Synergistically Better than One: Co-Application of Grasshopper-Derived +ssRNA Virus and Imidacloprid Induces Acute Toxicity in Locusta migratoria
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects, Virus, and Chemical Reagents
2.2. GPV and Imidacloprid Treatments of Nymphs
- (1)
- GPV only: Nymphs were intracoelomically injected with 200 μL sterilized PBS containing 2 × 107 virions via the third and fourth abdominal segments. After 24 h, 2 μL acetone was applied to the pronotum.
- (2)
- Imidacloprid only: Nymphs were injected with 200 μL sterilized PBS. After 24 h, 2 µL imidacloprid (dissolved in 10 mg/L acetone) was applied to the pronotum.
- (3)
- GPV + imidacloprid: GPV-infected nymphs were incubated for 24 h, then received 2 µL imidacloprid (dissolved in 10 mg/L acetone) on the pronotum.
- (4)
- Controls: Nymphs were injected with 200 μL sterile PBS, and after 24 h, 2 µL acetone was placed on the pronotum.
2.3. Histopathological Observations of Midgut
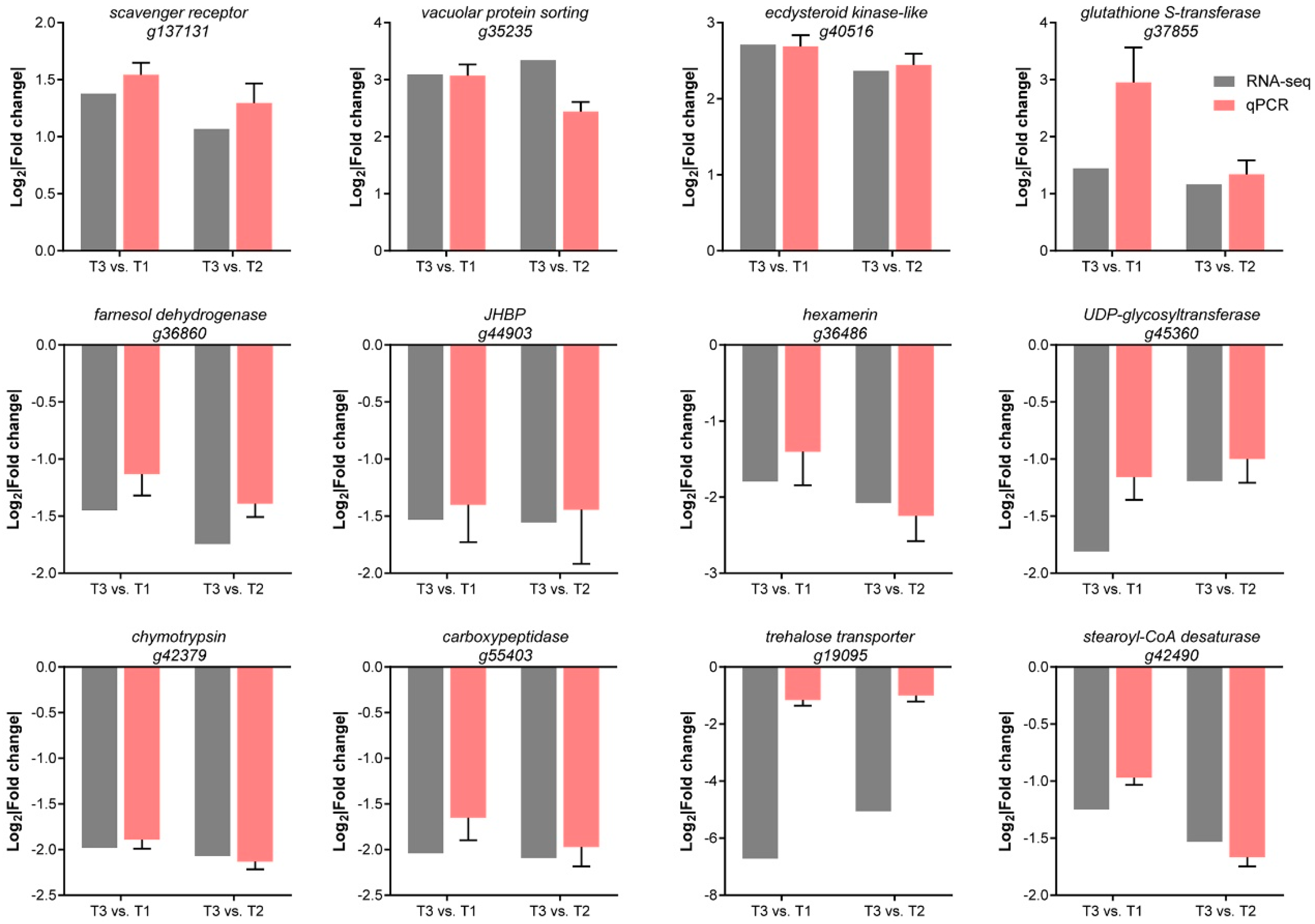
2.4. RNA-Seq Analyses and qPCR Validation
2.5. Quantification of Total Proteins, Total Sugars, Triglycerides, and ATP
2.6. Statistical Analysis
3. Results
3.1. Combined Treatment with GPV and Imidacloprid Caused Rapid Acute Toxicity
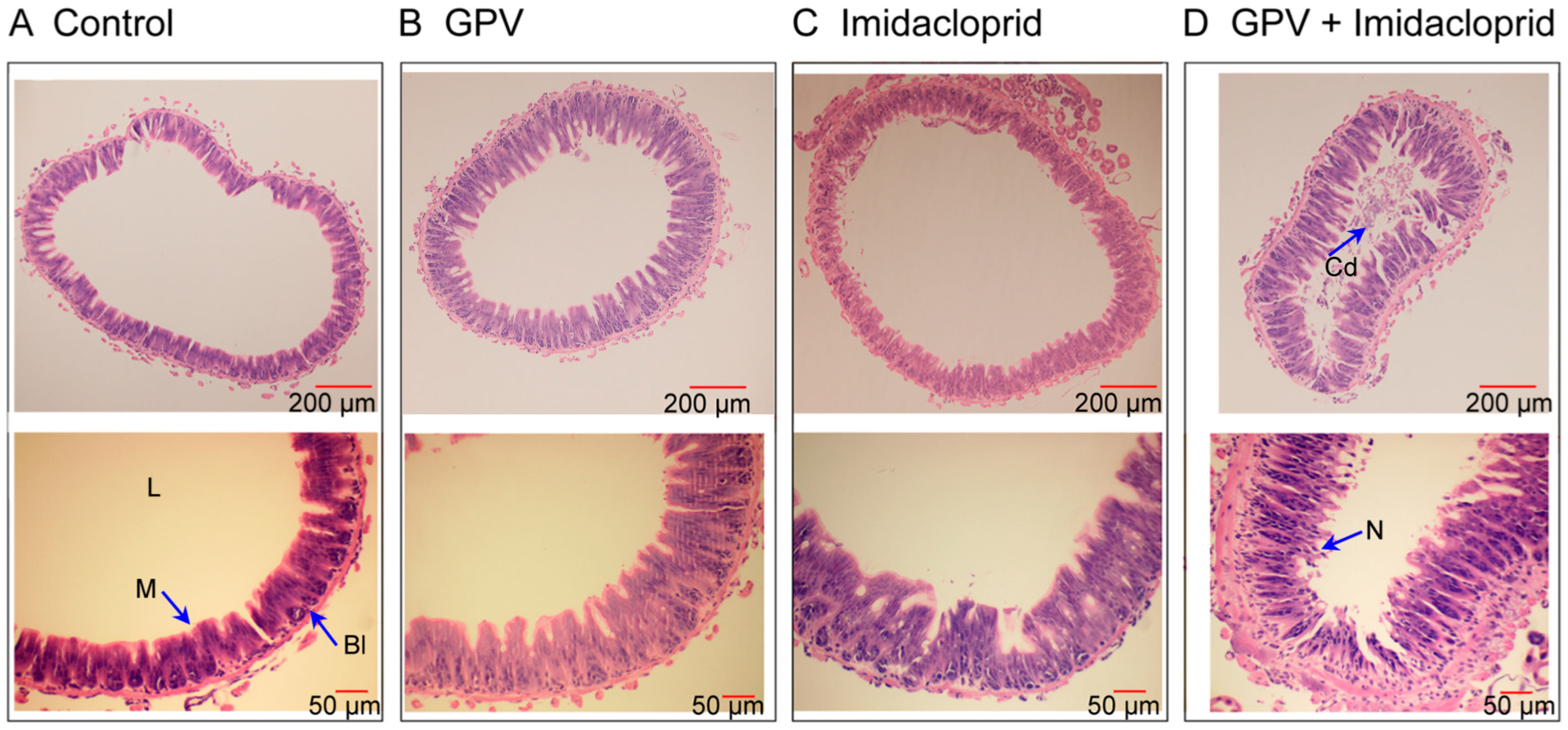
3.2. Combined GPV and Imidacloprid Treatment Induced Synergistic Midgut Epithelial Damage
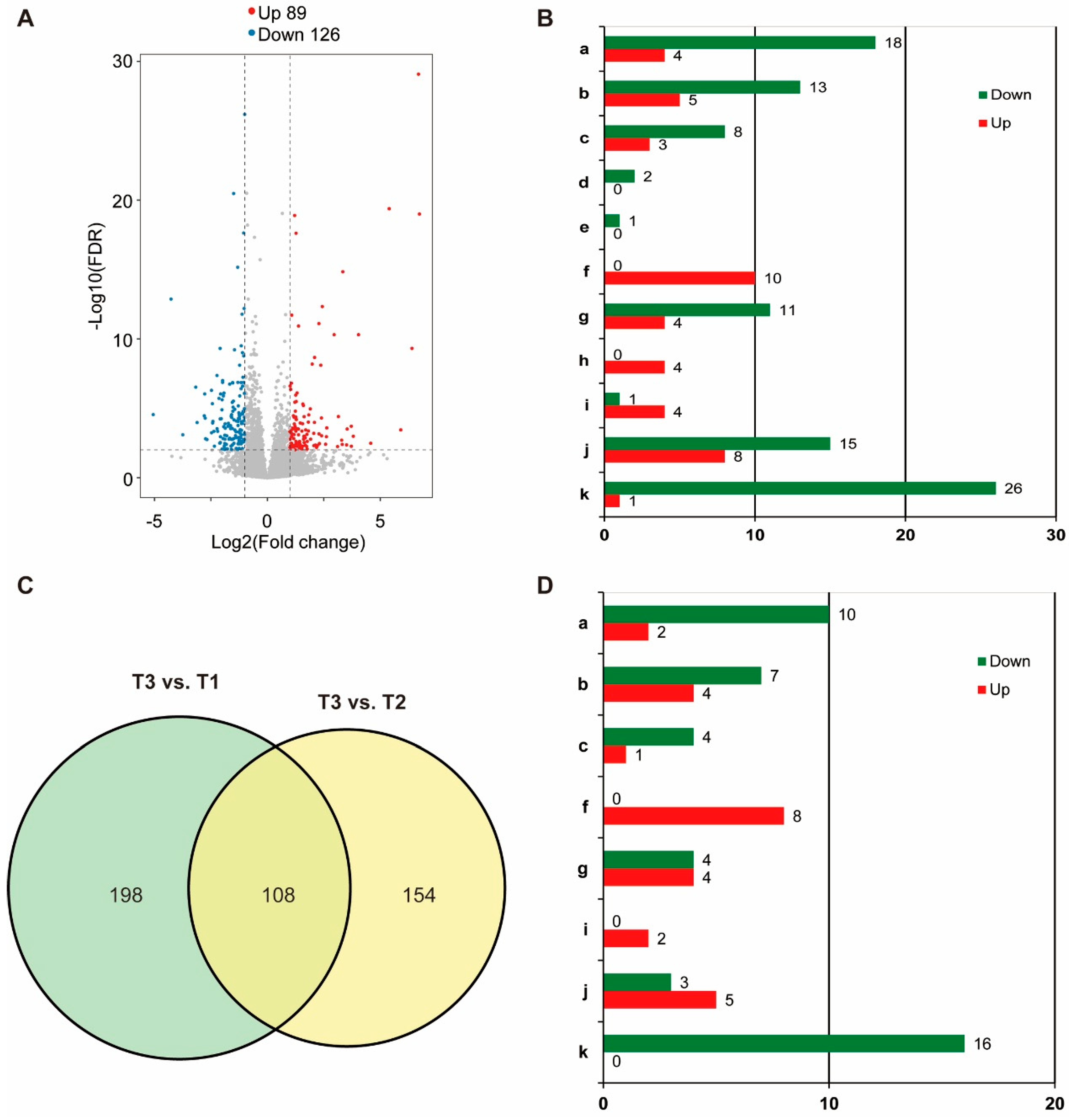
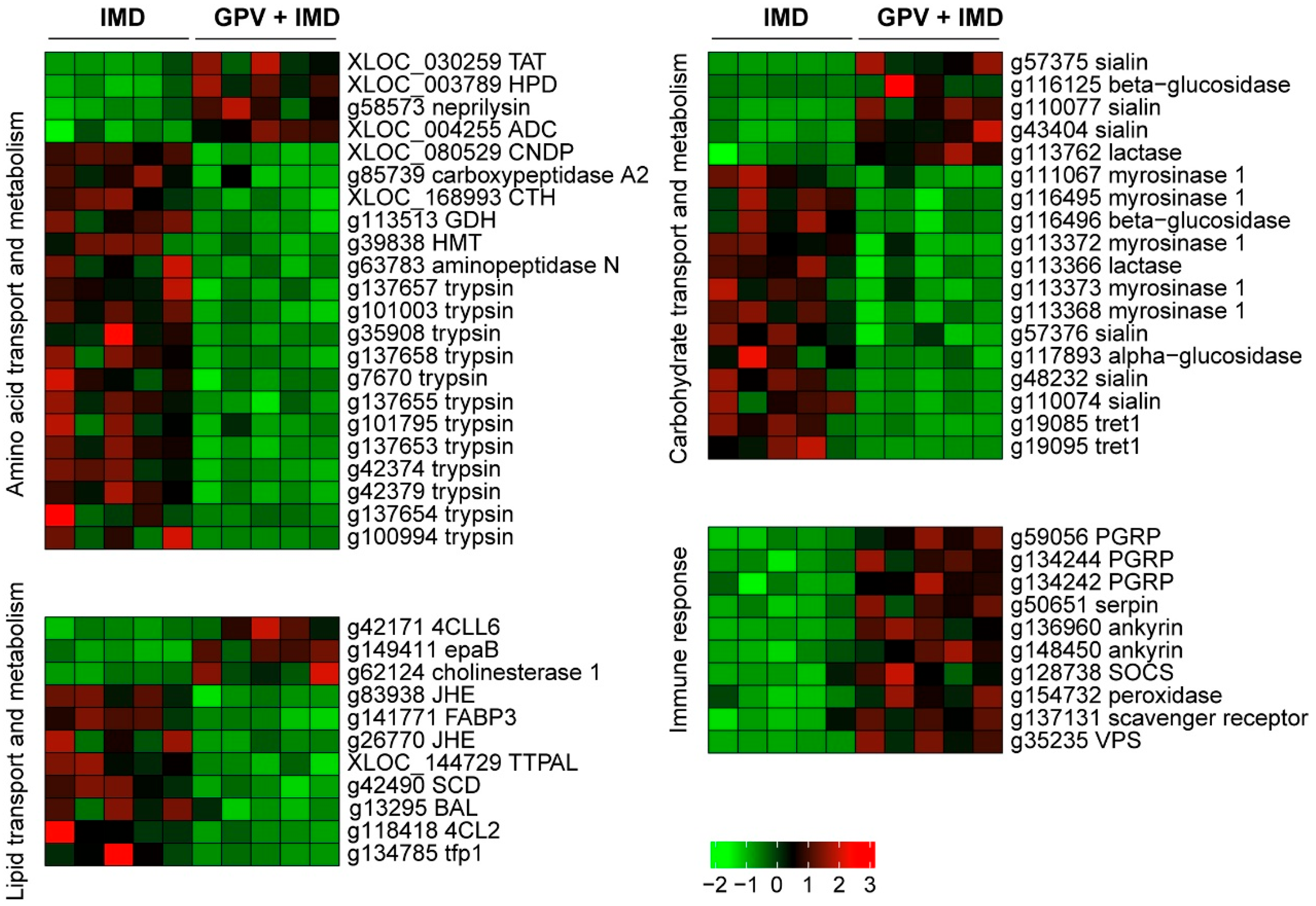
3.3. GPV + Imidacloprid Treatment Induced More Transcriptional Alterations in Nymphs
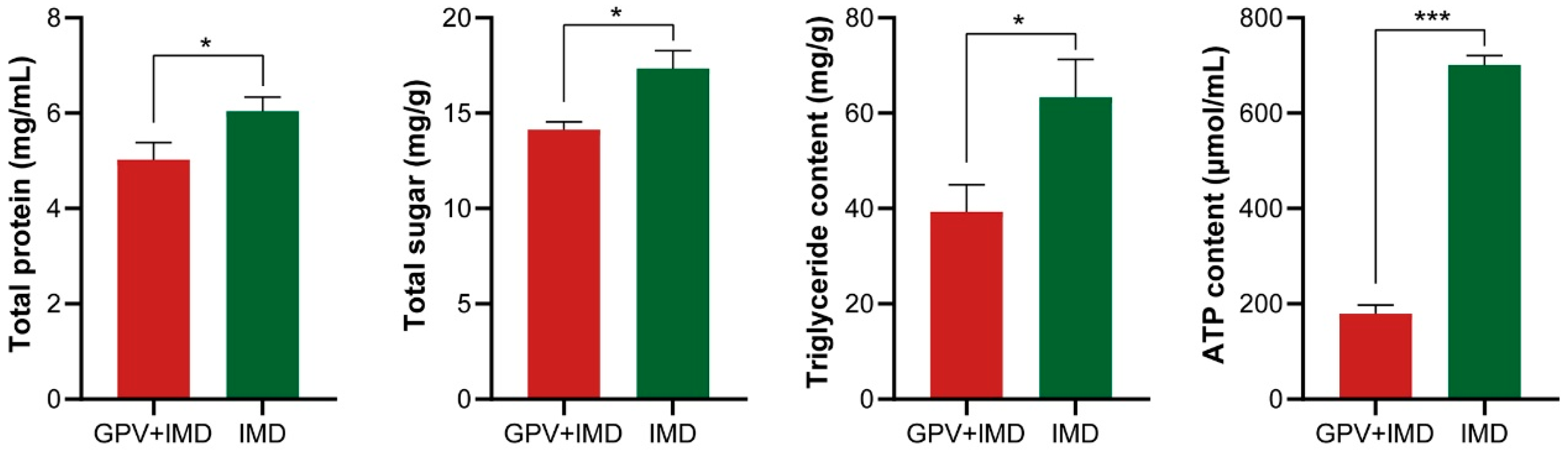
3.4. Imidacloprid + GPV Treatment Significantly Impairs Nymph Energy Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Yu, Q.; Chen, D.; Wei, J.; Yang, P.; Yu, J.; Wang, X.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.; Kraft, M.; Wilknitz, P.; Nowotny, M.; Schöneich, S. Flupyradifurone, imidacloprid and clothianidin disrupt the auditory processing in the locust CNS. J. Comp. Physiol. A 2025, 211, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Hua, M.; Jiang, M.; Jiang, C.; Xi, Y.; Deng, J.; Xu, H.; Zeng, B.; Zhou, S. Transgenic expression of mAChR-C dsRNA in maize confers efficient locust control. Plant Commun. 2025, 6, 101316. [Google Scholar] [CrossRef] [PubMed]
- Githae, E.W.; Kuria, E.K. Biological control of desert locust (Schistocerca gregaria Forskål). CABI Rev. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2021, 16, 13. [Google Scholar] [CrossRef]
- Gebregiorgis, D.; Asrat, A.; Birhane, E.; Tiwari, C.; Kiage, L.M.; Ramisetty-Mikler, S.; Kallam, S.; Kabengi, N.; Gebrekirstos, A.; Wanjiru, S.; et al. Critical gaps in the global fight against locust outbreaks and addressing emerging challenges. npj Sustain. Agric. 2025, 3, 29. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Yang, P.; Jiang, X.; Jiang, F.; Zhao, D.; Li, B.; Cui, F.; Wei, J.; Ma, C.; et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 2014, 5, 2957. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Lin, Y.; Li, X.; Liu, M.; Hafeez, M.; Huang, J.; Zhang, Z.; Chen, L.; Ren, X.; et al. Spodoptera exigua multiple nucleopolyhedrovirus increases the susceptibility to insecticides: A promising efficient way for pest resistance management. Biology 2023, 12, 260. [Google Scholar] [CrossRef]
- Lomer, C.; Bateman, R.; Johnson, D.; Langewald, J.; Thomas, M. Biological control of locusts and grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and grasshopper management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Dáder, B.; Aguirre, E.; Caballero, P.; Medina, P. Synergy of lepidopteran nucleopolyhedroviruses AcMNPV and SpliNPV with insecticides. Insects 2020, 11, 316. [Google Scholar] [CrossRef]
- Nagloo, N.; Rigosi, E.; Herbertsson, L.; O’Carroll, D.C. Comparability of comparative toxicity: Insect sensitivity to imidacloprid reveals huge variations across species but also within species. Proc. R. Soc. B Biol. Sci. 2024, 291, 20232811. [Google Scholar] [CrossRef]
- Du, Q.; Gao, F.; Cui, B.; Wang, T.; Chen, F.; Zeng, Z.; Sun, C.; Zhou, X.; Cui, H. Improving the stability, foliar utilization and biological activity of imidacloprid delivery systems: Size effect of nanoparticles. Environ. Res. 2024, 257, 119386. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Y.; Zhang, Y.; Wu, Y.; Wilson, K.; Xu, P. Native mid-gut bacterial community increases resistance to nucleopolyhedrovirus in the cotton leafworm. Pestic. Biochem. Physiol. 2025, 212, 106462. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, J.; Lin, X.; Shi, W.; Cao, C. Identification of diverse viruses associated with grasshoppers unveils the parallel relationship between host phylogeny and virome composition. Virus Evol. 2022, 8, veac057. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, J.; Wang, Z.; Tian, J.; Shi, W.; Cao, C. Characterization of a novel pathogenic reovirus in grasshoppers. Viruses 2022, 14, 2810. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Tan, S.; Guan, M.; Lin, X.; Shen, J.; Shi, W.; Wang, D. Nanocarrier-mediated transdermal delivery of Lmidgf4 dsRNA expedites biological control of locusts by Beauveria bassiana. J. Nanobiotechnol. 2025, 23, 272. [Google Scholar] [CrossRef]
- Illa-Bochaca, I.; Montuenga, L.M. The regenerative nidi of the locust midgut as a model to study epithelial cell differentiation from stem cells. J. Exp. Biol. 2006, 209, 2215–2223. [Google Scholar] [CrossRef]
- Li, X.; Mank, J.E.; Ban, L. The grasshopper genome reveals long-term gene content conservation of the X Chromosome and temporal variation in X Chromosome evolution. Genome Res. 2024, 34, 997–1007. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, K.; Zhao, X.; Cao, C.; Dong, X.; Liang, J.; Shi, W. Bt Cry1Ab/2Ab toxins disrupt the structure of the gut bacterial community of Locusta migratoria through host immune responses. Ecotoxicol. Environ. Saf. 2022, 238, 113602. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Ji, C.; Li, J.; Yin, M.; Shen, J.; Yan, S. HLDP nano-assembly boosts monosultap insecticidal activity against Asian corn borers through enhanced neurotoxicity and energy depletion. Pestic. Biochem. Physiol. 2025, 213, 106562. [Google Scholar] [CrossRef]
- García-Munguía, A.M.; García-Munguía, C.A.; Guerra-Ávila, P.L.; Sánchez-Mendoza, E.A.; Rubalcava-Castillo, F.A.; García-Munguía, A.; Robles-López, M.R.; Cisneros-Guzmán, L.F.; Martínez-Alba, M.G.; Olvera-Gonzalez, E.; et al. Baculovirus-based biocontrol: Synergistic and antagonistic interactions of PxGV, PxNPV, SeMNPV, and SfMNPV in integrative pest management. Viruses 2025, 17, 1077. [Google Scholar] [CrossRef]
- Wang, J.Y.; Fan, N.N.; Yuan, Y.; Bass, C.; Siemann, E.; Ji, X.Y.; Jiang, J.X.; Wan, N.F. Plant defense metabolites influence the interaction between an insect herbivore and an entomovirus. Curr. Biol. 2024, 34, 5758–5768.e5. [Google Scholar] [CrossRef]
- Kang, Y.; Guo, J.; Wu, T.; Han, B.; Liu, F.; Chu, Y.; Wang, Q.; Gao, J.; Dai, P. Insecticide and pathogens co-exposure induces histomorphology changes in midgut and energy metabolism disorders on Apis mellifera. Pestic. Biochem. Physiol. 2025, 211, 106414. [Google Scholar] [CrossRef]
- Huang, D.; Qi, H.; Liu, H.; Yuan, F.; Yang, C.; Liu, T. Two birds with one stone: Eco-friendly nano-formulation endows a commercial fungicide with excellent insecticidal activity. Adv. Funct. Mater. 2025, 35, 2420401. [Google Scholar] [CrossRef]
- Huang, X.; Ni, X.; Li, H.; Wei, Y.; Wang, Z.; Zhen, C.A.; Yin, M.; Shen, J.; Shi, W.; Zhang, Y.; et al. Synergistic mechanism of botanical pesticide camptothecin encapsulated in a nanocarrier against fall armyworm: Enhanced stability and amplified growth suppression. Ecotoxicol. Environ. Saf. 2024, 284, 116900. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Dias, R.O.; Oliveira, P.L.; Ferreira, C.; Venancio, T.M. Transcriptomic analyses uncover emerging roles of mucins, lysosome/secretory addressing and detoxification pathways in insect midguts. Curr. Opin. Insect Sci. 2018, 29, 34–40. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Qin, Q.; Chen, L.; Dang, X.; Ma, Z.; Zhou, Z. Imidacloprid disrupts larval molting regulation and nutrient energy metabolism, causing developmental delay in honey bee Apis mellifera. eLife 2024, 12, RP88772. [Google Scholar] [CrossRef]
- Gao, Z.; Rensing, C.; Wang, J.; Shen, C.; Elzaki, M.E.A.; Li, X.; Tan, J.; Jiang, X. Increased and synergistic RNAi delivery using MOF polydopamine nanoparticles for biopesticide applications. Nat. Commun. 2025, 16, 6384. [Google Scholar] [CrossRef]
- Li, Z.; Blissard, G. The vacuolar protein sorting genes in insects: A comparative genome view. Insect Biochem. Mol. Biol. 2015, 62, 211–225. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Vosshall, L.B. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 2007, 450, 289–293. [Google Scholar] [CrossRef]
- Yan, S.; Yin, H.; Li, N.; Chen, Y.; Ji, C.; Jiang, Q.; Du, J.; Yin, M.; Shen, J.; Zhang, J. Combination of a nanocarrier delivery system with genetic manipulation further improves pesticide efficiency: A case study with chlorfenapyr. Environ. Sci. Nano 2022, 9, 2020–2031. [Google Scholar] [CrossRef]
- Contreras, A.; Jones, M.K.; Eldon, E.D.; Klig, L.S. Inositol in disease and development: Roles of catabolism via myo-inositol oxygenase in drosophila melanogaster. Int. J. Mol. Sci. 2023, 24, 4185. [Google Scholar] [CrossRef]
- Huo, C.; Liu, S.; Chang, B.H.; Cheng, Z.; Zhang, Y.; Liu, W.; Zhang, J.; Zhao, X. Zinc finger protein rotund is essential for wings and ovarian development by regulating lipid homeostasis in Locusta migratoria. Int. J. Biol. Macromol. 2025, 286, 138448. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, P.C.; Wang, J.X.; Zhao, X.F. A BTB domain-containing gene is upregulated by immune challenge. Arch. Insect Biochem. Physiol. 2011, 77, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, N.; Guo, Y.; Chen, Y.; Ji, C.; Yin, M.; Shen, J.; Zhang, J. Chronic exposure to the star polycation (SPc) nanocarrier in the larval stage adversely impairs life history traits in Drosophila melanogaster. J. Nanobiotechnol. 2022, 20, 515. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, X.; Zhou, Y.; Liu, B.; Zheng, W.; Li, R.; Wang, J.; Yu, J. The analysis of large-scale gene expression correlated to the phase changes of the migratory locust. Proc. Natl. Acad. Sci. USA 2004, 101, 17611–17615. [Google Scholar] [CrossRef]
- Vieira, J.; Freitas, F.C.P.; Cristino, A.S.; Moda, L.M.R.; Martins, J.R.; Bitondi, M.M.G.; Simões, Z.L.P.; Barchuk, A.R. miRNA-34 and miRNA-210 target hexamerin genes enhancing their differential expression during early brain development of honeybee (Apis mellifera) castes. Insect Mol. Biol. 2021, 30, 594–604. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.; Wang, Y.; Zhou, Z.; Wan, F.; Guo, J. Induced thermotolerance and expression of three key Hsp genes (Hsp70, Hsp21, and sHsp21) and their roles in the high temperature tolerance of Agasicles hygrophila. Front. Physiol. 2020, 10, 1593. [Google Scholar] [CrossRef]
- Ferdous, Z.; Fuchs, S.; Behrends, V.; Trasanidis, N.; Waterhouse, R.M.; Vlachou, D.; Christophides, G.K. Anopheles coluzzii stearoyl-CoA desaturase is essential for adult female survival and reproduction upon blood feeding. PLoS Pathog. 2021, 17, e1009486. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Shin, S.W.; Jeon, J.H.; Kim, J.A.; Park, D.S.; Shin, Y.J.; Oh, H.W. Inducible expression of several Drosophila melanogaster genes encoding juvenile hormone binding proteins by a plant diterpene secondary metabolite, methyl lucidone. Insects 2022, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Rigden, D.J.; Ebrahimi, B.; Turner, P.C.; Rees, H.H. Regulation of ecdysteroid signalling during Drosophila development: Identification, characterization and modelling of ecdysone oxidase, an enzyme involved in control of ligand concentration. Biochem. J. 2005, 389, 637–645. [Google Scholar] [CrossRef]
- Kshatriya, K.; Gershenzon, J. Disarming the defenses: Insect detoxification of plant defense-related specialized metabolites. Curr. Opin. Plant Biol. 2024, 81, 102577. [Google Scholar] [CrossRef]
- Scanlan, J.L.; Robin, C. Genetic characterization of candidate ecdysteroid kinases in Drosophila melanogaster. G3 Genes Genomes Genet. 2024, 14, jkae204. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Dong, H.; Lu, T.; Deng, Y.; Li, Z.; Hu, B.; Tan, A. SV2B is a crucial factor for early larval development in the silkworm, Bombyx mori. Insect Sci. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Liu, K.; Dong, Y.; Huang, Y.; Rasgon, J.L.; Agre, P. Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc. Natl. Acad. Sci. USA 2013, 110, 17504–17509. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ding, Z.; Chen, X.; Yang, W.; Dong, J.; Xu, Y.; Wang, Z.; Cao, C.; Shi, W.; Huang, X. Synergistically Better than One: Co-Application of Grasshopper-Derived +ssRNA Virus and Imidacloprid Induces Acute Toxicity in Locusta migratoria. Agriculture 2025, 15, 2425. https://doi.org/10.3390/agriculture15232425
Li S, Ding Z, Chen X, Yang W, Dong J, Xu Y, Wang Z, Cao C, Shi W, Huang X. Synergistically Better than One: Co-Application of Grasshopper-Derived +ssRNA Virus and Imidacloprid Induces Acute Toxicity in Locusta migratoria. Agriculture. 2025; 15(23):2425. https://doi.org/10.3390/agriculture15232425
Chicago/Turabian StyleLi, Sisi, Zehui Ding, Xinxin Chen, Weiyue Yang, Jianxin Dong, Yao Xu, Zhen Wang, Chuan Cao, Wangpeng Shi, and Xinzheng Huang. 2025. "Synergistically Better than One: Co-Application of Grasshopper-Derived +ssRNA Virus and Imidacloprid Induces Acute Toxicity in Locusta migratoria" Agriculture 15, no. 23: 2425. https://doi.org/10.3390/agriculture15232425
APA StyleLi, S., Ding, Z., Chen, X., Yang, W., Dong, J., Xu, Y., Wang, Z., Cao, C., Shi, W., & Huang, X. (2025). Synergistically Better than One: Co-Application of Grasshopper-Derived +ssRNA Virus and Imidacloprid Induces Acute Toxicity in Locusta migratoria. Agriculture, 15(23), 2425. https://doi.org/10.3390/agriculture15232425





