Alterations in Serum Immune Parameters, Cytokines, Intestinal Permeability, Fecal Microbiota, and Short-Chain Fatty Acids in Healthy and Diarrheic Suckling Calves
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Sample Collection
2.3. Biochemical Analyses
2.3.1. Serum Biochemical Parameters
2.3.2. Serum Immunoglobulins and Cytokines
2.3.3. Intestinal Permeability Markers
2.3.4. Fecal Microbiota Analysis
2.3.5. Targeted Metabolomics of SCFAs
Preparation of Samples
GC/MS Analysis
2.4. Statistical Analysis
3. Results
3.1. Serum Levels of Biochemical Parameters
3.2. Serum Levels of Immunoglobulins and Cytokines
3.3. Indicators of Serum Intestinal Permeability
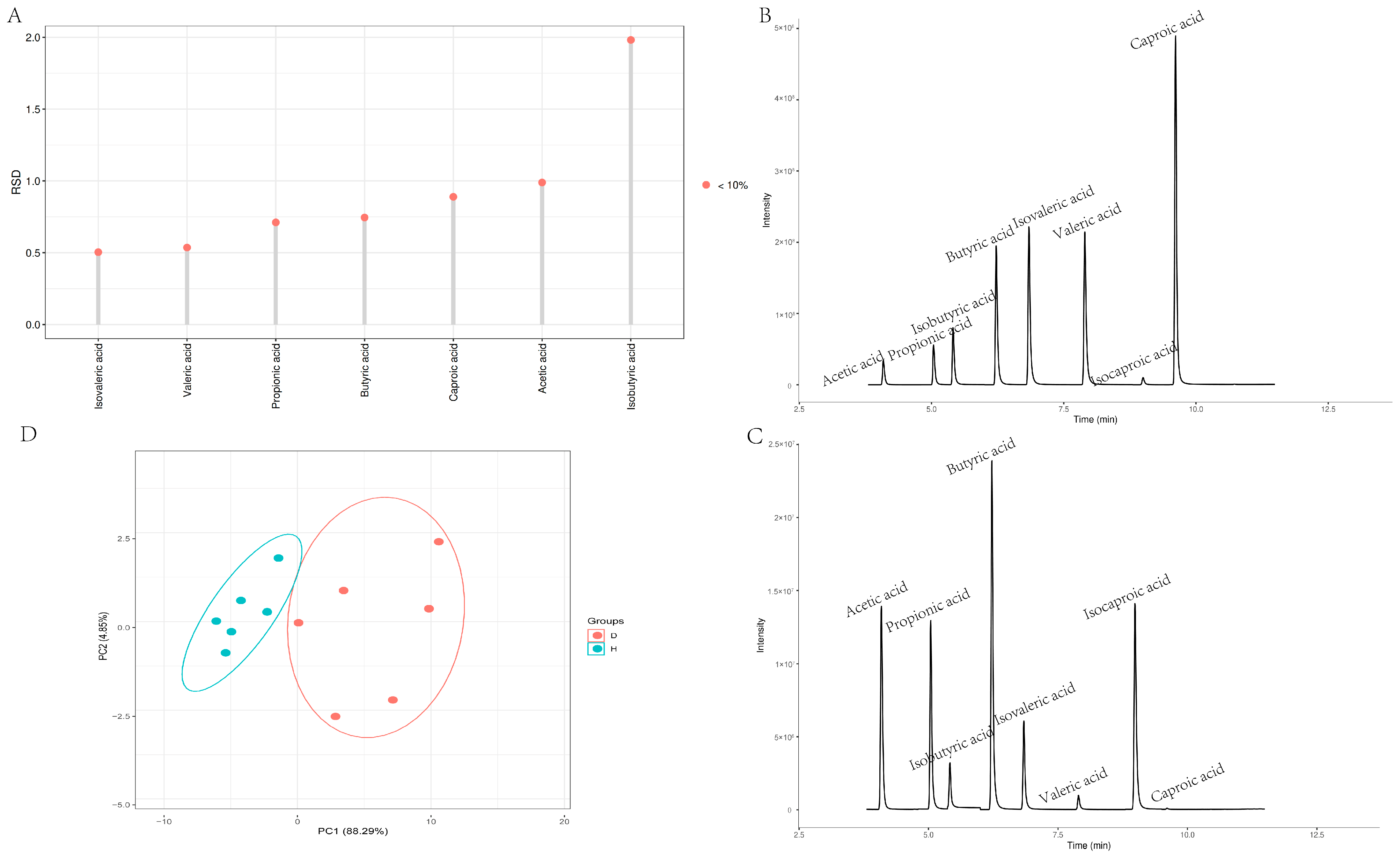
3.4. Multivariate Statistical Analysis of Short-Chain Fatty Acid Standards
3.5. Comparative Analysis of SCFAs
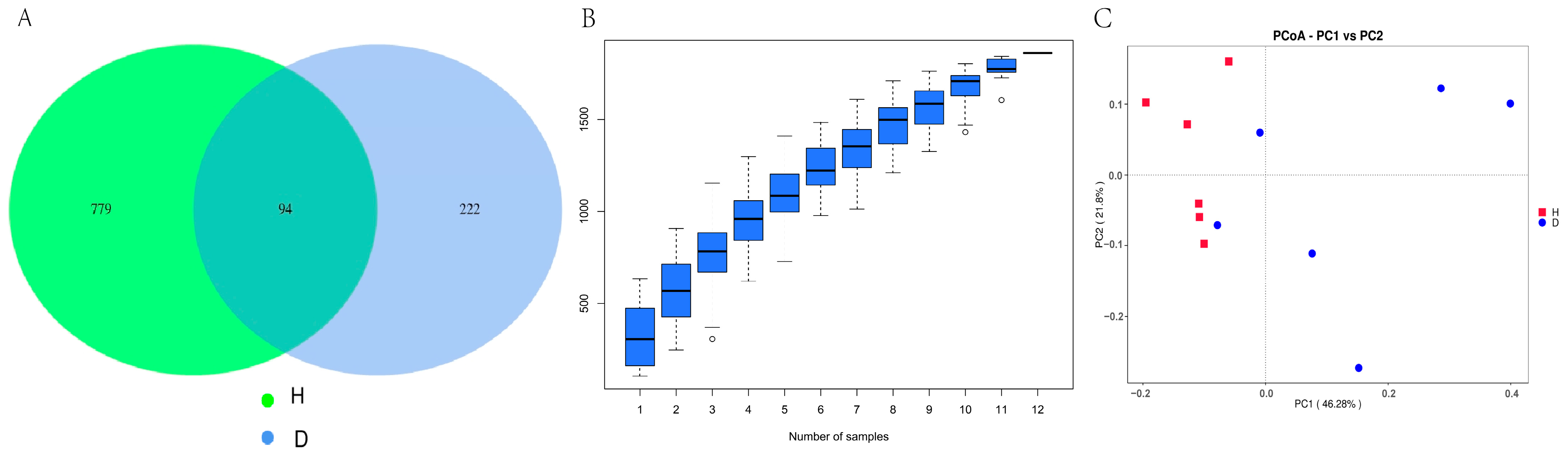
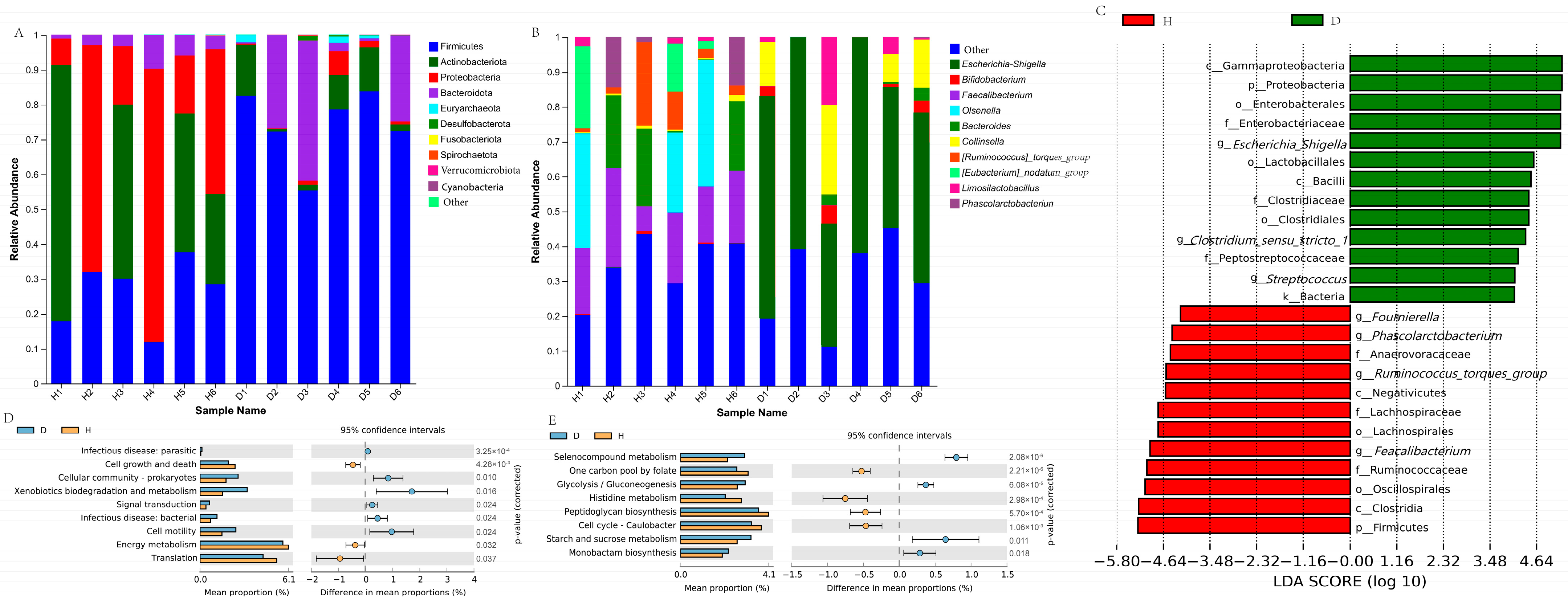
3.6. Fecal Microbial Changes
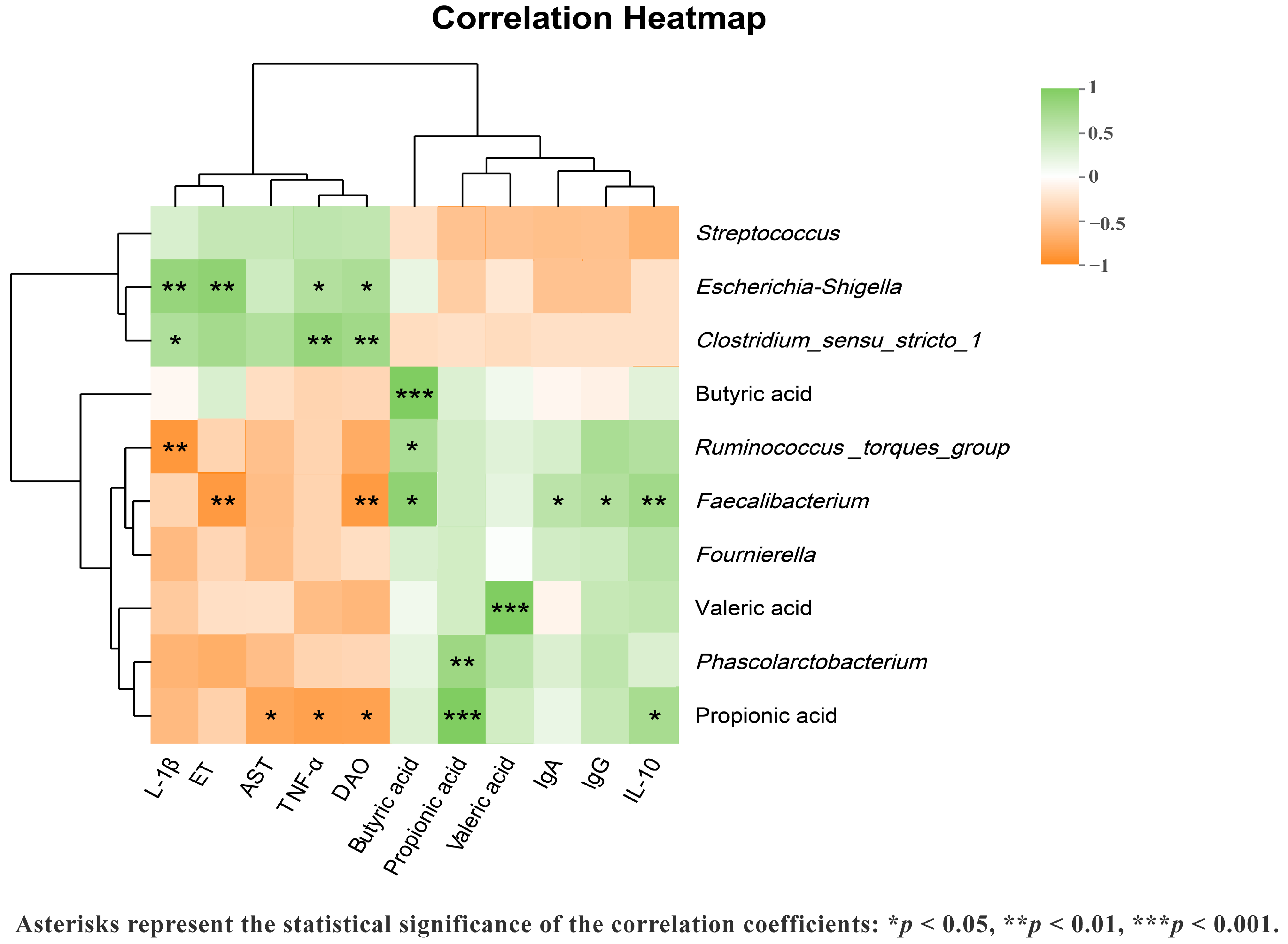
3.7. Correlation Analysis Among Immunological, Inflammatory, and Gut Permeability Markers with Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mee, J.F. Invited review: Bovine neonatal morbidity and mortality—Causes, risk factors, incidences, sequelae and prevention. Reprod. Domest. Anim. 2023, 58, 15–22. [Google Scholar] [CrossRef]
- Dall Agnol, A.M.; Lorenzetti, E.; Leme, R.A.; Ladeia, W.A.; Mainardi, R.M.; Bernardi, A.; Headley, S.A.; Freire, R.L.; Pereira, U.P.; Alfieri, A.F.; et al. Severe outbreak of bovine neonatal diarrhea in a dairy calf rearing unit with multifactorial etiology. Braz. J. Microbiol. 2021, 52, 2547–2553. [Google Scholar] [CrossRef]
- Alomari, M.M.M.; Dec, M.; Nowaczek, A.; Puchalski, A.; Wernicki, A.; Kowalski, C.; Urban-Chmiel, R. Therapeutic and prophylactic effect of the experimental bacteriophage treatment to control diarrhea caused by E. coli in newborn calves. ACS Infect. Dis. 2021, 7, 2093–2101. [Google Scholar] [CrossRef]
- Whon, T.W.; Kim, H.S.; Shin, N.R.; Sung, H.; Kim, M.S.; Kim, J.Y.; Kang, W.; Kim, P.S.; Hyun, D.W.; Seong, H.J.; et al. Calf diarrhea caused by prolonged expansion of autochthonous gut Enterobacteriaceae and their lytic bacteriophages. Msystems 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Yesilbag, K. Characterization of bovine rotavirus isolates from diarrheic calves in Türkiye. Mol. Biol. Rep. 2023, 50, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, M.; Roch, F.-F.; Conrady, B. Prevalence of worldwide neonatal calf diarrhoea caused by Bovine rotavirus in combination with Bovine coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-analysis. Animals 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The Role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef]
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: Prediction of diarrhea. ISME J. 2020, 14, 2223–2235. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4. [Google Scholar] [CrossRef]
- Sadler, R.; Cramer, J.V.; Heindl, S.; Kostidis, S.; Betz, D.; Zuurbier, K.R.; Northoff, B.H.; Heijink, M.; Goldberg, M.P.; Plautz, E.J.; et al. Short chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. 2020, 40, 1162–1173. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Han, D.; Pi, Y.; Tao, S.; Zhang, S.; Wang, S.; Zhang, J.; Chen, L.; Wang, J. Early life administration of milk fat globule membrane promoted SCFA-producing bacteria colonization, intestinal barriers and growth performance of neonatal piglets. Anim. Nutr. 2021, 7, 346–355. [Google Scholar] [CrossRef]
- Regassa, A.; Nyachoti, C.M. Application of resistant starch in swine and poultry diets with particular reference to gut health and function. Anim. Nutr. 2018, 4, 305–310. [Google Scholar] [CrossRef]
- Xin, H.; Ma, T.; Xu, Y.; Chen, G.; Chen, Y.; Villot, C.; Renaud, D.L.; Steele, M.A.; Guan, L.L. Characterization of fecal branched-chain fatty acid profiles and their associations with fecal microbiota in diarrheic and healthy dairy calves. J. Dairy Sci. 2021, 104, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Sun, C.; Wang, M.; Ji, X.; Li, S.; Jin, E.; Zhang, F. Linarin alleviates colonic barrier dysfunction induced by enterotoxic Escherichia coli in weaned piglets by regulating the gut microbiota and metabolic pathways. Front. Immunol. 2025, 16, 1631991. [Google Scholar] [CrossRef]
- Han, X.; Hu, X.; Jin, W.; Liu, G. Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets. Anim. Nutr. 2024, 17, 188–207. [Google Scholar] [CrossRef]
- Lee, H.J.; Khan, M.A.; Lee, W.S.; Yang, S.H.; Kim, S.B.; Ki, K.S.; Kim, H.S.; Ha, J.K.; Choi, Y.J. Influence of equalizing the gross composition of milk replacer to that of whole milk on the performance of Holstein calves. J. Anim. Sci. 2009, 87, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pang, S.; Wang, Q.; Tang, Y.; Li, Q.; Zhang, W.; Nie, C.; Ma, X.; Niu, J. Clostridium butyricum Supplementation Reduces Diarrhea in Preweaning Calves by Modulating Fecal Short-Chain Fatty Acids and Gut Microbiota. Microorganisms 2025, 13, 1993. [Google Scholar] [CrossRef]
- Wang, S.; Xu, P.; Zheng, L.; Qi, Y.; Liu, Z.; He, L.; Liu, C.; Hou, Y.; Guo, S.; Tong, Q.; et al. Effects of tannic acid on growth performance, intestinal barrier function and antioxidant capacity of piglets infected with porcine epidemic diarrhea virus. Chin. J. Anim. Nutr. 2023, 35, 4276–4286. Available online: https://link.cnki.net/urlid/11.5461.S.20230620.1634.076 (accessed on 21 October 2024).
- Megha, K.B.; Mohanan, P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian Decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef]
- Gandhar, J.S.; De, U.K.; Kala, A.; Malik, Y.S.; Yadav, S.; Paul, B.R.; Dixit, S.K.; Sircar, S.; Chaudhary, P.; Patra, M.K.; et al. Efficacy of microencapsulated probiotic as adjunct therapy on resolution of diarrhea, copper-zinc homeostasis, immunoglobulins, and inflammatory markers in serum of spontaneous rotavirus-infected diarrhoetic calves. Probiotics Antimicrob. Proteins 2022, 14, 1054–1066. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, A.; Bao, F.; He, M.; Gao, S.; Xu, J.; Zhang, X.; Niu, P.; Wang, C. Effect of nisin on microbiome-brain-gut axis neurochemicals by Escherichia coli-induced diarrhea in mice. Microb. Pathog. 2018, 119, 65–71. [Google Scholar] [CrossRef]
- De Araújo Farias, V.; Carrillo-Gálvez, A.B.; Martin, F.; Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Simujide, H.; Aricha, H.; Zhang, J.; Liu, B.; Zhang, C.; Cui, Y.; Aorigele, C. Effect of early pathogenic Escherichia coli infection on the intestinal barrier and immune function in newborn calves. Front. Cell. Infect. Microbiol. 2022, 12, 818276. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sánchez, C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Muñoz-Quezada, S.; Fontana, L.; Abadía-Molina, F. Microbial population changes and their relationship with human health and disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef]
- Markowiak-Kopec, P.; Slizewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Gao, K.; Peng, K.; Bi, S.; Cui, X.; Liu, Y. A Review of Nutritional Regulation of Intestinal Butyrate Synthesis: Interactions Between Dietary Polysaccharides and Proteins. Foods 2025, 14, 3649. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Poppe, J.; Baudot, A.; Dai Vu, L. Triacetin and a Mushroom Blend Restore Butyrate Production by IBS Microbiomes Ex Vivo, Thus Promoting Barrier Integrity. Int. J. Mol. Sci. 2025, 26, 9388. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, L.J.; Reyes, A.A.; Wolfe, C.A.; Weinroth, M.D.; Metcalf, J.L.; Delmore, R.J.; Belk, K.E.; Morley, P.S.; Engle, T.E. Bacteroidetes and firmicutes drive differing microbial diversity and community composition among micro-environments in the bovine rumen. Front. Vet. Sci. 2022, 9, 897996. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Zhang, T.; Si, H.; Nan, W.; Xu, C.; Guan, L.; Wright, A.D.G.; Li, G. The development of microbiota and metabolome in small intestine of sika deer (Cervus nippon) from birth to weaning. Front. Microbiol. 2018, 9, 4. [Google Scholar] [CrossRef]
- Foster, D.; Smith, G.W. Pathophysiology of diarrhea in calves. Vet. Clin. N. Am.-Food Anim. Pract. 2009, 25, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric pathogens and their toxin induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- Hansen, R.; Russell, R.K.; Reiff, C. Microbiota of de-novo pediatric IBD: Increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn’s but not in ulcerative colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shen, Q.; Lyu, W.; Lv, L.; Wang, W.; Yu, M.; Yang, H.; Tao, S.; Xiao, Y. Clostridium butyricum and its derived extracellular vesicles modulate gut homeostasis and ameliorate acute experimental colitis. Microbiol. Spectr. 2022, 10, e01368-22. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Xie, A.; Sun, J.; Yang, H.; Li, J.; Li, Y.; Chen, F.; Mei, Y.; Liang, Y. Lactobacillus rhamnosus and L. plantarum combination treatment ameliorated colitis symptoms in a mouse model by altering intestinal microbial composition and suppressing inflammatory response. Mol. Nutr. Food Res. 2023, 67, 2200340. [Google Scholar] [CrossRef]
- Zeineldin, M.M.; Aldridge, B.M.; Lowe, J. Dysbiosis of the fecal microbiota in feedlot cattle with hemorrhagic diarrhea. Microb. Pathog. 2018, 115, 123–130. [Google Scholar] [CrossRef]
- Ma, T.; O’hara, E.; Song, Y.; Fischer, A.J.; He, Z.; Steele, M.A.; Guan, L.L. Altered mucosa-associated microbiota in the ileum and colon of neonatal calves in response to delayed first colostrum feeding. J. Dairy Sci. 2019, 102, 7073–7086. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; Mcgregor, N.; Stapleton, D.I.; Butt, H.L.; De Meirleir, K.L. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. Vivo 2009, 23, 621–628. Available online: https://iv.iiarjournals.org/content/23/4/621 (accessed on 2 December 2024).
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Yang, X.; Lv, Z.; Li, P.; Zhang, M.; Wei, F.; Jin, X.; Hu, Y.; Guo, Y.; et al. Mining chicken ileal microbiota for immunomodulatory microorganisms. ISME J. 2023, 17, 758–774. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 4736. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Wan, F.; Wang, M.; Zhong, R.; Chen, L.; Han, H.; Liu, L.; Zhao, Y.; Lv, H.; Hou, F.; Yi, B.; et al. Supplementation with chinese medicinal plant extracts from Lonicera hypoglauca and Scutellaria baicalensis mitigates colonic inflammation by regulating oxidative stress and gut microbiota in a colitis mouse model. Front. Cell. Infect. Microbiol. 2021, 11, 798052. [Google Scholar] [CrossRef]
- Yan, S.; Qiao, L.; Dou, X.; Song, X.; Chen, Y.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles by Lactobacillus casei ATCC 393 alleviate the intestinal permeability, mitochondrial dysfunction and mitophagy induced by oxidative stress. Food Funct. 2021, 12, 7068–7080. [Google Scholar] [CrossRef]
- Zha, A.; Tu, R.; Qi, M.; Tan, B.; Liao, P.; Wu, C.; Yin, Y. Mannan oligosaccharides selenium ameliorates intestinal mucosal barrier and regulate intestinal microbiota to prevent Enterotoxigenic Escherichia coli-induced diarrhea in weaned piglets. Ecotoxicol. Environ. Saf. 2023, 264, 115448. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Feng, J.; Jie, Z. The role of hypoxia inducible factor-lα on inflammation mediated by HlNl infected macrophage. J. Microbes Infect. 2021, 16, 164–170. [Google Scholar] [CrossRef]
- Tomlinson, K.L.; Riquelme, S.A.; Baskota, S.U.; Drikic, M.; Monk, I.R.; Stinear, T.P.; Lewis, I.A.; Prince, A.S. Staphylococcus aureus stimulates neutrophil itaconate production that suppresses the oxidative burst. Cell Rep. 2023, 42, 112064. [Google Scholar] [CrossRef]
| Parameter | Unit | Treatments | p-Value | |
|---|---|---|---|---|
| Healthy (H) | Diarrheic (D) | |||
| Alanine aminotransferase (ALT) | U/L | 4.50 ± 0.73 | 5.00 ± 0.86 | 0.467 |
| Total protein (TP) | g/L | 65.73 ± 4.21 | 60.56 ± 3.74 | 0.841 |
| Albumin (ALB) | g/L | 37.1 ± 2.54 | 36.70 ± 2.98 | 0.566 |
| Globulin (GLB) | g/L | 28.46 ± 1.88 | 23.76 ± 2.04 | 0.232 |
| Aspartate aminotransferase (AST) | U/L | 40.71 ± 2.97 b | 48.33 ± 3.55 a | 0.021 |
| Alkaline phosphatase (ALP) | U/L | 182.55 ± 15.44 | 168.66 ± 48.97 | 0.059 |
| Urea nitrogen (BUN) | mmol/L | 2.46 ± 0.57 | 2.53 ± 0.93 | 0.250 |
| Glucose (GLU) | mmol/L | 5.50 ± 0.99 | 5.56 ± 0.74 | 0.658 |
| Triglycerides (TG) | mmol/L | 0.46 ± 0.001 | 0.48 ± 0.001 | 0.325 |
| Total cholesterol (TC) | mmol/L | 2.95 ± 0.32 | 2.35 ± 0.11 | 0.656 |
| Creatine kinase (CK) | U/L | 232.66 ± 33.77 | 149.66 ± 29.89 | 0.064 |
| Lactate dehydrogenase (LDH) | U/L | 652.66 ± 50.47 | 641.66 ± 54.32 | 0.489 |
| Uric creatinine (UCr) | mmol/L | 65.66 ± 4.15 | 71.00 ± 5.74 | 0.326 |
| Parameter | Unit | Treatments | p-Value | |
|---|---|---|---|---|
| Healthy (H) | Diarrheic (D) | |||
| Immunoglobulin A (IgA) | µg/mL | 783.25 ± 37.62 a | 574 ± 3.11 b | 0.019 |
| Immunoglobulin M (IgM) | µg/mL | 1715.22 ± 106.64 | 1699.86 ± 108.08 | 0.075 |
| Immunoglobulin G (IgG) | g/L | 18.28 ± 1.59 a | 12.46 ± 1.25 b | 0.002 |
| Interleukin-1β (IL-1β) | pg/mL | 247.30 ± 46.62 b | 455.78 ± 49.14 a | 0.005 |
| Interleukin-10 (IL-10) | pg/mL | 23.94 ± 3.12 a | 17.64 ± 2.38 b | 0.036 |
| Tumor necrosis factor-α (TNF-α) | pg/mL | 195.72 ± 19.17 b | 235.39 ± 9.09 a | 0.044 |
| Transforming growth factor-β (TGF-β) | pg/mL | 788.66 ± 58.08 | 483.19 ± 33.49 | 0.078 |
| Parameter | Unit | Treatments | p-Value | |
|---|---|---|---|---|
| Healthy (H) | Diarrheic (D) | |||
| endotoxin (ET) | EU/mL | 10.50 ± 35.14 b | 14.91 ± 30.48 a | 0.012 |
| diamine oxidase (DAO) | ng/mL | 4.98 ± 0.33 b | 6.11 ± 0.42 a | 0.034 |
| Parameter | Unit | Treatments | p-Value | |
|---|---|---|---|---|
| Healthy (H) | Diarrheic (D) | |||
| Acetic acid | μg/g | 1563.69 ± 109.82 | 1322.23 ± 105.78 | <0.001 |
| Propionic acid | μg/g | 729.04 ± 151.29 a | 233.54 ± 51.33 b | <0.001 |
| Butyric acid | μg/g | 447.12 ± 55.09 a | 265.48 ± 76.29 b | 0.026 |
| Isobutyric acid | μg/g | 91.74 ± 25.15 | 85.72 ± 1.19 | 0.605 |
| Valeric acid | μg/g | 26.60 ± 3.41 a | 7.69 ± 1.02 b | 0.021 |
| Isovaleric acid | μg/g | 69.66 ± 8.64 | 53.38 ± 4.79 | 0.22 |
| Caproic acid | μg/g | 3.62 ± 0.12 | 3.51 ± 0.34 | 0.218 |
| Parameter | Treatments | p-Value | |
|---|---|---|---|
| Healthy (H) | Diarrheic (D) | ||
| Chao 1 index | 238.13 ± 36.54 a | 86.85 ± 19.52 b | 0.001 |
| Observed_features index | 233.67 ± 26.21 a | 85.17 ± 16.66 b | 0.032 |
| Shannon index | 4.78 ± 0.35 | 3.34 ± 0.31 | 0.058 |
| Simpson index | 0.92 ± 0.02 | 0.80 ± 0.01 | 0.234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Pang, S.; Tang, Y.; Wang, Q.; Li, Q.; Zhang, W.; Nie, C.; Niu, J.; Lian, K. Alterations in Serum Immune Parameters, Cytokines, Intestinal Permeability, Fecal Microbiota, and Short-Chain Fatty Acids in Healthy and Diarrheic Suckling Calves. Agriculture 2025, 15, 2289. https://doi.org/10.3390/agriculture15212289
Gao P, Pang S, Tang Y, Wang Q, Li Q, Zhang W, Nie C, Niu J, Lian K. Alterations in Serum Immune Parameters, Cytokines, Intestinal Permeability, Fecal Microbiota, and Short-Chain Fatty Acids in Healthy and Diarrheic Suckling Calves. Agriculture. 2025; 15(21):2289. https://doi.org/10.3390/agriculture15212289
Chicago/Turabian StyleGao, Peiyun, Shaoyang Pang, Yaqin Tang, Qianqian Wang, Qiuyan Li, Wenju Zhang, Cunxi Nie, Junli Niu, and Kexun Lian. 2025. "Alterations in Serum Immune Parameters, Cytokines, Intestinal Permeability, Fecal Microbiota, and Short-Chain Fatty Acids in Healthy and Diarrheic Suckling Calves" Agriculture 15, no. 21: 2289. https://doi.org/10.3390/agriculture15212289
APA StyleGao, P., Pang, S., Tang, Y., Wang, Q., Li, Q., Zhang, W., Nie, C., Niu, J., & Lian, K. (2025). Alterations in Serum Immune Parameters, Cytokines, Intestinal Permeability, Fecal Microbiota, and Short-Chain Fatty Acids in Healthy and Diarrheic Suckling Calves. Agriculture, 15(21), 2289. https://doi.org/10.3390/agriculture15212289






