Economic Perspectives on Farm Biosecurity: Stakeholder Challenges and Livestock Species Considerations
Abstract
1. Introduction
2. Economic Barriers to Biosecurity Adoption
3. Stakeholder Knowledge Gaps and Perceptions
4. Comparative Regional Frameworks and Governance Contexts
5. Species-Specific Biosecurity Considerations Across Livestock Systems
| Species | Production System | Key Economic Drivers | Key Disease Risks | Adoption Barriers (Incl. Finance/Market Access) | Recommended Interventions | References |
|---|---|---|---|---|---|---|
| Cattle (beef and dairy) | Intensive | Market security and productivity: maintaining access to lucrative markets by preventing trade-disruption outbreaks; improved welfare/health boosts productivity and profitability. Risk-mitigation economics: herd-health/biosecurity programmes can yield net benefits when implemented well. | Transboundary and endemic: FMD trade shocks; endemic BVD, brucellosis, TB; emerging risks (e.g., lumpy skin disease). Historic crises (FMD/BSE) illustrate huge cull/export effects. | Costs and labour: upfront (fencing, facilities) and ongoing labour/time burdens. Knowledge/trust gaps: inconsistent advice; some producers unconvinced of costs and benefits without lived outbreak experience. | Incentives and education: tailored, trustworthy vet-led training; risk-based herd plans; conditional subsidies/insurance/compensation linked to verifiable biosecurity; simple checklists and farmer-led monitoring to sustain compliance. | Buchan et al., 2023 [1] Brennan & Christley 2013 [39] Renault et al., 2021 [40] |
| Smallholder/backyard | Household protection: reduce avoidable morbidity/mortality; incremental access to formal buyers. | Same as intensive; higher contact mixing through informal trade/movements. | Finance/indemnity exclusion; thin margins; information asymmetry; weak buyer linkages. | Micro-grants/micro-insurance; mobile extension; cooperative marketing rewarding compliance; compensation contingent on basic biosecurity. | OECD (2017) [23] | |
| Swine (pigs) | Intensive | Catastrophic-loss avoidance (ASF/CSF/PRRS); export access; supply-contract continuity. | ASF, CSF, PRRS; wildlife interface where relevant. | Cost/logistics of all-in/all-out and strict access control; compliance fatigue. | Fencing; controlled entry (visitor/vehicle logs and disinfection); all-in/all-out; company-supported upgrades; surge surveillance. | EFSA AHAW (2021) [60] |
| Smallholder/backyard | Livelihood preservation; avert whole-herd wipe-outs; keep local sales running. | ASF persistence in backyard/outdoor units with weak biosecurity; proximity to infected premises. | Finance/compensation gaps; swill feeding as a low-cost practice; weak enforcement; fatalism. | Phase-out or heat-treat swill with affordable alternatives; simple, auditable checklists; rapid reporting with fair, conditional compensation; local feed alternatives; community messaging on ASF consequences. | Boklund et al., 2020 [65]; Penrith 2020 [66] | |
| Poultry (broilers and layers) | Intensive | Avoid mass culls and trade bans; retailer standards; productivity gains from healthier birds. | HPAI (H5Nx), ND, Salmonella. | Protocol fatigue; uneven enforcement across firms/regions. | Company QA and audits; downtime and cleaning; contingency planning; vaccination per risk. | Ukita et al., 2025 [31] |
| Smallholder/backyard | Mortality reduction and income stability; potential on-ramps to better outlets. | HPAI/ND in backyard settings; mixed flocks; wild-bird contact. | Low training coverage; limited resources; no premium for safer product. | Community ND vaccination; simple entry control (handwashing, footbaths), isolation of new birds; targeted extension; routes into local certification to reward prevention. | Otieno et al., 2023 [6]; Samanta et al., 2015 [55]; Napit et al., 2023 [61]. | |
| Small ruminants (sheep and goats) | Intensive | Maintain output quality; disease-free status. | PPR; pox; brucellosis. | Distributed herds; movement patterns. | Scheduled vaccination; coordinated movements; record-keeping. | OIE-FAO. 9 (2015) [67] |
| Smallholder/pastoral | Safeguard “living bank” assets; avert high-mortality shocks; protect nutrition. | PPR with very high morbidity/mortality in naïve flocks. | Sparse veterinary reach; cash/credit limits; communal grazing; mobility. | Free mass vaccination with para-vet networks; community quarantine; micro-finance for pens/water points; targeted awareness. | Rahman et al., 2021 [62]; Govindaraj et al., 2023 [68]; FAO/WOAH 2025 [69]. | |
| Aquaculture | Intensive | Protect dense, high-value biomass; meet export certification. | ISA (salmon), vibriosis; rapid water-borne spread. | Water connectivity; input quality risks. | Certified seed; water treatment; area-based management; synchronised fallowing; early testing and removal. | World Bank 2014 [70]; Godoy et al., 2013 [71]. |
| Smallholder | Avoid pond wipe-outs; steady cash flow in village economies. | Same as intensive; amplified by shared water and fry trade. | Finance/coordination gaps; weak extension; neighbours’ non-compliance undermines private returns. | Cluster/zone biosecurity; hatchery accreditation; emergency funds/insurance with simple compliance proofs; adopt FAO/WOAH PMP/AB (stepwise, inclusive). | FAO (2023) [72]. |
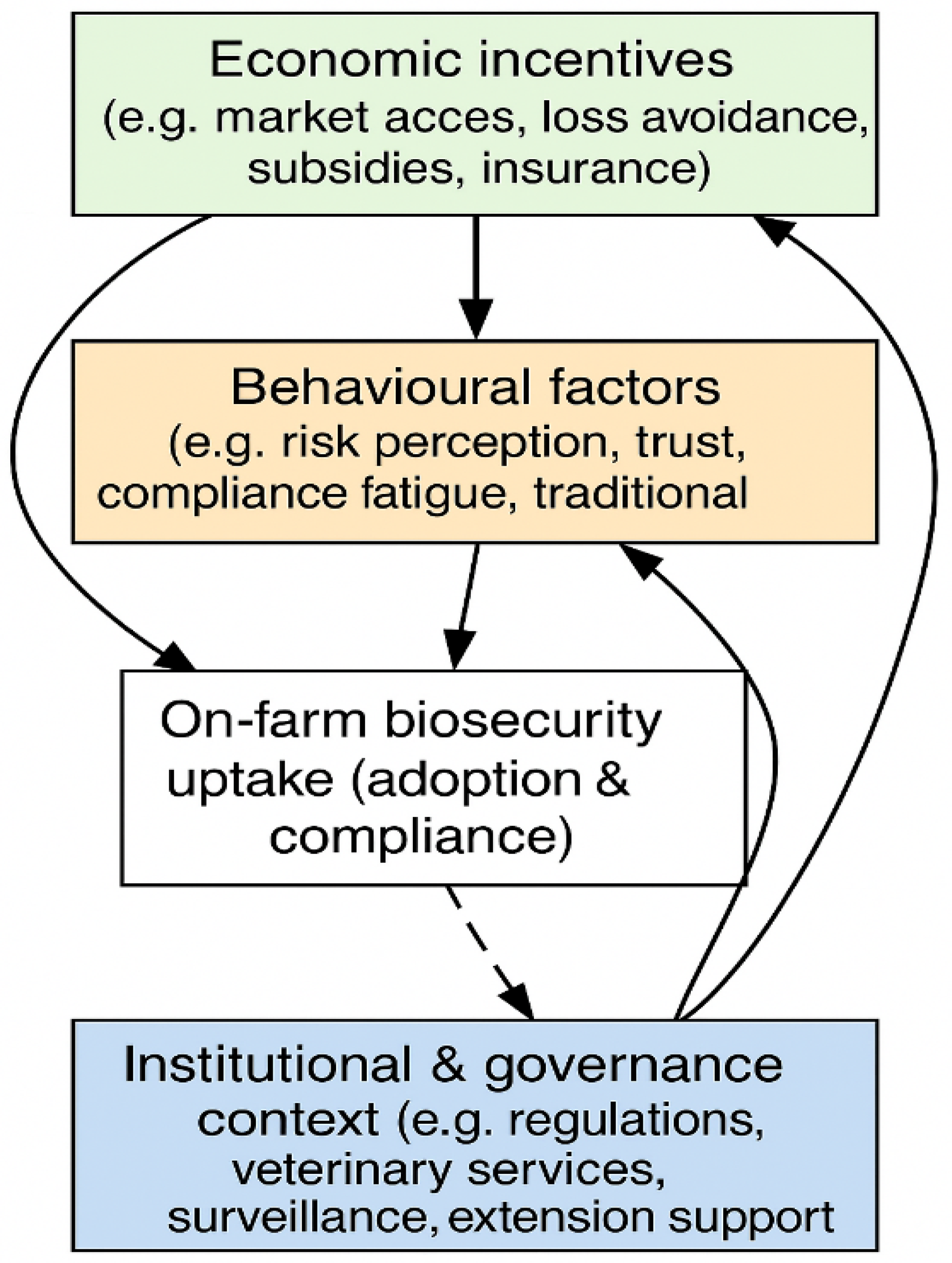
5.1. Behavioural (Farmer Attitudinal) Factors
5.2. Institutional and Governance Context
5.3. Interactions and Feedback Loops
6. Strategic Recommendations and Future Perspectives: Embedding Economic Incentives and Stakeholder Alignment into Farm Biosecurity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchan, M.S.; Lhermie, G.; Mijar, S.; Pajor, E.; Orsel, K. Individual drivers and barriers to adoption of disease control and welfare practices in dairy and beef cattle production: A scoping review. Front. Vet. Sci. 2023, 10, 1104754. [Google Scholar] [CrossRef] [PubMed]
- Militzer, N.; McLaws, M.; Rozstalnyy, A.; Li, Y.; Dhingra, M.; Auplish, A.; Mintiens, K.; Sabirovic, M.; von Dobschuetz, S.; Heilmann, M. Characterising biosecurity initiatives globally to support the development of a progressive management pathway for terrestrial animals: A scoping review. Animals 2023, 13, 2672. [Google Scholar] [CrossRef]
- Ogundijo, O.A.; Omotosho, O.O.; Al-Mustapha, A.I.; Abiola, J.O.; Awosanya, E.J.; Odukoya, A.; Owoicho, S.; Oyewo, M.; Ibrahim, A.; Orum, T.G.; et al. A multi-state survey of farm-level preparedness towards African swine fever outbreak in Nigeria. Acta Trop. 2023, 246, 106989. [Google Scholar] [CrossRef] [PubMed]
- Hallet, L. Les modes de collaboration entre vétérinaires officiels, vétérinaires privés et organisations d’éleveurs [Collaboration between official veterinarians, private veterinarians and livestock producer organisations]. Rev. Sci. Tech. 2003, 22, 523–532. [Google Scholar] [CrossRef]
- Lane, J.K.; Kelly, T.; Bird, B.; Chenais, E.; Roug, A.; Vidal, G.; Gallardo, R.; Zhou, H.; VanHoy, G.; Smith, W. A One Health approach to reducing livestock disease prevalence in developing countries: Advances, challenges, and prospects. Annu. Rev. Anim. Biosci. 2025, 13, 277–302. [Google Scholar] [CrossRef]
- Niemi, J.; Bennett, R.; Clark, B.; Frewer, L.; Jones, P.; Rimmler, T.; Tranter, R. A value chain analysis of interventions to control production diseases in the intensive pig production sector. PLoS ONE 2020, 15, e0231338. [Google Scholar] [CrossRef] [PubMed]
- Renault, V.; Lomba, M.; Delooz, L.; Ribbens, S.; Humblet, M.F.; Saegerman, C. Pilot study assessing the possible benefits of a higher level of implementation of biosecurity measures on farm productivity and health status in Belgian cattle farms. Transbound. Emerg. Dis. 2020, 67, 769–777. [Google Scholar] [CrossRef]
- Kovács, L.; Klaucke, C.R.; Farkas, M.; Bakony, M.; Jurkovich, V.; Könyves, L. The correlation between on-farm biosecurity and animal welfare indices in large-scale turkey production. Poult. Sci. 2025, 104, 104598. [Google Scholar] [CrossRef]
- Msimang, V.; Rostal, M.K.; Cordel, C.; Machalaba, C.; Tempia, S.; Bagge, W.; Burt, F.J.; Karesh, W.B.; Paweska, J.T.; Thompson, P.N. Factors affecting the use of biosecurity measures for the protection of ruminant livestock and farm workers against infectious diseases in central South Africa. Transbound. Emerg. Dis. 2022, 69, e1899. [Google Scholar] [CrossRef]
- Isomura, R.; Matsuda, M.; Sugiura, K. An epidemiological analysis of the level of biosecurity and animal welfare on pig farms in Japan and their effect on the use of veterinary antimicrobials. J. Vet. Med. Sci. 2018, 80, 1853–1860. [Google Scholar] [CrossRef]
- Lurette, A.; Touzeau, S.; Ezanno, P.; Hoch, T.; Seegers, H.; Fourichon, C.; Belloc, C. Within-herd biosecurity and Salmonella seroprevalence in slaughter pigs: A simulation study. J. Anim. Sci. 2011, 89, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Osawe, O.W.; Läpple, D.; Mee, J.F. Economic analysis of biosecurity adoption in dairy farming: Evidence from Ireland. J. Anim. Sci. 2022, 100, skac218. [Google Scholar] [CrossRef] [PubMed]
- Renault, V.; Hambe, H.A.; Van Vlaenderen, G.; Timmermans, E.; Mohamed, A.M.; Ethgen, O.; Saegerman, C. Economic impact of contagious caprine pleuropneumonia and cost-benefit analysis of vaccination programmes based on one-year continuous monitoring of flocks in arid and semi-arid lands of Kenya. Transbound. Emerg. Dis. 2019, 66, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, P.; Chantziaras, I.; Vijay, D.; Bedi, J.S.; Makovska, I.; Biebaut, E.; Dewulf, J. Can improved farm biosecurity reduce the need for antimicrobials in food animals? A scoping review. Antibiotics 2023, 12, 893. [Google Scholar] [CrossRef]
- Fasina, F.O.; Ali, A.M.; Yilma, J.M.; Thieme, O.; Ankers, P. The cost-benefit of biosecurity measures on infectious diseases in Egyptian household poultry. Prev. Vet. Med. 2012, 103, 178–191. [Google Scholar] [CrossRef]
- Otieno, W.A.; Nyikal, R.A.; Mbogoh, S.G.; Rao, E.J.O. Adoption of farm biosecurity practices among smallholder poultry farmers in Kenya: A latent class analysis. Prev. Vet. Med. 2023, 217, 105967. [Google Scholar] [CrossRef]
- Pao, H.; Jackson, E.; Yang, T.; Tsai, J.; Sung, W.H.; Pfeiffer, D.U. Determinants of farmers’ biosecurity mindset: A social-ecological model using systems thinking. Front. Vet. Sci. 2022, 9, 959934. [Google Scholar] [CrossRef]
- Chepkwony, M.C.; Makau, D.N.; Yoder, C.; Corzo, C.; Culhane, M.; Perez, A.; Perez Aguirreburualde, M.S.; Nault, A.J.; Mahero, M. A scoping review of knowledge, attitudes, and practices in swine farm biosecurity in North America. Front. Vet. Sci. 2025, 12, 1507704. [Google Scholar] [CrossRef]
- Di Francesco, J.; Isenhower, E.; Fausak, E.D.; Silva-Del-Rio, N.; Pires, A.F. A scoping review of studies reporting biosecurity practices in small and backyard farms raising livestock or poultry in developed countries, 2000–2022. Prev. Vet. Med. 2025, 236, 106423. [Google Scholar] [CrossRef]
- Moya, S.; Lamont, K.; Brennan, M.L.; Ciavarino, G.; Costa, M.; Allepuz, A.; Tamminen, L.-M.; Correia-Gomes, C.; de Carvalho Ferreira, H.; Dogusan, M.M.; et al. Stakeholders’ perspectives on communicating biosecurity to encourage behavior change in farmers. Front. Vet. Sci. 2025, 12, 1562648. [Google Scholar] [CrossRef]
- Nantima, N.; Davies, J.; Dione, M.; Ocaido, M.; Okoth, E.; Mugisha, A.; Bishop, R. Enhancing knowledge and awareness of biosecurity practices for control of African swine fever among smallholder pig farmers along the Kenya–Uganda border. Trop. Anim. Health Prod. 2016, 48, 727–734. [Google Scholar] [CrossRef]
- Buckel, A.; Afakye, K.; Koka, E.; Price, C.; Kabali, E.; Caudell, M.A. Understanding the factors influencing biosecurity adoption on smallholder poultry farms in Ghana: A qualitative analysis using the COM-B model and Theoretical Domains Framework. Front. Vet. Sci. 2024, 11, 1324233. [Google Scholar] [CrossRef] [PubMed]
- OECD. Producer Incentives in Livestock Disease Management; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Campler, M.R.; Hall, M.; Mills, K.; Galvis, J.A.; Machado, G.; Arruda, A.G. Description of swine producer biosecurity planning for foreign animal disease preparedness using the Secure Pork Supply framework. Front. Vet. Sci. 2024, 11, 1380623. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, K.; Boklund, A.E.; Podgórski, T.; Vergne, T.; Aminalragia-Giamini, R.; Abrahantes, J.C.; Papaleo, S.; Mur, L. Epidemiological analysis of African swine fever in the European Union during 2024. EFSA J. 2025, 23, e9436. [Google Scholar] [CrossRef]
- Niemi, J.K.; Sahlström, L.; Kyyrö, J.; Lyytikäinen, T.; Sinisalo, A. Farm characteristics and perceptions regarding costs contribute to the adoption of biosecurity in Finnish pig and cattle farms. Rev. Agric. Food Environ. Stud. 2016, 97, 215–222. [Google Scholar] [CrossRef][Green Version]
- Baye, R.S.; Zia, A.; Merrill, S.C.; Clark, E.M.; Koliba, C.; Smith, J.M. Biosecurity indemnification and attitudes of United States swine producers towards the prevention of an African swine fever outbreak. Prev. Vet. Med. 2024, 227, 106193. [Google Scholar] [CrossRef]
- Piao, S.; Jin, X.; Hu, S.; Lee, J. The impact of African swine fever on the efficiency of China’s pig farming industry. Sustainability 2023, 16, 7819. [Google Scholar] [CrossRef]
- Gren, I.; Andersson, H.; Jonasson, L. Benefits and costs of measures to tackle the outbreak of African swine fever in Sweden. Prev. Vet. Med. 2024, 236, 106353. [Google Scholar] [CrossRef]
- Koppes, P.; Guerrant, T.; Marks, D.; Balcerzak, E.; Brown, J.; Harman, M.; Shwiff, S. An economic evaluation of preventing vs. suppressing HPAI outbreaks: A case study from Iowa. Prev. Vet. Med. 2025, 244, 106651. [Google Scholar] [CrossRef]
- Ukita, M.; Yasuda, A.; Kikuchi, E.; Hinata, T.; Makita, K. Direct economic losses in farms and government compensation costs due to highly pathogenic avian influenza outbreaks in Japan during the 2022–23 season. J. Vet. Med. Sci. 2025, 87, 976–985. [Google Scholar] [CrossRef]
- Bureau for Food and Agricultural Policy (BFAP). Economic Impact of the 2017 Highly Pathogenic Avian Influenza Outbreak in South Africa. BFAP Reports 279770. 2018. Available online: https://www.bfap.co.za (accessed on 20 October 2025).
- Humphreys, J.M.; Stenfeldt, C.; King, D.P.; Knight-Jones, T.; Perez, A.M.; VanderWaal, K.; Sanderson, M.W.; Di Nardo, A.; Jemberu, W.T.; Pamornchainavakul, N.; et al. Epidemiology and economics of foot-and-mouth disease: Current understanding and knowledge gaps. Vet. Res. 2025, 56, 141. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The EU Animal Health Law; EUR-Lex: Brussels, Belgium, 2023; Available online: https://eur-lex.europa.eu/EN/legal-content/summary/the-eu-animal-health-law.html (accessed on 20 October 2025).
- Akazawa, N.; Alvial, A.; Baloi, A.P.; Blanc, P.-P.; Brummett, R.E.; Burgos, J.M.; Chamberlain, G.C.; Chamberlain, G.W.; Forster, J.; Hao, N.V.; et al. Reducing Disease Risk in Aquaculture; Agriculture and Environmental Services Discussion Paper No. 9; World Bank Group: Washington, DC, USA, 2014; Available online: http://documents.worldbank.org/curated/en/110681468054563438 (accessed on 21 October 2025).
- Persky, J. Retrospectives: The ethology of Homo economicus. J. Econ. Perspect. 1995, 9, 221–231. [Google Scholar] [CrossRef]
- Fasina, F.O.; Lazarus, D.D.; Spencer, B.T.; Makinde, A.A.; Bastos, A.D. Cost implications of African swine fever in smallholder farrow-to-finish units: Economic benefits of disease prevention through biosecurity. Transbound. Emerg. Dis. 2012, 59, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Suartana, D.P.; Suryanto, S.; Arzam, T.S. Mortality and economic impact of African swine fever (ASF) outbreak on pigs in Luwu Timur Regency. Influ. Int. J. Sci. Rev. 2024, 6, 259–268. [Google Scholar]
- Brennan, M.L.; Christley, R.M. Cattle producers’ perceptions of biosecurity. BMC Vet. Res. 2013, 9, 71. [Google Scholar] [CrossRef]
- Renault, V.; Humblet, M.F.; Pham, P.N.; Saegerman, C. Biosecurity at cattle farms: Strengths, weaknesses, opportunities and threats. Pathogens 2021, 10, 1315. [Google Scholar] [CrossRef]
- Lestari, V.; Sirajuddin, S.N.; Abdullah, A. Constraints of biosecurity adoption on beef cattle farms. Eur. J. Sustain. Dev. 2018, 7, 151–156. [Google Scholar] [CrossRef]
- Morris, G. U.S. small-scale livestock operation approach to biosecurity. Agriculture 2023, 13, 2086. [Google Scholar] [CrossRef]
- Siekkinen, K.M.; Heikkilä, J.; Tammiranta, N.; Rosengren, H. Measuring the costs of biosecurity on poultry farms: A case study in broiler production in Finland. Acta Vet. Scand. 2012, 54, 12. [Google Scholar] [CrossRef]
- Birhanu, M.Y.; Jensen, N. Dynamics of improved agricultural technologies adoption: The chicken and maize paradox in Ethiopia. Sustain. Futures 2023, 5, 100112. [Google Scholar] [CrossRef]
- Tsegaye, D.; Tamir, B.; Gebru, G. Assessment of biosecurity practices and its status in small- and medium-scale commercial poultry farms in Arsi and East Showa zones, Oromia, Ethiopia. Poultry 2023, 2, 334–348. [Google Scholar] [CrossRef]
- Arjmand, A.; Bani-Yaghoub, M.; Corkran, K.; Pandit, P.S.; Aly, S.S. Assessing the impact of biosecurity compliance on farmworker and livestock health within a One Health modelling framework. One Health 2025, 20, 101023. [Google Scholar] [CrossRef]
- Inamura, M.; Rushton, J.; Antón, J. Risk Management of Outbreaks of Livestock Diseases; OECD Food, Agriculture and Fisheries Papers, No. 91; OECD Publishing: Paris, France, 2015. [Google Scholar] [CrossRef]
- Rich, K.M.; Perry, B.D. The economic and poverty impacts of animal disease in developing countries: New roles, new demands for economics and epidemiology. Prev. Vet. Med. 2011, 101, 133–147. [Google Scholar] [CrossRef]
- Heikkilä, J.; Niemi, J.K.; Heinola, K.; Liski, E.; Myyrä, S. Anything left for animal disease insurance? A choice experiment approach. Rev. Agric. Food Environ. Stud. 2016, 97, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Toson, M.; Dalla Pozza, M.; Ceschi, P. Farmers’ biosecurity awareness in small-scale Alpine dairy farms and the crucial role of veterinarians. Animals 2023, 14, 2032. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.S. Taxonomy of Educational Objectives: The Classification of Educational Goals. Vol. 1: Cognitive Domain; David McKay Company: New York, NY, USA, 1956. [Google Scholar]
- Biebaut, E.; Štukelj, M.; Chantziaras, I.; Nunes, T.P.; Nedosekov, V.; Gomes, C.C.; Mehmedi, B.; Corrégé, I.; Ózsvári, L.; Svennesen, L.; et al. Large heterogeneity in biosecurity legislation in the intensive pig production across Europe. Prev. Vet. Med. 2025, 237, 106439. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Tilli, G.; Laconi, A.; Ngom, R.V.; Leite, M.; Prodanov-Radulović, J.; Allepuz, A.; Chantziaras, I.; Piccirillo, A. Implementation of biosecurity measures according to legislation in intensive poultry production: An overview across 22 EU and non-EU countries. Prev. Vet. Med. 2025, 242, 106571. [Google Scholar] [CrossRef]
- Dhollander, S.; Cattaneo, E.; Abrahantes, J.C.; Boklund, A.E.; Szczotka-Bochniarz, A.; Mihalca, A.D.; Papanikolaou, A.; Mur, L.; Balmoș, O.M.; Frant, M.; et al. Prospective case-control study of determinants for African swine fever introduction in commercial pig farms in Poland, Romania and Lithuania. Transbound. Emerg. Dis. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Samanta, I.; Joardar, S.N.; Ganguli, D.; Das, P.K.; Sarkar, U. Evaluation of egg production after adoption of biosecurity strategies by backyard poultry farmers in West Bengal. Vet. World 2015, 8, 177–182. [Google Scholar] [CrossRef]
- Shanta, I.S.; Hasnat, M.A.; Zeidner, N.; Gurley, E.S.; Azziz-Baumgartner, E.; Sharker, Y.; Hossain, K.; Khan, S.U.; Haider, N.; Bhuyan, A.A.; et al. Raising backyard poultry in rural Bangladesh: Financial and nutritional benefits, but persistent risky practices. Transbound. Emerg. Dis. 2017, 64, 1454–1464. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, X. African swine fever shock: China’s hog industry’s resilience and its influencing factors. Animals 2023, 13, 2817. [Google Scholar] [CrossRef]
- McLaws, M.; Tago Pacheco, D.; Auplish, A.; Heilmann, M.; Pica-Ciamarra, U.; Dhingra, M. FAO Progressive Management Pathway for Terrestrial Animal Biosecurity (FAO-PMP-TAB): Putting the Framework into Action; FAO Animal Production and Health Handbooks, No. 3; FAO: Rome, Italy, 2025. [Google Scholar]
- World Organisation for Animal Health. Terrestrial Animal Health Code, 2024 ed.; World Organisation for Animal Health: Paris, France, 2024; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/2024/en_index.htm (accessed on 21 October 2025).
- EFSA AHAW Panel. African swine fever and outdoor farming of pigs in the EU. EFSA J. 2021, 19, e06639. [Google Scholar] [CrossRef]
- Napit, R.; Poudel, A.; Pradhan, S.M.; Manandhar, P.; Ghaju, S.; Sharma, A.N.; Joshi, J.; Tha, S.; Dhital, K.; Rajbhandari, U.; et al. Newcastle disease burden in Nepal and efficacy of Tablet I2 vaccine. PLoS ONE 2023, 18, e0280688. [Google Scholar] [CrossRef]
- Rahman, A.K.M.A.; Islam, S.S.; Sufian, M.A.; Talukder, M.H.; Ward, M.P.; Martínez-López, B. Peste des petits ruminants risk factors and space-time clusters in Bangladesh. Front. Vet. Sci. 2020, 7, 572432. [Google Scholar] [CrossRef] [PubMed]
- Aina, I.V.; Ayinde, O.E.; Thiam, D.R.; Miranda, M.J. Climate risk adaptation through livestock insurance: Evidence from a pilot programme in Nigeria. Clim. Dev. 2024, 17, 383–394. [Google Scholar] [CrossRef]
- Mazviona, B.; Sølvsten, S.; Palwishah, R.I. Intensity of crop and livestock insurance adoption: Lessons from Mexico. Mitig. Adapt. Strateg. Glob. Change 2025, 30, 72. [Google Scholar] [CrossRef]
- Boklund, A.; Dhollander, S.; Vasile, T.C.; Abrahantes, J.C.; Bøtner, A.; Gogin, A.; Villeta, L.C.G.; Gortázar, C.; More, S.J. Risk factors for African swine fever incursion in Romanian domestic pig farms during 2019. Sci. Rep. 2020, 10, 10215. [Google Scholar] [CrossRef]
- Penrith, M. Management options to mitigate the risk of swill feeding. Bull. l’OIE 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- OIE; FAO. Global Strategy for the Control and Eradication of PPR (Peste Des Petits Ruminants); World Organisation for Animal Health & Food and Agriculture Organization: Paris, France, 2015; Available online: https://www.woah.org/app/uploads/2021/12/ppr-global-strategy-avecannexes-2015-03-28.pdf (accessed on 21 October 2025).
- Govindaraj, G.N.; Balamurugan, V.; Reddy, G.B.M.; Yogisharadhya, R.; Reddy, T.S.; Naveenkumar, G.S.; Kumar, K.V.; Chaithra, H.R.; Bi, A.Z.; Parida, S.; et al. Towards eradication of peste des petits ruminants (PPR): Disease status, economic cost and perception of veterinarians in Karnataka, India. Animals 2023, 13, 778. [Google Scholar] [CrossRef]
- FAO; WOAH. FAO and WOAH Call on Members to Strengthen Global Efforts to Eradicate Peste des Petits Ruminants (PPR), 9 October 2025. 2025. Available online: https://www.fao.org/ppr/news-and-events/news/detail/en/c/1743662/ (accessed on 22 October 2025).
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Godoy, M.G.; Kibenge, M.J.; Suarez, R.; Lazo, E.; Heisinger, A.; Aguinaga, J.; Bravo, D.; Mendoza, J.; Llegues, K.O.; Avendaño-Herrera, R.; et al. Infectious salmon anaemia virus (ISAV) in Chilean Atlantic salmon (Salmo salar) aquaculture: Emergence of low-pathogenic ISAV-HPR0 and re-emergence of virulent ISAV-HPR∆ (HPR3 and HPR14). Virol. J. 2013, 10, 344. [Google Scholar] [CrossRef]
- FAO. The Progressive Management Pathway for Aquaculture Biosecurity: Guidelines for Application; FAO Fisheries and Aquaculture Technical Paper No. 689; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://www.fao.org/3/cc6858en/cc6858en.pdf (accessed on 19 October 2025).
- Hernández-Jover, M.; Hayes, L.; Woodgate, R.; Rast, L.; Toribio, J.-A.L.M. Animal health management practices among smallholder livestock producers in Australia and their contribution to the surveillance system. Front. Vet. Sci. 2019, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Belisário-Moi, A. The Role of Biosecurity in Promoting Farm Animal Welfare in Low- and Middle-Income Countries (LMICs). In From Zoo to Farm—The Quest for Animal Welfare; Intechopen: London, UK, 2025. [Google Scholar] [CrossRef]
- Suit, B.Y.; Hassan, L.; Krauss, S.E.; Ooi, P.T.; Ramanoon, S.Z.; Yasmin, A.R.; Epstein, J.H. Mental model of Malaysian pig farmers in implementing disease prevention and control practices. Front. Vet. Sci. 2021, 8, 695702. [Google Scholar] [CrossRef]
- Mankad, A. Psychological influence on biosecurity and farmer decision-making: A review. Agron. Sustain. Dev. 2016, 36, 40. [Google Scholar] [CrossRef]
- Saegerman, C.; Niemi, J.K.; De Briyne, N.; Jansen, W.; Cantaloube, A.; Heylen, M.; Niine, T.; Jerab, J.G.; Allepuz, A.; Chantziaras, I.; et al. Scanning European Needs and Expectations Related to Livestock Biosecurity Training by Using the World Café Method. In Transboundary and Emerging Diseases; Wiley: Hoboken, NJ, USA, 2024; p. 6743691. [Google Scholar] [CrossRef]
- Marić, M.; Manghnani, V.; Niemi, J.K.; Niine, T.; De Briyne, N.; Jansen, W. Empowering Veterinary Herd Health Management: Insights into Education, Implementation, and Regulation Across Europe. Vet. Sci. 2024, 11, 528. [Google Scholar] [CrossRef]
- Iatrou, A.M.; Kastrati, B.M.; Gecaj, R.M.; Batikas, G.; Niemi, J.K.; Saegerman, C.; Allepuz, A.O.; Jansen, W.; De Briyne, N.; De Meneghi, D.; et al. What are desirable biosecurity trainings for veterinary practitioners and farmers? J. Biosaf. Biosecurity 2025, 7, 91–106. [Google Scholar] [CrossRef]
- Mehmedi, B.; Niemi, J.; Saegerman, C.; De Meneghi, D.; Iatrou, A.M.; Yildiz, R.; Chantziaras, I.; Allepuz, A.; Toppari, I.; Batikas, G.; et al. Tailored Biosecurity Training for Veterinarians and Farmers: Bridging Knowledge and Practice Gaps. Front. Vet. Sci. 2025, 12, 1643029. [Google Scholar] [CrossRef]
- Yildiz, R.; Batikas, G.; Iatrou, A.M.; Allepuz, A.O.; Mehmedi, B.; Gecaj, R.M.; De Nardi, M.; Toppari, I.; Wielick, C.; Saegerman, C.; et al. What is a “good” website for communicating biosecurity information to farmers and veterinarians? Res. Vet. Sci. 2025, 2025, 105938. [Google Scholar] [CrossRef]
| Identified Gap/Challenge | Recommendation (Future Perspective) | Target Audience |
| Farmers’ limited knowledge or misperceptions of biosecurity | Provide context-specific, economically framed education through on-farm demonstrations, peer mentoring, and accessible materials linking actions to profit. | F, V, E |
| One-size-fits-all messaging is failing to change behaviour | Use co-created biosecurity plans and collaborative farm visits framed as supportive coaching rather than enforcement. | F, V, G, E |
| High perceived costs and unclear benefits of biosecurity | Deliver robust cost–benefit analyses, decision-support tools, and real farm case studies; introduce market rewards, conditional insurance, and targeted subsidies. | F, V, G, I, F/Ins |
| Labour and time constraints on small farms | Simplify protocols into routine-compatible “easy wins” and promote low-cost interventions using locally available materials. | F, V, E |
| Lack of standardised metrics for small/backyard farms | Created digital tools and developed tiered checklists for different scales/species to enable incremental monitoring. | F, V, G, E, R |
| Smallholders and backyard producers are largely unreachable | Integrate them into national surveillance and support programmes; provide free outreach, vaccination drives, and community-based agreements on movement controls. | F, V, G, E, I |
| Data and transparency gaps | Establish anonymised data-sharing frameworks and multi-stakeholder reporting platforms to improve surveillance and trust. | G, V, I, R |
| Stakeholder trust and alignment issues | Foster a collaborative biosecurity culture: include farmers in policy design, recognise exemplary farms publicly, and create regular multi-actor forums for problem-solving. | F, V, G, I, C |
| Stakeholder | Module Title | Key Learning Objectives | Format and Delivery | Bloom’s Taxonomy Level and Cognitive Focus |
|---|---|---|---|---|
| Veterinary Practitioners | Economic risk assessment in livestock health | Quantify farm-level financial risks of disease incursions. Prioritise cost-effective preventive strategies using risk models. | Interactive workshops using real datasets; scenario simulations. | Understanding: recall and explain key economic and epidemiological parameters and principles. Applying: use quantitative tools to estimate financial impacts. Analysing: interpret patterns and prioritise cost-effective interventions. |
| Cost–benefit analysis of biosecurity investments | Evaluate returns on infrastructure (e.g., fencing, hygiene stations) and routine labour. Advise clients on capital vs. recurrent costs. | Spreadsheet exercises; group case discussions. | Understanding: describe core economic concepts (costs, returns). Applying: perform practical cost–benefit calculations. Evaluating: justify investment decisions based on evidence. | |
| Insurance, compensation, and moral hazard | Understand compensation policies and insurance products. Explain how conditional indemnities influence farmers’ decisions. Define “moral hazard” in the veterinary context (when coverage/payment designs blunt prevention incentives for vets or clients and compensation provider cannot observe this) Identify policy levers that restore prevention incentives (e.g., deductibles/co-pays, risk-based premiums, conditional compensation tied to documented biosecurity). Specify practical compliance evidence (checklists, logs, photos) and oversight (random audits/peer review of attestations). | Short courses with policy briefs; expert Questions and answers panels; case exercises and template checklists | Remembering: recall definitions of indemnity and moral hazard. Understanding: explain relationships between insurance design and behaviour. Evaluating: assess how financial tools influence preventive behaviour. | |
| Market incentives and certification | Interpret how certification programmes (e.g., secure pork supply) and preferential sourcing generate revenue advantages. Guide producers through compliance steps. | Webinars with industry speakers; compliance checklists. | Understanding: describe the market rationale behind certification. Applying: support producers in meeting certification criteria. | |
| Communicating economic evidence | Translate financial analyses into persuasive, farmer-friendly messages. Integrate profitability narratives into extension work. | Role-play training; peer review of advisory scripts. | Applying: use communication strategies effectively in real contexts. Creating: design customised communication materials and narratives. | |
| Farmers | Profitability through prevention | Link outbreak prevention to stable income and market access. Calculate avoided losses versus preventive investments and enable asset valuation. | Farmer field schools; participatory budgeting sessions. | Understanding: explain the basic economic concepts and how and when prevention sustains income and trade. Applying: calculate economic trade-offs using simple tools and how economic value of assets is generated. |
| Low-cost, high-impact measures | Identify affordable, high-return interventions (e.g., do it yourself footbaths, barn partitions). Compare cost of inaction vs. small investments. | On-farm demos; peer discussion circles. | Remembering: recall feasible low-cost biosecurity options. Applying: implement and evaluate those practices on-farm. | |
| Reducing losses and risk | Quantify production losses and cash-flow risk for priority hazards using farm records and simple expected-loss estimates. Prioritise the top three preventive measures with an avoided-loss calculator and outline a lean continuity plan to sustain operations and market access during disruptions. | Small-group clinic with real farm numbers; worksheet budgeting (paper or mobile); facilitator-led discussion and quick on-farm simulations. | Understanding: interpret production and financial records. Applying: use simple models to estimate risk. Evaluating: identify which measures best sustain business continuity. | |
| Accessing subsidies, grants, and insurance | Navigate finance and financial support options. Plan incremental investments using external funding mechanisms. | Community workshops; step-by-step guidance handouts. | Remembering: list available support programmes. Applying: develop investment plans using external funding. | |
| Collective economic action for community biosecurity | Recognise shared financial risk within producer networks. Form cooperatives or pooled funds for shared infrastructure. | Community meetings; participatory mapping of economic risk. | Understanding: describe shared risk concepts and collective benefit. Creating: design cooperative or pooled funding mechanisms. | |
| Market access and consumer trust | Understand how strong biosecurity protects trade continuity and earns premiums. Learn from real farms that maintained sales during crises. | Peer-led storytelling; visual case study booklets. | Understanding: explain the relationship between biosecurity and consumer trust. Analysing: compare successful and unsuccessful market-access cases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmedi, B.; Iatrou, A.M.; Yildiz, R.; Lamont, K.; da Costa, M.R.; De Nardi, M.; Allepuz, A.; Niine, T.; Niemi, J.K.; Saegerman, C. Economic Perspectives on Farm Biosecurity: Stakeholder Challenges and Livestock Species Considerations. Agriculture 2025, 15, 2288. https://doi.org/10.3390/agriculture15212288
Mehmedi B, Iatrou AM, Yildiz R, Lamont K, da Costa MR, De Nardi M, Allepuz A, Niine T, Niemi JK, Saegerman C. Economic Perspectives on Farm Biosecurity: Stakeholder Challenges and Livestock Species Considerations. Agriculture. 2025; 15(21):2288. https://doi.org/10.3390/agriculture15212288
Chicago/Turabian StyleMehmedi, Blerta, Anna Maria Iatrou, Ramazan Yildiz, Kate Lamont, Maria Rodrigues da Costa, Marco De Nardi, Alberto Allepuz, Tarmo Niine, Jarkko K. Niemi, and Claude Saegerman. 2025. "Economic Perspectives on Farm Biosecurity: Stakeholder Challenges and Livestock Species Considerations" Agriculture 15, no. 21: 2288. https://doi.org/10.3390/agriculture15212288
APA StyleMehmedi, B., Iatrou, A. M., Yildiz, R., Lamont, K., da Costa, M. R., De Nardi, M., Allepuz, A., Niine, T., Niemi, J. K., & Saegerman, C. (2025). Economic Perspectives on Farm Biosecurity: Stakeholder Challenges and Livestock Species Considerations. Agriculture, 15(21), 2288. https://doi.org/10.3390/agriculture15212288








