Ecogeographic Characterization of Potential Tectona grandis L.f. (Teak) Exploitation Areas in Ecuador
Abstract
1. Introduction
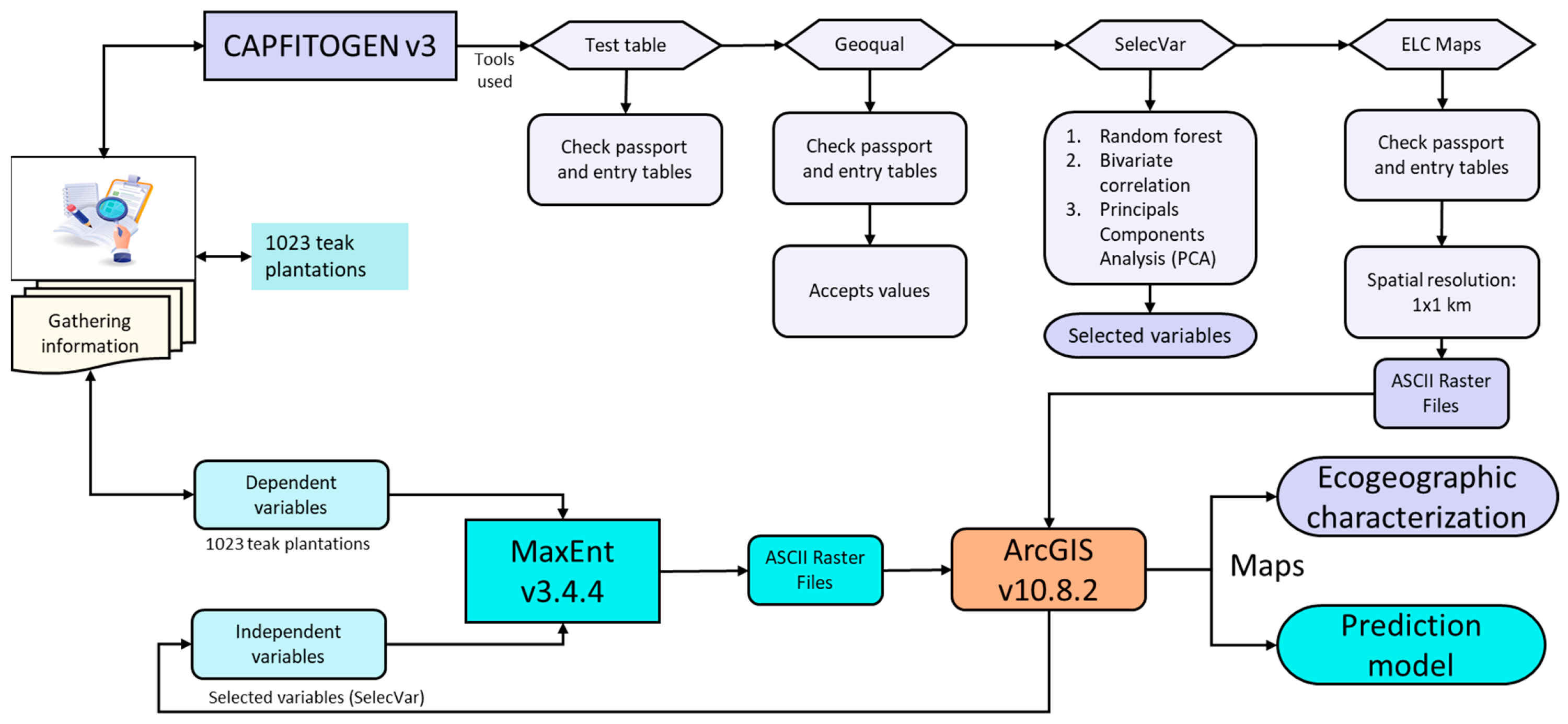
2. Materials and Methods
2.1. Data Sources
2.2. Study Area
2.3. Ecogeographic Characterization
2.4. Predictive Model
3. Results
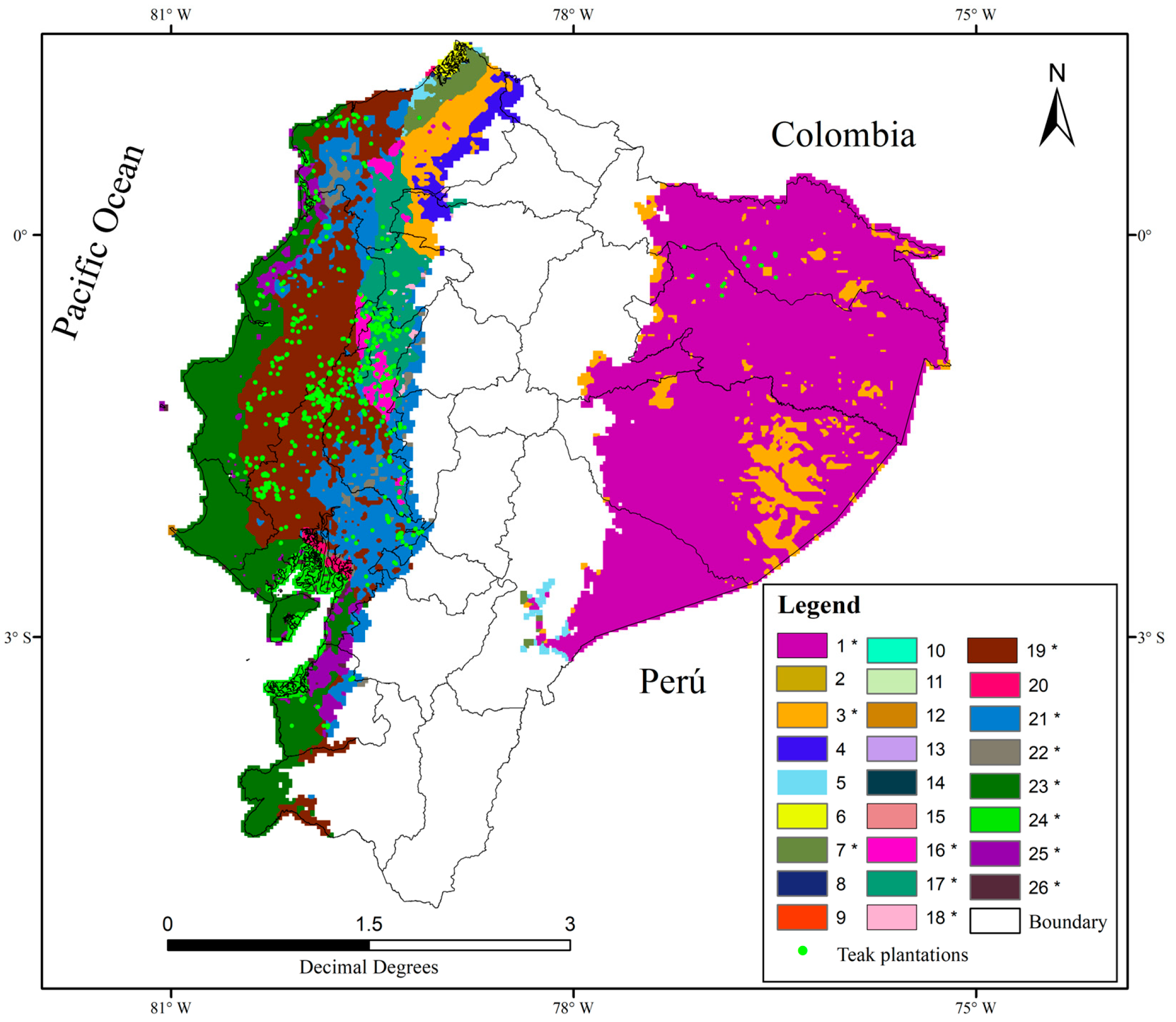
3.1. Ecogeographic Characterization
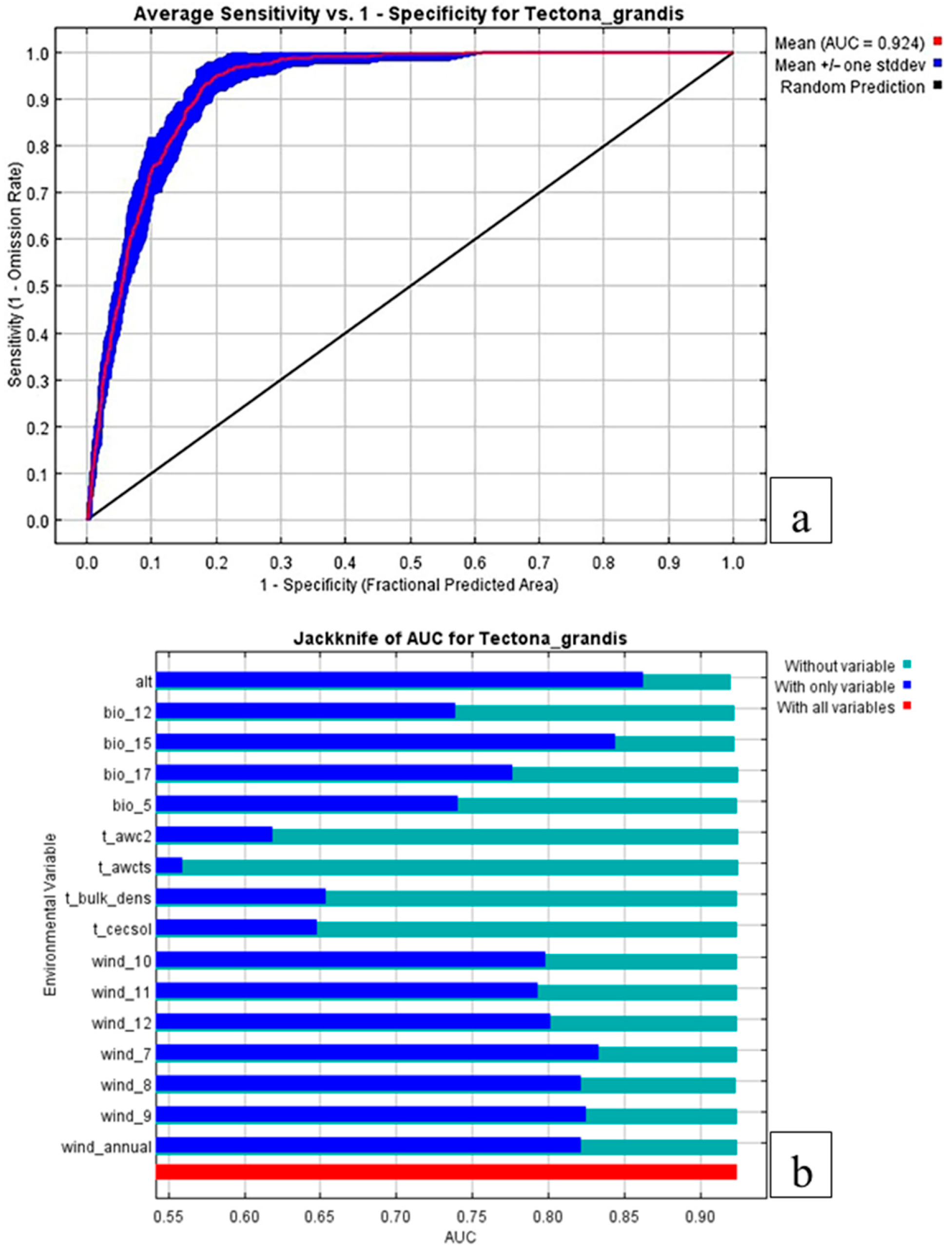
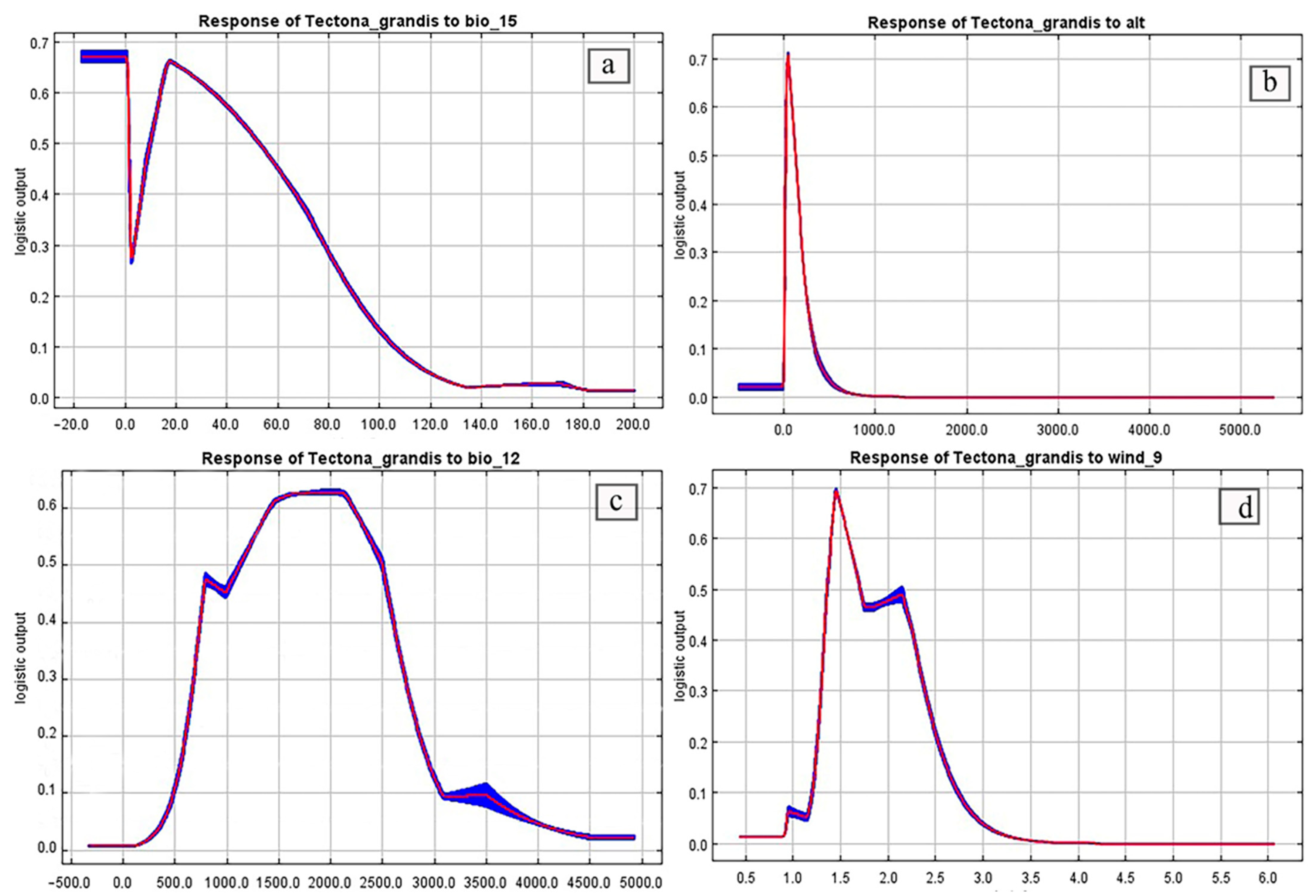
3.2. Predictive Model Validation and Importance of the Variables
3.3. Identification of Potential Areas for Teak Exploitation in Ecuador
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, D.; Brown, C. Teak: A Global Overview; Unasylva-FAO: Rome, Italy, 2000; pp. 3–13. [Google Scholar]
- Kollert, W.; Kleine, M. The Global Teak Study. Analysis, Evaluation and Future Potential of Teak Resources; IUFRO World Series; IUFRO: Vienna, Austria, 2017; p. 108. [Google Scholar]
- Kaosa-ard, A. Teak (Tectona grandis Linn. f.) Its Natural Distribution And Related factors; Teak Seed Centre, Royal Forest Department: Chiang Mai, Thailand, 1989. [Google Scholar]
- Home, J. Teak in Nigeria; Federal Department of Forest Research: Ibadan, Nigeria, 1966.
- Keogh, R.M. Does teak have a future in tropical America? A survey of Tectona grandis in the Caribbean, Central America, Venezuela and Colombia. Unasylva 1979, 31, 13–19. [Google Scholar]
- Kollert, W.; Sandeep, S.; Sreelakshmy, M.; Kokutse, A.; Reis, C.A.; Bedijo, N.G.; Murillo, O.; Thulasidas, P. Global Teak Resources and Market Assessment 2022; IUFRO World Series; IUFRO: Vienna, Austria, 2024; p. 92. [Google Scholar]
- Kollert, W.; Cherubini, L. Teak Resources and Market Assessment 2010; Planted Forests and Trees Working Paper; FP/47/E; FAO of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Jácome Mena, A.; Herrera Ramirez, B.; Belezaca Pinargote, C.E.; Salvatierra Pilozo, D.; Jiménez Romero, E.; Cuasquer Fuel, E.; Veloz Aguirre, L.; Murillo, O. Reservorio Genético de Teca en Ecuador. Proyecto La Mayronga; Universidad Técnica Estatal de Quevedo: Quevedo, Ecuador, 2016. [Google Scholar]
- Ministerio del Ambiente. Acuerdo Ministerial N°065 Expídese el Plan Nacional de Restauración Forestal 2019–2030; Ministerio del Ambiente MAE: Quito, Ecuador, 2019.
- Loayza-León, J.; Manjarrés-Tete, A.E.; Rivera-Ríos, J.R. Gestión financiera basada en la potencialidad de exportación de la industria de teca ecuatoriana, período 2019–2023. Polo Conoc. 2024, 9, 197–221. Available online: https://www.researchgate.net/publication/389943906_Gestion_financiera_basada_en_la_potencialidad_de_exportacion_de_la_industria_de_teca_ecuatoriana_periodo_2019-2023 (accessed on 4 November 2025). [CrossRef]
- Vera, W.A. Análisis Económico de la Producción de Madera Teca (Tectona grandis) en el Cantón El Empalme, Provincia del Guayas. Bachelor’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2014. Available online: https://repositorio.uteq.edu.ec/items/0c4056ea-297b-4f5d-92ee-333104300842 (accessed on 16 May 2025).
- Cañadas, Á.; Andrade-Candell, J.; Domínguez, J.M.; Molina, C.; Schnabel, O.; Vargas-Hernández, J.J.; Wehenkel, C. Growth and yield models for teak planted as living fences in coastal Ecuador. Forests 2018, 9, 55. [Google Scholar] [CrossRef]
- Aguilar, F.J.; Nemmaoui, A.; Peñalver, A.; Rivas, J.R.; Aguilar, M.A. Developing allometric equations for teak plantations located in the coastal region of Ecuador from terrestrial laser scanning data. Forests 2019, 10, 1050. [Google Scholar] [CrossRef]
- Ministerio del Ambiente. Acuerdo 0125 Las Normas para el Manejo Sostenible de los Bosques. Ministerio del Ambiente MAE. 2015. Available online: https://faolex.fao.org/docs/pdf/ecu162523.pdf (accessed on 6 August 2025).
- Tomaselli, I. Country Report Ecuador. 2019. Available online: https://www.itto.int/files/itto_project_db_input/3212/technical/E-4-Ecuador%20Country%20Report.pdf?v=1709520336 (accessed on 24 August 2025).
- Salazar, J.C. Plantaciones de teca en Ecuador. In Las Plantaciones de Teca de América Latina: Mitos y Realidades; Camino, R., Pierre Morales, J., Eds.; CATIE: Turrialba, Costa Rica, 2013. [Google Scholar]
- Sánchez, M.A.; Fernández, A.A.; Illera, P. Los sistemas de información geográfica en la gestión forestal. In TELEDETECCIÓN. Avances y Aplicaciones, Proceedings of the VIII Congreso Nacional de Teledetección, Albacete, España; Asociación Española de Teledetección: Valencia, Spain, 1999; pp. 96–99. [Google Scholar]
- Nieto Masot, A. The didactic use of geographical information systems in the European Higher Education Area. Tejuelo 2010, 9, 136–161. [Google Scholar]
- Tohme, J.M.; Beebe, S.E.; Iwanaga, M. The combined use of agroecological and characterisation data to establish the CIAT Phaseolus vulgaris core collection. In Core Collections of Plant Genetic Resources; Hodgkin, T., Brown, A.D.H., van Hintum, T.J.L., Morales, E.A.V., Eds.; International Plant Genetic Resources Institute IBPGRI; John Wiley and Sons: Chichester, UK, 1995. [Google Scholar]
- Hijmans, R.J.; Spooner, D.M. Geographic distribution of wild potato species. Am. J. Bot. 2001, 88, 2101–2112. [Google Scholar] [CrossRef]
- Bortolon, E.S.O.; Mielniczuk, J.; Tornquist, C.G.; Lopes, F.; Giasson, É.; Bergamaschi, H. Potencial de uso do modelo century e sig para avaliar o impacto da agricultura sobre estoques regionais de carbono orgânico do solo. Rev. Bras. Cienc. Solo 2012, 36, 831–850. [Google Scholar] [CrossRef]
- Holness, S.; Hamer, M.; Brehm, J.M.; Raimondo, D. Priority areas for the in situ conservation of crop wild relatives in South Africa. Plant Genet. Resour. 2019, 17, 115–127. [Google Scholar] [CrossRef]
- Tezel, D.; Inam, S.; Kocaman, S. GIS-based assessment of habitat networks for conservation planning in Kas-Kekova protected area (Turkey). Int. J. Geo-Inf. 2020, 9, 91. [Google Scholar] [CrossRef]
- Brown, M.J.; Walker, B.E.; Black, N.; Govaerts, R.H.; Ondo, I.; Turner, R.; Nic Lughadha, E. rWCVP: A companion R package for the World Checklist of Vascular Plants. New Phytol. 2023, 240, 1355–1365. [Google Scholar] [CrossRef]
- Borja, E. Caracterización Eco-Geográfica de Prunus serotina Ehrh subsp. capuli (Cav.) McVaugh (capulí), en la Región Andina de Ecuador. Master’s Thesis, Universidad de Valencia, Valencia, Spain, 2017. Available online: https://repositorio.iniap.gob.ec/server/api/core/bitstreams/414ca753-6c6e-4ceb-87bc-428811df8528/content (accessed on 2 November 2024).
- Naranjo, E.J.; Tapia Bastidas, C.G.; Velázquez, R.J.; Cruz Pérez, Y.; Delgado Pilla, A.H.; Borja, E.J.; Paredes Andrade, N.J. Caracterización eco-geográfica de Melloco (Ullucus tuberosus C.) en la región Alto Andina del Ecuador. Téc. Rev. Agrocienc. 2018, 1, 31–46. [Google Scholar]
- Monteros-Altamirano, Á.; Tapia, C.; Paredes, N.; Alulema, V.; Tacán, M.; Roura, A.; Lima, L.; Sørensen, M. Morphological and ecogeographic study of the diversity of cassava (Manihot esculenta Crantz) in Ecuador. Agronomy 2021, 11, 1844. [Google Scholar] [CrossRef]
- Chalampuente-Flores, D.; Mosquera-Losada, M.R.; Ron, A.M.D.; Tapia Bastidas, C.; Sørensen, M. Morphological and ecogeographical diversity of the Andean lupine (Lupinus mutabilis Sweet) in the High Andean region of Ecuador. Agronomy 2023, 13, 2064. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Uribe Botero, E. El Cambio Climático y sus Efectos en la Biodiversidad en América Latina; Comisión Económica para América Latina y el Caribe (CEPAL), Unión Europea: Santiago, Chile, 2015. [Google Scholar]
- Cedillo, H.; García-Montero, L.G.; Cabrera, O.; Rocano, M.; Arciniegas, A.; Jadán, O. Modeling the Potential Distribution of Aulonemia queko: Historical, Current, and Future Scenarios in Ecuador and Other Andean Countries. Diversity 2025, 17, 167. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Mas, B.; Riera, S.; Allué, E. Modelling Mediterranean oak palaeolandscapes using the MaxEnt model algorithm: The case of the NE Iberia under the Middle Holocene climatic scenario. Ecol. Inform. 2023, 74, 101984. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Gu, D.; Cheng, Y.; Lv, X.; Huang, Y.; Ye, P.; Zhang, X.; Zhang, J.; Jian, W. Investigation of the impact of diverse climate conditions on the cultivation suitability of Cinnamomum cassia using the MaxEnt model, HPLC and chemometric methods in China. Sci. Rep. 2024, 14, 25686. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Miao, G.; Zhou, X.; Yu, C.; Cao, Y. Predicting the potential distribution of Dendrolimus punctatus and its host Pinus massoniana in China under climate change conditions. Front. Plant Sci. 2024, 15, 1362020. [Google Scholar] [CrossRef]
- Chavez, D.; Nénger, N.; Bolaños-Carriel, C.; Espinosa Marín, J.; Bastidas, W.; García, L. Ecological Modeling of the Potential Distribution of the Mistletoe Phoradendron nervosum (Viscaceae) Parasitism in Ecuador. Agriculture 2025, 15, 1732. [Google Scholar] [CrossRef]
- Parra Quijano, M.; Iriondo, J.M.; Torres, M.E.; López, F.; Phillips, J.; Kell, S. CAPFITOGEN 3: A Toolbox for the Conservation and Promotion of the Use of Agricultural Biodiversity; Universidad Nacional de Colombia: Bogota, Colombia, 2021. [Google Scholar]
- Phillips, J.; Asdal, Å.; Magos Brehm, J.; Rasmussen, M.; Maxted, N. In situ and ex situ diversity analysis of priority crop wild relatives in Norway. Divers. Distrib. 2016, 22, 1112–1126. [Google Scholar] [CrossRef]
- Magos Brehm, J.; Gaisberger, H.; Kell, S.; Parra-Quijano, M.; Thormann, I.; Dulloo, M.E.; Maxted, N. Planning complementary conservation of crop wild relative diversity in southern Africa. Divers. Distrib. 2022, 28, 1358–1372. [Google Scholar] [CrossRef]
- Nduche, M.U.; Magos Brehm, J.; Parra-Quijano, M.; Maxted, N. In situ and ex situ conservation gap analyses of West African priority crop wild relatives. Genet. Resour. Crop Evol. 2023, 70, 333–351. [Google Scholar] [CrossRef]
- Borja, E.; Guara Requena, M.; Vera, D. Situación actual, problemas fitosanitarios y alternativas de manejo de la teca en Ecuador. Cienc. Tecnol. 2021, 14, 27–32. [Google Scholar] [CrossRef]
- FAO. Ecuador en una Mirada; FAO en Ecuador: Quito, Ecuador, 2025; Available online: https://www.fao.org/ecuador/fao-en-ecuador/ecuador-en-una-mirada/ar/ (accessed on 3 May 2025).
- IGM. Atlas Geográfico de la República del Ecuador; Instituto Geográfico Militar (IGM), Secretaría Nacional de Planificación y Desarrollo (SEMPLADES): Quito, Ecuador, 2013; p. 356.
- Winckell, A. Presentación general de los grandes rasgos del relieve del Ecuador. In Los Paisajes Naturales del Ecuador. Las Condiciones Generales del Medio Natural; Winckell, A., Marocco, R., Winter, T., Huttel, C., Pourrut, P., Zebrowski, C., Sourdat, M., Eds.; Centro Ecuatoriano de Investigación Geográfica: Quito, Ecuador, 1997; Volume 1, pp. 208–319. [Google Scholar]
- Portilla, F. Agroclimatología del Ecuador; Editorial Abya-Yala: Quito, Ecuador, 2018; p. 647. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- FAO; IIASA. Harmonized World Soil Database Version 2.0; FAO: Rome, Italy; IIASA: Laxenburg, Austria, 2023. [Google Scholar] [CrossRef]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L. The shuttle radar topography mission. Rev. Geophys. 2007, 45, RG2004. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.I.; Nelson, H.I.; Guevara, E. Hole-Filed Seamless SRTM Data, Version 4; International Centre for Tropical Agriculture (CIAT): Palmira, Colombia, 2008; Available online: https://srtm.csi.cgiar.org (accessed on 25 October 2025).
- Posit Team. RStudio; Version 2024.03; Posit: Boston, MA, USA, 2024; Available online: https://posit.co/download/rstudio-desktop/ (accessed on 6 December 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: London, UK, 2019. [Google Scholar]
- Wickham, H. ggplot2: Elegant graphics for data analysis. In ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Houston, TX, USA, 2016; pp. 189–201. [Google Scholar]
- Gross, J.; Ligges, U. nortest: Tests for Normality. R Package, Version 1.0-4. 2015. Available online: https://cran.r-project.org/web/packages/nortest/index.html (accessed on 16 January 2025).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. 2023. Available online: https://cran.r-project.org/web/packages/FSA/index.html (accessed on 16 January 2025).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar] [CrossRef]
- Mohammad, N.; Rahaman, S.M.; Khatun, M.; Rajkumar, M.; Garai, S.; Ranjan, A.; Tiwari, S. Teak (Tectona grandis L.f.) demonstrates robust adaptability to climate change scenarios in central India. Vegetos 2023, 36, 795–804. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 2010, 3, 54–89. [Google Scholar] [CrossRef]
- Scheldeman, X.; van Zonneveld, M. Training Manual on Spatial Analysis of Plant Diversity and Distribution; Bioversity International: Rome, Italy, 2010. [Google Scholar]
- Zhang, S.; Wang, Z.; Liao, S. Environmental drivers of the current and future distribution of high-yielding lacquer trees (Toxicodendron vernicifluum (stokes) FA Barkley). For. Int. J. For. Res. 2023, 96, 763–774. [Google Scholar] [CrossRef]
- Newbold, T.; Gilbert, F.; Zalat, S.; El--Gabbas, A.; Reader, T. Climate--based models of spatial patterns of species richness in Egypt’s butterfly and mammal fauna. J. Biogeogr. 2009, 36, 2085–2095. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop; Version 10.8.2; Environmental Systems Research Institute: Redlands, CA, USA, 2022; Available online: https://www.esri.com (accessed on 10 November 2024).
- Deb, J.C.; Phinn, S.; Butt, N.; McAlpine, C.A. Climatic-induced shifts in the distribution of teak (Tectona grandis) in tropical Asia: Implications for forest management and planning. Environ. Manag. 2017, 60, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Patasaraiya, M.K.; Devi, R.M.; Sinha, B.; Bisaria, J. Predicting impacts of climate change on teak and sal forests in central India using maximum entropy modeling: An approach for future conservation and silvicultural strategies. For. Sci. 2023, 69, 463–472. [Google Scholar] [CrossRef]
- Herrera-Feijoo, R.J.; Torres, B.; López-Tobar, R.; Tipán-Torres, C.; Toulkeridis, T.; Heredia-R, M.; Mateo, R.G. Modelling climatically suitable areas for Mahogany (Swietenia macrophylla King) and their shifts across Neotropics: The role of Protected Areas. Forests 2023, 14, 385. [Google Scholar] [CrossRef]
- Vistín, D.A.; Salas, E.M.; Balseca, J.E.; Lara, N.X. Distribución potencial de Polylepis incana en los Andes ecuatorianos para estudios de fisiología vegetal y planes de rehabilitación forestal. Ecol. Aust. 2023, 33, 001–012. [Google Scholar] [CrossRef]
- Vera, D.; Cañarte, E.; Navarrete, J.; Espinoza, G.; Borja, E. Manejo Integrado de la Enfermedad “MUERTE REGRESIVA” EN TECA (Tectona grandis Lf); Instituto Nacional de Investigaciones Agropecuarias: Quito, Ecuador, 2021; p. 124. [Google Scholar]
- De Camino, R.; Sage, L.; Alfaro, M.; Varmola, M. Teak (Tectona grandis) in Central America; FAO: Rome, Italy, 2002. [Google Scholar]
- Kaosa-ard, A. Physiological Studies of Sprouting of Teak (Tectona grandis Linn. f.) Planting Stumps. Ph.D. Thesis, The Australian National University, Canberra, Australia, 1977. Available online: https://openresearch-repository.anu.edu.au/items/600f6735-ff39-447c-b781-7efe0550b523 (accessed on 22 July 2025).
- Singh Ramesh, A.; Cheesman, A.W.; Flores-Moreno, H.; Preece, N.D.; Crayn, D.M.; Cernusak, L.A. Temperature, nutrient availability, and species traits interact to shape elevation responses of Australian tropical trees. Front. For. Glob. Change 2023, 6, 1089167. [Google Scholar] [CrossRef]
- Martínez, H. Teca (Tectona grandis L. f.): Condiciones para su Cultivo “Fomento de la Reforestación Comercial para la Mejora y Conservación de las Reservas de Carbono”; FONAFIFO (Fondo Nacional de Fomento Forestal): Moravia, Costa Rica, 2015; p. 39. [Google Scholar]
- Fonseca, W. Manual para Productores de teca (Tectona grandis L.f.) en Costa Rica; Fondo Nacional de Financiamiento Forestal FONAFIFO: Heredia, Costa Rica, 2003. [Google Scholar]
- Weaver, L. Tectona grandis L-f. Teak: SOITF-SM-64; Deparment of Agriculture, Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1993.
- Kolmert, A. Teak in Northern Laos: Sustainability and Livelihood; Swedish University of Agricultural Sciences, International Office: Uppsala, Sweden, 2001. [Google Scholar]
- Tanaka, N.; Hamazaki, T.; Vacharangkura, T. Distribution, growth and site requirements of teak. Jpn. Agric. Res. Q. 1998, 32, 65–77. [Google Scholar]
- Wu, C.; Chen, D.; Shen, J.; Sun, X.; Zhang, S. Estimating the distribution and productivity characters of Larix kaempferi in response to climate change. J. Environ. Manag. 2021, 280, 111633. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Huang, S.; Zhang, Y.; Fang, H.; Jiang, J. Prediction of suitable habitats for Sapindus delavayi based on the MaxEnt model. Forests 2022, 13, 1611. [Google Scholar] [CrossRef]
- Kadambi, K. Silviculture and Management of Teak; Austin State University, School of Forestry: Nacogdoches, TX, USA, 1972; p. 137. [Google Scholar]
- Potterf, M.; Eyvindson, K.; Blattert, C.; Burgas, D.; Burner, R.; Stephan, J.G.; Mönkkönen, M. Interpreting wind damage risk–how multifunctional forest management impacts standing timber at risk of wind felling. Eur. J. For. Res. 2022, 141, 347–361. [Google Scholar] [CrossRef]
- Rau, E.-P.; Gardiner, B.A.; Fischer, F.J.; Maréchaux, I.; Joetzjer, E.; Sun, I.-F.; Chave, J. Wind speed controls forest structure in a subtropical forest exposed to cyclones: A case study using an individual-based model. Front. For. Glob. Change 2022, 5, 753100. [Google Scholar] [CrossRef]
- Ennos, A. Wind as an ecological factor. Trends Ecol. Evol. 1997, 12, 108–111. [Google Scholar] [CrossRef]
- Suzuki, S.; Miyashita, A. Measuring the dynamic wind load acting on standing trees in the field without destroying them. PLoS ONE 2025, 20, e0323532. [Google Scholar] [CrossRef]
- Watt, M.; Moore, J.; McKinlay, B. The influence of wind on branch characteristics of Pinus radiata. Trees 2005, 19, 58–65. [Google Scholar] [CrossRef]
- Bonnesoeur, V.; Constant, T.; Moulia, B.; Fournier, M. Forest trees filter chronic wind-signals to acclimate to high winds. New Phytol. 2016, 210, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hua, J.; Kang, M.; Wang, X.; Fan, X.-R.; Fourcaud, T.; De Reffye, P. Stronger wind, smaller tree: Testing tree growth plasticity through a modeling approach. Front. Plant Sci. 2022, 13, 971690. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, E.; Stokes, A.; Lasserre, B.; Danjon, F.; Berthier, S.; Fourcaud, T.; Chiatante, D. Influence of wind loading on root system development and architecture in oak (Quercus robur L.) seedlings. Trees 2005, 19, 374–384. [Google Scholar] [CrossRef]
- Camarero, J.J.; Colangelo, M.; Gazol, A.; Pizarro, M.; Valeriano, C.; Igual, J.M. Effects of windthrows on forest cover, tree growth and soil characteristics in drought-prone pine plantations. Forests 2021, 12, 817. [Google Scholar] [CrossRef]
- Smith, G. Wind Damage was related to Thinning Intensity in a 10 year-old Teak Plantation. In Proceedings of the 3rd Word Teak Conference—Strengthening Global Teak Resources and Markets for Sustainable Development, Guayaquil, Ecuador, 11–16 May 2015; p. 61. [Google Scholar]
- Galeano, E.; Vasconcelos, T.S.; Novais de Oliveira, P.; Carrer, H. Physiological and molecular responses to drought stress in teak (Tectona grandis Lf). PLoS ONE 2019, 14, e0221571. [Google Scholar] [CrossRef]
- Trisurat, Y.; Chitechote, A.; Vongkhamsao, V. Predicted climate change impact on natural teak forests in the Greater Mekong sub-region. In Proceedings of the XV World Forestry Congress, Seoul, Republic of Korea, 2–6 May 2022; Available online: https://openknowledge.fao.org/handle/20.500.14283/cc1833en (accessed on 5 May 2025).
- Soto Vivar, R.D. Propuestas para Incentivar la Comercialización de Fibra Natural de la Planta de Abacá a Partir del Análisis de su Cadena Agroproductiva para Pequeños Productores de la Zona de Monterrey. Bachelor’s Thesis, Pontificia Universidad Católica del Ecuador-Sede Esmeraldas PUCE-SE, Esmeraldas, Ecuador, 2023. Available online: https://repositorio.puce.edu.ec/server/api/core/bitstreams/e44a7bf7-00a5-4b88-9193-61f04d43b509/content (accessed on 16 January 2025).
- Ministerio de Agricultura Ganadería Acuacultura y Pesca. Programa de Incentivos para la Reforestación con Fines Comerciales; Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP): Guayaquil, Ecuador, 2016.
- Pérez, D.; Kanninen, M. Stand growth scenarios for Tectona grandis plantations in Costa Rica. For. Ecol. Manag. 2005, 210, 425–441. [Google Scholar] [CrossRef]
- ESPAC. Encuesta de Superficie y Producción Agropecuaria Continua; ESPAC: Dammam, Saudi Arabia, 2023; Available online: https://app.powerbi.com/view?r=eyJrIjoiZTEyY2NiZDItYjIzYi00ZGQ1LTlkNGEtNDE1OGViM2Q1N2VlIiwidCI6ImYxNThhMmU4LWNhZWMtNDQwNi1iMGFiLWY1ZTI1OWJkYTExMiJ9&pageName=ReportSection (accessed on 19 March 2025).
- Loor, R.G.; Sotomayor Cantos, I.A.; Jiménez Barragán, J.C.; Tarqui Freire, O.M.; Rodríguez Zamora, G.A.; Casanova Mendoza, T.d.J.; Quijano Rivadeneira, G.C. INIAP-EETP-800 e INIAP-EETP-801 Nuevos Clones de Cacao Fino y de Aroma con alto Rendimiento; Instituto Nacional de Investigaciones Agropecuarias: Quito, Ecuador, 2018. Available online: https://www.semanticscholar.org/paper/INIAP-EETP-800-e-INIAP-EETP-801-nuevos-clones-de-y-Sol%C3%B3rzano-Cantos/9e04b567219524c07afc5fb51fff8617679b79fc (accessed on 10 May 2025).
- Acción Ecológica. Balsa en Ecuador# 6: Plantaciones, Poblaciones Silvestres y Nuevos Espacios Ocupados por la Balsa; Acción Ecológica: Quito, Ecuador, 2021; Available online: https://www.accionecologica.org/balsa-en-ecuador-6-plantaciones-poblaciones-silvestres-y-nuevos-espacios-ocupados-por-la-balsa-2/ (accessed on 30 April 2025).
- MAG. Ecuador Producirá con un Enfoque de Mercado para Sustituir las Importaciones; Ministerio de Agricultura y Ganadería (MAG): Santa Tecla, El Salvador, 2019. Available online: https://www.agricultura.gob.ec/ecuador-producira-con-un-enfoque-de-mercado-y-para-sustituir-las-importaciones/ (accessed on 21 May 2025).
- Jadán Sánchez, V.M.; Belduma Pizarro, N.A.; Elizalde Orellana, M.V. Evolución y proyección de la producción agrícola (Banano y Café) en Ecuador en el período 2012–2025. Rev. InveCom 2024, 4, 1–9. [Google Scholar] [CrossRef]
- Mendoza Briones, N.C. Incidencia de Factores Determinantes en el Sector Agrícola en Ecuador, Banano, Cacao, Café y Palma Africana. Bachelor’s Thesis, Universidad Católica de Santiago de Guayaquil, Guayaquil, Ecuador, 2018. Available online: http://repositorio.ucsg.edu.ec/bitstream/3317/11674/1/T-UCSG-PRE-ECO-CECO-252.pdf (accessed on 6 August 2025).
- Sierra, R.; Calva, O.; Guevara, A. La deforestación en el Ecuador, 1990–2018. Factores Promotores y Tendencias Recientes; Ministerio de Ambiente y Agua del Ecuador, Ministerio de Agricultura del Ecuador: Quito, Ecuador, 2021; p. 216.
- Ramos Veintimilla, R.A.; MacFarlane, D.; Cooper, L. The carbon sequestration potential of ‘analog’forestry in Ecuador: An alternative strategy for reforestation of degraded pastures. For. Int. J. For. Res. 2020, 94, 102–114. [Google Scholar] [CrossRef]
- Krishnan, S.N.; Balasubramanian, A.; Sivaprakash, M.; Ravi, R.; Sivakumar, B.; Prasath, C.H.; Swathiga, G.; Manimaran, V.; Anjali, K. Climatic and edaphic influences on productivity and carbon sequestration of farm grown teak (Tectona grandis, linn. f) in Tamil Nadu, India. HORIZON 2024, 12, 1–7. [Google Scholar] [CrossRef]
- Patel, S.K.; Naik, M. Assessment of Biomass, Carbon Stock and Sequestered CO2 in Teak (Tectona grandis L. f.) Plantation at Pt. Ravishankar Shukla University Campus, Raipur, Chhattisgarh, India. J. Glob. Ecol. Environ. 2024, 4, 102–114. [Google Scholar] [CrossRef]
- Amusa, T.O.; Aminu, M.; Moshood, F. Assessment of carbon sequestration of Teak (Tectona grandis Linn. F.) plantation on the campus of University of Ilorin, Nigeria. Reforesta 2023, 29, 27–42. [Google Scholar] [CrossRef]
- Asigbaase, M.; Annan, M.; Adusu, D.; Abugre, S.; Nsor, C.A.; Kumi, S.; Acheamfour, S.A. Teak-Soil Interaction: Teak (Tectona grandis) Plantations Impact and are Impacted by Soil Properties and Fertility in Southwestern Ghana. Appl. Environ. Soil Sci. 2024, 2024, 7931830. [Google Scholar] [CrossRef]
- Chayaporn, P.; Sasaki, N.; Venkatappa, M.; Abe, I. Assessment of the overall carbon storage in a teak plantation in Kanchanaburi province, Thailand–Implications for carbon-based incentives. Clean. Environ. Syst. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Niskanen, A. Value of external environmental impacts of reforestation in Thailand. Ecol. Econ. 1998, 26, 287–297. [Google Scholar] [CrossRef]
- Suwethaasri, D.; Baranidharan, K.; Ravi, R.; Tilak, M.; Kalpana, M.; Ragunath, K.; Hemalatha, P.; Vijayabhama, M.; Kabinesh, V.; Bargavi, S.; et al. Assessing the impact of climate change on forest biomass and carbon sequestration in India: A systematic review. Plant Sci. Today 2025, 12, 1–12. [Google Scholar] [CrossRef]
- Holguín Burgos, B.P.; Delgado Delgado, D.D. Estudio económico del comportamiento de la madera en el Ecuador en los últimos años. 2009–2017. OIDLES Obs. Iberoam. Desarro. Local Econ. Soc. 2018, 25, 1–14. Available online: https://www.eumed.net/rev/oidles/25/madera-ecuador.html (accessed on 4 November 2025).
- Kleemann, J.; Zamora, C.; Villacis-Chiluisa, A.B.; Cuenca, P.; Koo, H.; Noh, J.K.; Fürst, C.; Thiel, M. Deforestation in continental Ecuador with a focus on protected areas. Land 2022, 11, 268. [Google Scholar] [CrossRef]
- Sánchez, D.; Merlo, J.; Haro, R.; Acosta, M.; Gustavo, B. Suelos del Oriente. In Suelos del Ecuador Clasificación, Uso y Manejo; Espinosa, J., Moreno, J., Bernal, G., Eds.; Instituto Geográfico Militar (IGM): Quito, Ecuador, 2022; pp. 157–188. [Google Scholar]
- Mulyoutami, E.; Tata, H.L.; Silvianingsih, Y.A.; van Noordwijk, M. Agroforests as the intersection of instrumental and relational values of nature: Gendered, culture-dependent perspectives? Curr. Opin. Environ. Sustain. 2023, 62, 101293. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Rohadi, D.; Perdana, A.; Sabastian, G.; Nuryartono, N.; Pramono, A.A.; Widyani, N.; Manalu, P.; Fauzi, M.A.; Sumardamto, P. Teak agroforestry systems for livelihood enhancement, industrial timber production, and environmental rehabilitation. For. Trees Livelihoods 2013, 22, 241–256. [Google Scholar] [CrossRef]
- Cooper, K. Amazon Rainforest: The Slow Disappearance of Earth’s Green Lungs. J. Ecosyst. Ecography 2023, 13, 431. Available online: https://www.omicsonline.org/open-access/amazon-rainforest-the-slow-disappearance-of-earths-green-lungs-126107.html (accessed on 4 November 2025). [CrossRef]
- Pinnschmidt, A.; Yousefpour, R.; Nölte, A.; Hanewinkel, M. Close-to-nature management of tropical timber plantations is economically viable and provides biodiversity benefits. For. Int. J. For. Res. 2025, 98, 99–116. [Google Scholar] [CrossRef]
- Duarte Ritter, C.; Muñoz, J.; Fabrício Machado, A.; Albert, J.S.; Ribas, C.C.; Carnaval, A.C.; Ulloa Ulloa, C.; Carrillo, J.D.; Tuomisto, H.; Armenteras, D. Indigenous territories and protected areas are crucial for ecosystem connectivity in the Amazon basin. Proc. Natl. Acad. Sci. USA 2025, 122, e2418189122. [Google Scholar] [CrossRef] [PubMed]
- Beltran, K. Effects of Climate Change on Key Ecosystem Services Provided by the Ecuadorian Páramo Ecosystems. Ph.D. Thesis, University of York, York, UK, 2018. Available online: https://etheses.whiterose.ac.uk/id/eprint/23567/1/Thesis_KBeltran_2019_Final.pdf (accessed on 2 November 2025).
- de Meyer, A.P.; Ortega-Andrade, H.M.; Moulatlet, G.M. Assessing the conservation of eastern Ecuadorian cloud forests in climate change scenarios. Perspect. Ecol. Conserv. 2022, 20, 159–167. [Google Scholar] [CrossRef]
- Ames-Martínez, F.N.; Romero, I.C.; Guerra, A.; Guillen, J.G.I.; Quispe-Melgar, H.R.; Galeano, E.; Rodríguez-Ramírez, E.C. Climate change and tree cover loss affect the habitat suitability of Cedrela angustifolia: Evaluating climate vulnerability and conservation in Andean montane forests. PeerJ 2025, 13, e18799. [Google Scholar] [CrossRef]
- de Morais, I.L.L.; de Lima, A.A.; Santos, I.N.L.d.; Meneses, C.; da Silva, R.F.; Lopes, R.; Ramos, S.L.F.; de Aguiar, A.V.; Wrege, M.S.; Lopes, M.T.G. Climate change impact on the distribution of forest species in the Brazilian Amazon. Sustainability 2024, 16, 3458. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.; Killeen, T.J.; Laurance, W.F.; Peres, C.A.; Guevara, J.E.; Salomão, R.P.; Castilho, C.V.; Amaral, I.L.; de Almeida Matos, F.D. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 2015, 1, e1500936. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, L. Can Agroforestry Chocolate Help Save the World’s Most Endangered Rainforest? Mongaby. 12 September 2023. Available online: https://news.mongabay.com/2023/09/can-agroforestry-chocolate-help-save-the-worlds-most-endangered-rainforest/ (accessed on 4 November 2025).
- Middendorp, R.S.; Vanacker, V.; Lambin, E.F. Impacts of shaded agroforestry management on carbon sequestration, biodiversity and farmers income in cocoa production landscapes. Landsc. Ecol. 2018, 33, 1953–1974. [Google Scholar] [CrossRef]
- Charney, N.D.; Record, S.; Gerstner, B.E.; Merow, C.; Zarnetske, P.L.; Enquist, B.J. A test of species distribution model transferability across environmental and geographic space for 108 western North American tree species. Front. Ecol. Evol. 2021, 9, 689295. [Google Scholar] [CrossRef]
- Sist, P.; Peña-Claros, M.; Baldiviezo Calles, J.P.; Derroire, G.; Kanashiro, M.; Mendoza Ortega, K.; Piponiot, C.; Roopsind, A.; Verissimo, A.; Vidal, E. Forest Management for Timber Production and Forest Landscape Restoration in the Amazon: The Way Towards Sustainability; SPA: New York, NY, USA, 2023. [Google Scholar] [CrossRef]


| Province/Region | N° of Plantations | Area | Plantation Age (Years) | |||
|---|---|---|---|---|---|---|
| Ha | Mean | Min–Max | Average | Min–Max | ||
| Littoral Region | 984 | 21,647.53 | 22.00 | 0.01–563.0 | 4.6 | 1.0–22.0 |
| El Oro | 21 | 523.7 | 24.94 | 0.31–267.42 | 3.6 | 2.6–5.6 |
| Esmeraldas | 48 | 773.61 | 16.12 | 0.12–78.58 | 4.9 | 2.5–18 |
| Guayas | 350 | 6647.54 | 18.99 | 0.04–500.0 | 4.4 | 2.3–18 |
| Los Ríos | 251 | 6875.87 | 27.39 | 0.01–563.83 | 5 | 1.0–12.0 |
| Manabí | 265 | 3373.14 | 12.73 | 0.04–300.0 | 4.4 | 2.0–18.0 |
| Santo Domingo | 49 | 3453.08 | 70.47 | 0.09–2700.00 | 5.4 | 3.3–22.0 |
| Andean Region | 20 | 251.03 | 12.55 | 0.49–100.0 | 4.9 | 2.0–15.0 |
| Azuay | 3 | 2.13 | 0.71 | 0.49–0.96 | 5.6 | 5.6–5.6 |
| Bolívar | 6 | 24.5 | 4.08 | 0.5–12.0 | 6 | 2.0–15.0 |
| Cañar | 6 | 110.11 | 3.06 | 4.39–52.15 | 4 | 3.4–5.6 |
| Chimborazo | 2 | 9.43 | 4.72 | 2.14–7.29 | 3.6 | 3.6–3.6 |
| Cotopaxi | 2 | 4.86 | 2.43 | 1.92–2.95 | 5.2 | 5.2–5.2 |
| Pichincha | 1 | 100 | 100.00 | 100.0–100.0 | 5 | 5.0–5.0 |
| Amazonian Region | 19 | 1442.6 | 75.60 | 0.61–1369.0 | 7 | 2.8–14.0 |
| Orellana | 5 | 1390 | 278.00 | 3.0–1369.0 | 9.8 | 3.0–14.0 |
| Sucumbíos | 14 | 46.4 | 3.31 | 0.61–14.24 | 4.2 | 2.8–14.0 |
| Total | 1023 | 23,334.38 | 22.81 | 0.01–1369.0 | 5.5 | 1.0–22.0 |
| Variable | CV | Mean ± S.D. | Min–Max | Q1–Q3 |
|---|---|---|---|---|
| Precipitation of the driest quarter (bio_17) | 81.47 | 340.57 ± 277.45 | 0–841 | 38–617 |
| Precipitation seasonality (coefficient of variation) (bio_15) | 75.75 | 55.73 ± 42.22 | 12–189 | 18–99 |
| Elevation (alt) | 61.61 | 226.03 ± 139.26 | 0–600 | 112–305 |
| August wind speed (wind_8) | 54.83 | 1.62 ± 0.89 | 0.8–5.3 | 1–2 |
| July wind speed (wind_7) | 54.82 | 1.58 ± 0.87 | 0.8–5.3 | 1–1.9 |
| September wind speed (wind_9) | 52.83 | 1.67 ± 0.88 | 0.9–5.2 | 1.1–2 |
| October wind speed (wind_10) | 50.82 | 1.69 ± 0.86 | 0.9–5 | 1.1–2 |
| November wind speed (wind_11) | 49.8 | 1.68 ± 0.84 | 0.9–4.9 | 1.1–2 |
| Annual wind speed (wind_annual) | 49.39 | 1.56 ± 0.77 | 0.87–4.75 | 1.03–1.84 |
| December wind speed (wind_12) | 48.53 | 1.63 ± 0.79 | 0.9–4.8 | 1.1–1.9 |
| Annual precipitation (bio_12) | 47.06 | 2243.31 ± 1055.67 | 103–4374 | 1231–3125 |
| Cation exchange capacity of soil in cmolc/kg—topsoil (t_cecsol) | 46.95 | 16.1 ± 7.56 | 4–102.82 | 10.5–20.33 |
| Available soil water capacity (volumetric fraction) for h2—topsoil (t_awc2) | 11.88 | 10.11 ± 1.2 | 5.2–20.95 | 9.45–10.68 |
| Bulk density (fine earth) in kg/m3—topsoil (t_bulk_dens) | 7.02 | 1333.55 ± 93.61 | 492.33–1579.8 | 1299.75–1386.9 |
| Saturated water content (volumetric fraction) for tS—topsoil (t_awcts) | 6.68 | 45.37 ± 3.03 | 38.05–71.57 | 43.6–46.45 |
| Maximum temperature of the warmest month (bio_5) | 2.73 | 30.54 ± 0.83 | 26.4–33.1 | 30.1–31.1 |
| Categories | Study Area | Studied Farms | ||||
|---|---|---|---|---|---|---|
| Number of Grid Cells (km2) | Percentage of the Number of Grid Cells | Number | Percentage | Area (ha) | Percentage | |
| 1 * | 74,378 | 43.15 | 18 | 1.76 | 1427.40 | 6.12 |
| 2 | 2 | 0.00 | 0 | 0.00 | 0.00 | 0.00 |
| 3 * | 13,414 | 7.78 | 1 | 0.10 | 9.00 | 0.04 |
| 4 | 1994 | 1.16 | 0 | 0.00 | 0.00 | 0.00 |
| 5 | 416 | 0.24 | 0 | 0.00 | 0.00 | 0.00 |
| 6 | 265 | 0.15 | 0 | 0.00 | 0.00 | 0.00 |
| 7 * | 1881 | 1.09 | 1 | 0.10 | 1.95 | 0.01 |
| 8 | 84 | 0.05 | 0 | 0.00 | 0.00 | 0.00 |
| 9 | 161 | 0.09 | 0 | 0.00 | 0.00 | 0.00 |
| 10 | 680 | 0.39 | 0 | 0.00 | 0.00 | 0.00 |
| 11 | 197 | 0.11 | 0 | 0.00 | 0.00 | 0.00 |
| 12 | 1918 | 1.11 | 0 | 0.00 | 0.00 | 0.00 |
| 13 | 85 | 0.05 | 0 | 0.00 | 0.00 | 0.00 |
| 14 | 2870 | 1.67 | 0 | 0.00 | 0.00 | 0.00 |
| 15 | 1760 | 1.02 | 0 | 0.00 | 0.00 | 0.00 |
| 16 * | 1822 | 1.06 | 30 | 2.93 | 281.47 | 1.21 |
| 17 * | 6828 | 3.96 | 174 | 17.01 | 9091.72 | 38.96 |
| 18 * | 365 | 0.21 | 14 | 1.37 | 24.06 | 0.10 |
| 19 * | 25,169 | 14.60 | 597 | 58.36 | 8975.52 | 38.46 |
| 20 | 411 | 0.24 | 0 | 0.00 | 0.00 | 0.00 |
| 21 * | 13,829 | 8.02 | 132 | 12.90 | 2436.70 | 10.44 |
| 22 * | 1589 | 0.92 | 1 | 0.10 | 7.84 | 0.03 |
| 23 * | 17,056 | 9.90 | 41 | 4.01 | 823.38 | 3.53 |
| 24 * | 1553 | 0.90 | 3 | 0.29 | 85.12 | 0.36 |
| 25 * | 2937 | 1.70 | 7 | 0.68 | 63.38 | 0.27 |
| 26 * | 704 | 0.41 | 4 | 0.39 | 106.85 | 0.46 |
| Total | 172,368 | 100 | 1023 | 100 | 23,334.38 | 100.00 |
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Precipitation seasonality (bio_15) | 42 | 37.8 |
| Elevation (alt) | 34.4 | 35.2 |
| Annual precipitation (bio_12) | 8.4 | 7.2 |
| September wind speed (wind_9) | 5.3 | 4.5 |
| July wind speed (wind_7) | 1.9 | 2.1 |
| Precipitation of the driest quarter (bio_17) | 1.8 | 1.2 |
| August wind speed (wind_8) | 1.6 | 5.4 |
| Maximum temperature of the warmest month (bio_5) | 1.1 | 0.2 |
| Annual wind speed (wind_annual) | 1 | 1 |
| December wind speed (wind_12) | 0.6 | 1.3 |
| Cation exchange capacity of soil in cmolc/kg—topsoil (t_cecsol) | 0.5 | 0.6 |
| November wind speed (wind_11) | 0.5 | 0 |
| October wind speed (wind_10) | 0.4 | 2.9 |
| Available soil water capacity (volumetric fraction) for h2—topsoil (t_awc2) | 0.3 | 0.3 |
| Bulk density (fine earth) in kg/m3—topsoil (t_bulk_dens) | 0.1 | 0.3 |
| Saturated water content (volumetric fraction) for tS—topsoil (t_awcts) | 0.1 | 0.1 |
| Province/Probability | Null | Low | Moderate | Elevated | High | Total |
|---|---|---|---|---|---|---|
| Littoral region | 30,360.00 | 12,065.04 | 11,565.20 | 9576.21 | 6600.43 | 70,166.90 |
| El Oro | 4577.26 | 699.52 | 387.91 | 66.82 | 0.00 | 5731.51 |
| Esmeraldas | 9425.72 | 3286.10 | 2591.42 | 583.03 | 0.00 | 15,886.27 |
| Guayas | 5470.19 | 1805.00 | 2099.25 | 2866.64 | 3029.22 | 15,270.30 |
| Los Ríos | 359.57 | 1041.79 | 1289.69 | 1746.85 | 2765.29 | 7203.20 |
| Manabí | 5615.92 | 4079.47 | 4437.82 | 4002.36 | 797.57 | 18,933.13 |
| Santo Domingo | 1898.45 | 824.10 | 513.22 | 202.03 | 8.35 | 3446.15 |
| Santa Elena | 3012.89 | 329.06 | 245.90 | 108.50 | 0.00 | 3696.35 |
| Andean region | 57,282.29 | 1313.45 | 795.88 | 578.49 | 137.39 | 60,107.49 |
| Azuay | 7985.57 | 132.88 | 168.42 | 38.79 | 0.00 | 8325.67 |
| Bolívar | 3442.78 | 148.52 | 181.40 | 121.98 | 50.18 | 3944.86 |
| Cañar | 2726.90 | 51.37 | 101.35 | 260.57 | 6.77 | 3146.95 |
| Carchi | 3528.73 | 0.00 | 0.00 | 0.00 | 0.00 | 3528.73 |
| Cotopaxi | 5634.76 | 139.93 | 149.84 | 104.15 | 80.45 | 6109.12 |
| Chimborazo | 6469.30 | 7.81 | 4.13 | 19.38 | 0.00 | 6500.62 |
| Imbabura | 4574.16 | 9.31 | 0.00 | 0.00 | 0.00 | 4583.48 |
| Loja | 10,553.53 | 472.74 | 18.14 | 0.00 | 0.00 | 11,044.40 |
| Pichincha | 8980.77 | 350.89 | 172.59 | 33.62 | 0.00 | 9537.87 |
| Tungurahua | 3385.78 | 0.00 | 0.00 | 0.00 | 0.00 | 3385.78 |
| Amazonian region | 115,788.93 | 692.81 | 8.35 | 0.00 | 0.00 | 116,490.08 |
| Morona Santiago | 24,012.17 | 0.00 | 0.00 | 0.00 | 0.00 | 24,012.17 |
| Napo | 12,542.42 | 0.00 | 0.00 | 0.00 | 0.00 | 12,542.42 |
| Pastaza | 29,628.77 | 0.00 | 0.00 | 0.00 | 0.00 | 29,628.77 |
| Zamora Chinchipe | 10,532.66 | 0.00 | 0.00 | 0.00 | 0.00 | 10,532.66 |
| Sucumbíos | 17,787.62 | 317.86 | 0.00 | 0.00 | 0.00 | 18,105.48 |
| Orellana | 21,285.29 | 374.94 | 8.35 | 0.00 | 0.00 | 21,668.59 |
| Total | 203,431.20 | 14,071.29 | 12,369.43 | 10,154.70 | 6737.83 | 246,764.47 |
| Probability/Categories | Null | Low | Moderate | Elevated | High | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells | % | Cells | % | Cells | % | Cells | % | Cells | % | ||
| 1 * | 73,594.00 | 61.44 | 766.00 | 4.78 | 12.00 | 0.09 | 6.00 | 0.05 | 0.00 | 0.00 | 74,378.00 |
| 2 | 2.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | |
| 3 * | 12,237.00 | 10.22 | 672.00 | 4.19 | 328.00 | 2.34 | 177.00 | 1.47 | 0.00 | 0.00 | 13,414.00 |
| 4 | 1744.00 | 1.46 | 195.00 | 1.22 | 49.00 | 0.35 | 6.00 | 0.05 | 0.00 | 0.00 | 1994.00 |
| 5 | 339.00 | 0.28 | 49.00 | 0.31 | 25.00 | 0.18 | 3.00 | 0.02 | 0.00 | 0.00 | 416.00 |
| 6 | 240.00 | 0.20 | 25.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 265.00 |
| 7 * | 1634.00 | 1.36 | 101.00 | 0.63 | 134.00 | 0.96 | 12.00 | 0.10 | 0.00 | 0.00 | 1881.00 |
| 8 | 75.00 | 0.06 | 9.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 84.00 |
| 9 | 161.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 161.00 |
| 10 | 680.00 | 0.57 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 680.00 |
| 11 | 197.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 197.00 |
| 12 | 1918.00 | 1.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1918.00 |
| 13 | 85.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 85.00 |
| 14 | 2870.00 | 2.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2870.00 |
| 15 | 1760.00 | 1.47 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1760.00 |
| 16 * | 68.00 | 0.06 | 137.00 | 0.85 | 237.00 | 1.69 | 433.00 | 3.59 | 1007.00 | 13.75 | 1882.00 |
| 17 * | 499.00 | 0.42 | 1701.00 | 10.61 | 1917.00 | 13.67 | 1508.00 | 12.49 | 1203.00 | 16.42 | 6828.00 |
| 18 * | 107.00 | 0.09 | 109.00 | 0.68 | 115.00 | 0.82 | 25.00 | 0.21 | 9.00 | 0.12 | 365.00 |
| 19 * | 2351.00 | 1.96 | 4591.00 | 28.63 | 6225.00 | 44.38 | 7954.00 | 65.89 | 4048.00 | 55.26 | 25,169.00 |
| 20 | 212.00 | 0.18 | 143.00 | 0.89 | 56.00 | 0.40 | 0.00 | 0.00 | 0.00 | 411.00 | |
| 21 * | 3684.00 | 3.08 | 3764.00 | 23.47 | 3536.00 | 25.21 | 1813.00 | 15.02 | 1032.00 | 14.09 | 13,829.00 |
| 22 * | 896.00 | 0.75 | 553.00 | 3.45 | 113.00 | 0.81 | 3.00 | 0.02 | 24.00 | 0.33 | 1589.00 |
| 23 * | 10,484.00 | 8.75 | 2311.00 | 14.41 | 915.00 | 6.52 | 116.00 | 0.96 | 3.00 | 0.04 | 13,829.00 |
| 24 * | 1489.00 | 1.24 | 91.00 | 0.57 | 9.00 | 0.06 | 0.00 | 0.00 | 0.00 | 1589.00 | |
| 25 * | 1850.00 | 1.54 | 716.00 | 4.46 | 355.00 | 2.53 | 16.00 | 0.13 | 0.00 | 2937.00 | |
| 26 * | 599.00 | 0.50 | 105.00 | 0.65 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 704.00 | |
| Total | 119,775.00 | 100.00 | 16,038.00 | 100.00 | 14,026.00 | 100.00 | 12,072.00 | 100.00 | 7326.00 | 100.00 | 169,237.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borja, E.; Guara-Requena, M.; Tapia, C.; Vera, D. Ecogeographic Characterization of Potential Tectona grandis L.f. (Teak) Exploitation Areas in Ecuador. Agriculture 2025, 15, 2328. https://doi.org/10.3390/agriculture15222328
Borja E, Guara-Requena M, Tapia C, Vera D. Ecogeographic Characterization of Potential Tectona grandis L.f. (Teak) Exploitation Areas in Ecuador. Agriculture. 2025; 15(22):2328. https://doi.org/10.3390/agriculture15222328
Chicago/Turabian StyleBorja, Edwin, Miguel Guara-Requena, César Tapia, and Danilo Vera. 2025. "Ecogeographic Characterization of Potential Tectona grandis L.f. (Teak) Exploitation Areas in Ecuador" Agriculture 15, no. 22: 2328. https://doi.org/10.3390/agriculture15222328
APA StyleBorja, E., Guara-Requena, M., Tapia, C., & Vera, D. (2025). Ecogeographic Characterization of Potential Tectona grandis L.f. (Teak) Exploitation Areas in Ecuador. Agriculture, 15(22), 2328. https://doi.org/10.3390/agriculture15222328










