Technol. 2017, 24, 319–325. [Google Scholar] [CrossRef]
- Zhao, Q.F. Research on directional flame injection of weeder based on DEM-CFD coupling. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2024. [Google Scholar]
- Sivesind, E.C.; Leblanc, M.L.; Cloutier, D.C.; Seguin, P.; Stewart, K.A. Weed Response to Flame Weeding at Different Developmental Stages. Weed Technol. 2009, 23, 438–443. [Google Scholar] [CrossRef]
- Ulloa, S.M.; Datta, A.; Bruening, C.; Neilson, B.; Miller, J.; Gogos, G.; Knezevic, S.Z. Maize response to broadcast flaming at different growth stages: Effects on growth, yield and yield components. Eur. J. Agron. 2011, 34, 10–19. [Google Scholar] [CrossRef]
- Carrington, M.E. Effects of Soil Temperature during Fire on Seed Survival in Florida Sand Pine Scrub. Int. J. For. Res. 2010, 2010. [Google Scholar] [CrossRef]
- Sivesind, E.C.; Leblanc, M.L.; Cloutier, D.C.; Seguin, P.; Stewart, K.A. Impact of selective flame weeding on onion yield, pungency, flavonoid concentration, and weeds. Crop Prot. 2012, 39, 45–51. [Google Scholar] [CrossRef]
- Chehade, L.A.; Fontanelli, M.; Martelloni, L.; Frasconi, C.; Raffaelli, M.; Peruzzi, A. Effects of Flame Weeding on Organic Garlic Production. HortTechnology 2018, 28, 502–508. [Google Scholar] [CrossRef]
- Rajković, M.; Malidža, G.; Stepanović, S.; Kostić, M.; Petrović, K.; Urošević, M.; Vrbničanin, S. Influence of Burner Position on Temperature Distribution in Soybean Flaming. Agric. Food Sci. 2020, 10, 391. [Google Scholar] [CrossRef]
- Taylor, E.C.; Sprague, R.C.L. Organic Weed Management in Field Crops with a Propane Flamer and Rotary Hoe. Weed Technol. 2012, 26, 793–799. [Google Scholar] [CrossRef]
- Upadhyay, A.; Singh, K.P.; Jhala, K.B.; Kumar, M.; Salem, A. Non-chemical weed management: Harnessing flame weeding for effective weed control. Heliyon 2024, 10, 1–16. [Google Scholar] [CrossRef]
- Wang, X.H. Effects of Temperatures and Diets on Occurrence of Chaetolonchaea alliumi Zhang et Xue. Master’s Thesis, Shandong Agricultural University, Taian, China, 2019. [Google Scholar]
- Banfi, D.; Bianchi, T.; Mastore, M.; Brivio, M.F. The role of heat shock proteins in insect stress response, immunity, and climate adaptation. Insects 2025, 16, 741. [Google Scholar] [CrossRef]
- Li, B.; Cai, H.; Chen, Y. Insect heat shock response and heat shock proteins. Acta Entomol. Sin. 1997, 40, 417–427. [Google Scholar]
- Duman, J. The inhibition of ice nucleators by insect antifreeze proteins is enhanced by glycerol and citrate. J. Comp. Physiol. B 2002, 172, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.X. Studies on Biological Characteristics and Cold Hardiness of Bradysia odoriphaga. Ph.D. Thesis, Northwest Sci-tech University of Agriculture and Forestry, Shanxi, China, 2003. [Google Scholar]
- Yang, Y.T.; Liang, L.; Shi, C.H.; Xie, W.; Zhang, Y.J. Age-stage, two-sex life table of laboratory populations of Bradysia odoriphaga Yang et Zhang under different temperatures. Plant Prot. 2019, 45, 51–57. [Google Scholar]
- Wang, Y.P.; Tian, L.L. The occurrence pattern and green prevention and control technology of chive root maggots in Gangu County. Agric. Sci. Technol. Inf. 2019, 2, 38–39. [Google Scholar]
- Hu, J.R.; Shi, C.H.; Li, C.R.; Zhang, Y.J. Integrated Control Technology and Application of Garlic Rootworms. China Veg. 2020, 1, 93–96. [Google Scholar]
- Shen, Y.Y.; Re, J.C.; Qin, M.G.; Lai, Y.P. Preliminary study on the main harmful characteristics of Ledu purple Allium sativum L. and the toxicidal activity of insecticides against it. J. Qinghai Univ. 2023, 41, 36–40+101. [Google Scholar]
- Gao, F.; Zhang, S.A.; Jiang, Z.X. Winter cultivation techniques for garlic in solar greenhouses in Liaocheng, Shandong Province. Agric. Eng. Technol. 2024, 44, 65–66. [Google Scholar]
- Chen, C.Q.; Xue, J.G.; Li, H.Q. Control measures for the occurrence of underground pests such as grubs, wireworms, and mole crickets in autumn. Nong Jia Can Mou 2022, 3, 72–74. [Google Scholar]
- Yang, J.Q.; Chen, Q.J.; Chen, J.H.; Zhang, Y.H. Effect of temperature on laboratory population of black cutworm. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2000, 29, 337–341. [Google Scholar]
- Xiang, Y.Y.; Yang, K.L.; Liao, Q.R.; Yang, M.F.; Li, Z.Z. Effects of temperature on the development and reproduction of the black cutworm. J. Anhui Agric. Univ. 2009, 36, 365–368. [Google Scholar]
- Fang, W.S.; Cao, A.C.; Wang, Q.X.; Yan, D.D.; Li, Y.; Jin, X.; Zhao, Q.; Qiu, Y.K.; Zhao, H.M. A New Integrated Soil Disinfection Machine Improves the Uniformity of Dazomet in Soil. Sci. Agric. Sin. 2021, 54, 2570–2580. [Google Scholar]
- Han, Q.L.; Han, X.Y.; Liu, Z.L.; Tian, J.J.; Yang, S.B.; Xu, Y.C.; Chen, Y.Q. Effects of 3 Different Soil Fumigation on Control of Soil-borne Diseases and Agronomic Traits of Flue-cured Tobacco. J. Southwest For. Univ. (Nat. Sci.) 2025, 45, 177–182. [Google Scholar]
- Fang, W.S.; Wang, Q.X.; Yan, D.D.; Li, Y.; Cao, B.W.; Xu, J.; Jin, Q.; Cao, A.C. Research progresses and future development trends of soil fumigant dazomet in control of soil-borne diseases. J. Plant Prot. 2023, 50, 40–49. [Google Scholar]
- Kita, N. Physical Soil Sterilization for Soil-Borne Disease Control. Proc. Veg. Tea Sci. 2006, 7–15. [Google Scholar]
- Mao, L.; Wang, Q.; Yan, D.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Flame soil disinfestation: A novel, promising, non-chemical method to control soilborne nematodes, fungal and bacterial pathogens in China. Crop Prot. 2016, 83, 90–94. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Xiao, Y.; Gu, Y.; Liu, H.; Liang, Y.; Liu, X.; Hu, J.; Meng, D.; Yin, H. Integrated Insight into the Relationship between Soil Microbial Community and Tobacco Bacterial Wilt Disease. Front. Microbiol. 2017, 8, 2179. [Google Scholar] [CrossRef] [PubMed]
- Checinska, A.; Paszczynski, A.; Burbank, M. Bacillus and other spore-forming genera: Variations in responses and mechanisms for survival. Annu. Rev. Food Sci. Technol. 2015, 6, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.Q.; Kulkarni, M.; Bencomo, A.; Faiez, T.S.; Hardwick, J.M.; Casadevall, A. Environmental Fungi from Cool and Warm Neighborhoods in the Heat Island of Baltimore City Show Differences in Thermal Susceptibility and Pigmentation. ISME Commun. 2025, 5, ycaf177. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, E.; Francis, E.K.; Šlapeta, J. In vitro effect of burned pasture soil on eggs and free-living larvae of ruminant gastrointestinal nematodes. Vet. Parasitol. 2023, 319, 109953. [Google Scholar] [CrossRef]
- Huang, W.; González, G.; Barberena-Arias, M.F.; Liu, W.; Zou, X. Changes in soil arthropods and litter nutrients after prescribed burn in a subtropical moist pastureland. Pedobiologia 2024, 106, 150990. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Liu, Q.; HAO, Y.Y.; Chen, J.; Lei, R.X.; Wang, X.C. Design and Experiment of an Integrated Machine for Precision Rotary, Flame Soil Disinfection and Ridging in Greenhouses. Trans. Chin. Soc. Agric. Eng. 2025, 41, 5. [Google Scholar]
- Gao, D.; Wu, Y.; Lu, Y.; Yang, Z.; Zhang, H.; Shi, Y.; Xie, H.; Fu, S.; Wang, H.; Han, Z.; et al. Fumigation-driven restructuring of soil microbial ecosystems enhances endangered Fritillaria cirrhosa productivity and secondary metabolite biosynthesis: Insights from metabolomics and microbiome profiling. Ind. Crops Prod. 2025, 236, 121938. [Google Scholar] [CrossRef]
- Liu, W.; Bo, T.; Zhao, N.; Hu, B.; Li, G.; Xiao, Z. Small mammal activities structure fine-scale soil microbial communities and coordinate rodenticide effects in the typical steppes of China. Ecotoxicol. Environ. Saf. 2025, 302, 118560. [Google Scholar] [CrossRef]
- Shlevin, E.; Mahrer, Y.; Kritzman, G.; Katan, J. Survival of plant pathogens under structural solarization. Phytoparasitica 2004, 32, 470–478. [Google Scholar] [CrossRef]
- Pullman, G.S.; DeVay, J.E.; Garber, R.H. Soil solarization and thermal death: A logarithmic relationship between time and temperature for four soilborne plant pathogens. Phytopathology 1981, 71, 959–964. [Google Scholar] [CrossRef]
- Wu, W.; Ogawa, F.; Ochiai, M.; Yamada, K.; Fukui, H. Common strategies to control pythium disease. Rev. Agric. Sci. 2020, 8, 58–69. [Google Scholar] [CrossRef]
- Jung, T.; Milenkovic, I.; Balci, Y.; Janousek, J.; Kudlacek, T.; Nagy, Z.A.; Baharuddin, B.; Bakonyi, J.; Broders, K.D.; Caccio-la, S.O.; et al. Worldwide forest surveys reveal forty-three new species in Phytophthora major Clade 2 with fundamental implications for the evolution and biogeography of the genus and global plant biosecurity. Stud. Mycol. 2024, 107, 251. [Google Scholar] [CrossRef]
- Hu, R.H.; Yu, C.P.; Ren, M.H.; Li, X.Y.; Liu, Q.G. Isolation and biological characteristics of bacteriophages against Ralstonia solanacearum in tobacco. Guangdong Agric. Sci. 2019, 46, 78–84. [Google Scholar]
- Hu, J.R.; Shi, C.H.; Shi, L.L.; Wei, Q.W.; Wu, Q.J.; Fang, S.G.; Li, C.R.; Zhang, Y.J. Application and prospect of soil solarization in green plant protection. Plant Prot. 2019, 45, 27–35. [Google Scholar]
- Zhong, L.K.; Xu, C.H.; Huang, Z.M.; An, Q.L.; Liang, Y. Construction and application of lux luminescent strain of Pectobacterium carotovorum. J. Zhejiang Univ. (Agric. Life Sci.) 2021, 47, 566–576. [Google Scholar]
- Brakke, M.K. Soil-borne wheat mosaic virus. CMI/AAB Descr. Plant Viruses 1971, 77, 905–910. [Google Scholar]
- Ebrahim, G.J. Dictionary of Microbiology and Molecular Biology, 3rd ed.; Sigleton, P., Sainsbury, D., Eds.; John Wiley & Sons, Ltd.: London, UK, 2006; Volume 53, p. 145. [Google Scholar]
- Yang, Z.; Chen, Q.; Wang, R.; Lin, Y.; Kong, D.; Wang, Z.; He, X.; Han, Z.; Guo, Y.; Xia, H.; et al. Responses of root physiological characteristics and resistance gene expression to infection by Meloidogyne incognita at different temperatures in tobacco. Front. Plant Sci. 2025, 16, 1592335. [Google Scholar] [CrossRef]
- Li, H.; Shu, Y.; Wei, J.; Zhao, P.; Zhou, M.; Jia, W. Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China. Forests 2024, 15, 664. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Lu, L.; Gui, H.; Wan, S. Global recovery patterns of soil microbes after fire. Soil Biol. Biochem. 2023, 183, 109057. [Google Scholar] [CrossRef]
- Flores-Piña, A.; Valencia-Cantero, E.; Santoyo, G. Underground fires shape the structure of microbial communities and select for thermophilic bacteria through a temperature gradient. Microbiol. Res. 2025, 292, 127996. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Chavez, M.F.; Randolph, J.W.J.; Zalman, C.; Larios, L.; Homyak, P.M.; Glassman, S.I. Rapid bacterial and fungal successional dynamics in first year after chaparral wildfire. Mol. Ecol. 2023, 32, 1685–1707. [Google Scholar] [CrossRef]
- Birch, J.D.; Lutz, J.A.; Dickinson, M.B.; Franklin, J.; Larson, A.J.; Swanson, M.E.; Miesel, J.R. Small-scale fire refugia increase soil bacterial and fungal richness and increase community cohesion nine years after fire. Sci. Total Environ. 2025, 966, 178677. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Fu, Y.; Jeewani, P.H.; Tang, C.; Pan, S.; Reid, B.J.; Gunina, A.; Li, Y.; Li, Y.; Cai, Y.; et al. Organic matter chemistry and bacterial community structure regulate decomposition processes in post-fire forest soils. Soil Biol. Biochem. 2021, 160, 108311. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, Y.; Li, A.; Hu, M.; Liu, W. Fire alters soil bacterial and fungal communities and intensifies seasonal variation in subtropical forest ecosystem. Eur. J. Soil Biol. 2024, 123, 103677. [Google Scholar] [CrossRef]
- Nocentini, C.; Guenet, B.; Di Mattia, E.; Certini, G.; Bardoux, G.; Rumpel, C. Charcoal mineralisation potential of microbial inocula from burned and unburned forest soil with and without substrate addition. Soil Biol. Biochem. 2010, 42, 1472–1478. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Frossard, E. Biological cycling of inorganic nutrients and metals in soils and role in soil biogeochemistry. In Soil Microbiology, Ecology, and Biochemistry; Academic Press: Cambridge, MA, USA, 2015; pp. 471–503. [Google Scholar]
- Klose, S.; Acosta-Martínez, V.; Ajwa, H.A. Microbial community composition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides. Soil Biol. Biochem. 2006, 38, 1243–1254. [Google Scholar] [CrossRef]
- Soliman, E.R.S.; Abdelhameed, R.E.; Metwally, R.A. Role of arbuscular mycorrhizal fungi in drought-resilient soybeans (Glycine max L.): Unraveling the morphological, physio-biochemical traits, and expression of polyamine biosynthesis genes. Bot. Stud. 2025, 66, 9. [Google Scholar] [CrossRef]
- Yang, J.; He, M.; Zhang, B.; Guo, M.; Zhou, L.; Yin, J. Shifts in biogeochemical cycles driven by soil microbial functional genes due to habitat fragmentation in urban remnant forests. Appl. Soil Ecol. 2025, 216, 106506. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Z.; Teng, Y.; Christie, P.; Wang, J.; Ren, W.; Luo, Y.; Li, Z. Non-target effects of repeated chlorothalonil application on soil nitrogen cycling: The key functional gene study. Sci. Total Environ. 2016, 543, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; de Goede, R.; Li, Y.; Zhang, J.; Wang, G.; Zhang, J.; Creamer, R. Unlocking soil health: Are microbial functional genes effective indicators? Soil Biol. Biochem. 2025, 204, 109768. [Google Scholar] [CrossRef]
- Birthisel, S.K.; Gallandt, E.R. Trials evaluating solarization and tarping for improved stale seedbed preparation in the Northeast USA. Org. Farming 2019, 5, 52–65. [Google Scholar] [CrossRef]
- Ascard, J. Thermal weed control by flaming: Biological and technical aspects. In Rapport—Sveriges lantbruksuniversitet; Institutionen för lantbruksteknik: Ultuna, Uppsala, 1995. [Google Scholar]
- Han, X.; Wang, L.; Li, R.; Han, Q. The Effect of Flame Sterilization on the Microorganisms in Continuously Cultivated Soil and the Yield and Quality of Tobacco Leaves. Agriculture 2024, 14, 1868. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Liu, Q.; Hao, Y.; Chen, J.; Lei, R.; Wang, X. Design and test of greenhouse fine spiral flame soil disinfection and ridge forming machine. Trans. Chin. Soc. Agric. Eng. 2025, 41, 1–10, (In Chinese with English abstract). [Google Scholar]
- Weng, X.; Shi, X.; Huang, Y.; Zhao, J.; Lin, Y. Design of Flame Rotary Cultivator Fueled by Biomass Pellets. Agric. Eng. 2019, 9, 84–87. [Google Scholar]
- Yang, Y. Design and Effect Test of a Fire Insecticide Precision Rotary Cultivator. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2017. [Google Scholar]
- Chen, Y. The Design of Flame Micro Plowing Machine. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. [Google Scholar]
- Guan, P. Research and Realization of Key Technology of Flame Weeding Machine for Chinese Medicinal Materials. Master’s Thesis, Shanxi Agricultural University, Shanxi, China, 2019. [Google Scholar]
- He, X.L.; Ran, H.Y.; Lan, X.M.; Chen, S.H.; Ye, Z.H. Effect of High-Temperature Flame on Weeding in Young Tea Garden. J. Weeds 2022, 40, 60–65. [Google Scholar]
- Kang, W.S. Development of a Flame Weeder. Trans. ASAE 2001, 44, 1065–1070. [Google Scholar] [CrossRef]
- Frasconi, C.; Martelloni, L.; Fontanelli, M.; Raffaelli, M.; Emmi, L.; Pirchio, M.; Peruzzi, A. Design and Full Realization of Physical Weed Control (PWC) Automated Machine within the RHEA Project. In Proceedings of the International Conference of Agricultural Engineering, Zurich, Switzerland, 6–10 July 2014. [Google Scholar]
- Stepanovic, S.; Datta, A.; Neilson, B.; Bruening, C.; Shapiro, C.; Gogos, G.; Knezevic, S.Z. Effectiveness of flame weeding and cultivation for weed control in organic maize. Biol. Agric. Hortic. 2016, 36, 47–62. [Google Scholar] [CrossRef]
- Xiong, K.; Gui, J.P.; Xu, H.C.; Jin, H. Evaluation and Improvement of Thermal Conductivity Model of Room Temperature Soil. Water Sav. Irrig. 2021, 4, 86–91. [Google Scholar]
- Duan, Y. Experimental Study on Testing and Variation of Soil Thermophysical Parameters. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2015. [Google Scholar]
- Bu, F. Optimization Test of Soil Heating Characteristics and Fire Spray Parameters for Flame Weeding. Master’s Thesis, Yangzhou University, Yangzhou, China, 2022. [Google Scholar]

| Category | Major Pathogen/Pest Types | References |
|---|---|---|
| Oomycetes and Fungi | Phytophthora, Verticillium, Rhizoctonia, etc. (>10,000 species) | [13] |
| Bacteria | Ralstonia solanacearum, soft rot bacteria, etc. (several thousand species) | [14] |
| Soil Protozoa | Parasitic (Apicomplexa), fungivorous, omnivorous (>100,000 species, 1700 described) | [15] |
| Nematodes | Root-knot nematodes, cyst nematodes, and other plant-parasitic types (25,000 species described) | [16] |
| Soil Insects | Beetle larvae, springtails (Collembola), etc. (tens of thousands of species) | [17] |
| Soil-Borne Disease Management | ||||
|---|---|---|---|---|
| Agriculture Control | Biological Control | Physical Control | Chemical Control | Integrated Management |
| Resistant varieties and grafting | Biofumigation | Solarization | allyl isothiocyanate, AITC | Solarization-chemical |
| Blocking Pathogen Transmission | Anaerobic Soil Disinfestation | Soil flame disinfection | dimethyl disulfide, DMDS | Fumigant–non-chemical rotation |
| Deep plowing | Biocontrol formulations | Microwave sterilization | ethanedinitrile, EDN | Fumigant–contact pesticide |
| Crop rotation | Radio Frequency Disinfection | ethylicin | Fumigant–contact fungicide | |
| Soilless cultivation | Soil Electrolytic Disinfection | Fumigant–Grafting Combined | ||
| Ecosystem | Flame Intensity/Duration | Soil Organic Matter | Nitrogen Content | Phosphorus Content | Potassium Content | Microbial Biomass | Source |
|---|---|---|---|---|---|---|---|
| Chinese Fir Plantation | Low intensity, ≤60 min | Increased | Initial increase | Initial decrease, subsequent increase | No significant effect | – | [54] |
| Typical Grassland | Winter burning | Increased (0–10 cm) | Increased (0–10 cm) | – | – | – | [55] |
| Chinese Pine Forest | High intensity | Decreased by 25.3% | – | – | – | – | [56] |
| Leymus chinensis Grassland | Simulated burning | Decreased | Available N increased | No significant change | Available K decreased | Decreased | [57] |
| Larix gmelinii forest | Moderate-severe burning | – | – | – | Significantly increased | – | [58] |
| Type | Target Organism | Lethal Temperature Threshold | Critical Exposure Time | Source |
|---|---|---|---|---|
| Fungi | Fusarium | >60 | 30 min | [93] |
| Rhizoctonia solani | 50 | 10 min | [94] | |
| Pythium | 50–55 | 10–30 min | [95] | |
| Phytophthora | 30–35 | 30 min | [96] | |
| Bacteria | Ralstonia solanacearum | 52–53 | 10 min | [97] |
| Agrobacterium tumefaciens | 50 | 10 min | [98] | |
| Pectobacterium carotovorum | 50–53 | 30 min | [99] | |
| Streptomyces scabies | 50 | 30 min | [100] | |
| Virus | Soit-borne wheat mosaic virus | 60–65 | 30–60 min | [101] |
| Tobacco mosaic virus | 95 | 10 min | [102] | |
| Parasite | Meloidogyne incognita | 55 | 10 min | [103] |
| Heterodera spp. | 50 | 30–60 min | [90] |
| Category | Device Name | Fuel Type | Working Width (m) | Working Depth (cm) | Pest/Weed Control Efficiency (%) | Intelligence Level | Source |
|---|---|---|---|---|---|---|---|
| Chinese | Precision Rotary Flame Sterilization and Ridging Machine | Liquefied Petroleum Gas | 1.2 | 22 | 82.9 (Pest) | L2 | [122] |
| Chinese | Biomass Pellet Flame Sterilization Rotary Tiller | Biomass Pellets | 1.0 | 18 | 85 (Pest/Weed) | L1 | [123] |
| Chinese | Tea Garden High-Temperature Flame Weeding Device | Liquefied Petroleum Gas | 0.6 | 5–8 | 80 (Weed) | L0 | [124] |
| International | RHEA Automatic Cross-Combustion Weeder | Propane | 1.8 | 10–12 | 90 (Weed) | L3 | [125] |
| International | Stepanovic Parallel Torch System | Propane | 2 × 0.3 | 5–10 | 88 (Weed) | L2 | [126] |
| International | Kang Flame Weeder | Liquefied Petroleum Gas | Not specified | Not specified | – | L1 | [127] |
| Disinfection Target | Target Temperature | Depth Range | Exposure Time | Conditions |
|---|---|---|---|---|
| Pathogen | 55–70 °C | 0–20 cm | 10 min | A sufficient heat flux and action time are required. |
| Weed | 60–80 °C | 0–5 cm | 5 min | The heat is concentrated on the surface; the required temperature is high but the duration is relatively short. |
| Pest | >55 °C | 0–15 cm | 8 min | Balancing the depth of heat penetration and the duration of temperature maintenance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Chen, J.; Zhang, Y. A Review on the Mechanism of Soil Flame Disinfection and the Precise Control Technology of the Device. Agriculture 2025, 15, 2447. https://doi.org/10.3390/agriculture15232447
Zhang Y, Wang Y, Chen J, Zhang Y. A Review on the Mechanism of Soil Flame Disinfection and the Precise Control Technology of the Device. Agriculture. 2025; 15(23):2447. https://doi.org/10.3390/agriculture15232447
Chicago/Turabian StyleZhang, Yunhe, Ying Wang, Jinshi Chen, and Yu Zhang. 2025. "A Review on the Mechanism of Soil Flame Disinfection and the Precise Control Technology of the Device" Agriculture 15, no. 23: 2447. https://doi.org/10.3390/agriculture15232447
APA StyleZhang, Y., Wang, Y., Chen, J., & Zhang, Y. (2025). A Review on the Mechanism of Soil Flame Disinfection and the Precise Control Technology of the Device. Agriculture, 15(23), 2447. https://doi.org/10.3390/agriculture15232447





