Application of Exogenous 24-Epibrassinolide at the Silking Stage Alleviates the Effects of Post-Silking Heat Stress on Photosynthetic Performance of Waxy Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Sites and Materials
2.2. Sampling, Measurement and Analysis
2.2.1. Yield Determination and Dry Matter Accumulation/Translocation
2.2.2. Photosynthetic Parameter Measurement
2.2.3. Leaf Anatomical Microstructure Observation
2.2.4. Mesophyll Cell Ultrastructure Observation
2.2.5. Photosynthetic Pigment, Osmotic Adjustment Substances Quantification
2.2.6. Enzyme Activities, Peroxides, and Endogenous Hormones
2.3. Statistical Analyses
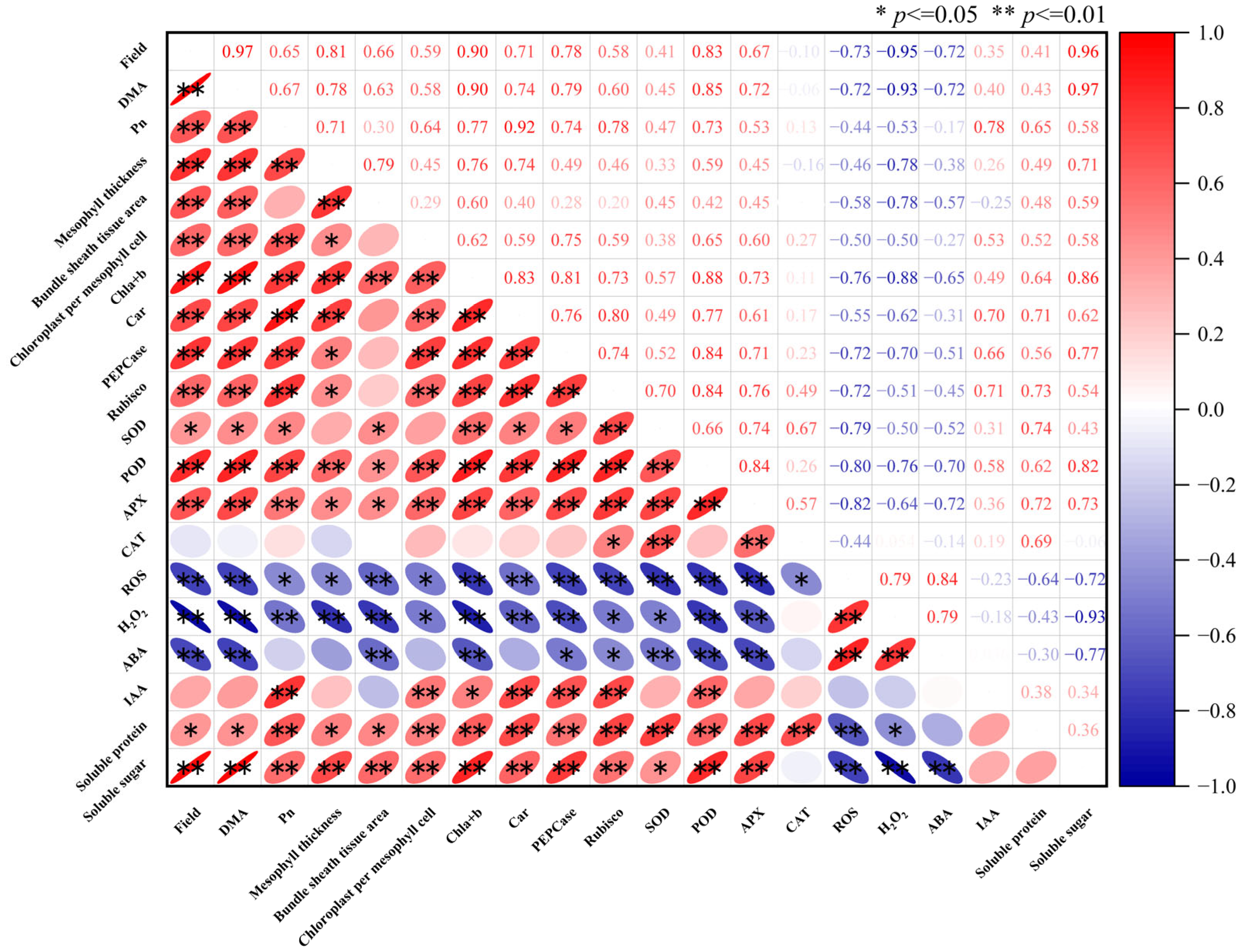
3. Results
3.1. Maturity Yield
3.2. Dry Matter Accumulation and Translocation
3.3. Photosynthetic Parameters
3.4. Leaf Anatomical Microstructure
3.5. Mesophyll Cell Ultrastructure
3.6. Photosynthetic Pigment
3.7. Photosynthetic Key Enzyme Activity
3.8. Antioxidant System
3.9. Endogenous Hormone Content
3.10. Soluble Protein and Sugar Content
4. Discussion
4.1. Effects of Exogenous BR on the Yield, Dry Matter Accumulation and Translocation of Waxy Maize Under HS
4.2. Effects of Exogenous BR on Photosynthetic Performance of Waxy Maize Leaves Under HS
4.3. Effects of Exogenous BR on Anatomical Microstructure and Mesophyll Cell Ultrastructure of Waxy Maize Leaves Under HS
4.4. Effects of Exogenous BR on Antioxidant System and Osmotic Regulators of Waxy Maize Under HS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bongaarts, J. IPCC, 2023: Climate Change 2023: Synthesis Report. Popul. Dev. Rev. 2024, 50, 577–580. [Google Scholar] [CrossRef]
- Sallam, A.; Amro, A.; El-Akhdar, A.; Dawood, M.F.A.; Kumamaru, T.; Stephen Baenziger, P. Genetic diversity and genetic variation in morpho-physiological traits to improve heat tolerance in Spring barley. Mol. Biol. Rep. 2018, 45, 2441–2453. [Google Scholar] [CrossRef]
- Magana Ugarte, R.; Escudero, A.; Gavilan, R.G. Metabolic and physiological responses of Mediterranean high-mountain and alpine plants to combined abiotic stresses. Physiol. Plant 2019, 165, 403–412. [Google Scholar] [CrossRef]
- Hao, Z.C.; AghaKouchak, A.; Phillips, T.J. Changes in concurrent monthly precipitation and temperature extremes. Environ. Res. Lett. 2013, 8, 034014. [Google Scholar] [CrossRef]
- Liu, B.; Martre, P.; Ewert, F.; Porter, J.R.; Challinor, A.J.; Muller, C.; Ruane, A.C.; Waha, K.; Thorburn, P.J.; Aggarwal, P.K.; et al. Global wheat production with 1.5 and 2.0 °C above pre-industrial warming. Glob. Change Biol. 2019, 25, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, M.; Ahmed, M.; Gill, K.S.; Akram, Z. Association analysis for agronomic traits in wheat under terminal heat stress. Saudi J. Biol. Sci. 2021, 28, 7404–7415. [Google Scholar] [CrossRef] [PubMed]
- Tigchelaar, M.; Battisti, D.S.; Naylor, R.L.; Ray, D.K. Future warming increases probability of globally synchronized maize production shocks. Proc. Natl. Acad. Sci. USA 2018, 115, 6644–6649. [Google Scholar] [CrossRef]
- Hou, P.; Liu, Y.E.; Liu, W.M.; Yang, H.S.; Xie, R.Z.; Wang, K.R.; Ming, B.; Liu, G.Z.; Xue, J.; Wang, Y.H.; et al. Quantifying maize grain yield losses caused by climate change based on extensive field data across China. Resour. Conserv. Recycl. 2021, 174, 105811. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.J.; Li, Y.; Tang, J.X.; Li, X.; Zhang, Y.D.; Han, D.C.; Zhao, Y.C.; Wang, Q.; Yang, Z.Q. Identifying trigger thresholds for heat damage at different growth stages of summer maize: A case study of the Huang-Huai-Hai Plain, China. Eur. J. Agron. 2025, 170, 127729. [Google Scholar] [CrossRef]
- Nelson, O.; Pan, D. Starch synthesis in maize endosperms. In Annual Review of Plant Biology; Jones, R.L., Ed.; Annual Review of Plant Physiology and Plant Molecular Biology; Annual Reviews: Palo Alto, CA, USA, 1995; Volume 46, pp. 475–496. [Google Scholar]
- Guo, Y.Q.; Sun, L.L.; Chen, L.R.; Wang, X.Y.; Wang, C.G.; Gong, K.J. Applications of waxy corn flour based on physicochemical and processing properties: Comparison with waxy rice flour and waxy corn starch. Int. J. Food Eng. 2021, 17, 355–363. [Google Scholar] [CrossRef]
- Luo, Y.; Han, X.; Shen, M.; Yang, J.; Ren, Y.; Xie, J. Mesona chinensis polysaccharide on the thermal, structural and digestibility properties of waxy and normal maize starches. Food Hydrocoll. 2021, 112, 106317. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Edreira, J.I.R.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Differences in crop growth, biomass partitioning and reserves use. Field Crops Res. 2012, 130, 87–98. [Google Scholar] [CrossRef]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Bommert, P.; Whipple, C. Grass inflorescence architecture and meristem determinacy. Semin. Cell. Dev. Biol. 2018, 79, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum). BBA-Bioenergetics 2011, 1807, 22–29. [Google Scholar] [CrossRef]
- Posch, B.C.; Hammer, J.; Atkin, O.K.; Bramley, H.; Ruan, Y.L.; Trethowan, R.; Coast, O. Wheat photosystem II heat tolerance responds dynamically to short- and long-term warming. J. Exp. Bot. 2022, 73, 3268–3282. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Suzuki, N.; Katano, K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; IntechOpen: London, UK, 2013; pp. 169–205. [Google Scholar]
- Shao, J.Y.; Wang, Q.H.; Liu, P.; Zhao, B.; Han, W.; Zhang, J.W.; Ren, B.Z. The complex stress of waterlogging and high temperature accelerated maize leaf senescence and decreased photosynthetic performance at different growth stages. J. Agron. Crop Sci. 2024, 210, e12689. [Google Scholar] [CrossRef]
- Lisong, C.; Xinghui, L. Effect of high temperature stress on cell membrane permeability and photosynthetic pigment in leaves of peach and pomelo. Plant Sci. J. 1997, 15, 233–237. [Google Scholar]
- Zhang, P.; Sharwood, R.E.; Carroll, A.; Estavillo, G.M.; von Caemmerer, S.; Furbank, R.T. Systems analysis of long-term heat stress responses in the C4 grass Setaria viridis. Plant Cell 2025, 37, 4. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, I.; Pati, P.K. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front. Plant Sci. 2015, 6, 950. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S.; et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Brassinosteroids and the tolerance of cereals to low and high temperature stress: Photosynthesis and the physicochemical properties of cell membranes. Int. J. Mol. Sci. 2022, 23, 342. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Khan, R.; Wu, X.Y.; Zhou, L.; Xu, N.; Du, S.S.; Ma, X.H. Exogenous application of brassinosteroids regulates tobacco leaf size and expansion via modulation of endogenous hormones content and gene expression. Physiol. Mol. Biol. Plants 2021, 27, 847–860. [Google Scholar] [CrossRef]
- Rattan, A.; Kapoor, D.; Kapoor, N.; Bhardwaj, R.; Sharma, A. Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. J. Plant Growth Regul. 2020, 39, 1465–1475. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.R.; Chen, S.C. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant steroidal hormone epibrassinolide regulate—Heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Dhaubhadel, S.; Browning, K.S.; Gallie, D.R.; Krishna, P. Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 2002, 29, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xu, H.; Zhang, P.; Su, X.; Zhao, H. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2016, 81, 377–384. [Google Scholar] [CrossRef]
- Quintero-CalderÓN, E.H.; SÁNchez-Reinoso, A.D.; ChÁVez-Arias, C.C.; Garces-Varon, G.; Restrepo-DÍAz, H. Rice seedlings showed a higher heat tolerance through the foliar application of biostimulants. Not. Bot. Horti. Agrobot. 2021, 49, 12120. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, X.-G.; Zhang, L.; Lin, S.; Zhao, X.; Zhou, L.-L.; Shen, S.; Zhou, S.-L. Spraying exogenous 6-benzyladenine and brassinolide at tasseling increases maize yield by enhancing source and sink capacity. Field Crops Res. 2017, 211, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.L.; Jin, M.; Yu, H.X.; Jiang, D.; Wollenweber, B.; Dai, T.B.; Cao, W.X. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 2012, 55, 331–336. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Saıdou, A.; Janssen, B.H.; Temminghoff, E.J.M. Effects of soil properties, mulch and NPK fertilizer on maize yields and nutrient budgets on ferralitic soils in southern Benin. Agric. Ecosyst. Environ. 2003, 100, 265–273. [Google Scholar] [CrossRef]
- Wang, W.; Cai, C.; Lam, S.K.; Liu, G.; Zhu, J. Elevated CO2 cannot compensate for japonica grain yield losses under increasing air temperature because of the decrease in spikelet density. Eur. J. Agron. 2018, 99, 21–29. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- McIntyre, C.L.; Seung, D.; Casu, R.E.; Rebetzke, G.J.; Shorter, R.; Xue, G.P. Genotypic variation in the accumulation of water soluble carbohydrates in wheat. Funct. Plant Biol. 2012, 39, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Tatar, Ö.; Brück, H.; Asch, F. Photosynthesis and remobilization of dry matter in wheat as affected by progressive drought stress at stem elongation stage. J. Agron. Crop Sci. 2016, 202, 292–299. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Q.; Hu, S.J.; Wang, R.Y.; Wang, H.L.; Zhang, K.; Zhao, H.; Ren, S.X.; Yang, Y.; Zhao, F.N.; et al. Effects of high temperature and drought stresses on growth and yield of summer maize during grain filling in north China. Agriculture 2022, 12, 1948. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Zhen, F.X.; Zhou, J.J.; Mahmood, A.; Wang, W.; Chang, X.N.; Liu, B.; Liu, L.L.; Cao, W.X.; Zhu, Y.; Tang, L. Quantifying the effects of short-term heat stress at booting stage on nonstructural carbohydrates remobilization in rice. Crop J. 2020, 8, 194–212. [Google Scholar] [CrossRef]
- Zamani, M.M.; Nabipour, M.; Meskarbashee, M. Stem water soluble carbohydrate remobilization in wheat under heat stress during the grain filling. Int. J. Agric. Biol. 2014, 16, 401–405. [Google Scholar]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- De la Haba, P.; De la Mata, L.; Molina, E.; Agüera, E. High temperature promotes early senescence in primary leaves of sunflower (Helianthus annuus L.) plants. Can. J. Plant Sci. 2014, 94, 659–669. [Google Scholar] [CrossRef]
- Lv, X.K.; Li, T.; Wen, X.X.; Liao, Y.C.; Liu, Y. Effect of potassium foliage application post-anthesis on grain filling of wheat under drought stress. Field Crops Res. 2017, 206, 95–105. [Google Scholar] [CrossRef]
- Lv, X.K.; Han, J.; Liao, Y.C.; Liu, Y. Effect of phosphorus and potassium foliage application post-anthesis on grain filling and hormonal changes of wheat. Field Crops Res. 2017, 214, 83–93. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, H.Z.; Xiang, J.; Zhang, Y.K.; Wang, Z.G.; Zhu, D.F.; Wang, J.K.; Zhang, Y.P.; Wang, Y.L. Rice spikelet formation inhibition caused by decreased sugar utilization under high temperature is associated with brassinolide decomposition. Environ. Exp. Bot. 2021, 190, 104585. [Google Scholar] [CrossRef]
- Li, Q.; Ye, Y.C.; Wang, D.M.; Wang, Y.J.; Zhang, M.; Cai, R.G.; Zhao, G.C.; Yang, Y.S.; Chang, X.H.; Liu, X.W. Temporal dynamics of pre-flowering dry matter remobilization and post-flowering photosynthetic compensation in wheat under combined high temperature and drought stress. Plant Growth Regul. 2025, 105, 1307–1321. [Google Scholar] [CrossRef]
- Ji, D.L.; Xiao, W.H.; Sun, Z.W.; Liu, L.J.; Gu, J.F.; Zhang, H.; Harrison, M.T.; Liu, K.; Wang, Z.Q.; Wang, W.L.; et al. Translocation and distribution of carbon-nitrogen in relation to rice yield and grain quality as affected by high temperature at early panicle initiation stage. Rice Sci. 2023, 30, 598–612. [Google Scholar] [CrossRef]
- Diao, H.; Cernusak, L.A.; Saurer, M.; Gessler, A.; Siegwolf, R.T.W.; Lehmann, M.M. Uncoupling of stomatal conductance and photosynthesis at high temperatures: Mechanistic insights from online stable isotope techniques. New Phytol. 2024, 241, 2366–2378. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Kruler, V.; Winkelmuller, T.M.; Wang, Y.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjarvi, J. Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 2016, 171, 1569–1580. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Meng, J.; Xi, Z. Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol. Plant. 2014, 37, 1729. [Google Scholar] [CrossRef]
- da Fonseca, S.S.; da Silva, B.R.S.; Lobato, A.K.d.S. 24-Epibrassinolide positively modulate leaf structures, antioxidant system and photosynthetic machinery in rice under simulated acid rain. J. Plant Growth Regul. 2020, 39, 1559–1576. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Guo, H. New advances in the regulation of leaf senescence by classical and peptide hormones. Front. Plant Sci. 2022, 13, 923136. [Google Scholar] [CrossRef]
- Jespersen, D.; Zhang, J.; Huang, B.R. Chlorophyll loss associated with heat-induced senescence in bentgrass. Plant Sci. 2016, 249, 1–12. [Google Scholar] [CrossRef]
- Siddiqui, H.; Hayat, S.; Bajguz, A. Regulation of photosynthesis by brassinosteroids in plants. Acta Physiol. Plant. 2018, 40, 59. [Google Scholar] [CrossRef]
- Dias, M.C.; Brüggemann, W. Differential inhibition of photosynthesis under drought stress in species with different degrees of development of the C4 syndrome. Photosynthetica 2007, 45, 75–84. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Hendrickson, L.; Quinn, V.; Vella, N.; Millgate, A.G.; Furbank, R.T. Reductions of Rubisco activase by antisense RNA in the C4 plant Flaveria bidentis reduces Rubisco carbamylation and leaf photosynthesis. Plant Physiol. 2005, 137, 747–755. [Google Scholar] [CrossRef]
- Galmes, J.; Aranjuelo, I.; Medrano, H.; Flexas, J. Variation in Rubisco content and activity under variable climatic factors. Photosynth. Res. 2013, 117, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, X.Y.; Gu, L.M.; Liu, P.; Zhao, B.; Zhang, J.W.; Ren, B.Z. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 2023, 289, 108525. [Google Scholar] [CrossRef]
- Fujii, S.; Nishida, K.; Akitsu, T.K.; Kume, A.; Hanba, Y.T. Variation in leaf mesophyll anatomy of fern species imposes significant effects on leaf gas exchange, light capture, and leaf hydraulic conductance. Photosynthetica 2023, 61, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.G. Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environ. Exp. Bot. 2000, 44, 171–183. [Google Scholar] [CrossRef]
- Hao, L.; Guo, L.; Li, R.; Cheng, Y.; Huang, L.; Zhou, H.; Xu, M.; Li, F.; Zhang, X.; Zheng, Y. Responses of photosynthesis to high temperature stress associated with changes in leaf structure and biochemistry of blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2019, 246, 251–264. [Google Scholar] [CrossRef]
- Aguraijuja, K.; Kloseiko, J.; Ots, K.; Lukjanova, A. Effect of wood ash on leaf and shoot anatomy, photosynthesis and carbohydrate concentrations in birch on a cutaway peatland. Environ. Monit. Assess. 2015, 187, 444. [Google Scholar] [CrossRef]
- Vedenicheva, N.; Futorna, O.; Shcherbatyuk, M.; Kosakivska, I. Effect of seed priming with zeatin on Secale cereale L. growth and cytokinins homeostasis under hyperthermia. J. Crop Improv. 2021, 36, 656–674. [Google Scholar] [CrossRef]
- Flori, S.; Jouneau, P.-H.; Bailleul, B.; Gallet, B.; Estrozi, L.F.; Moriscot, C.; Bastien, O.; Eicke, S.; Schober, A.; Bártulos, C.R.; et al. Plastid thylakoid architecture optimizes photosynthesis in diatoms. Nat. Commun. 2017, 8, 15885. [Google Scholar] [CrossRef]
- Packer, L.; Siegenthaler, P.A.; Nobel, P.S. Light-induced volume changes in spinach chloroplasts. J. Cell Biol. 1965, 26, 593–599. [Google Scholar] [CrossRef]
- Gounaris, K.; Brain, A.R.R.; Quinn, P.J.; Williams, W.P. Structural reorganisation of chloroplast thylakoid membranes in response to heat-stress. BBA–Bioenergetics 1984, 766, 198–208. [Google Scholar] [CrossRef]
- Zhang, R.; Wise, R.R.; Struck, K.R.; Sharkey, T.D. Moderate heat stress of Arabidopsis thaliana leaves causes chloroplast swelling and plastoglobule formation. Photosynth. Res. 2010, 105, 123–134. [Google Scholar] [CrossRef]
- Ben Salem-Fnayou, A.; Bouamama, B.; Ghorbel, A.; Mliki, A. Investigations on the leaf anatomy and ultrastructure of grapevine (vitis vinifera) under heat stress. Microsc. Res. Tech. 2011, 74, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Devadasu, E.; Susanto, F.A.; Schilmiller, A.L.; Mahey, M.; Johnny, C.; Lundquist, P.K. Dynamic changes to the plastoglobule lipidome and proteome in maize during heat stress and recovery. J. Exp. Bot. 2025, eraf452. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Corral, R.; Schwenkert, S.; Lundquist, P.K. Molecular changes of Arabidopsis thaliana plastoglobules facilitate thylakoid membrane remodeling under high light stress. Plant J. 2021, 106, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.B.; Cai, K.F.; Zhang, G.P.; Zeng, F.R. Brassinolide alleviates the drought-induced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul. 2017, 82, 447–455. [Google Scholar] [CrossRef]
- Lai, H.J.; Li, X.D.; Chen, Y.L.; Liu, Z.X. Mitigating heat-induced yield loss in peanut: Insights into 24-epibrassino-lide-mediated improvement in antioxidant capacity, photosynthesis, and kernel weight. Field Crops Res. 2024, 316, 109521. [Google Scholar] [CrossRef]
- Dobrikova, A.G.; Vladkova, R.S.; Rashkov, G.D.; Todinova, S.J.; Krumova, S.B.; Apostolova, E.L. Effects of exogenous 24-epibrassinolide on the photosynthetic membranes under non-stress conditions. Plant Physiol. Biochem. 2014, 80, 75–82. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Tian, S.; Lu, L.; Shohag, M.; Yang, X. Metallothionein 2 (SaMT2) from sedum alfredii hance confers increased cd tolerance and accumulation in yeast and tobacco. PLoS ONE 2014, 9, e102750. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Tiwari, Y.K.; Yadav, S.K. High temperature stress tolerance in maize (Zea mays L.): Physiological and molecular mechanisms. J. Plant Biol. 2019, 62, 93–102. [Google Scholar] [CrossRef]
- Ahammed, G.J.; He, B.B.; Qian, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Yu, J.Q.; Xia, X.J. 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ. Pollut. 2017, 229, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Kohila, S.; Gomathi, R. Adaptive physiological and biochemical response of sugarcane genotypes to high-temperature stress. Indian J. Plant Physiol. 2018, 23, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chang, Q.; Hou, X.; Wang, J.; Chen, S.; Zhang, Q.; Wang, Z.; Yin, Y.; Liu, J. The effect of high-temperature stress on the physiological indexes, chloroplast ultrastructure, and photosystems of two herbaceous peony cultivars. J. Plant Growth Regul. 2022, 42, 1631–1646. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Zhang, F.; Li, C. Effect of nitrogen application on enhancing high-temperature stress tolerance of tomato plants during the flowering and fruiting stage. Front. Plant Sci. 2023, 14, 1172078. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboinska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Bao, G.; Tang, W.; He, F.; Chen, W.; Zhu, Y.; Fan, C.; Zhang, M.; Chang, Y.; Sun, J.; Ding, X. Physiological response in the leaf and stolon of white clover under acid precipitation and freeze-thaw stress. Funct. Plant Biol. 2019, 47, 50–57. [Google Scholar] [CrossRef]
- Xu, F.; Xi, Z.M.; Zhang, H.; Zhang, C.J.; Zhang, Z.W. Brassinosteroids are involved in controlling sugar unloading in Vitis vinifera ‘Cabernet Sauvignon’ berries during veraison. Plant Physiol. Biochem. 2015, 94, 197–208. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Ma, X.R.; Tang, D.D.; Chen, Y.D.; Chen, G.; Zou, J.F.; Tan, L.Q.; Tang, Q.; Chen, W. Effects of brassinosteroid on the physiological changes on two varieties of tea plants under salt stress. Int. J. Mol. Sci. 2024, 25, 13445. [Google Scholar] [CrossRef]
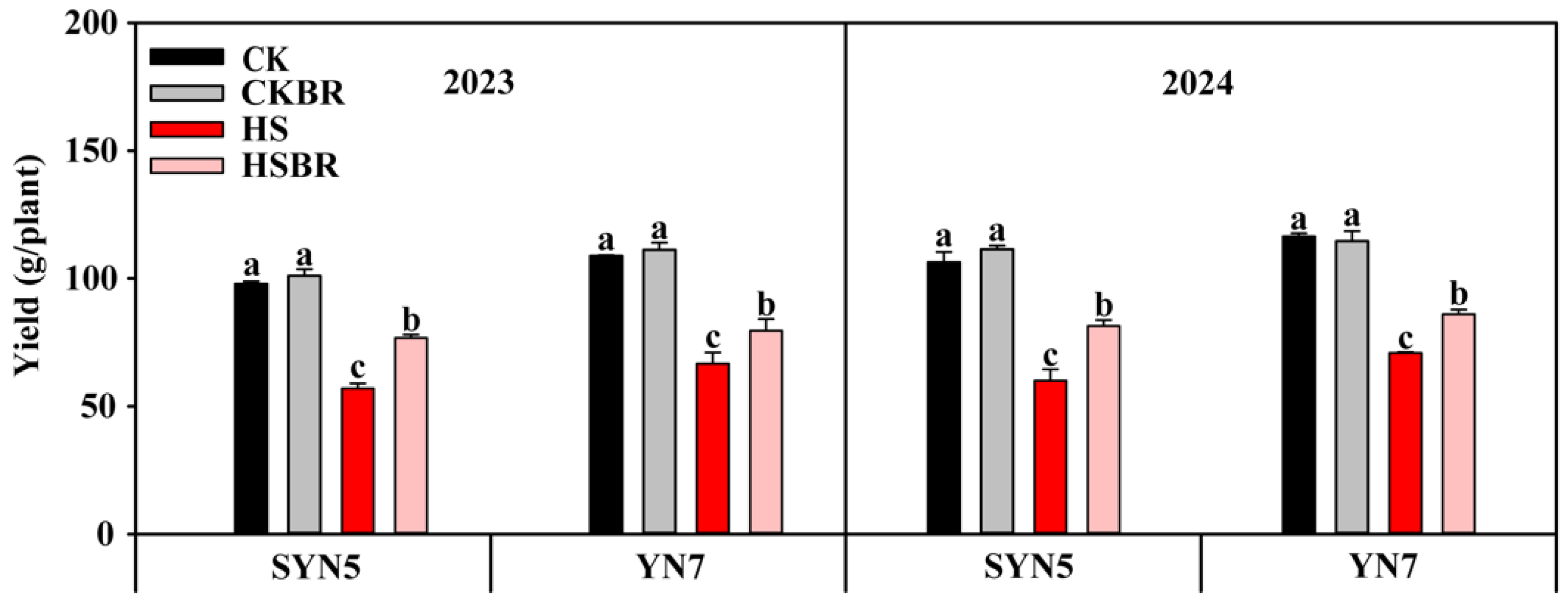
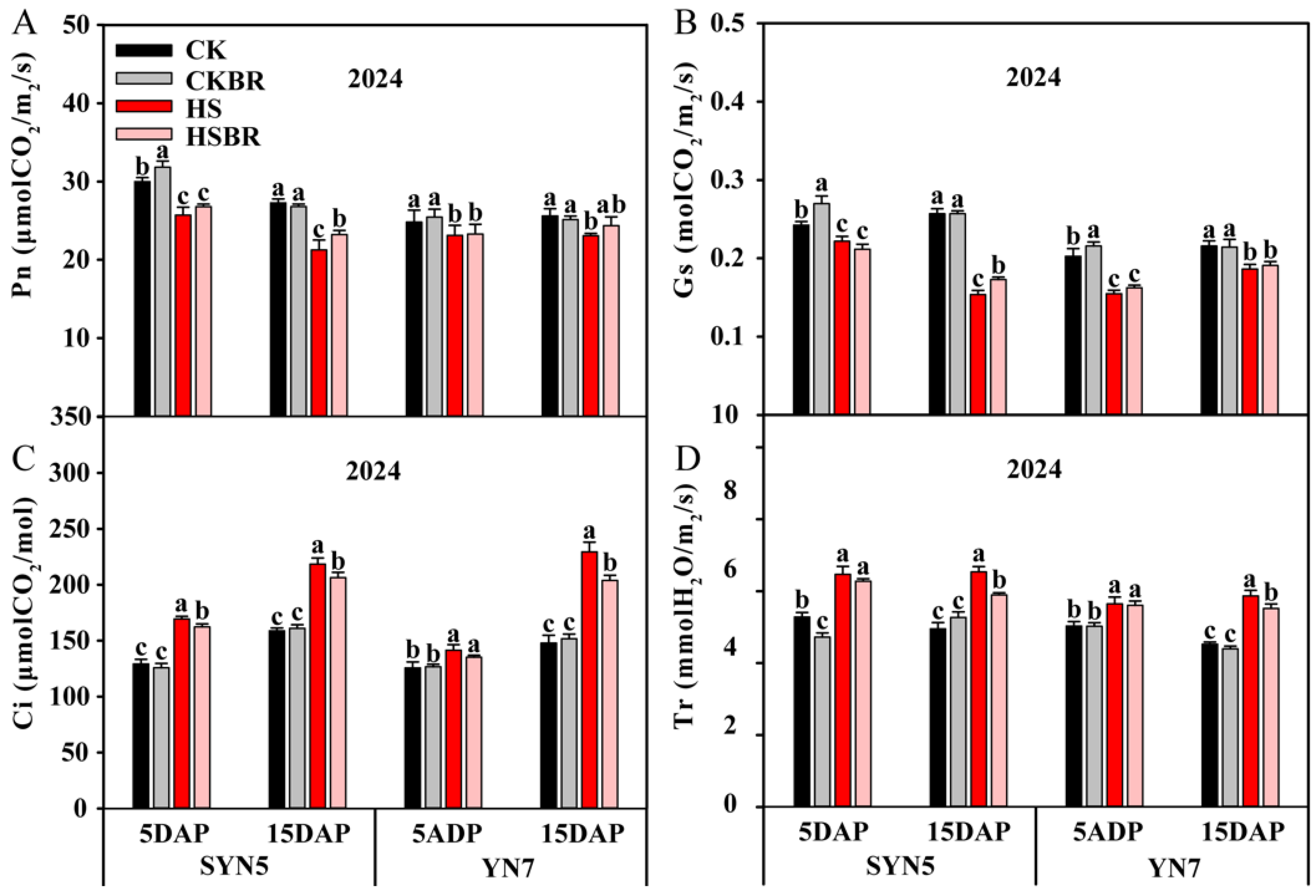

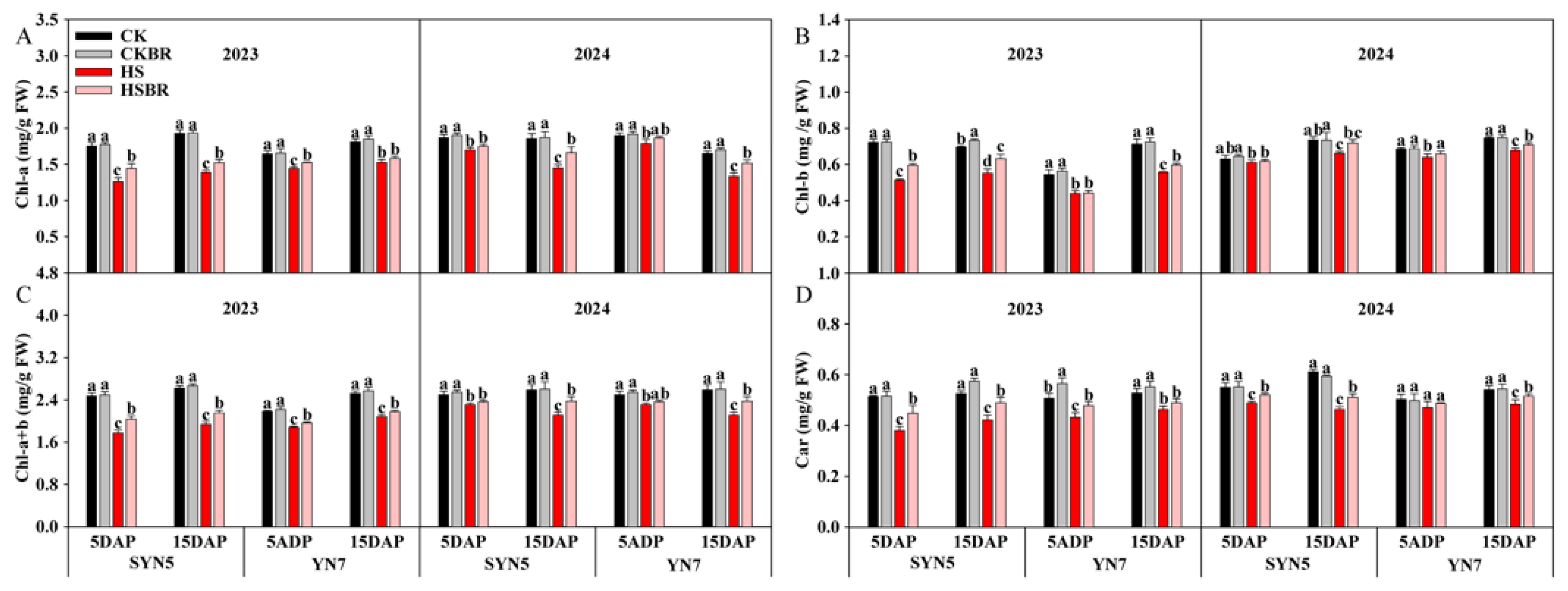


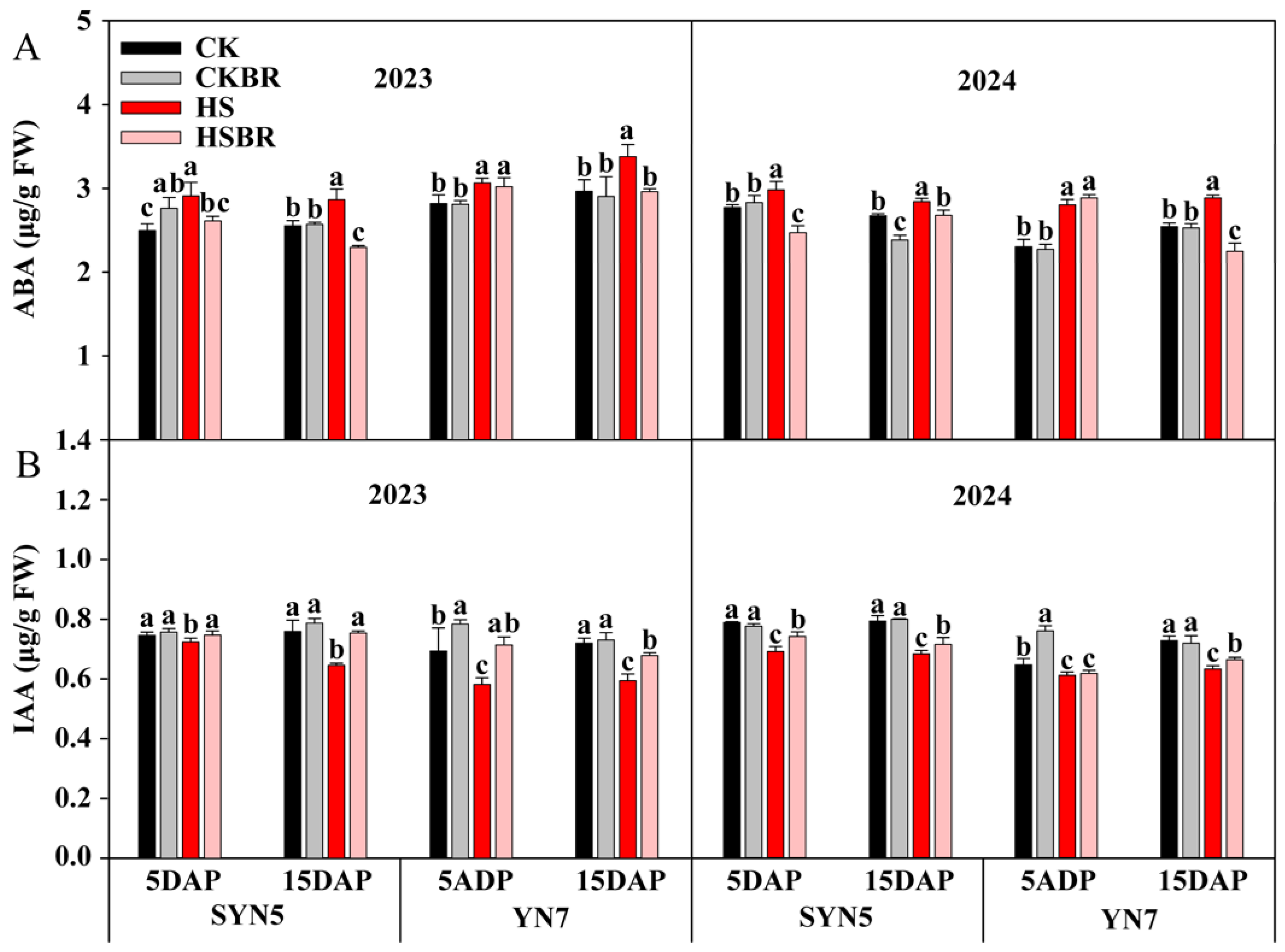

| Year | Hybrid | Treatment | TAP (g/Plant) | TRP (%) | CRP (%) | TAA (g/Plant) | CRA (%) | DMA (g/Plant) |
|---|---|---|---|---|---|---|---|---|
| 2023 | SYN5 | CK | 8.5c | 3.6c | 10.3d | 74.3b | 89.7a | 112.9b |
| CKBR | 9.0c | 3.9c | 10.5d | 76.5b | 89.5a | 112.2b | ||
| HS | 26.9a | 16.3a | 56.1a | 21.1e | 43.9d | 44.5d | ||
| HSBR | 20.8b | 10.9b | 32.1c | 44.0c | 67.9b | 70.3c | ||
| YN7 | CK | 9.0c | 3.5c | 9.7d | 83.1a | 90.3a | 118.9ab | |
| CKBR | 8.9c | 3.5c | 9.5d | 85.3a | 90.5a | 121.2a | ||
| HS | 28.6a | 15.1a | 50.7b | 27.7d | 49.3c | 52.1d | ||
| HSBR | 22.0b | 10.5b | 31.8c | 47.7c | 68.2b | 72.9c | ||
| 2024 | SYN5 | CK | 11.8d | 4.5e | 13.1e | 78.6b | 86.9a | 127.8b |
| CKBR | 8.9e | 3.3f | 9.4e | 85.9a | 90.6a | 131.7b | ||
| HS | 29.6a | 16.7a | 58.2a | 21.4f | 41.8e | 43.4e | ||
| HSBR | 27.0b | 12.8c | 39.0c | 42.3d | 61.0c | 77.7c | ||
| YN7 | CK | 12.0d | 4.3ef | 12.1e | 87.0a | 87.9a | 138.3ab | |
| CKBR | 10.6de | 3.7ef | 10.9e | 86.9a | 89.1a | 143.6a | ||
| HS | 28.8ab | 14.6b | 47.9b | 31.4e | 52.1d | 56.8d | ||
| HSBR | 20.3c | 9.3d | 27.8d | 52.8c | 72.2b | 77.8c |
| Hybrid | Treatment | Leaf Thickness (μm) | Upper Epidermis Thickness (μm) | Under Epidermis Thickness (μm) | Mesophyll Thickness (μm) | Vascular Bundle Tissue Area (μm2) | Bundle Sheath Tissue Area (μm2) |
|---|---|---|---|---|---|---|---|
| SYN5 | CK | 239.0a | 29.0a | 25.7a | 184.2a | 2545.7a | 7650.4b |
| CKBR | 235.9a | 28.7a | 27.8a | 179.4a | 2810.0a | 9192.2a | |
| HS | 155.2c | 24.9b | 20.5b | 109.8b | 906.8c | 4250.8d | |
| HSBR | 164.7b | 26.6a | 21.4b | 115.0b | 1262.0b | 4810.2c | |
| YN7 | CK | 212.9a | 30.7b | 23.2a | 159.1ab | 3233.2a | 7876.3b |
| CKBR | 215.0a | 27.5b | 23.3a | 164.3a | 3265.6a | 9251.9a | |
| HS | 204.1b | 36.1a | 25.0a | 143.0c | 746.0c | 6797.2c | |
| HSBR | 205.4b | 26.7b | 25.8a | 152.9b | 2218.2b | 9870.1a |
| Variety | Treatment | Chloroplast per Mesophyll Cell | Chloroplast Size | Grana per Unit Area of Chloroplast (μm−2) | Lamellae per Grana | ||
|---|---|---|---|---|---|---|---|
| Length (μm) | Width (μm) | Area (μm2) | |||||
| SYN5 | CK | 8.3ab | 5.5a | 4.4b | 25.3c | 1.2a | 20.4bc |
| CKBR | 9.0a | 5.2a | 3.2c | 22.9c | 1.1a | 28.5a | |
| HS | 5.3b | 5.6a | 5.1a | 34a | 0.5b | 15.0c | |
| HSBR | 6.0b | 5.5a | 4.8a | 29.8b | 1.0a | 26.0ab | |
| YN7 | CK | 6.7a | 5.3ab | 4.5bc | 24.7b | 1.0a | 22.9a |
| CKBR | 7.0a | 5.5a | 4.1c | 24.9b | 1.0a | 25.0a | |
| HS | 5.3b | 5.4ab | 5.2a | 31.5a | 0.7b | 18.9b | |
| HSBR | 5.7b | 5.3b | 4.7b | 26.3b | 0.9ab | 22.1a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, J.; Guo, J.; Yang, H.; Li, G.; Lu, D. Application of Exogenous 24-Epibrassinolide at the Silking Stage Alleviates the Effects of Post-Silking Heat Stress on Photosynthetic Performance of Waxy Maize. Agriculture 2025, 15, 2445. https://doi.org/10.3390/agriculture15232445
Liu J, Li J, Guo J, Yang H, Li G, Lu D. Application of Exogenous 24-Epibrassinolide at the Silking Stage Alleviates the Effects of Post-Silking Heat Stress on Photosynthetic Performance of Waxy Maize. Agriculture. 2025; 15(23):2445. https://doi.org/10.3390/agriculture15232445
Chicago/Turabian StyleLiu, Jiawei, Jing Li, Jian Guo, Huan Yang, Guanghao Li, and Dalei Lu. 2025. "Application of Exogenous 24-Epibrassinolide at the Silking Stage Alleviates the Effects of Post-Silking Heat Stress on Photosynthetic Performance of Waxy Maize" Agriculture 15, no. 23: 2445. https://doi.org/10.3390/agriculture15232445
APA StyleLiu, J., Li, J., Guo, J., Yang, H., Li, G., & Lu, D. (2025). Application of Exogenous 24-Epibrassinolide at the Silking Stage Alleviates the Effects of Post-Silking Heat Stress on Photosynthetic Performance of Waxy Maize. Agriculture, 15(23), 2445. https://doi.org/10.3390/agriculture15232445








