Microclimate Condition Influence on the Physicochemical Properties and Antioxidant Activity of Pomegranate (Punica granatum L.): A Case Study of the East Adriatic Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Climatic Condition
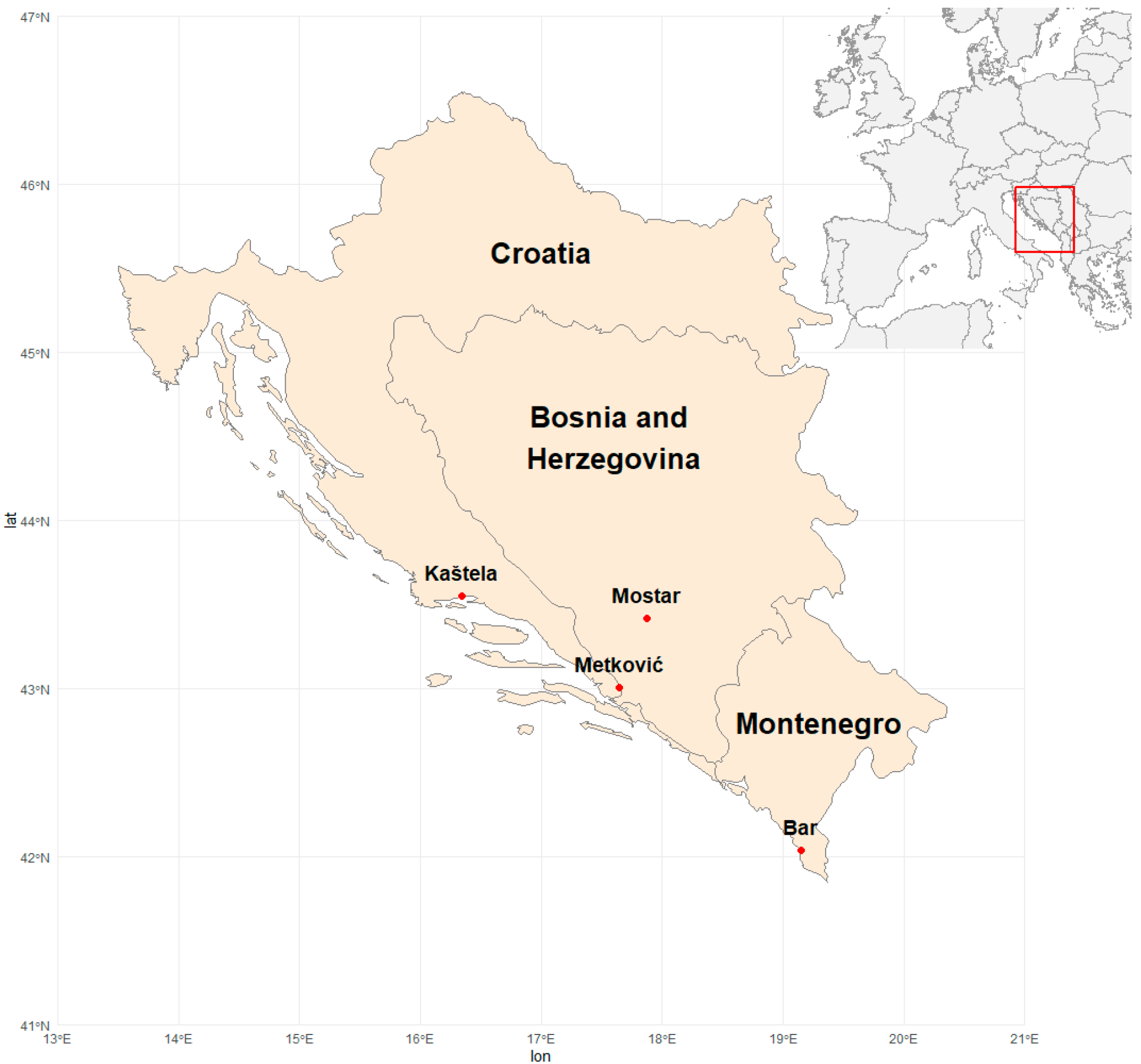
- Kaštela is situated on the coastal part of Croatia (CRO), Middle Dalmatia (43°33′05″ N, 16°20′23″ E, 9 altitude). The main climatic characteristics were an average annual temperature of 16.4 °C, an absolute minimum of −3.7 °C (December), and an absolute maximum of 37.2 °C (July). Annual rainfall in this location was 1428 mm, but during April to October, it was 712 mm (50% of the total). Average sunny hours totaled 2700.
- Metković is situated in the lower part of the Neretva river valley, South Dalmatia, Croatia (CRO) (43°00′29″ N, 17°38′47″ E, 5 m altitude). The main climatic characteristics were a mean daily temperature of 15.6 °C, an absolute minimum of −6.4 °C (December), and an absolute maximum of 36.5 °C (August). Annual rainfall in this location was 1555.4 mm, with 646 mm (42% of total) recorded from April to October. Average sunny hours totaled 2700.
- Mostar is situated in the upper part of the Neretva Valley, in the southern part of Bosnia and Herzegovina (BIH) (43°25′13″ N, 17°52′26″ E, 100 m altitude). Mostar has a temperate Mediterranean climate with milder but colder winters (characterized by little to no snow) and hot summers. Average annual temperature was 15.7 °C, with an absolute minimum of −7.8 °C (December) and an absolute maximum of 38.9 °C (July). Annual rainfall was 1848.9 mm, with 741.6 mm (40% of total) recorded from April to September. Annual sunny hours totaled 2285.
- Bar is situated on the southeast coastal part of Montenegro (MNE) (42°02′22″ N, 19°09′09″ E, 143 m altitude). The climate of Bar is determined by the proximity of the Adriatic Sea and Lake Skadar and the mountain massif of Rumia. The average annual temperature of 16.9 °C characterizes it, with an absolute minimum of 0 °C (February) and an absolute maximum of 35.4 °C (August). Annual rainfall was 1826.4 mm, with 641.8 mm (35% of total) recorded during April to October. Annual sunny hours totaled 2555.
2.3. Physical Properties
2.4. Chemical Analysis
2.4.1. Total Soluble Solids, Titratable Acidity, TSS/TA Ratio, pH, and Total Sugar Content
2.4.2. Total Phenolic Content, Anthocyanin Content, and Antioxidant Activity
Determination of Total Phenolic Content
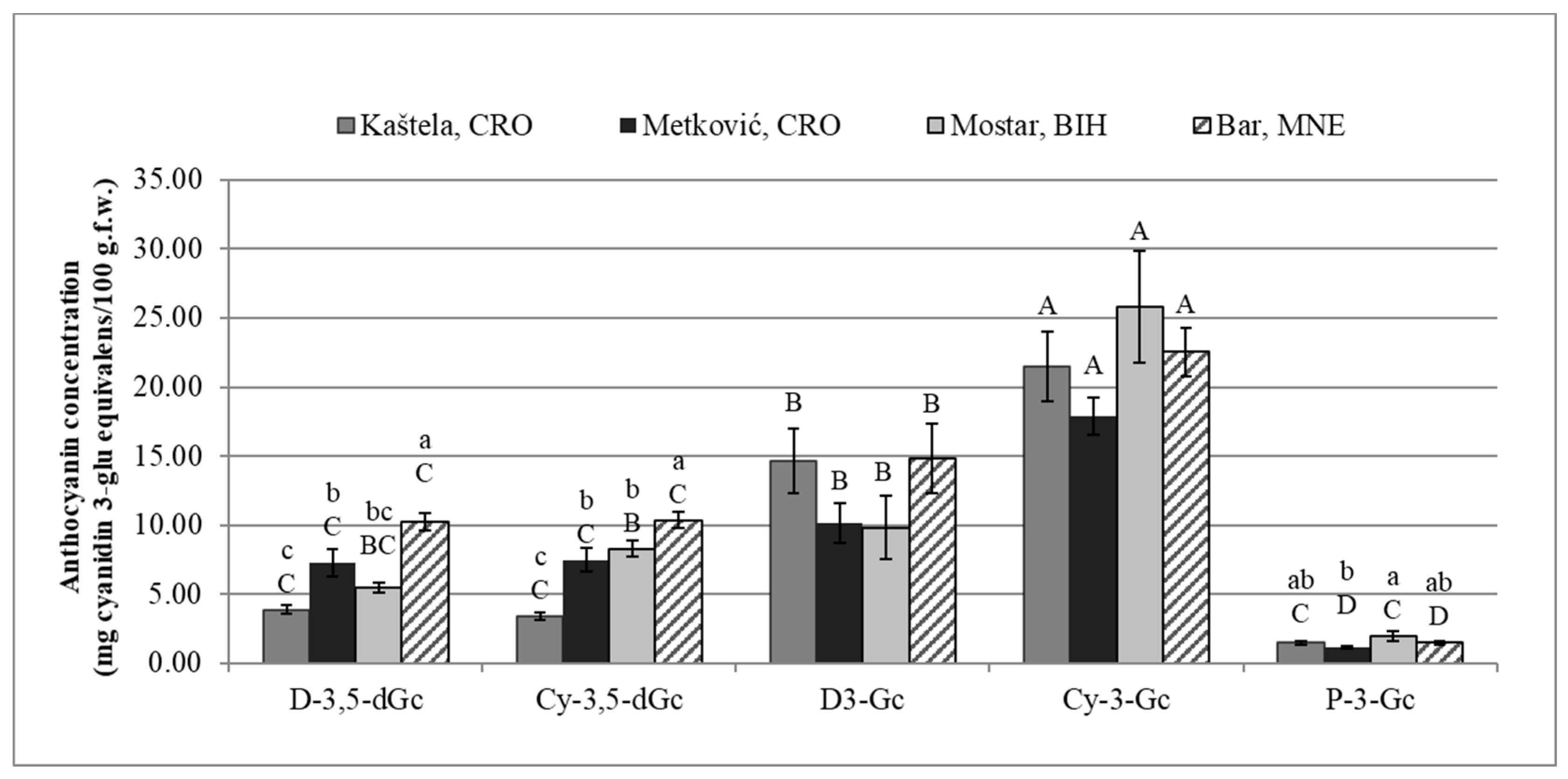
Identification and Quantification of Anthocyanin Compounds
Determination of Antioxidant Activities
2.5. Statistical Analysis
3. Results
3.1. Physical Properties
3.2. Chemical Properties
4. Discussion
4.1. Microclimate Condition Influence on Physical Properties
4.2. Microclimate Condition Influence on Chemical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çiçek, M.; Pakyürek, M.; Çelik, F. Determination of Morphological and Pomological Characteristics of Diyarbakır Region Pomegranates (Punica granatum L.). Int. J. Agric. Environ. Food Sci. 2019, 3, 197–203. [Google Scholar] [CrossRef]
- Boussaa, F.; Zaouay, F.; Burlo-Carbonell, F.; Noguera-Artiaga, L.; Carbonell-Barrachina, A.; Melgarejo, P.; Hernandez, F.; Mars, M. Growing Location Affects Physical Properties, Bioactive Compounds, and Antioxidant Activity of Pomegranate Fruit (Punica granatum L. Var. Gabsi). Int. J. Fruit Sci. 2020, 20, 508–523. [Google Scholar] [CrossRef]
- Özgüven, A.I.; Yılmaz, C. Pomegranate Growing in Turkey. In Proceedings of the Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology, Orihuela, Spain, 15–17 October 1998; Melgarejo-Moreno, P., Martinez-Nicolas, J.J., Martinez-Tome, J., Eds.; CIHEAM-IAMZ: Zaragoza, Spain, 1998; pp. 41–48. [Google Scholar]
- Özcan, E.; Ünaldi, Ü.E. Ecology of Pomegranate and Its Economics in Turkey. In Proceedings of the International Symposium on Geography Environment and Culture in the Mediterranean Region, Marseille, France, 27–29 March 2007; Available online: https://web.deu.edu.tr/geomed2010/2007/Ozcan-Unaldi.pdf (accessed on 18 August 2025).
- Davarpanah, S.; Tehranifar, A.; Abadía, J.; Val, J.; Davarynejad, G.; Aran, M.; Khorassani, R. Foliar Calcium Fertilization Reduces Fruit Cracking in Pomegranate (Punica granatum Cv. Ardestani). Sci. Hortic. 2018, 230, 86–91. [Google Scholar] [CrossRef]
- Czieczor, L.; Bentkamp, C.; Damerow, L.; Blanke, M. Non-Invasive Determination of the Quality of Pomegranate Fruit. Postharvest Biol. Technol. 2018, 136, 74–79. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal Changes of Mineral Nutrients and Phenolics in Pomegranate (Punica granatum L.) Fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Linus Opara, U.; Witthuhn, C.R. Modeling the Effect of Time and Temperature on Respiration Rate of Pomegranate Arils (Cv. “Acco” and “Herskawitz”). J. Food Sci. 2012, 77, E80–E87. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Caleb, O.J.; Fawole, O.A.; Opara, U.L. Effects of Different Maturity Stages and Growing Locations on Changes in Chemical, Biochemical and Aroma Volatile Composition of ‘Wonderful’ Pomegranate Juice. J. Sci. Food Agric. 2016, 96, 1002–1009. [Google Scholar] [CrossRef]
- AL-Kalbani, B.S.; Al-Yahyai, R.A.; Al-Sadi, A.M.; Al-Mamari, A.-G.H. Physical and Chemical Fruit Quality Attributes of Two Pomegranate Cultivars Grown at Varying Altitudes of Al-Hajar Mountains in Oman. J. Agric. Mar. Sci. 2021, 26, 42–50. [Google Scholar] [CrossRef]
- Gómez-Devia, L.; Nevo, O. Effects of Temperature Gradient on Functional Fruit Traits: An Elevation-for-Temperature Approach. BMC Ecol. Evol. 2024, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Welles, G.W.H.; Buitelaar, K. Factors Affecting Soluble Solids Content of Muskmelon (Cucumis melo L.). Wagening. J. Life Sci. 1988, 36, 3. [Google Scholar] [CrossRef]
- Koshita, Y. Effect of Temperature on Fruit Color Development. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 47–58. [Google Scholar]
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate Changes and Potential Impacts on Postharvest Quality of Fruit and Vegetable Crops: A Review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Fischer, G.; Parra-Coronado, A.; Balaguera-López, H.E. Altitude as a Determinant of Fruit Quality with Emphasis on the Andean Tropics of Colombia. A Review. Agron. Colomb. 2022, 40, 212–227. [Google Scholar] [CrossRef]
- Radunić, M.; Goreta Ban, S.; Gadže, J.; MacLean, D.; Martino, K.; Scherm, H.; Horton, D. Šipak-Pomegranate; Institute for Adriatic Crops and Karst Reclamation Split: Split, Croatia, 2012. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Mousavinejad, G.; Emam-Djomeh, Z.; Rezaei, K.; Khodaparast, M.H.H. Identification and Quantification of Phenolic Compounds and Their Effects on Antioxidant Activity in Pomegranate Juices of Eight Iranian Cultivars. Food Chem. 2009, 115, 1274–1278. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Und Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R. A Modified Total Oxyradical Scavenging Capacity Assay for Antioxidants in Plant Tissues. Postharvest Biol. Technol. 2003, 29, 183–194. [Google Scholar] [CrossRef]
- TIBCO. Statistica, v. 14.0.0.15; TIBCO Software Inc.: San Ramon, CA, USA, 2020. [Google Scholar]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Kaplan, M.; Hayek, T.; Raz, A.; Coleman, R.; Dornfeld, L.; Vaya, J.; Aviram, M. Pomegranate Juice Supplementation to Atherosclerotic Mice Reduces Macrophage Lipid Peroxidation, Cellular Cholesterol Accumulation and Development of Atherosclerosis. J. Nutr. 2001, 131, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate Juice Consumption for 3 Years by Patients with Carotid Artery Stenosis Reduces Common Carotid Intima-Media Thickness, Blood Pressure and LDL Oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- de Nigris, F.; Williams-Ignarro, S.; Lerman, L.O.; Crimi, E.; Botti, C.; Mansueto, G.; D’Armiento, F.P.; De Rosa, G.; Sica, V.; Ignarro, L.J.; et al. Beneficial Effects of Pomegranate Juice on Oxidation-Sensitive Genes and Endothelial Nitric Oxide Synthase Activity at Sites of Perturbed Shear Stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4896–4901. [Google Scholar] [CrossRef]
- Bahari, H.; Pourreza, S.; Goudarzi, K.; Mirmohammadali, S.N.; Asbaghi, O.; Kolbadi, K.S.H.; Naderian, M.; Hosseini, A. The Effects of Pomegranate Consumption on Obesity Indices in Adults: A Systematic Review and Meta-analysis. Food Sci. Nutr. 2024, 12, 641–660. [Google Scholar] [CrossRef]
- Attanayake, R.; Eeswaran, R.; Rajapaksha, R.; Weerakkody, P.; Bandaranayake, P.C.G. Biochemical Composition and Expression of Anthocyanin Biosynthetic Genes of a Yellow Peeled and Pinkish Ariled Pomegranate (Punica granatum L.) Cultivar Are Differentially Regulated in Response to Agro-Climatic Conditions. J. Agric. Food Chem. 2018, 66, 8761–8771. [Google Scholar] [CrossRef]
- Levin, G.M. Pomegranate; Third Millennium Publishing: Tempe, AZ, USA, 2006. [Google Scholar]
- Shwartz, E.; Glazer, I.; Bar-Ya’akov, I.; Matityahu, I.; Bar-Ilan, I.; Holland, D.; Amir, R. Changes in Chemical Constituents during the Maturation and Ripening of Two Commercially Important Pomegranate Accessions. Food Chem. 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Verma, N.; Mohanty, A.; Lal, A. Invited Mini-Review Fruit, Vegetable and Cereal Science and Biotechnology Pomegranate Genetic Resources and Germplasm Conservation: A Review. Fruit Veg. Cereal Sci. Biotechnol 2010, 4, 120–125. [Google Scholar]
- Mditshwa, A.; Fawole, O.A.; Al-Said, F.; Al-Yahyai, R.; Opara, U.L. Phytochemical Content, Antioxidant Capacity and Physicochemical Properties of Pomegranate Grown in Different Microclimates in South Africa. S. Afr. J. Plant Soil 2013, 30, 81–90. [Google Scholar] [CrossRef]
- Borochov-Neori, H.; Judeinstein, S.; Tripler, E.; Harari, M.; Greenberg, A.; Shomer, I.; Holland, D. Seasonal and Cultivar Variations in Antioxidant and Sensory Quality of Pomegranate (Punica granatum L.) Fruit. J. Food Compos. Anal. 2009, 22, 189–195. [Google Scholar] [CrossRef]
- Ghasemi-Soloklui, A.A.; Kordrostami, M.; Gharaghani, A. Environmental and Geographical Conditions Influence Color, Physical Properties, and Physiochemical Composition of Pomegranate Fruits. Sci. Rep. 2023, 13, 15447. [Google Scholar] [CrossRef]
- Feng, L.; Yin, Y.; Yang, X.; Tang, H. Evaluation of Physiochemical Properties of Different Pomegranate Cultivars in China. Acta Hortic. 2020, 1281, 89–96. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, Horticulture, Breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar] [CrossRef]
- Babu, K.D. Invited Mini-Review Fruit, Vegetable and Cereal Science and Biotechnology Floral Biology of Pomegranate (Punica granatum L.). Fruit Veg. Cereal Sci. Biotechnol 2010, 4, 45–50. [Google Scholar]
- Chandra, R.; Jadhav, V.T.; Sharma, J. Global Scenario of Pomegranate (Punica granatum L.) Culture with Special Reference to India. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 7–18. [Google Scholar]
- Wetzstein, H.Y.; Yi, W.; Porter, J.A.; Ravid, N. Flower Position and Size Impact Ovule Number per Flower, Fruitset, and Fruit Size in Pomegranate. J. Am. Soc. Hortic. Sci. 2013, 138, 159–166. [Google Scholar] [CrossRef]
- Evreinoff, V.A. Contribution à l’étude Du Grenadier. J. Agric. Trop. Bot. Appl. 1957, 4, 124–138. [Google Scholar] [CrossRef]
- Gozlekcİ, S.; Kaynak, L. Physical and Chemical Changes during Fruit Development and Flowering in Pomegranate (Punica granatum L.) Cultivar Hicaz Nar Grow in Antalya Region. In Proceedings of the Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology, Orihuela, Spain, 15–17 October 1998; Melgarejo, P., Martínez-Nicolás, J.J., Martínez-Tomé, J., Eds.; CIHEAM Options Méditerranéennes: Série A. Séminaires Méditerranéens. CIHEAM: Zaragoza, Spain, 2000; Volume 42, pp. 79–85. [Google Scholar]
- Rodríguez, P.; Mellisho, C.D.; Conejero, W.; Cruz, Z.N.; Ortuño, M.F.; Galindo, A.; Torrecillas, A. Plant Water Relations of Leaves of Pomegranate Trees under Different Irrigation Conditions. Environ. Exp. Bot. 2012, 77, 19–24. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in Physical Properties, Chemical and Elemental Composition and Antioxidant Capacity of Pomegranate (Cv. Ruby) Fruit at Five Maturity Stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Preharvest and Postharvest Factors Influencing Bioactive Compounds in Pomegranate (Punica granatum L.)—A Review. Sci. Hortic. 2014, 178, 114–123. [Google Scholar] [CrossRef]
- Radunić, M.; Jukić Špika, M.; Goreta Ban, S.; Gadže, J.; Díaz-Pérez, J.C.; Maclean, D. Physical and Chemical Properties of Pomegranate Fruit Accessions from Croatia. Food Chem. 2015, 177, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.M.; Umar, I.; Mir, S.A.; Rehman, M.U.; Rather, G.H.; Banday, S.A. Quality Evaluation of Pomegranate Crop—A Review. Int. J. Agric. Biol. 2012, 14, 658–667. [Google Scholar]
- Kulkarni, A.P.; Aradhya, S.M. Chemical Changes and Antioxidant Activity in Pomegranate Arils during Fruit Development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Jacobo, C.M. Cactus Pear Domestication and Breeding; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 20, pp. 135–166. [Google Scholar]
- de Wit, M.; Nel, P.; Osthoff, G.; Labuschagne, M.T. The Effect of Variety and Location on Cactus Pear (Opuntia Ficus-Indica) Fruit Quality. Plant Foods Hum. Nutr. 2010, 65, 136–145. [Google Scholar] [CrossRef]
- Shulman, Y.; Fainberstein, L.; Lavee, S. Pomegranate Fruit Development and Maturation. J. Hortic. Sci. 1984, 59, 265–274. [Google Scholar] [CrossRef]
- Zarei, M.; Azizi, M.; Bashir-Sadr, Z. Evaluation of Physicochemical Characteristics of Pomegranate (Punica granatum L.) Fruit during Ripening. Fruits 2011, 66, 121–129. [Google Scholar] [CrossRef]
- Ozgen, M.; Durgaç, C.; Serçe, S.; Kaya, C. Chemical and Antioxidant Properties of Pomegranate Cultivars Grown in the Mediterranean Region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Poyrazoǧlu, E.; Gökmen, V.; Artik, N. Organic Acids and Phenolic Compounds in Pomegranates (Punica granatum L.) Grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Wang, S.Y. Effect of Pre-Harvest Conditions on Antioxidant Capacity in Fruits. Acta Hortic. 2006, 712, 299–306. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total Phenolic Content and Antioxidant Capacity of Extracts Obtained from Six Important Fruit Residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Molassiotis, A.; Diamantidis, G.; Vasilakakis, M. Antioxidant and Free Radical-Scavenging Activities of Phenolic Extracts of Olive Fruits. Food Chem. 2010, 120, 1097–1103. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Liber, Z.; Montemurro, C.; Miazzi, M.M.; Ljubenkov, I.; Soldo, B.; Žanetić, M.; Vitanović, E.; Politeo, O.; Škevin, D. Quantitatively Unraveling Hierarchy of Factors Impacting Virgin Olive Oil Phenolic Profile and Oxidative Stability. Antioxidants 2022, 11, 594. [Google Scholar] [CrossRef]
- Branchereau, C.; Hardner, C.; Dirlewanger, E.; Wenden, B.; Le Dantec, L.; Alletru, D.; Parmentier, J.; Ivančič, A.; Giovannini, D.; Brandi, F.; et al. Genotype-by-Environment and QTL-by-Environment Interactions in Sweet Cherry (Prunus avium L.) for Flowering Date. Front. Plant Sci. 2023, 14, 1142974. [Google Scholar] [CrossRef]
- Deloire, A.; Rogiers, S.; Šuklje, K.; Antalick, G.; Zeyu, X.; Pellegrino, A. Grapevine Berry Shrivelling, Water Loss and Cell Death: An Increasing Challenge for Growers in the Context of Climate Change. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Ubi, B.E. External Stimulation of Anthocyanin Biosynthesis in Apple Fruit. Food Agric. Environ. 2004, 2, 65–70. [Google Scholar]
- Borochov-Neori, H.; Judeinstein, S.; Harari, M.; Bar-Ya’akov, I.; Patil, B.S.; Lurie, S.; Holland, D. Climate Effects on Anthocyanin Accumulation and Composition in the Pomegranate (Punica granatum L.) Fruit Arils. J. Agric. Food Chem. 2011, 59, 5325–5334. [Google Scholar] [CrossRef] [PubMed]
- Zaouay, F.; Mars, M. Phenotypic Variation and Estimation of Genetic Parameters to Improve Fruit Quality in Tunisian Pomegranate (Punica granatum L.) Accessions. J. Hortic. Sci. Biotechnol. 2014, 89, 221–228. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of Latitude on Flavonoid Biosynthesis in Plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef]
| Location | Kaštela, Croatia (CRO) | Metković, Croatia (CRO) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month | Temperature (°C) | Rainfall (mm) | Humidity (%) | Temperature (°C) | Rainfall (mm) | Humidity (%) | ||||
| Mean | Max | Min | Mean | Max | Min | |||||
| January | 8.1 | 15.9 | −3.0 | 228.4 | 65 | 7.1 | 16.7 | −4.0 | 243.1 | 68 |
| February | 7.1 | 15.1 | −3.0 | 81.8 | 57 | 6.0 | 17.5 | −3.9 | 71.0 | 63 |
| March | 10.2 | 19.0 | 2.0 | 88.2 | 58 | 9.8 | 20.7 | −0.2 | 132.0 | 60 |
| April | 15.2 | 24.5 | 8.5 | 111.2 | 70 | 15.1 | 27.1 | 6.9 | 54.4 | 68 |
| May | 20.9 | 34.7 | 9.7 | 17.4 | 54 | 20.8 | 34.0 | 8.2 | 33.0 | 67 |
| June | 22.2 | 31.9 | 13.7 | 242.4 | 61 | 21.5 | 32.8 | 12.6 | 203.1 | 69 |
| July | 26.3 | 37.2 | 17.4 | 34.0 | 52 | 25.6 | 36.4 | 14.7 | 25.8 | 69 |
| August | 26.8 | 36.1 | 18.0 | 14.6 | 50 | 25.9 | 36.5 | 15.5 | 34.7 | 69 |
| September | 22.5 | 31.0 | 14.6 | 90.4 | 58 | 21.8 | 32.0 | 12.8 | 52.9 | 67 |
| October | 15.7 | 26.2 | 5.5 | 202.0 | 59 | 14.0 | 27.7 | 1.7 | 242.2 | 66 |
| November | 12.0 | 19.7 | 3.1 | 119.2 | 76 | 10.8 | 19.6 | 0.3 | 130.4 | 72 |
| December | 9.4 | 18.6 | −3.7 | 198.6 | 66 | 8.3 | 21.0 | −6.4 | 332.8 | 73 |
| Average | 16.4 | 15.6 | ||||||||
| Summa | 1428.2 | 1555.4 | ||||||||
| Location | Mostar, Bosnia and Herzegovina (BIH) | Bar, Monte Negro (MNE) | ||||||||
| Month | Temperature (°C) | Rainfall (mm) | Humidity (%) | Temperature (°C) | Rainfall (mm) | Humidity (%) | ||||
| Mean | Max | Min | Mean | Max | Min | |||||
| January | 6.3 | 16.3 | −3.6 | 336.9 | 63 | 9.6 | 17.2 | 1.0 | 351.1 | 69 |
| February | 5.9 | 18.0 | −3.0 | 117.4 | 55 | 8.1 | 20.1 | 0.0 | 141.9 | 54 |
| March | 9.5 | 20.0 | 1.6 | 173.6 | 60 | 10.8 | 22.2 | 2.7 | 188.7 | 61 |
| April | 15.6 | 26.4 | 7.3 | 68.1 | 65 | 15.5 | 25.2 | 8.2 | 52.5 | 72 |
| May | 21.3 | 35.0 | 7.6 | 35.7 | 55 | 20.5 | 30.8 | 10.7 | 39.7 | 66 |
| June | 22.0 | 36.1 | 12.0 | 190.0 | 62 | 22.3 | 32.0 | 14.7 | 175.8 | 68 |
| July | 26.3 | 38.9 | 15.0 | 15.8 | 55 | 25.5 | 33.8 | 17.7 | 27.0 | 66 |
| August | 26.8 | 37.9 | 18.1 | 57.8 | 51 | 25.8 | 35.4 | 19.9 | 11.8 | 66 |
| September | 22.3 | 34.8 | 14.2 | 127.8 | 56 | 22.8 | 30.8 | 15.6 | 103.1 | 62 |
| October | 14.8 | 28.6 | 3.6 | 246.6 | 62 | 16.9 | 26.5 | 6.7 | 231.9 | 65 |
| November | 10.4 | 19.4 | 2.9 | 164.2 | 75 | 13.1 | 21.1 | 5.9 | 236.7 | 79 |
| December | 7.5 | 18.2 | −7.8 | 315.0 | 68 | 11.9 | 19.9 | 1.1 | 265.9 | 70 |
| Average | 15.7 | 16.9 | ||||||||
| Summary | 1848.9 | 1826.4 | ||||||||
| Location | ||||
|---|---|---|---|---|
| Fruit Properties | Kaštela, CRO | Metković, CRO | Mostar, BIH | Bar, MNE |
| Fruit weight (FW; g) | 506.8 * ± 18.5 a ** | 421.6 ± 20.09 ab | 453.9 ± 26.4 ab | 386.9 ± 31.9 b |
| Fruit volume (FV; mL) | 531.1 ± 12.9 a | 448.9 ± 20.9 ab | 477.2 ± 27.6 ab | 407.2 ± 33.8 b |
| Fruit length (FL; mm) | 84.6 ± 0.8 | 79.6 ± 1.2 | 83.5 ± 2.2 | 79.7 ± 2.6 |
| Fruit diameter (FD; mm) | 91.5 ± 1.2 b | 86.4 ± 0.8 c | 96.1 ± 0.7 a | 92.6 ± 2.5 ab |
| Fruit shape index (FL/FD) | 0.92 ± 0.02 a | 0.92 ± 0.01 a | 0.87 ± 0.02 b | 0.86 ± 0.02 b |
| Calix length (CL; mm) | 17.0 ± 0.5 b | 17.2 ± 0.6 b | 18.9 ± 0.3 a | 19.2 ± 0.3 a |
| Calix diameter (CD; mm) | 23.9 ± 1.2 | 23.6 ± 0.9 | 26.3 ± 0.4 | 23.6 ± 1.1 |
| Peel thickness (PT; mm) | 4.2 ± 0.4 | 4.1 ± 0.4 | 3.5 ± 0.2 | 4.4 ± 0.4 |
| Number of arils per fruit (NoA/F) | 749 ± 41 a | 607 ± 27 b | 611 ± 46 b | 561 ± 23 b |
| Total aril weight (TAW; g) | 309.2 ± 14.8 a | 261.1 ± 17.1 bc | 294.3 ± 10.9 ab | 235.0 ± 17.9 c |
| Aril weight (AW; g) | 0.41 ± 0.02 b | 0.42 ± 0.02 ab | 0.47 ± 0.02 a | 0.39 ± 0.03 b |
| Aril yield (AY; %) | 60.8 ± 0.9 | 60.9 ± 1.7 | 58.6 ± 1.9 | 61.1 ± 1.4 |
| Juice yield (JY; %) | 71.8 ± 1.7 | 74.2 ± 0.4 | 73.3 ± 1.6 | 71.0 ± 2.6 |
| Location | ||||
|---|---|---|---|---|
| Juice Properties | Kaštela, CRO | Metković, CRO | Mostar, BIH | Bar, MNE |
| Total soluble solids (TSS; °Brix) | 14.1 * ± 0.1 b ** | 15.0 ± 0.2 a | 13.1 ± 0.0 c | 12.8 ± 0.4 c |
| Total acidity (TA; %) | 0.53 ± 0.01 bc | 0.59 ± 0.03 a | 0.48 ± 0.01 c | 0.55 ± 0.01 ab |
| TSS/TA ratio | 26.4 ± 0.5 | 25.7 ± 0.9 | 27.0 ± 0.3 | 23.2 ± 1.1 |
| Total sugar content (TSC; %) | 11.8 ± 0.4 b | 12.1 ± 0.2 b | 12.6 ± 0.1 b | 13.9 ± 0.5 a |
| pH | 3.57 ± 0.02 a | 3.52 ± 0.05 ab | 3.60 ± 0.03 a | 3.42 ± 0.03 b |
| Location | ||||
|---|---|---|---|---|
| Aril Properties | Kaštela, CRO | Metković, CRO | Mostar, BiH | Bar, MNE |
| Total phenol content (mg L−1 GAE) | 3101.9 * ± 35.7 a ** | 2603.0 ± 124.1 b | 2725.5 ± 150.2 b | 2772.3 ± 108.3 b |
| Total anthocyanin content (mg/100 g) | 44.9 ± 5.58 | 43.99 ± 4.67 | 51.3 ± 6.71 | 59.36 ± 4.03 |
| DPPH (%) | 70.5 ± 0.8 | 67.7 ± 1.2 | 69.1 ± 1.3 | 69.3 ± 2.5 |
| TOSC (umol Trolox eq/kg) | 414.3 ± 12.4 | 425.8 ± 10.0 | 420.3 ± 8.2 | 436.4 ± 10.4 |
| Location | ||||
|---|---|---|---|---|
| Color Parameters | Kaštela, CRO | Metković, CRO | Mostar, BIH | Bar, MNE |
| L* | 32.95 * ± 0.57 a ** | 35.47 ± 2.26 a | 32.91 ± 2.09 a | 26.36 ± 1.03 b |
| a* | 6.47 ± 0.41 c | 8.34 ± 1.83 bc | 10.66 ± 0.83 ab | 11.92 ± 1.62 a |
| b* | 12.07 ± 0.65 a | 14.56 ± 3.84 a | 4.20 ± 1.54 b | 8.61 ± 2.18 ab |
| C | 14.33 ± 0.31 ab | 17.23 ± 3.24 a | 11.62 ± 0.97 b | 15.06 ± 1.05 ab |
| h° | 58.27 ± 2.40 a | 56.90 ± 9.30 a | 19.96 ± 5.53 b | 36.07 ± 10.42 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radunić, M.; Jukić Špika, M.; Gadže, J.; Goreta Ban, S.; Díaz-Pérez, J.C.; MacLean, D. Microclimate Condition Influence on the Physicochemical Properties and Antioxidant Activity of Pomegranate (Punica granatum L.): A Case Study of the East Adriatic Coast. Agriculture 2025, 15, 2210. https://doi.org/10.3390/agriculture15212210
Radunić M, Jukić Špika M, Gadže J, Goreta Ban S, Díaz-Pérez JC, MacLean D. Microclimate Condition Influence on the Physicochemical Properties and Antioxidant Activity of Pomegranate (Punica granatum L.): A Case Study of the East Adriatic Coast. Agriculture. 2025; 15(21):2210. https://doi.org/10.3390/agriculture15212210
Chicago/Turabian StyleRadunić, Mira, Maja Jukić Špika, Jelena Gadže, Smiljana Goreta Ban, Juan Carlos Díaz-Pérez, and Dan MacLean. 2025. "Microclimate Condition Influence on the Physicochemical Properties and Antioxidant Activity of Pomegranate (Punica granatum L.): A Case Study of the East Adriatic Coast" Agriculture 15, no. 21: 2210. https://doi.org/10.3390/agriculture15212210
APA StyleRadunić, M., Jukić Špika, M., Gadže, J., Goreta Ban, S., Díaz-Pérez, J. C., & MacLean, D. (2025). Microclimate Condition Influence on the Physicochemical Properties and Antioxidant Activity of Pomegranate (Punica granatum L.): A Case Study of the East Adriatic Coast. Agriculture, 15(21), 2210. https://doi.org/10.3390/agriculture15212210









