Use of Microbial and Enzymatic Additives on the Nutritional Quality, Fermentation Profile, and In Vitro Digestibility of Mixed Silages of Amaranth and Sweet Potato Vines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Experimental Design
2.3. Analysis of Fermentation Indicators and Chemical Compositions in Mixed Silage
2.4. Measurement of Microbial Number and Aerobic Stability in Mixed Silage
2.5. In Vitro Fermentation, Nutrient Digestibility and Fermentation Characteristic of Mixed Silage
| Ingredients (%) | Nutritional Levels (%) | ||
|---|---|---|---|
| Alfalfa hay | 9.58 | CP | 14.61 |
| Sweet potato vine | 25.42 | EE | 2.87 |
| Maize | 33.75 | NDF | 33.20 |
| Wheat bran | 6.26 | ADF | 20.07 |
| Soybean meal | 6.78 | Ca | 0.91 |
| Rapeseed meal | 4.82 | P | 0.48 |
| Cottonseed meal | 5.64 | ME b, MJ/kg | 9.52 |
| NaCl | 0.52 | ||
| Limestone | 0.99 | ||
| CaHPO4 | 0.34 | ||
| NaHCO3 | 0.90 | ||
| Premix a | 5.00 |
2.6. Statistical Analysis
3. Results
3.1. Fermentation Indicators of Amaranth and Sweet Potato Vine Mixed Silage
3.2. Chemical Components of Amaranth and Sweet Potato Vine Mixed Silage
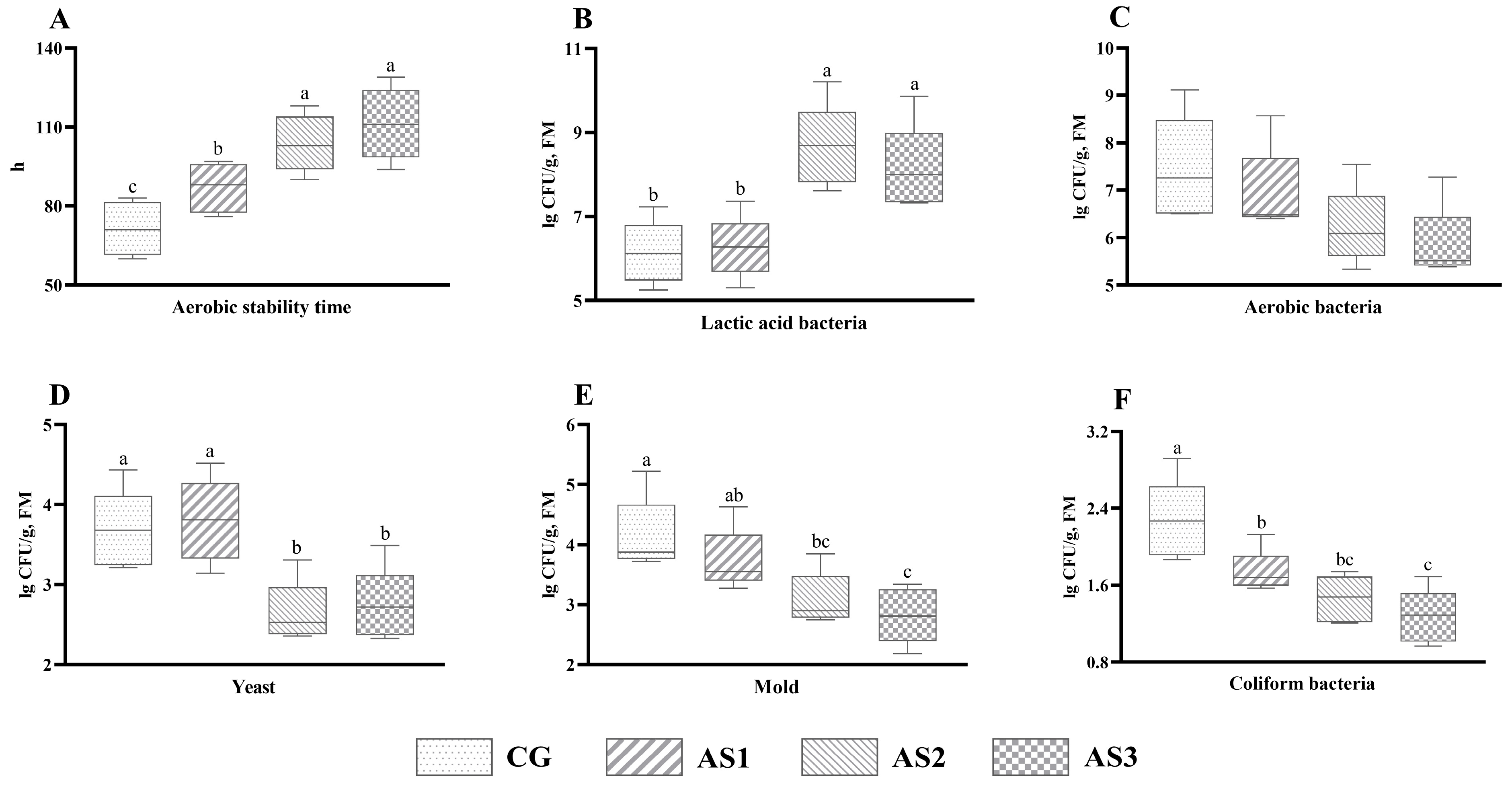
3.3. Microbial Counts of Amaranth and Sweet Potato Vine Mixed Silage
3.4. Aerobic Stability of Amaranth and Sweet Potato Vine Mixed Silage
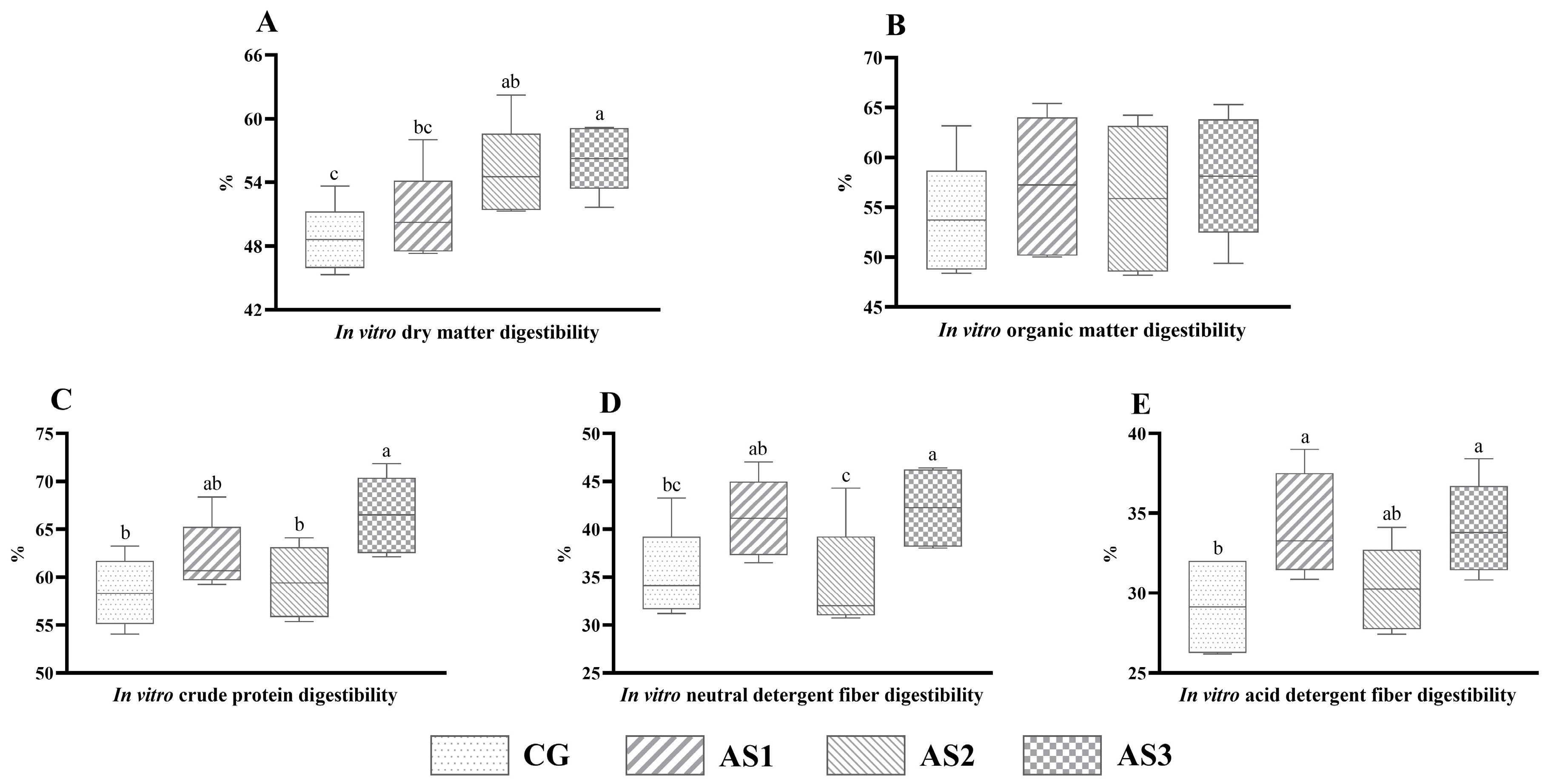
3.5. In Vitro Nutrient Digestibility of Amaranth and Sweet Potato Vine Mixed Silage
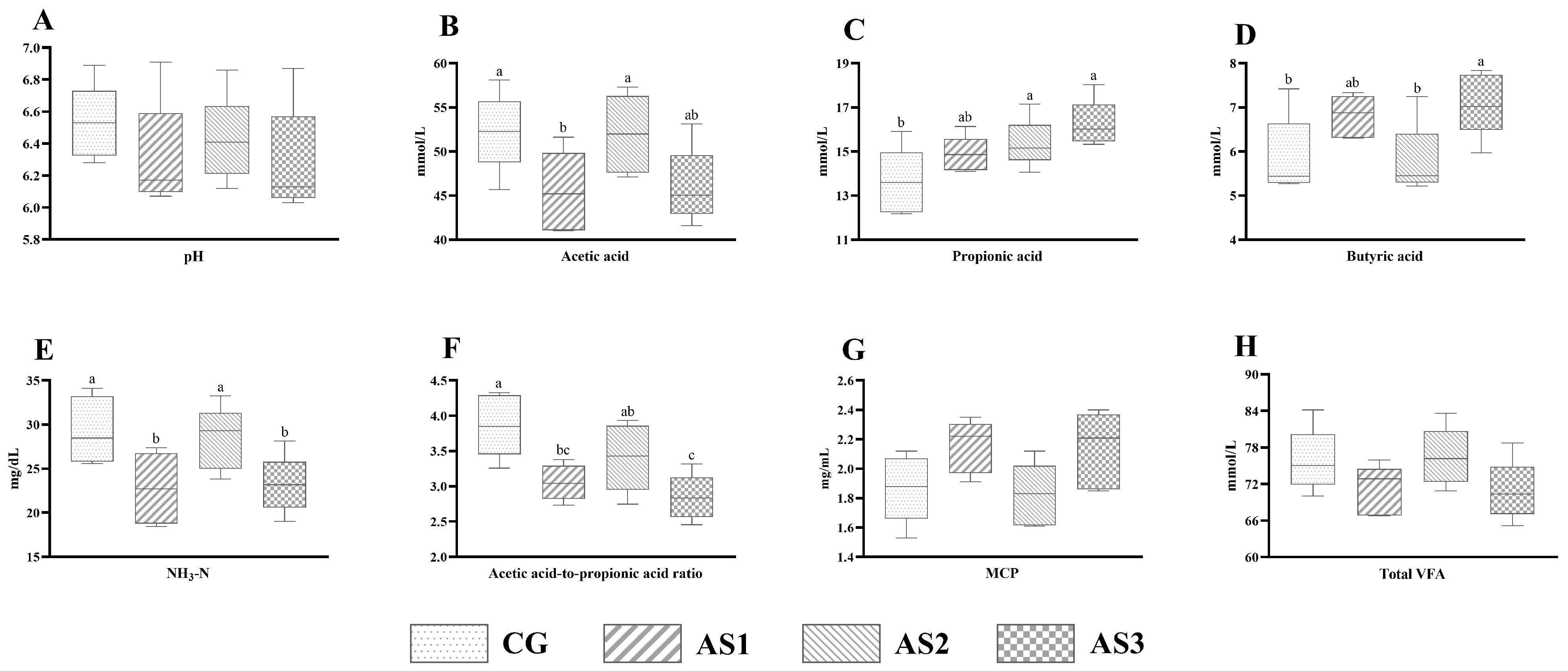
3.6. In Vitro Gas Production and Ruminal Fermentation Characteristics of Amaranth and Sweet Potato Vine Mixed Silage
4. Discussion
4.1. Effects of Additives on the Chemical Components of Mixed Silage
4.2. Effects of Additives on the Fermentation Parameters of Mixed Silage
4.3. Effects of Additives on the Microbial Population of Mixed Silage
4.4. Effects of Additives on the Aerobic Stability of Mixed Silage
4.5. Effects of Additives on the In Vitro Digestibility of Mixed Silage
4.6. Effects of Additives on the In Vitro Gas Production and Rumen Fermentation of Mixed Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sasu, P.; Attoh-Kotoku, V.; Akorli, D.E.; Adjei-Mensah, B.; Tankouano, R.A.; Kwaku, M. Nutritional evaluation of the leaves of Oxytenanthera abyssinica, Bambusa balcooa, Moringa oleifera, Terminalia catappa, Blighia sapida, and Mangifera indica as non-conventional green roughages for ruminants. J. Agric. Food Res. 2023, 11, 100466. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, M.; Sun, P.; Zhu, L.; Yan, X.; Hao, J.; Si, Q.; Wang, Z.; Jia, Y.; Wang, M.; et al. Effects of different additives on fermentation characteristics, nutrient composition and microbial communities of Leymus chinensis silage. BMC Microbiol. 2025, 25, 296. [Google Scholar] [CrossRef]
- Seven, P.T.; Yıldırım, E.N.; Seven, I.; Kaya, C.A.; Mutlu, S.I. An evaluation of the effectiveness of sumac and molasses as additives for alfalfa silage: Influence on nutrient composition, in vitro degradability and fermentation quality. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Garcia, S.C.; Islam, M.A.; Bashar, M.K.; Roy, A.; Roy, B.K.; Sarker, N.R.; Clark, C.E.F. Ruminant production from Napier grass (Pennisetum purpureum Schum): A review. Animals 2024, 14, 467. [Google Scholar] [CrossRef]
- Pulvento, C.; Sellami, M.H.; Lavini, A. Yield and quality of Amaranthus hypochondriacus grain amaranth under drought and salinity at various phenological stages in southern Italy. J. Sci. Food Agric. 2022, 102, 5022–5033. [Google Scholar] [CrossRef]
- Ma, J.; Sun, G.; Shah, A.M.; Fan, X.; Li, S.; Yu, X. Effects of different growth stages of amaranth silage on the rumen degradation of dairy cows. Animals 2019, 9, 793. [Google Scholar] [CrossRef]
- Oteri, M.; Gresta, F.; Costale, A.; Lo Presti, V.; Meineri, G.; Chiofalo, B. Amaranthus hypochondriacus L. as a sustainable source of nutrients and bioactive compounds for animal feeding. Antioxidants 2021, 10, 876. [Google Scholar] [CrossRef]
- Lotfi, S.; Rouzbehan, Y.; Fazaeli, H.; Feyzbakhsh, M.T.; Rezaei, J. The nutritional value and yields of amaranth (Amaranthus hypochondriacus) cultivar silages compared to silage from corn (Zea mays) harvested at the milk stage grown in a hot-humid climate. Anim. Feed Sci. Technol. 2022, 289, 115336. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, L.; Zhou, S.; Li, Y.; Wang, Y.; Yang, K.; Chen, W.; Zhao, S. Effects of different proportions of Amaranthus hypochondriacus stem and leaf powder inclusions on growth performance, carcass traits, and blood biochemical parameters of broilers. Animals 2023, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fan, X.; Sun, G.; Yin, F.; Zhou, G.; Zhao, Z.; Gan, S. Replacing alfalfa hay with amaranth hay: Effects on production performance, rumen fermentation, nutrient digestibility and antioxidant ability in dairy cow. Anim. Biosci. 2024, 37, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, A.; Titze, N.; Rodehutscord, M.; Melesse, A. Effect of substituting concentrate mix with sweet potato vines on growth performances and carcass components of yearling rams and its potential in mitigating methane production. Vet. Med. Int. 2025, 2025, 1054348. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Wang, Y.; Lin, Y.; Ni, K.; Yang, F. Effects of lactic acid bacteria and cellulase additives on the fermentation quality, antioxidant activity, and metabolic profile of oat silage. Bioresour. Bioprocess. 2024, 11, 92. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Zhu, L.; Guo, W.; Guo, X.; Zhu, B.; Yang, M. Effect of additive cellulase on fermentation quality of whole-plant corn silage ensiling by a Bacillus inoculant and dynamic microbial community analysis. Front. Microbiol. 2024, 14, 1330538. [Google Scholar] [CrossRef]
- Nazar, M.; Tian, J.; Wang, X.; Wang, S.; Khan, N.A.; Cheng, Y.; Zhang, W.; Xu, N.; Liu, B.; Ding, C. Effect of biological lignin depolymerization on rice straw enzymatic hydrolysis, anerobic fermentation characteristics and in vitro ruminal digestibility. Int. J. Biol. Macromol. 2025, 305, 141664. [Google Scholar] [CrossRef]
- Sánchez-Guerra, N.A.; Gonzalez-Ronquillo, M.; Anderson, R.C.; Hume, M.E.; Ruiz-Albarrán, M.; Bautista-Martínez, Y.; Zúñiga-Serrano, A.; Nájera-Pedraza, O.G.; Salinas-Chavira, J. Improvements in fermentation and nutritive quality of elephant grass [Cenchrus purpureus (Schumach.) Morrone] silages: A review. Trop. Anim. Health Prod. 2024, 56, 171. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, L.; Zhao, J.; Dong, Z.; Li, J.; Shao, T. Synergistic effects of exogenous lactobacillus plantarum and fibrolytic enzymes on fermentation quality, fiber degradation, and in vitro digestibility of napiergrass (Pennisetum purpureum) silage. Agronomy 2025, 15, 340. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Y.; Zhao, J.; Dong, Z.; Li, J.; Nazar, M.; Shao, T. Assessment of inoculating various epiphytic microbiota on fermentative profile and microbial community dynamics in sterile Italian ryegrass. J. Appl. Microbiol. 2020, 129, 509–520. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Association of Official Analytical Chemists Official Methods of Analysis; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Murphy, R.P. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z. Impact of packing density on the bacterial community, fermentation, and in vitro digestibility of whole-crop barley silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wu, Y.; Wang, Y.; Gao, L.; Li, X.; Jiang, J. Fermentation profile, aerobic stability, and microbial community dynamics of corn straw ensiled with Lactobacillus buchneri PC-C1 and Lactobacillus plantarum PC1-1. Microbiol. Res. 2023, 270, 127329. [Google Scholar] [CrossRef]
- GB/T 6436-2018; Determination of Calcium in Feeds. China Standards Press: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds—Spectrophotometry. China Standards Press: Beijing, China, 2018.
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Bai, J.; Li, Y.; Li, T.; Zhang, W.; Fan, M.; Zhang, K.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. Comparison of different soluble dietary fibers during the in vitro fermentation process. J. Agric. Food Chem. 2021, 61, 7446–7457. [Google Scholar] [CrossRef] [PubMed]
- Øskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighed according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef] [PubMed]
- NY/T 816-2021; Nutrient Requirements of Meat-Type Sheep and Goats. China Agriculture Press: Beijing, China, 2021.
- Xu, J.; Zhang, K.; Lin, Y.; Li, M.; Wang, X.; Yu, Q.; Sun, H.; Cheng, Q.; Xie, Y.; Wang, C.; et al. Effect of cellulase and lactic acid bacteria on the fermentation quality, carbohydrate conversion, and microbial community of ensiling oat with different moisture contents. Front. Microbiol. 2022, 13, 1013258. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Kumari, N.; Mishra, D.B. Dynamic changes in microbial succession and fermentation profiles of sugarcane tops silage treated with exogenous enzymes and lactic acid bacteria following various duration of ensiling. Sugar Tech 2023, 25, 592–602. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Vicente, F.; Rodríguez, M.L.; Martínez-Fernández, A.; Soldado, A.; Argamentería, A.; Peláez, M.; de la Roza-Delgado, B. Subclinical ketosis on dairy cows in transition period in farms with contrasting butyric acid contents in silages. Sci. World J. 2014, 2014, 279614. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of cellulase and Lactobacillus plantarum on fermentation quality, chemical composition, and microbial community of mixed silage of whole-plant corn and peanut vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 2018, 101, 4132–4142. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Wang, Y. Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef]
- Jia, T.; Yun, Y.; Yu, Z. Propionic Acid and sodium benzoate affected biogenic amine formation, microbial community, and quality of oat silage. Front. Microbiol. 2021, 12, 750920. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Liu, N.; Diao, X.; He, L.; Zhou, H.; Zhang, W. Effects of cellulase and xylanase on fermentation characteristics, chemical composition and bacterial community of the mixed silage of king grass and rice straw. Microorganisms 2024, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, S.; Chen, X.; Sun, Z.; Sun, Y.; Zhen, Y.; Qin, G.; Wang, T.; Demelash, N.; Zhang, X. Effects of Lactiplantibacillus plantarum inoculation on the quality and bacterial community of whole-crop corn silage at different harvest stages. Chem. Biol. Technol. Agric. 2022, 9, 57. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Ma, G.; Jiang, X.; Yang, J.; Lv, J.; Zhang, Y. Cellulase interacts with lactic acid bacteria to affect fermentation quality, microbial community, and ruminal degradability in mixed silage of soybean residue and corn stover. Animals 2021, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Jia, Y.; Ge, G.; Du, S. Revealing the underlying potential mechanisms of lactic acid bacteria-mediated anaerobic fermentation of native grass by microbiome and metagenomic. J. Agric. Food Res. 2025, 23, 102283. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, H.; Li, Y.; Li, L.; He, J.; Yang, X.; Xie, X. Fermentation characteristics and microbial community composition of wet brewer’s grains and corn stover mixed silage prepared with cellulase and lactic acid bacteria supplementation. Anim. Biosci. 2024, 37, 84–94. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Z.; Du, S.; Sun, L.; Bao, J.; Hao, J.; Ge, G. Lactobacillus plantarum and propionic acid improve the fermentation quality of high-moisture amaranth silage by altering the microbial community composition. Front. Microbiol. 2022, 13, 1066641. [Google Scholar] [CrossRef] [PubMed]
- Kung Jr, L.; Schmidt, R.J.; Ebling, T.E.; Hu, W. The effect of Lactobacillus buchneri 40788 on the fermentation and aerobic stability of ground and whole high-moisture corn. J. Dairy Sci. 2007, 90, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, G.; Yuan, X.; Zhang, J.; Li, J.; Shao, T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 2016, 96, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shen, Y.; Ma, H. Silibinin reduces in vitro methane production by regulating the rumen microbiome and metabolites. Front. Microbiol. 2023, 14, 1225643. [Google Scholar] [CrossRef]
- Dai, T.; Dong, D.; Wang, J.; Yin, X.; Zong, C.; Jia, Y.; Shao, T. Effects of wet brewers grains on fermentation quality and in vitro ruminal digestibility of mixed silage prepared with corn stalk, sweet potato peel and dried apple pomace in southeast China. J. Anim. Physiol. Anim. Nutr. 2022, 107, 340–349. [Google Scholar] [CrossRef]
- Tian, J.; Yu, Y.; Yu, Z.; Shao, T.; Na, R.; Zhao, M. Effects of lactic acid bacteria inoculants and cellulase on fermentation quality and in vitro digestibility of Leymus chinensis silage. Grassl. Sci. 2014, 60, 199–205. [Google Scholar] [CrossRef]
- Bai, B.; Qiu, R.; Wang, Z.; Liu, Y.; Bao, J.; Sun, L.; Liu, T.; Ge, G.; Jia, Y. Effects of cellulase and lactic acid bacteria on ensiling performance and bacterial community of Caragana korshinskii silage. Microorganisms 2023, 11, 337. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, G.; Xiao, M.; Dong, C.; Zhang, R.; Du, L.; Zheng, Y.; Wei, M.; Wei, M.; Wu, B. Effects of cellulase and Lactiplantibacillus plantarum on the fermentation quality, microbial diversity, gene function prediction, and in vitro rumen fermentation parameters of Caragana korshinskii silage. Front. Food Sci. Technol. 2023, 2, 1108043. [Google Scholar] [CrossRef]
- Xia, T.; Tahir, M.; Wang, T.; Wang, Y.; Zhang, X.; Liu, S.; Teng, K.; Fu, Z.; Yun, F.; Wang, S. Lactobacillus cocktail and cellulase synergistically improve the fiber transformation rate in Sesbania cannabina and sweet sorghum mixed silage. Chem. Biol. Technol. Agric. 2024, 11, 81. [Google Scholar] [CrossRef]
- Huang, K.; Chen, H.; Liu, Y.; Hong, Q.; Yang, B.; Wang, J. Lactic acid bacteria strains selected from fermented total mixed rations improve ensiling and in vitro rumen fermentation characteristics of corn stover silage. Anim. Biosci. 2022, 35, 1379–1389. [Google Scholar] [CrossRef]
- Vargas, J.; Ungerfeld, E.; Muñoz, C.; DiLorenzo, N. Feeding strategies to mitigate enteric methane emission from ruminants in grassland systems. Animals 2022, 12, 1132. [Google Scholar] [CrossRef]
- Gao, S.T.; Girma, D.D.; Bionaz, M.; Ma, L.; Bu, D.P. Hepatic transcriptomic adaptation from prepartum to postpartum in dairy cows. J. Dairy Sci. 2021, 104, 1053–1072. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; Wang, Z.; Cao, G.; Hu, R.; Wang, X.; Zou, H.; Kang, K.; Peng, Q.; Xue, B.; et al. Active dry yeast supplementation improves the growth performance, rumen fermentation, and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Maake, T.W.; Aiyegoro, O.A.; Adeleke, M.A. Effects of Lactobacillus rhamnosus and Enterococcus faecalis Supplementation as Direct-Fed Microbials on Rumen Microbiota of Boer and Speckled Goat Breeds. Vet. Sci. 2021, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yamasaki, S.; Oya, T.; Cai, Y. Cellulase-lactic acid bacteria synergy action regulates silage fermentation of woody plant. Biotechnol. Biofuels Bioprod. 2023, 16, 125. [Google Scholar] [CrossRef]

| Items | DM (% Fresh Matter) | CP (% DM) | NDF (% DM) | ADF (% DM) | OM (% DM) | WSC (% DM) |
|---|---|---|---|---|---|---|
| Sweet potato vine | 88.45 | 10.78 | 44.20 | 33.83 | 91.27 | 8.24 |
| Amaranth | 18.86 | 11.04 | 56.24 | 39.12 | 89.14 | 3.82 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | AS1 | AS2 | AS3 | |||
| pH | 4.21 a | 4.12 ab | 3.92 bc | 3.80 c | 0.055 | 0.017 |
| NH3-N (% of total N) | 4.30 a | 3.49 bc | 3.84 ab | 3.15 c | 0.129 | 0.003 |
| Acetic acid (% DM) | 2.32 a | 1.94 b | 1.36 c | 1.44 c | 0.101 | <0.001 |
| Propionic acid (% DM) | 0.025 a | 0.016 ab | 0.007 b | 0.008 b | 0.002 | 0.015 |
| Butyric acid (% DM) | 0.003 | 0.001 | 0.001 | - | 0.001 | 0.214 |
| Lactic acid (% DM) | 5.16 b | 5.53 b | 6.85 a | 7.28 a | 0.240 | <0.001 |
| Lactic acid/Acetic acid | 2.22 b | 2.88 b | 5.09 a | 5.15 a | 0.322 | <0.001 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | AS1 | AS2 | AS3 | |||
| DM (% fresh matter) | 26.09 bc | 25.27 c | 31.09 a | 29.89 ab | 0.842 | 0.020 |
| WSC (% DM) | 2.29 | 2.48 | 2.18 | 1.97 | 0.088 | 0.226 |
| CP (% DM) | 9.02 c | 10.28 ab | 9.31 bc | 10.68 a | 0.238 | 0.027 |
| NDF (% DM) | 54.38 a | 49.08 b | 53.48 ab | 49.24 b | 0.863 | 0.034 |
| ADF (% DM) | 36.16 a | 32.61 b | 35.79 a | 31.95 b | 0.618 | 0.013 |
| OM (% DM) | 88.03 | 88.63 | 87.48 | 89.53 | 0.740 | 0.815 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | AS1 | AS2 | AS3 | |||
| 2 h | 16.72 | 17.32 | 18.08 | 17.55 | 0.365 | 0.651 |
| 4 h | 19.15 b | 20.05 ab | 22.26 ab | 23.29 a | 0.614 | 0.046 |
| 8 h | 24.24 b | 24.86 b | 30.66 a | 29.45 a | 0.813 | 0.001 |
| 12 h | 32.10 c | 34.18 bc | 38.56 ab | 39.23 a | 0.991 | 0.014 |
| 24 h | 48.15 | 50.74 | 54.12 | 54.34 | 1.006 | 0.076 |
| 48 h | 65.94 b | 71.07 ab | 73.31 a | 74.48 a | 1.178 | 0.035 |
| 72 h | 71.20 | 75.61 | 75.18 | 78.68 | 1.035 | 0.071 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | AS1 | AS2 | AS3 | |||
| A (mL/g) | 1.49 b | 1.58 b | 1.64 ab | 1.78 a | 0.038 | 0.034 |
| B (mL/g) | 64.56 b | 71.22 a | 70.98 a | 72.45 a | 1.068 | 0.025 |
| A + B (mL/g) | 66.05 b | 72.79 a | 72.62 a | 74.23 a | 1.092 | 0.022 |
| C (%/h) | 0.074 | 0.070 | 0.056 | 0.052 | 0.006 | 0.469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Su, M.; Wu, S.; Xu, W.; Weng, B.; Feng, Y.; Zhang, W.; Ma, J. Use of Microbial and Enzymatic Additives on the Nutritional Quality, Fermentation Profile, and In Vitro Digestibility of Mixed Silages of Amaranth and Sweet Potato Vines. Agriculture 2025, 15, 2276. https://doi.org/10.3390/agriculture15212276
Fang L, Su M, Wu S, Xu W, Weng B, Feng Y, Zhang W, Ma J. Use of Microbial and Enzymatic Additives on the Nutritional Quality, Fermentation Profile, and In Vitro Digestibility of Mixed Silages of Amaranth and Sweet Potato Vines. Agriculture. 2025; 15(21):2276. https://doi.org/10.3390/agriculture15212276
Chicago/Turabian StyleFang, Liuyan, Mengrong Su, Shaoyan Wu, Wenhui Xu, Beiyu Weng, Yaochang Feng, Wenjie Zhang, and Jian Ma. 2025. "Use of Microbial and Enzymatic Additives on the Nutritional Quality, Fermentation Profile, and In Vitro Digestibility of Mixed Silages of Amaranth and Sweet Potato Vines" Agriculture 15, no. 21: 2276. https://doi.org/10.3390/agriculture15212276
APA StyleFang, L., Su, M., Wu, S., Xu, W., Weng, B., Feng, Y., Zhang, W., & Ma, J. (2025). Use of Microbial and Enzymatic Additives on the Nutritional Quality, Fermentation Profile, and In Vitro Digestibility of Mixed Silages of Amaranth and Sweet Potato Vines. Agriculture, 15(21), 2276. https://doi.org/10.3390/agriculture15212276







