A Novel Laboratory Protocol for Pollen Viability Assessment to Inform Biosafety Evaluation of Transgenic Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Pollen Collection
2.2. Experimental Materials
2.3. Preparation and Optimization of Culture Media
2.4. Temperature Treatment of Pollen
2.5. Pollen In Vitro Treatment
2.6. Pollen Germination and Data Analysis
2.7. Microscopic Examination of Pollen Morphology
3. Results
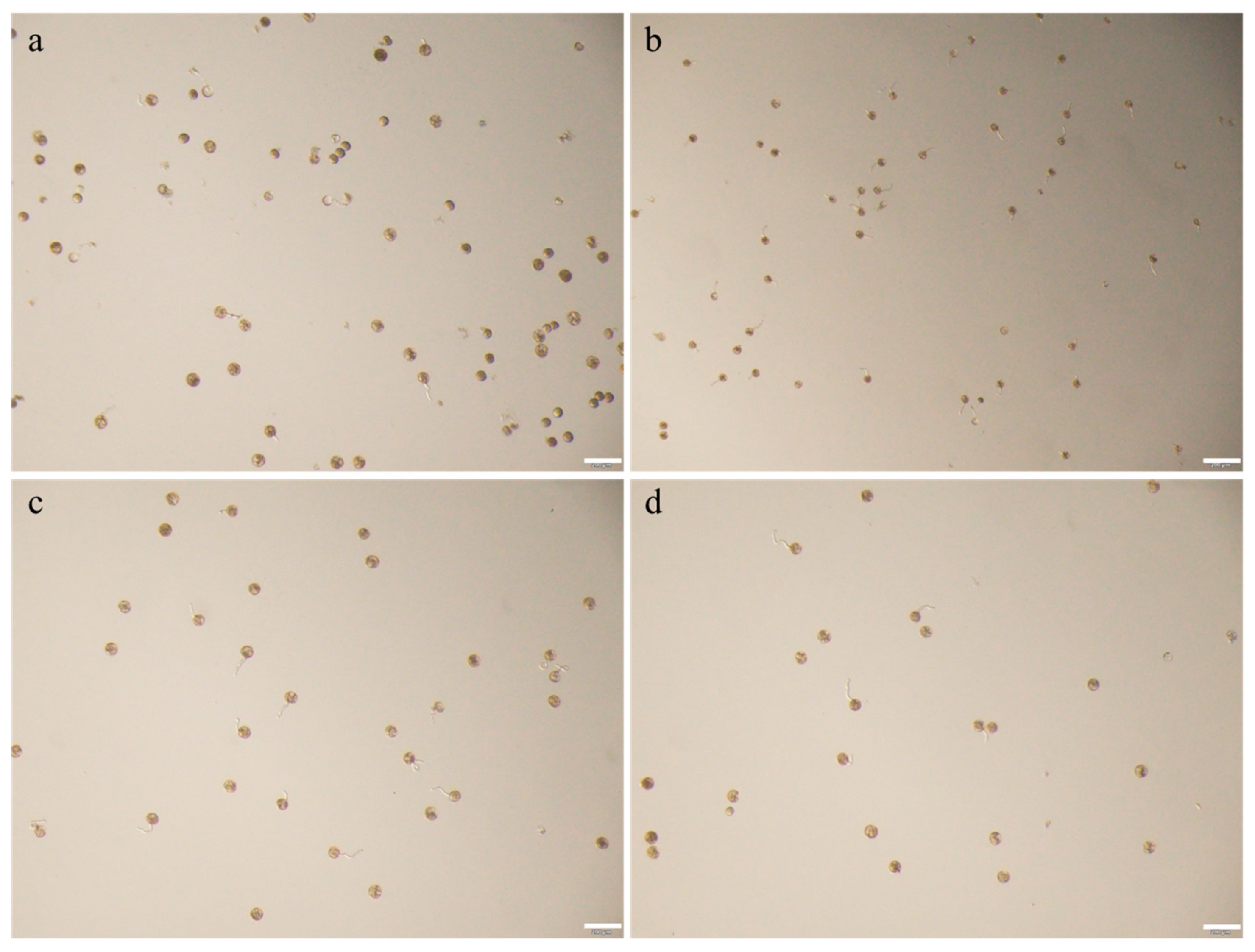
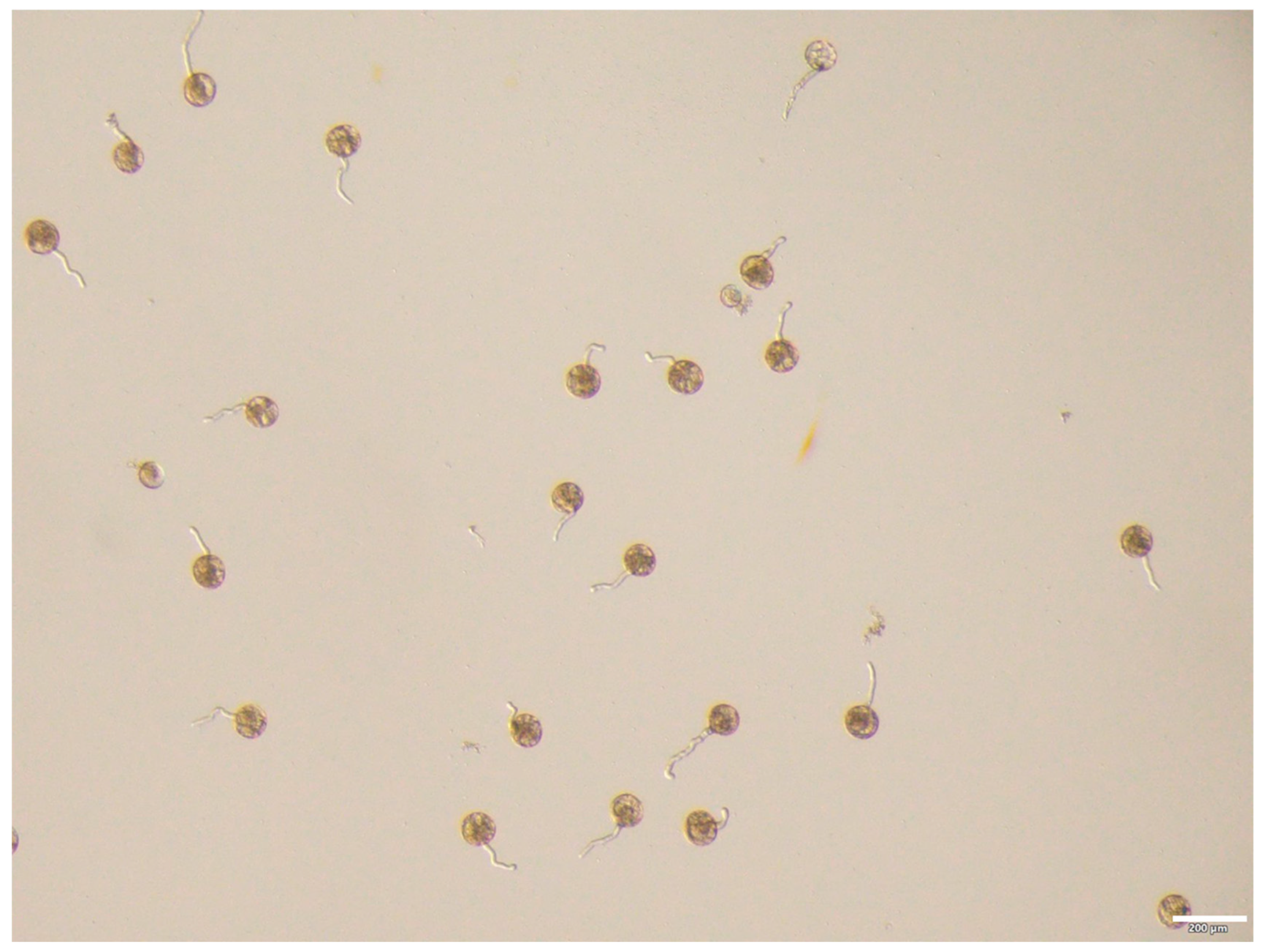
3.1. Microscopic Observation of Pollen Morphology
3.2. Screening of the Initial Medium
3.3. Further Optimization of Pollen Germination Medium
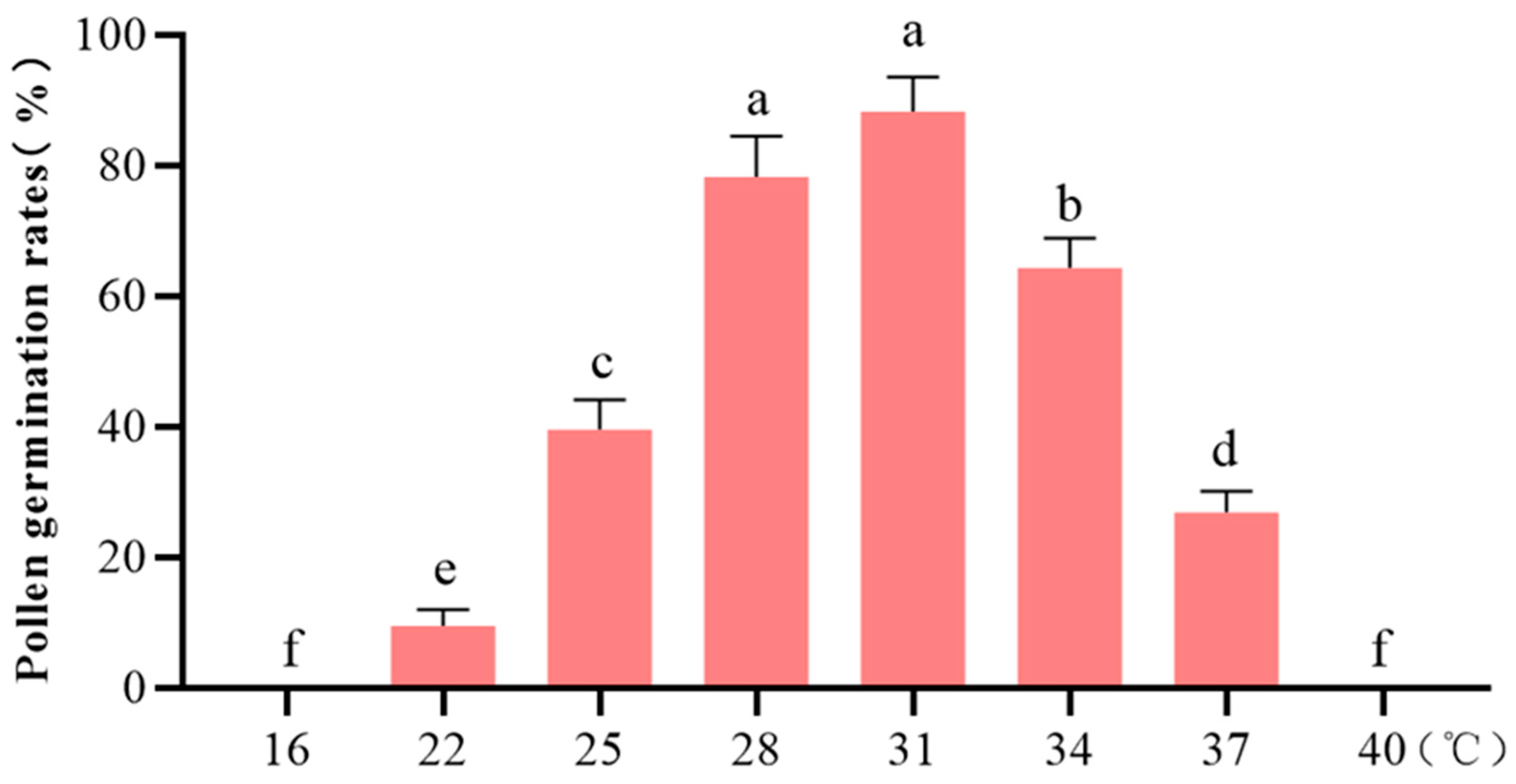
3.4. Determination of Pollen Culture Temperature
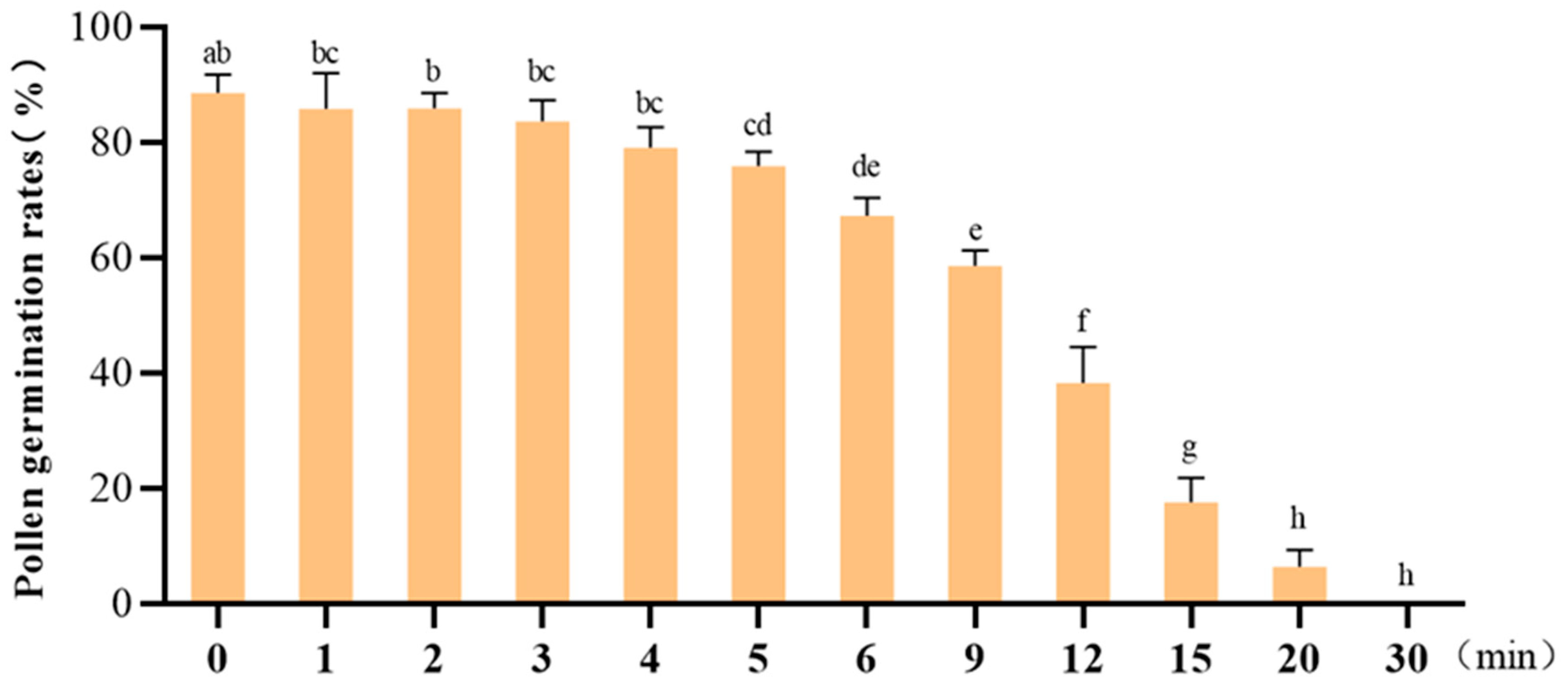
3.5. Determination of Post-Excision Time for Pollen
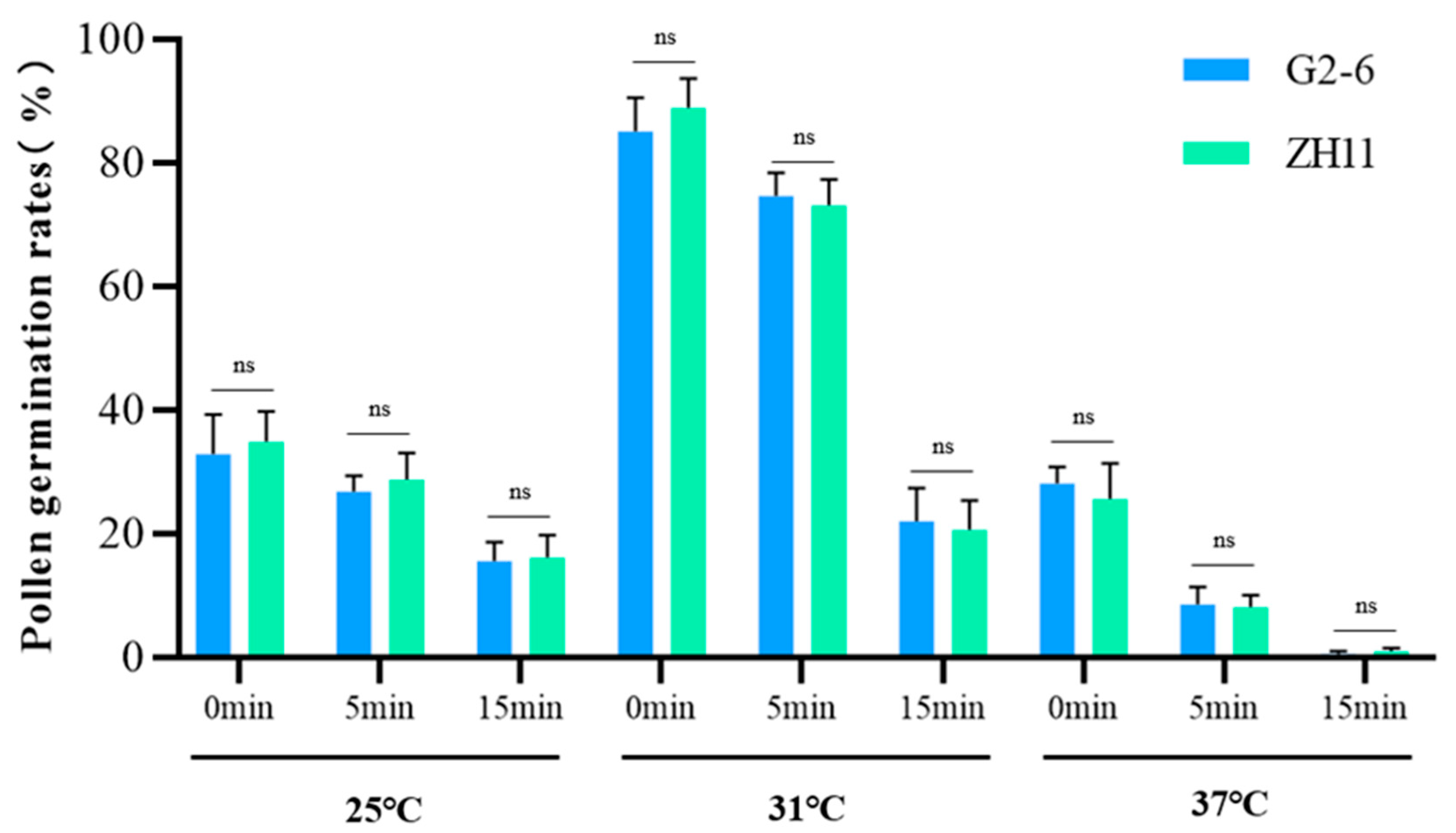
3.6. The Transgenic Event in G2-6 Did Not Affect Pollen Viability Relative to ZH11 Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| H3BO3 | Boric acid |
| CaCl2 | Directory of open access journals |
| KH2PO4 | Monopotassium phosphate |
| MgSO4 | Magnesium sulfate |
| GM | Genetically modified |
| VB1 | Vitamin B1 |
Appendix A

References
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Grafton, R.Q.; Williams, J.; Jiang, Q. Food and water gaps to 2050: Preliminary results from the global food and water system (GFWS) platform. Food Secur. 2015, 7, 209–220. [Google Scholar] [CrossRef]
- Hu, H.H.; Dai, M.Q.; Yao, J.L.; Xiao, B.Z.; Li, X.H.; Zhang, Q.F.; Xiong, L.Z. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef]
- Shepherd, D.N.; Mangwende, T.; Martin, D.P.; Bezuidenhout, M.; Kloppers, F.J.; Carolissen, C.H.; Monjane, A.L.; Rybicki, E.P.; Thomson, J.A. Maize streak virus-resistant transgenic maize: A first for Africa. Plant Biotechnol. J. 2007, 5, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Li, P.C.; Li, X.Y.; Wen, N.; Wang, Y.X.; Lu, W.; Lin, M.; Lang, Z.H. In maize, co-expression of GAT and GR79-EPSPS provides high glyphosate resistance, along with low glyphosate residues. Abiotech 2023, 4, 277–290. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops: 2019—ISAAA Brief 55-2019; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Lubieniechi, S.A.; Van Eenennaam, A.L.; Smyth, S.J. Regulation of animal and plant agricultural biotechnology. Trends Biotechnol. 2025, 43, 511–521. [Google Scholar] [CrossRef]
- Ludlow, K.; Falck-Zepeda, J.; Smyth, S.J. Risk-appropriate, science-based innovation regulations are important. Trends Biotechnol. 2025, 43, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, K.L.; Yao, Y. China’s regulatory change toward genome-edited crops. Trends Biotechnol. 2024, 42, 801–806. [Google Scholar] [CrossRef]
- Jank, B.; Gaugitsch, H. Assessing the environmental impacts of transgenic plants. Trends Biotechnol. 2001, 19, 371–372. [Google Scholar] [CrossRef]
- Lu, B.R.; Yang, C. Gene flow from genetically modified rice to its wild relatives: Assessing potential ecological consequences. Biotechnol. Adv. 2009, 27, 1083–1091. [Google Scholar] [CrossRef]
- Chandler, S.; Dunwell, J.M. Gene flow, risk assessment and the environmental release of transgenic plants. Crit. Rev. Plant Sci. 2008, 27, 25–49. [Google Scholar] [CrossRef]
- Noack, F.; Engist, D.; Gantois, J.; Gaur, V.; Hyjazie, B.F.; Larsen, A.; M’Gonigle, L.K.; Missirian, A.; Qaim, M.; Sargent, R.D.; et al. Environmental impacts of genetically modified crops. Science 2024, 385, eado9340. [Google Scholar] [CrossRef]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.K.; Deng, Y.T.; Tang, F.; Zhao, L.K.; Zhao, L.X.; Wang, Y.; Dai, X.B.; Zhou, Z.L.; Cao, Q.H. Screening and optimisation of in vitro pollen germination medium for sweetpotato (Ipomoea batatas). Plant Methods 2023, 19, 93. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T.J. The estimation of pollen viability in rice. J. Exp. Bot. 1995, 46, 151–154. [Google Scholar] [CrossRef]
- Gealy, D.R.; Mitten, D.H.; Rutger, J.N. Gene flow between red rice (Oryza sativa) and herbicide-resistant rice (O-sativa): Implications for weed management. Weed Technol. 2003, 17, 627–645. [Google Scholar] [CrossRef]
- Dong, Y.F.; Jin, X.; Tang, Q.L.; Zhang, X.; Yang, J.T.; Liu, X.J.; Cai, J.F.; Zhang, X.B.; Wang, X.J.; Wang, Z.X. Development and Event-specific Detection of Transgenic Glyphosate-resistant Rice Expressing the G2-EPSPS Gene. Front. Plant Sci. 2017, 8, 885. [Google Scholar] [CrossRef]
- Jia, W.Q.; Wang, Y.L.; Mi, Z.R.; Wang, Z.; He, S.L.; Kong, D.Z. Optimization of culture medium for in vitro germination and storage conditions of Exochorda racemosa pollen. Front. Plant Sci. 2022, 13, 994214. [Google Scholar] [CrossRef]
- Castillo, S.E.; Tovar, J.C.; Shamin, A.; Gutirerrez, J.; Pearson, P.; Gehan, M.A. A protocol for Chenopodium quinoa pollen germination. Plant Methods 2022, 18, 65. [Google Scholar] [CrossRef]
- Wu, Y.L.; Gao, W.Y.; Zhou, Y.Z.; Guo, H.Y. Optimization of In Vitro Germination and storage of Armeniaca sibirica Pollen. Sci. Hortic. 2022, 304, 111309. [Google Scholar] [CrossRef]
- Announcement No. 423 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: https://www.moa.gov.cn/govpublic/ncpzlaq/202107/t20210714_6371842.htm (accessed on 26 October 2025).
- Song, Z.P.; Lu, B.R.; Zhu, Y.G.; Chen, J.K. Gene flow from cultivated rice to the wild species Oryza rufipogon under experimental field conditions. N. Phytol. 2003, 157, 657–665. [Google Scholar] [CrossRef]
- Wang, S.H.; Chen, F.; Zhou, K.D. In vitro germination of rice pollen. Crop Sci. 2000, 5, 609–612. (In Chinese) [Google Scholar]
- Tian, C.; Lu, H.; Wang, F.; Zhang, C.Y.; Lin, J.X. Effects of medium components on in vitro pollen germination and pollen tube growth of Picea wilsonii Mast. J. Beijing For. Univ. 2007, 29, 47–52. [Google Scholar]
- Kwack, B.H. Studies on cellular site of calcium action in promoting pollen growth. Physiol. Plant. 1967, 20, 825–833. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lu, L.D.; Wu, X.Q.; Li, Y.Q.; Lin, J.X. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Shipley, A.M.; Rivers, B.A.; Cresti, M.; Hepler, P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 1994, 6, 1815–1828. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Z.; Liu, M.X.; Xie, Z.W.; Wang, H.H. Pollen Grain Germination and Pollen Tube Growth in Pistil of Rice. Rice Sci. 2008, 15, 125–130. [Google Scholar] [CrossRef]
- Ye, L.; Lv, D.; Jian, M.; Tian, H. Isolation of sperm cells of Allium tuberosum Roxb. J. Mol. Cell Biol. 2008, 41, 323–328. [Google Scholar]
- Zhao, R.; Hu, X.; Yuan, D.Y.; Masabni, J.; Xiong, H.; Zou, F. Orthogonal test design for optimizing culture medium for in vitro pollen germination of interspecific oil tea hybrids. An. Acad. Bras. Cienc. 2021, 93, e20190431. [Google Scholar] [CrossRef]
- Moor, A. The effect of magnesium sulphate (MgSO4) on capsicum pollen quality: A magneziumszulfat (MgSO4) hatasa a paprikapollen ninosegere. Zoldsegtermesztesi Kut. Intez. Bull. 1993, 25, 71–74. [Google Scholar]
- Brunet, J.; Ziobro, R.; Osvatic, J.; Clayton, M.K. The effects of time, temperature and plant variety on pollen viability and its implications for gene flow risk. Plant Biol. 2019, 21, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Boavida, L.C.; McCormick, S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007, 52, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Kakani, V.G.; Reddy, K.R.; Koti, S.; Wallace, T.P.; Prasad, P.V.V.; Reddy, V.R.; Zhao, D. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann. Bot. 2005, 96, 59–67. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Sucrose (g/L) | H3BO3 (mg/L) | CaCl2 (mg/L) | Germination Rate (%) |
|---|---|---|---|---|
| M1 | 50 | 40 | 20 | 9.27 ± 2.42 d |
| M2 | 100 | 40 | 20 | 29.34 ± 4.18 c |
| M3 | 150 | 40 | 20 | 62.67 ± 6.30 b |
| M4 | 175 | 40 | 20 | 84.72 ± 4.79 a |
| M5 | 200 | 40 | 20 | 58.47 ± 5.13 b |
| M6 | 400 | 40 | 20 | 0 ± 0 e |
| Treatment | Sucrose (g/L) | H3BO3 (mg/L) | CaCl2 (mg/L) | Germination Rate (%) |
|---|---|---|---|---|
| M7 | 175 | 20 | 20 | 63.05 ± 4.38 bc |
| M8 | 175 | 20 | 40 | 72.31 ± 3.08 bc |
| M9 | 175 | 20 | 60 | 65.44 ± 4.45 bc |
| M10 | 175 | 40 | 20 | 85.03 ± 3.70 a |
| M11 | 175 | 40 | 40 | 80.51 ± 2.35 ab |
| M12 | 175 | 40 | 60 | 61.86 ± 4.80 c |
| M13 | 175 | 60 | 20 | 67.35 ± 2.97 bc |
| M14 | 175 | 60 | 40 | 69.74 ± 7.32 bc |
| M15 | 175 | 60 | 60 | 65.13 ± 4.98 bc |
| Treatment | Sucrose (g/L) | H3BO3 (mg/L) | CaCl2 (mg/L) | KH2PO4 (mg/L) | MgSO4 (mg/L) | Germination Rate (%) |
|---|---|---|---|---|---|---|
| M16 | 175 | 40 | 20 | 0 | 0 | 85.45 ± 2.11 ab |
| M17 | 175 | 40 | 20 | 10 | 10 | 90.38 ± 4.31 a |
| M18 | 175 | 40 | 20 | 10 | 20 | 85.64 ± 2.35 ab |
| M19 | 175 | 40 | 20 | 20 | 10 | 83.33 ± 3.03 ab |
| M20 | 175 | 40 | 20 | 20 | 20 | 81.31 ± 3.81 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, C.; Zhang, X.; Dong, Y.; Yang, J.; Wang, D.; Wang, Z.; Wang, X. A Novel Laboratory Protocol for Pollen Viability Assessment to Inform Biosafety Evaluation of Transgenic Rice (Oryza sativa L.). Agriculture 2025, 15, 2420. https://doi.org/10.3390/agriculture15232420
Chen Y, Liu C, Zhang X, Dong Y, Yang J, Wang D, Wang Z, Wang X. A Novel Laboratory Protocol for Pollen Viability Assessment to Inform Biosafety Evaluation of Transgenic Rice (Oryza sativa L.). Agriculture. 2025; 15(23):2420. https://doi.org/10.3390/agriculture15232420
Chicago/Turabian StyleChen, Yuxiao, Caiyue Liu, Xiaochun Zhang, Yufeng Dong, Jiangtao Yang, Dongmei Wang, Zhixing Wang, and Xujing Wang. 2025. "A Novel Laboratory Protocol for Pollen Viability Assessment to Inform Biosafety Evaluation of Transgenic Rice (Oryza sativa L.)" Agriculture 15, no. 23: 2420. https://doi.org/10.3390/agriculture15232420
APA StyleChen, Y., Liu, C., Zhang, X., Dong, Y., Yang, J., Wang, D., Wang, Z., & Wang, X. (2025). A Novel Laboratory Protocol for Pollen Viability Assessment to Inform Biosafety Evaluation of Transgenic Rice (Oryza sativa L.). Agriculture, 15(23), 2420. https://doi.org/10.3390/agriculture15232420






