Characterization and Evaluation of Biomass Waste Biochar for Turfgrass Growing Medium Enhancement in a Pot Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar Material
2.2. Analysis Methods Used for Characterization of PL and PLB
FTIR and SEM/EDX Analysis
2.3. Turfgrass Planting Experiment and Chemical Characterization of Grass and Growing Media Residue
- -
- Two watering regimes (Regular irrigation—Series 1 and Limited irrigation—Series 2);
- -
- Four different treatment groups by soil amendment composition (0—control without amendments; B—with biochar PLB; BF—Combined treatment with biochar PLB and nitrogen fertilizer; and F—with nitrogen fertilizer only);
- -
- Two replicates per treatment per watering regime.
2.4. Electrical Conductivity (EC) and Cation Exchange Capacity (CEC) of Biochar and Residual Growing Medium
2.5. Chlorophyll Content as Indicator of Biofertilizer Efficiency
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Biochar
3.1.1. FTIR Spectra of PL and PLB
3.1.2. SEM/EDX Analysis of PL and PLB
3.2. Grass Planting Experiment
3.2.1. Measuring Moisture and pH Values of the Residual Growing Medium
3.2.2. Electrical Conductivity (EC) and Cation Exchange Capacity (CEC) of Residual Growing Medium (S)
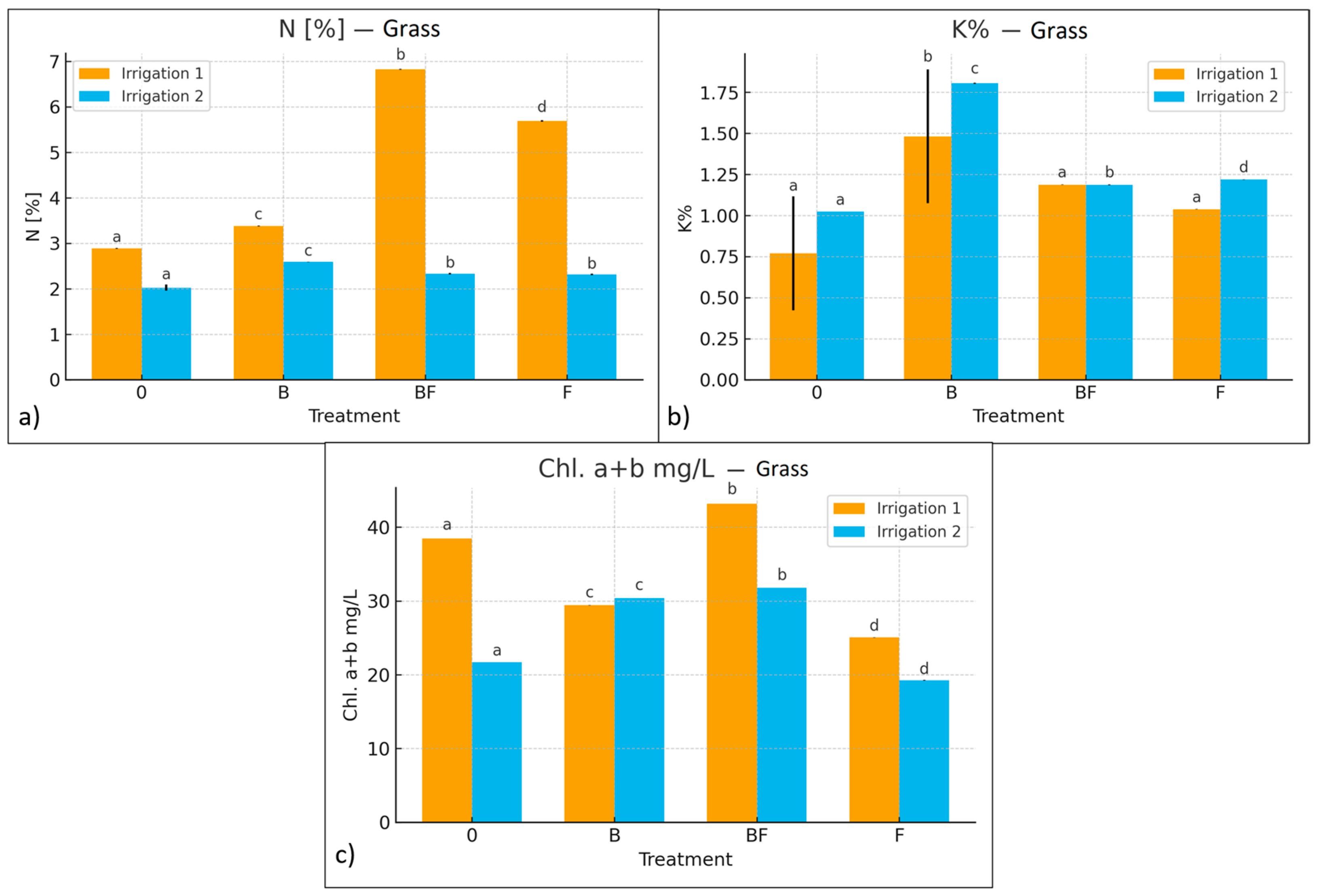
3.2.3. Chlorophyll Content as Indicator of Biofertilizer Efficiency
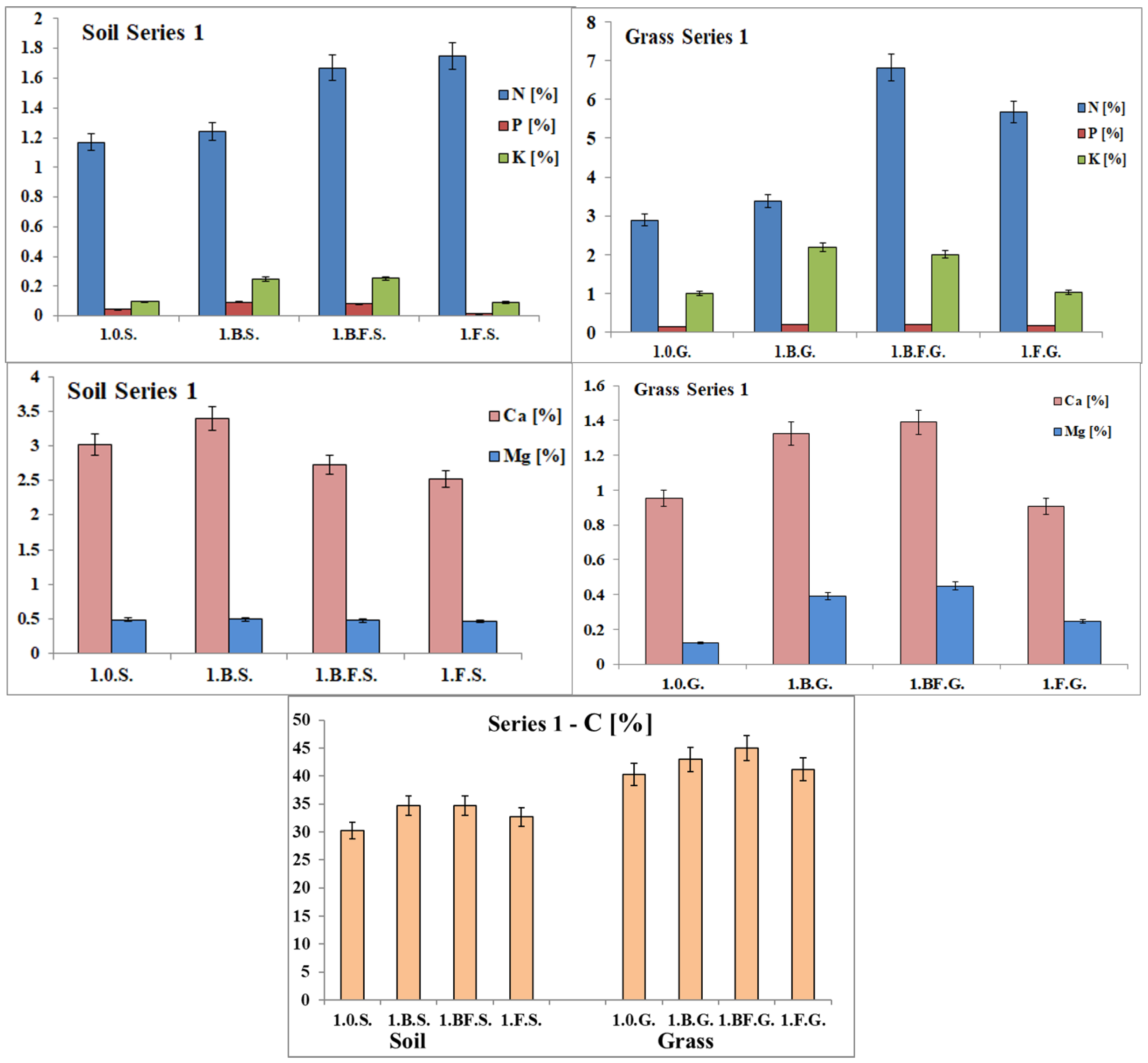
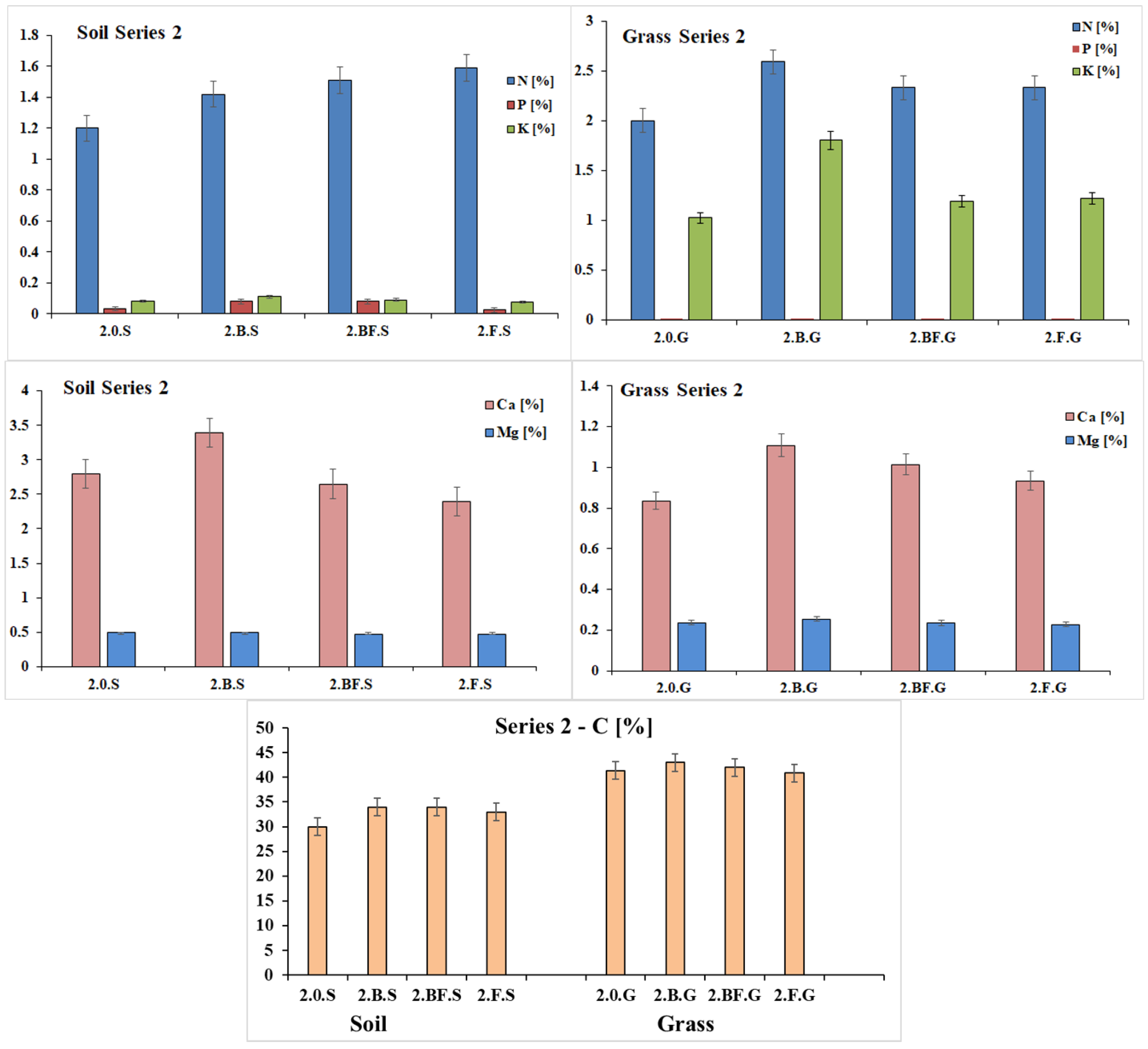
3.2.4. Chemical Characterization of Residual Growing Medium and Turfgrass Samples
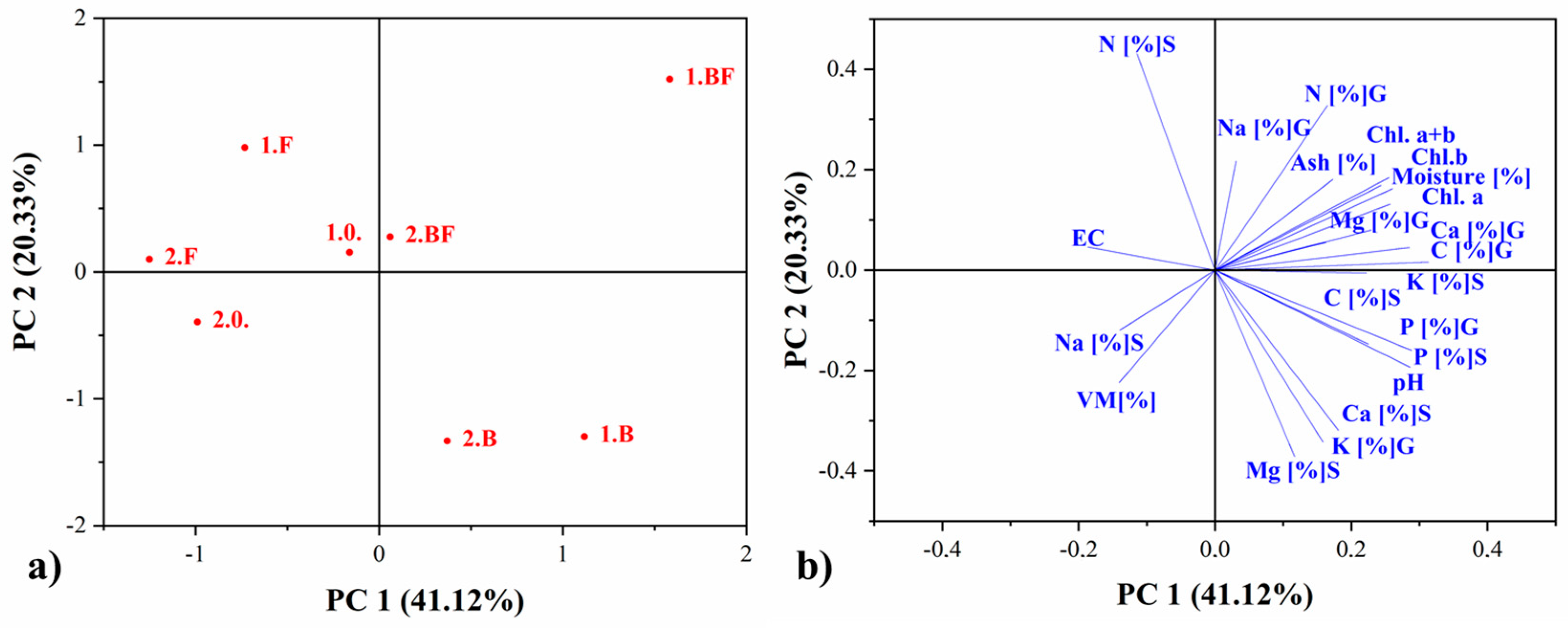
3.2.5. Statistical Analysis of Pot Experiments Under Outdoor Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bock, H.; Wickings, K. Collembola (Isotomidae) and mowing management practices control distinct aspects of thatch decomposition in a lawn mesocosm experiment. Pedobiologia 2023, 101, 150896. [Google Scholar] [CrossRef]
- Wang, R.; Mattox, C.M.; Phillips, C.L.; Kowalewski, A.R. Carbon Sequestration in Turfgrass-Soil Systems. Plants 2022, 11, 2478. [Google Scholar] [CrossRef]
- Ali, L.; Xiukang, W.; Naveed, M.; Ashraf, S.; Nadeem, S.M.; Haider, F.U.; Mustafa, A. Impact of Biochar Application on Germination Behavior and Early Growth of Maize Seedlings: Insights from a Growth Room Experiment. Appl. Sci. 2021, 11, 11666. [Google Scholar] [CrossRef]
- Sisay, A.; Girma, E. Biochar: Usage, Potential as Alternative to Chemical Fertilizer and Impact of Biochar on Soil-Microbial-Plant, Root Interaction. Ind. Eng. Manag. 2021, 10, 311. [Google Scholar] [CrossRef]
- Rombel, A.; Krasucka, P.; Oleszczuk, P. Sustainable biochar-based soil fertilizers and amendments as a new trend in biochar research. Sci. Total Environ. 2022, 816, 151588. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch´ng, H.Y. Soil Nitrogen Sorption Using Charcoal and Wood Ash. Agronomy 2021, 11, 1801. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Yu, Y.; Ma, X. Biochar, grass, and cross-ridge reshaped the surface runoff nitrogen under consecutive rainstorms in loessial sloping lands. Agric. Water Manag. 2022, 261, 107354. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Yuan, R.; Wang, P. Biomass pyrolysis with alkaline-earth-metal additive for co-production of bio-oil and biochar-based soil amendment. Sci. Total Environ. 2020, 743, 140760. [Google Scholar] [CrossRef]
- Hale, L.; Curtis, D.; Azeem, M.; Montgomery, J.; Crowley, D.; McGiffen, M., Jr. Influence of compost and biochar on soil biological properties under turfgrass supplied deficit irrigation. Appl. Soil Ecol. 2021, 168, 104134. [Google Scholar] [CrossRef]
- Wilson, R.; Suk Kim, H.; Bontchev, R.P.; Wahlgren, N.C.; Traxler, V.; Thompson, T.A.; Buege, B.; Jarand, M. Method for Application of Biochar in Turf Grass and Landscaping Environments. U.S. Patent No. 10,392,313, 25 February 2021. [Google Scholar]
- Koprivica, M.; Petrović, J.; Ercegović, M.; Simić, M.; Milojković, J.; Šoštarić, T.; Dimitrijević, J. Improvement of combustible characteristics of Paulownia leaves via hydrothermal carbonization. Biomass Conv. Bioref. 2024, 14, 3975–3985. [Google Scholar] [CrossRef]
- Koprivica, M.; Simić, M.; Petrović, J.; Ercegović, M.; Dimitrijević, J. Evaluation of Adsorption Efficiency on Pb(II) Ions Removal Using Alkali-Modified Hydrochar from Paulownia Leaves. Processes 2023, 11, 1327. [Google Scholar] [CrossRef]
- Domingues, R.; Trugilho, P.; Silva, C.; De Melo, I.C.; Melo, L.; Magriotis, Z.; Sánchez-Monedero, M. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Peng, Y.; Xiang, P.; Zhou, Y.; Yao, B.; Zhou, Y.; Sun, C. Co-application of biochar and organic fertilizer promotes the yield and quality of red pitaya (Hylocereus polyrhizus) by improving soil properties. Chemosphere 2022, 294, 133619. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 1984.
- Petrović, J.; Perišić, N.; Dragišić Maksimović, J.; Maksimović, V.; Kragović, M.; Stojanović, M.; Laušević, M.; Mihajlović, M. Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase. J. Anal. Appl. Pyrol. 2016, 118, 267–277. [Google Scholar] [CrossRef]
- U.S. EPA Method 3052. Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices. In Test Methods for Evaluating Solid Wastes: Physical/Chemical Methods, EPA SW-846; U.S. Environmental Protection Agency: Washington, DC, USA, 1996.
- ISO 11466:1995; Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. International Organization for Standardization: Geneva, Switzerland, 1995.
- Awwad, N.S.; El-Nadi, Y.A.; Hamed, M.M. Successive processes for purificationand extraction of phosphoric acid produced by wet process. Chem. Eng. Process. 2013, 74, 69–74. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, electrical conductivity and liming potential. In Chapter 3. in Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; Csiro Publishing: Clayton, Australia, 2017; pp. 23–28. ISBN 1486305105/9781486305100. [Google Scholar]
- Milićević, T.; Relić, D.; Škrivanj, S.; Tešić, Ž.; Popović, A. Assessment of major and trace element bioavailability in vineyard soil applying different single extraction procedures and pseudo-total digestion. Chemosphere 2017, 171, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Haq, N.; Farhan, A.; Waseem, A.; Junaid, A.; Zahid, M.; Hudood, U.; Noor, U.B. Soil Electrical conductivity as Affected by Biochar Under Summer Crops. Int. J. Environ. Sci. Nat. Res. 2018, 14, 555887. [Google Scholar]
- Nel, T.; Bruneel, Y.; Smolders, E. Comparison of five methods to determine the cation exchange capacity of soil. J. Plant Nutr. Soil Sci. 2023, 186, 311–320. [Google Scholar] [CrossRef]
- Ngcobo, S.; Bada, S.O.; Ukpong, A.M.; Risenga, I. Optimal chlorophyll extraction conditions and postharvest stability in Moringa (M. Oleifera) leaves. J. Food Meas. Charact 2024, 18, 1611–1626. [Google Scholar] [CrossRef]
- McBeath, A.; Wurster, C.; Bird, M. Influence of feedstock properties and pyrolysis conditions on biochar carbon stability as determined by hydrogen pyrolysis. Biomass Bioenergy 2015, 73, 155–173. [Google Scholar] [CrossRef]
- Murillo-Acevedo, Y.; Grande, C. Grass clippings to biochar: A promising soil amendment for sustainable agriculture under drip irrigation. Environ. Chem. Eng. 2024, 12, 114856. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Svec, J.; Trudicova, M.; Hajzler, J.; Kubikova, L.; Enev, V. The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner. Materials 2022, 15, 8855. [Google Scholar] [CrossRef]
- Shyam, S.; Ahmed, A.; Joshi, S.; Sarma, H. Biochar as a Soil amendment: Implications for soil health, carbon sequestration, and climate resilience. Discover Soil 2025, 2, 18. [Google Scholar] [CrossRef]
- Pandian, K.; Vijayakumar, S.; Mustafa, M.R.A.F.; Subramanian, P.; Chitraputhirapillai, S. Biochar—A sustainable soil conditioner for improving soil health, crop production and environment under changing climate: A review. Front. Soil Sci. 2024, 4, 1376159. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrol. 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Jassal, R.; Johnson, M.; Molodovskaya, M.; Black, A.; Jollymore, A.; Sveinson, K. Nitrogen enrichment potential of biochar in relation to pyrolysis temperature and feedstock quality. J. Environ. Manag. 2015, 152, 140–144. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Li, J.; Song, M.; Yin, J.; Fenton, O.; Gao, L.; Tian, Y. The beneficial effects of biochar on overall soil quality and plant performance are dose-dependent and are closely associated with soil pH. Pedosphere 2025. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilizing Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Available online: https://www.lexaris.de/library/tableofcontents/2056005 (accessed on 26 May 2025).
- Liao, J.; Hao, G.; Guo, H.; Chen, H. Effects of biochar on plant and microbial communities in landfill soil. Appl. Soil Ecol. 2024, 204, 105749. [Google Scholar] [CrossRef]
- Bolan, N.; Sarmah, A.; Bordoloi, S.; Bolan, S.; Padhye, L.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Hong, Z.-N.; Li, J.-Y.; Jiang, J.; Baquy, M.; Xu, R.-K.; Qian, W. Mechanisms for Increasing the pH Buffering Capacity of an Acidic Ultisol by Crop Residue-Derived Biochars. J. Agric. Food Chem. 2017, 65, 8111–8119. [Google Scholar] [CrossRef]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The key role of biochar in amending acidic soil: Reducing soil acidity and improving soil acid buffering capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Wu, B.; Yang, H.; Li, S.; Tao, J. The effect of biochar on crop productivity and soil salinity and its dependence on experimental conditions in salt-affected soils: A meta-analysis. Carbon Res. 2024, 3, 56. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef] [PubMed]
- Mihajlović, M.; Petrović, J.; Maletić, S.; Kragulj Isakovski, M.; Stojanović, M.; Lopičić, Z.; Trifunović, S. Hydrothermal carbonization of Miscanthus×giganteus: Structural and fuel properties of hydrochars and organic profile with the ecotoxicological assessment of the liquid phase. Energy Convers. Manag. 2018, 159, 254–263. [Google Scholar] [CrossRef]
- Lee, M.-E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. Int. J. Environ. Res. Public Health 2017, 14, 1528. [Google Scholar] [CrossRef]
- Florio, G.; Zwier, T.; Myshakin, E.; Jordan, K.; Sibert, E. Theoretical modeling of the OH stretch infrared spectrum of carboxylic acid dimers based on first-principles anharmonic couplings. J. Chem. Phys. 2003, 118, 1735–1746. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef]
- Yan, L.; Gao, G.; Lu, M.; Riaz, M.; Zhang, M.; Tong, K.; Yu, H.; Yang, Y.; Hao, W.; Niu, Y. Insight into the Amelioration Effect of Nitric Acid-Modified Biochar on Saline Soil Physicochemical Properties and Plant Growth. Plants 2024, 13, 3434. [Google Scholar] [CrossRef]
- He, K.; Liu, Q.; Zhang, J.; Zhang, G.; Li, G. Biochar Enhances the Resistance of Legumes and Soil Microbes to Extreme Short-Term Drought. Plants 2023, 12, 4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, W.; Luo, H. Effect of Biochar Amendment on the Growth and Photosynthetic Traits of Plants Under Drought Stress: A Meta-Analysis. Agronomy 2024, 14, 2952. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A review on biochar’s effect on soil properties and crop growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzhieva, S.; et al. Sustainable Approach and Safe Use of Biochar and Its Possible Consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Song, X.; Chen, C.; Arthur, E.; Tuller, M.; Zhou, H.; Shang, J.; Ren, T. Cation exchange capacity and soil pore system play key roles in water vapour sorption. Geoderma 2022, 424, 116017. [Google Scholar] [CrossRef]
- Rodgers, E.; Nicolson, E.; Lauder, S.; Hodge, S. Response of Pasture Grasses to Organic Fertilizer Produced from Black Soldier Fly Frass. Plants 2024, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, H.; Gao, D.; Wang, Y.; Jin, K.; Liu, J.; Xue, D.; Chen, Y.; Li, Y.; Gao, T.; et al. Comparative study on the effects of soil quality improvement between urban spontaneous groundcover and lawn. Ecol. Indic. 2023, 148, 110056. [Google Scholar] [CrossRef]
- Yu, L.; Yu, M.; Lu, X.; Tang, C.; Liu, X.; Brookes, P.C.; Xu, J. Combined application of biochar and nitrogen fertilizer benefits nitrogen retention in the rhizosphere of soybean by increasing microbial biomass but not altering microbial community structure. Sci Total Environ. 2018, 640, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Basalirwa, D.; Sudo, S.; Wacal, C.; Zaw, O.A.; Sasagawa, D.; Yamamoto, S.; Masunaga, T.; Nishihara, E. Impact of fresh and aged palm shell biochar on N2O emissions, soil properties, nutrient content and yield of Komatsuna (Brassica rapa var. perviridis) under sandy soil conditions. Soil Sci. Plant Nutr. 2020, 66, 328–343. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Tao, W.; Zhang, X.; Xu, Z.; Xu, C. The Biological Effects of Biochar on Soil’s Physical and Chemical Characteristics: A Review. Sustainability 2025, 17, 2214. [Google Scholar] [CrossRef]
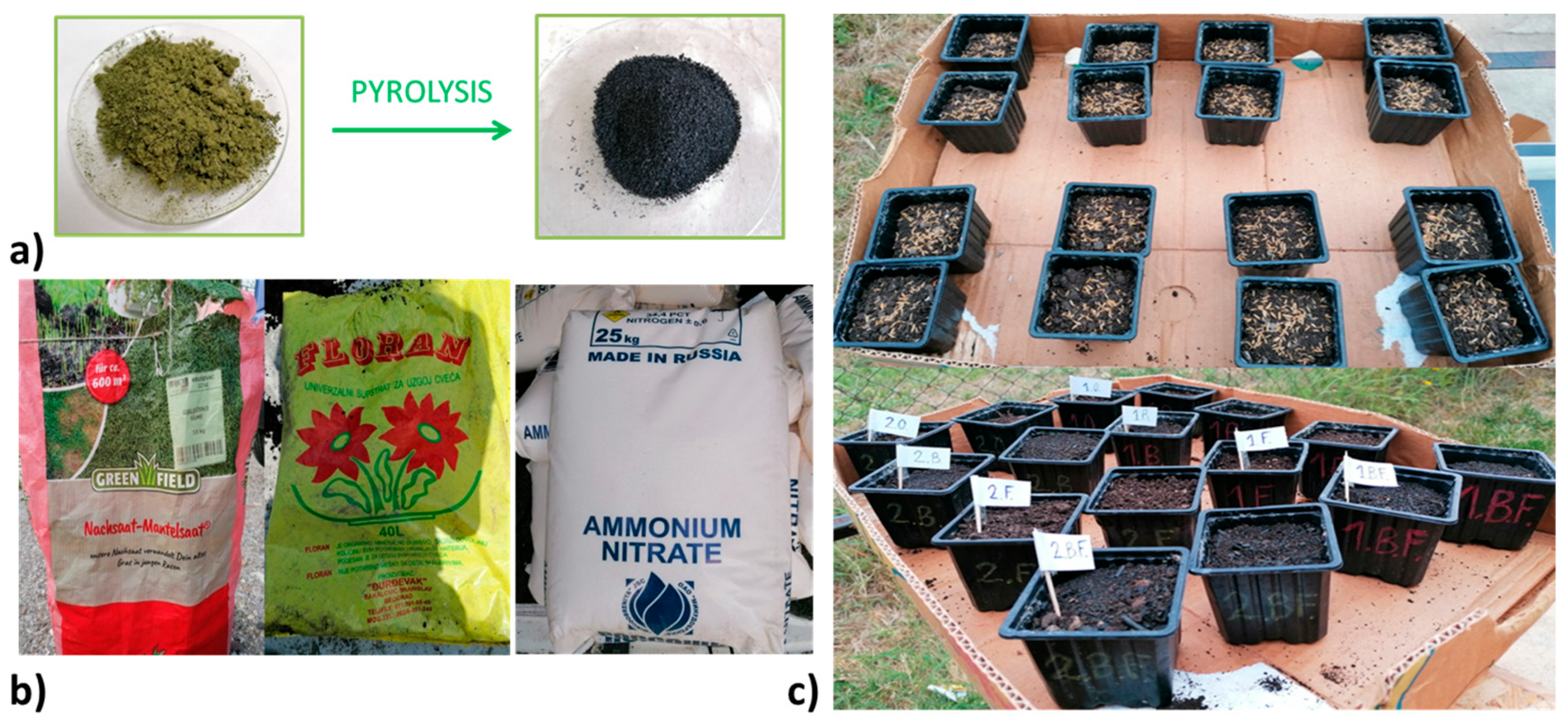






| Parameter | PL | PLB | |
|---|---|---|---|
| Proximate Analysis (wt%) | MY a (wt%) | - | 38.95 ± 0.22 |
| Moisture | 8.71 ± 0.04 | 6.19 ± 0.07 | |
| Volatiles | 77.01 ± 0.65 | 35.81 ± 0.71 | |
| Ash | 5.69 ± 0.20 | 17.92 ± 0.31 | |
| Cfix b | 8.89 ± 0.15 | 40.06 ± 0.35 | |
| Elemental analysis (%) | Carbon | 45.71 ± 0.19 | 63.38 ± 0.42 |
| Hydrogen | 6.05 ± 0.02 | 4.77 ± 0.01 | |
| H/C atomic | 1.57 | 0.90 | |
| Oxygen b | 38.22 ± 0.14 | 8.86 ± 0.03 | |
| Nitrogen | 3.79 ± 0.01 | 4.84 ± 0.02 | |
| Sulfur | 0.53 ± 0.02 | 0.23 ± 0.03 | |
| K | 1.02 ± 0.03 | 1.57 ± 0.04 | |
| Na | 0.060 ± 0.02 | 0.02 ± 0.00 | |
| Ca | 1.74 ± 0.01 | 3.76 ± 0.03 | |
| Mg | 0.32 ± 0.00 | 0.55 ± 0.04 | |
| P | 0.22 ± 0.01 | 0.57 ± 0.01 | |
| Si | 0.32 ± 0.03 | 0.34 ± 0.02 | |
| Fe | 0.03 ± 0.01 | 0.10 ± 0.02 | |
| Al | 0.023 ± 0.050 | 0.075 ± 0.032 | |
| Mn | 0.004 ± 0.021 | 0.010 ± 0.001 | |
| Ti | <0.01 | <0.01 | |
| pH in water | 5.70 ± 0.01 | 8.67 ± 0.03 |
| Metal | PL (mg/kg) | PLB (mg/kg) | EBC AgroBio Limit (mg/kg) | EBC Agro Limit (mg/kg) | IBI Limit (mg/kg) |
|---|---|---|---|---|---|
| Pb | <0.01 | <0.01 | 45 | 120 | 150 |
| Cd | <0.01 | 1.13 ± 0.00 | 0.7 | 1.5 | 1.5 |
| Cu | <0.01 | 6.35 ± 0.02 | 70 | 100 | 300 |
| Ni | 1.12 ± 0.00 | 7.24 ± 0.00 | 25 | 50 | 100 |
| Hg | <0.000001 | <0.000001 | 0.4 | 1.0 | 1.0 |
| Zn | 7.38 ± 0.00 | 14.41 ± 0.01 | 200 | 400 | 500 |
| Cr | 0.53 ± 0.02 | 1.92 ± 0.01 | 70 | 90 | 120 |
| As | <0.000001 | <0.000001 | 13 | 13 | 13 |
| pHpzc | 7.02 ± 0.06 |
| pH buff. [mmol/g] | 0.30 ± 0.01 |
| ANC [mmol/g] | 6.38 ± 0.09 |
| CaCO3eq [%] | 34.4 ± 0.91 |
| EC [mS/cm] | 1.74 ± 0.02 |
| CEC [cmolc/kg] | 74.42 ± 0.83 |
| Field Measurements | Laboratory Measurements | |||
|---|---|---|---|---|
| Moisture [Unit on the Scale] * | pH | Moisture [%] | pH | |
| 1.0.S | 6.0 ± 0.1 | 6.0 ± 0.5 | 13.77 ± 0.22 d | 6.45 ± 0.14 b |
| 1.B.S | 8.0 ± 0.1 | 7.0 ± 0.1 | 14.03 ± 0.61 c | 7.30 ± 0.27 a |
| 1.BF.S | 7.5 ± 0.2 | 6.5 ± 0.2 | 15.40 ± 0.80 a | 6.90 ± 0.73 b |
| 1.F.S | 6.0 ± 0.1 | 5.5 ± 0.6 | 11.54 ± 0.60 f | 5.92 ± 0.51 c |
| 2.0.S | 5.0 ± 0.1 | 6.0 ± 0.5 | 10.51 ± 0.07 g | 6.31 ± 1.02 b |
| 2.B.S | 6.0 ± 0.2 | 7.0 ± 0.1 | 12.87 ± 0.31 e | 7.05 ± 0.90 a |
| 2.BF.S | 7.0 ± 0.2 | 6.5 ± 0.2 | 12.05 ± 0.83 e | 6.74 ± 0.04 b |
| 2.F.S | 4.0 ± 0.1 | 6.0 ± 0.5 | 11.71 ± 0.70 f | 5.54 ± 0.08 c |
| Soil Samples | EC [mS/cm] | CEC [cmolc/kg] |
|---|---|---|
| 1.0.S | 0.43 ± 0.05 d | 115.43 ± 0.34 c |
| 1.B.S | 0.39 ± 0.03 d | 120.76 ± 0.12 b |
| 1.BF.S | 0.38 ± 0.04 d | 110.18 ± 0.28 e |
| 1.F.S | 0.65 ± 0.01 c | 106.88 ± 0.51 e |
| 2.0.S | 0.98 ± 0.05 b | 142.60 ± 0.43 a |
| 2.B.S | 0.48 ± 0.02 d | 111.63 ± 0.55 d |
| 2.BF.S | 2.14 ± 0.02 a | 114.13 ± 0.17 c |
| 2.F.S | 2.66 ± 0.03 a | 114.94 ± 0.62 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koprivica, M.; Petrović, J.; Simić, M.; Dimitrijević, J.; Ercegović, M.; Trifunović, S. Characterization and Evaluation of Biomass Waste Biochar for Turfgrass Growing Medium Enhancement in a Pot Experiment. Agriculture 2025, 15, 2206. https://doi.org/10.3390/agriculture15212206
Koprivica M, Petrović J, Simić M, Dimitrijević J, Ercegović M, Trifunović S. Characterization and Evaluation of Biomass Waste Biochar for Turfgrass Growing Medium Enhancement in a Pot Experiment. Agriculture. 2025; 15(21):2206. https://doi.org/10.3390/agriculture15212206
Chicago/Turabian StyleKoprivica, Marija, Jelena Petrović, Marija Simić, Jelena Dimitrijević, Marija Ercegović, and Snežana Trifunović. 2025. "Characterization and Evaluation of Biomass Waste Biochar for Turfgrass Growing Medium Enhancement in a Pot Experiment" Agriculture 15, no. 21: 2206. https://doi.org/10.3390/agriculture15212206
APA StyleKoprivica, M., Petrović, J., Simić, M., Dimitrijević, J., Ercegović, M., & Trifunović, S. (2025). Characterization and Evaluation of Biomass Waste Biochar for Turfgrass Growing Medium Enhancement in a Pot Experiment. Agriculture, 15(21), 2206. https://doi.org/10.3390/agriculture15212206









