Biogas Production from Olive Oil Mill Byproducts: A Comparative Study of Two Treatments for Pursuing a Biorefinery Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Procedures, and Analysis
2.1.1. Analytic Methods for Biomass Characterization
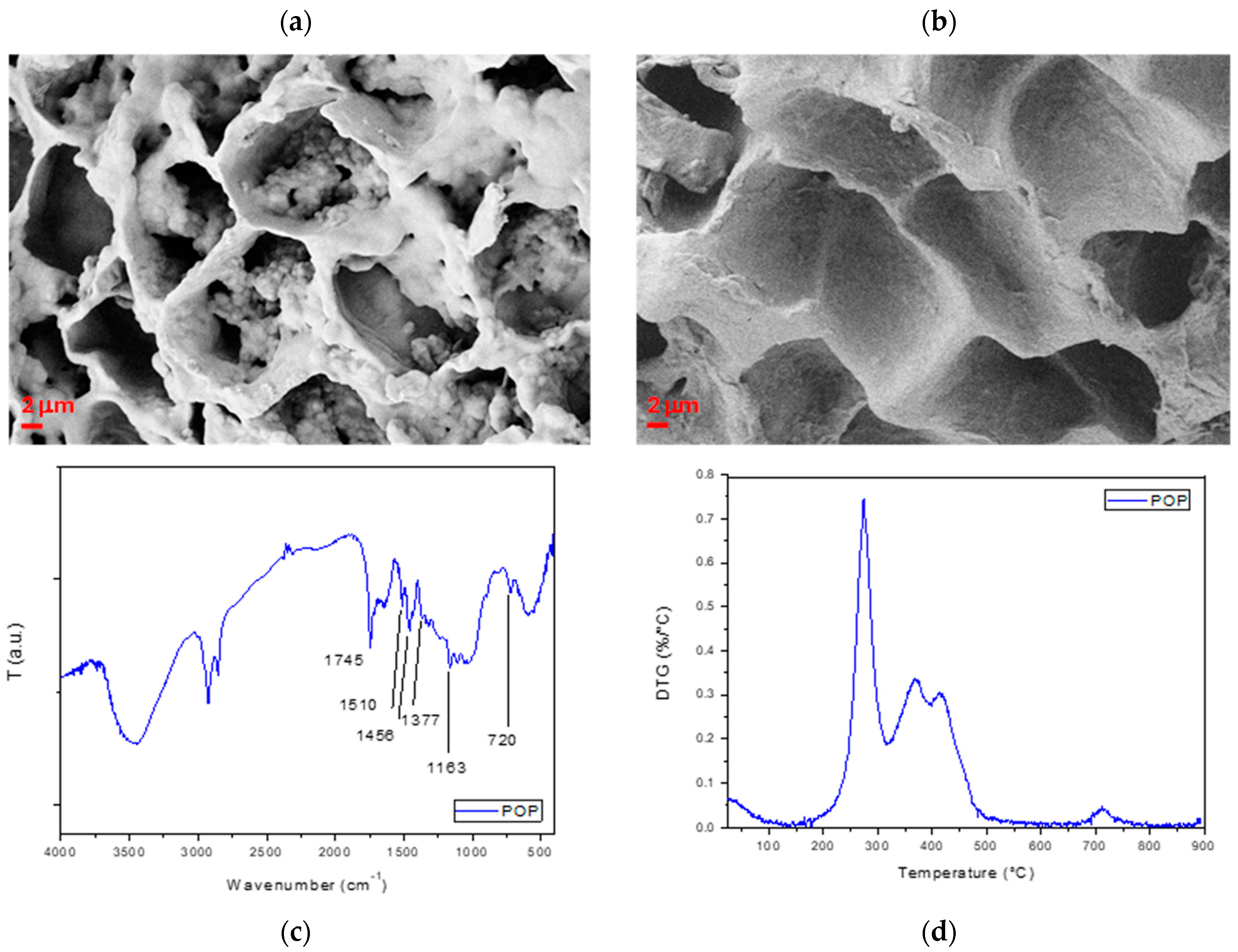
2.1.2. Production of Pomace Pulp
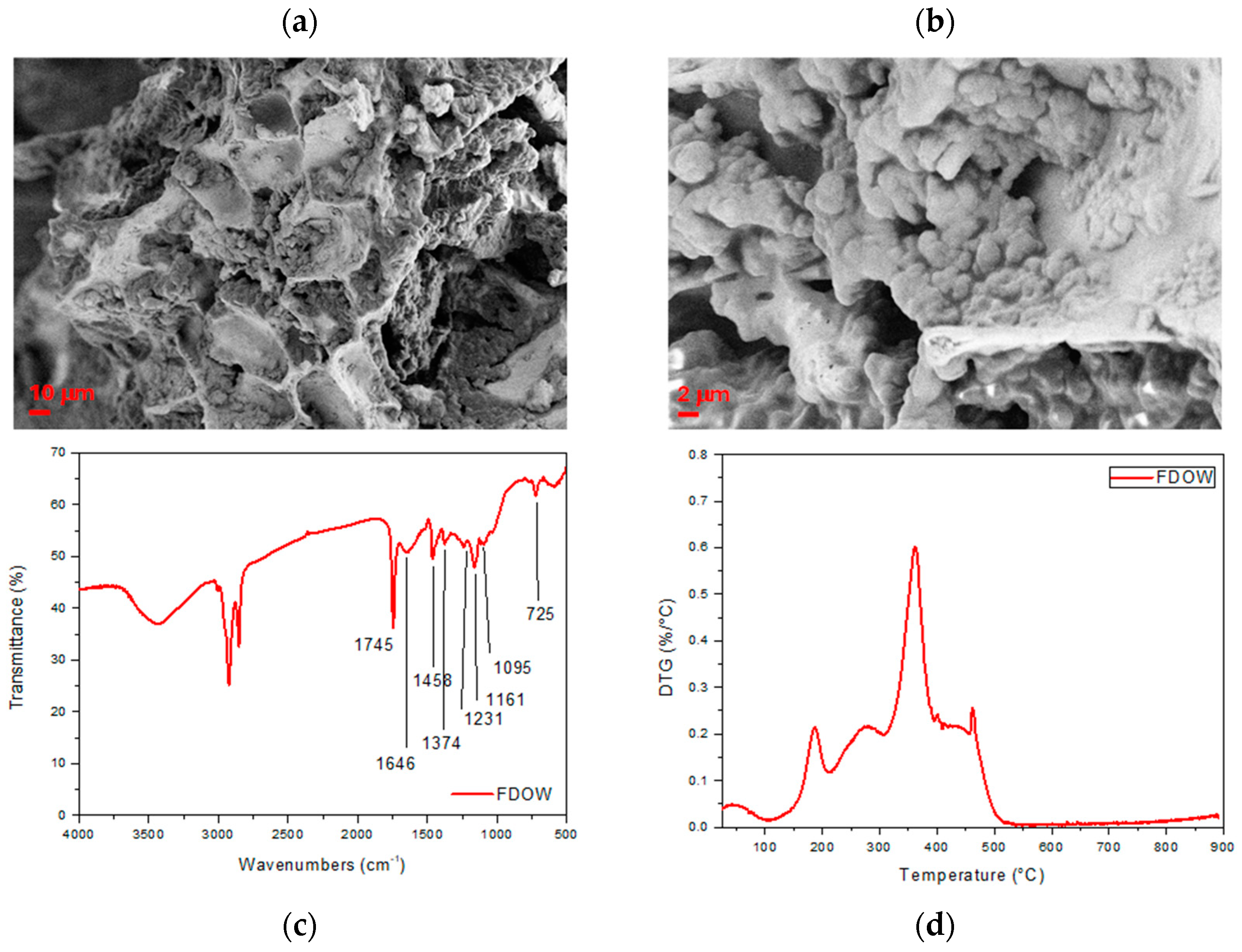
2.1.3. Characterization of Produced Pulp
2.1.4. Production of Freeze-Dried Olive Mill Wastewater
2.2. Anaerobic Bioreactors
- -
- Control consisting of sole inoculum;
- -
- Sample OW: composed of 3/4 (dw) inoculum and 1/4 (dw) OW;
- -
- Sample FDOW: composed of 3/4 (dw) inoculum and 1/4 (dw) freeze-dried OW (referred as FDOW);
- -
- Sample OP: composed of 3/4 (dw) inoculum and 1/4 (dw) OP;
- -
- Sample POP: composed of 3/4 (dw) inoculum and 1/4 (dw) POP;
- -
- Sample BSG: composed of 3/4 (dw) inoculum and 1/4 (dw) BSG;
- -
- Sample POP+BSG: composed of 3/4 (dw) inoculum and 1/4 (dw) POP+BSG.
- -
- Sample FDOW+BSG: composed of 3/4 (dw) inoculum and 1/4 (dw) FDOW+BSG.
2.3. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Biomasses
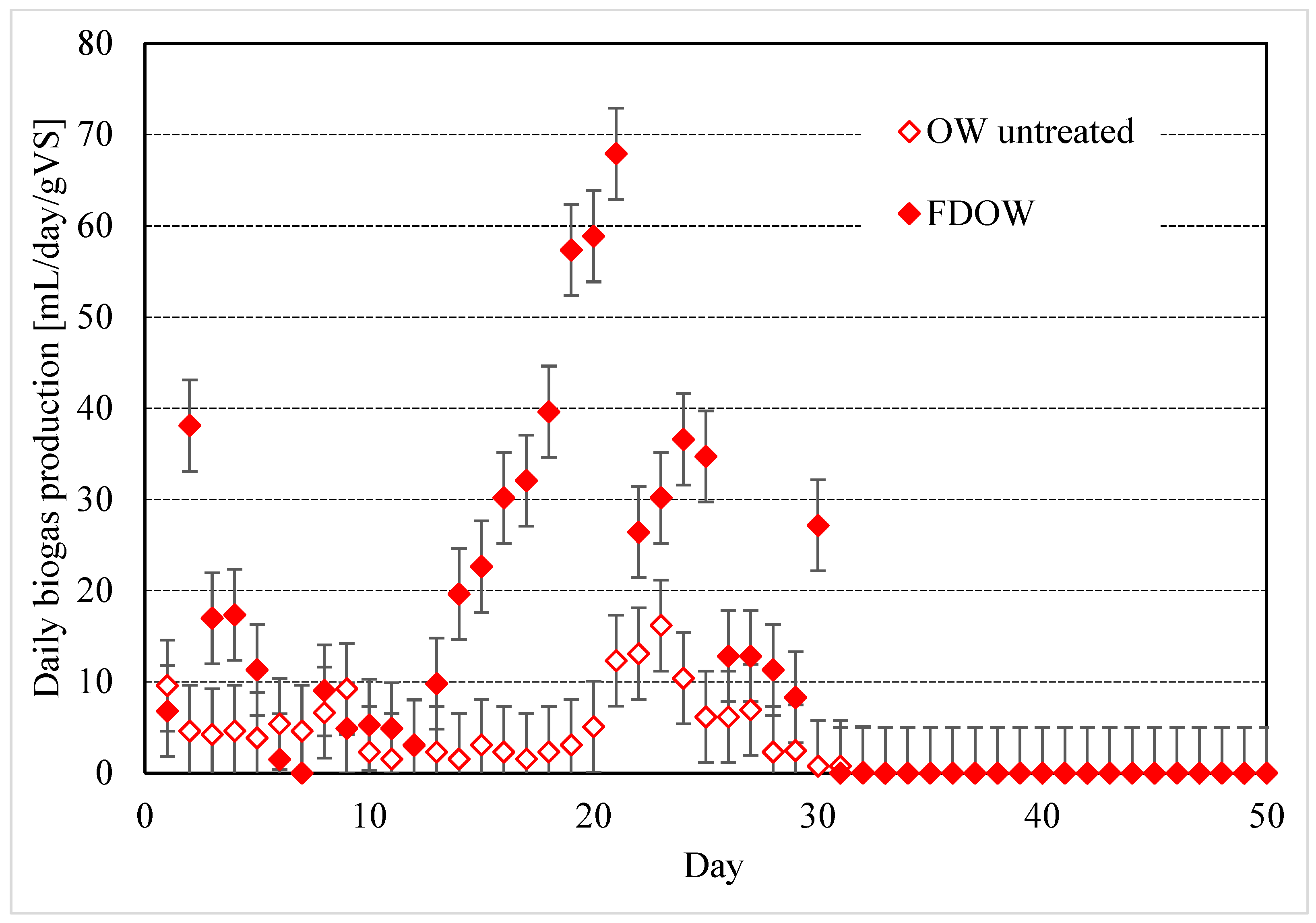
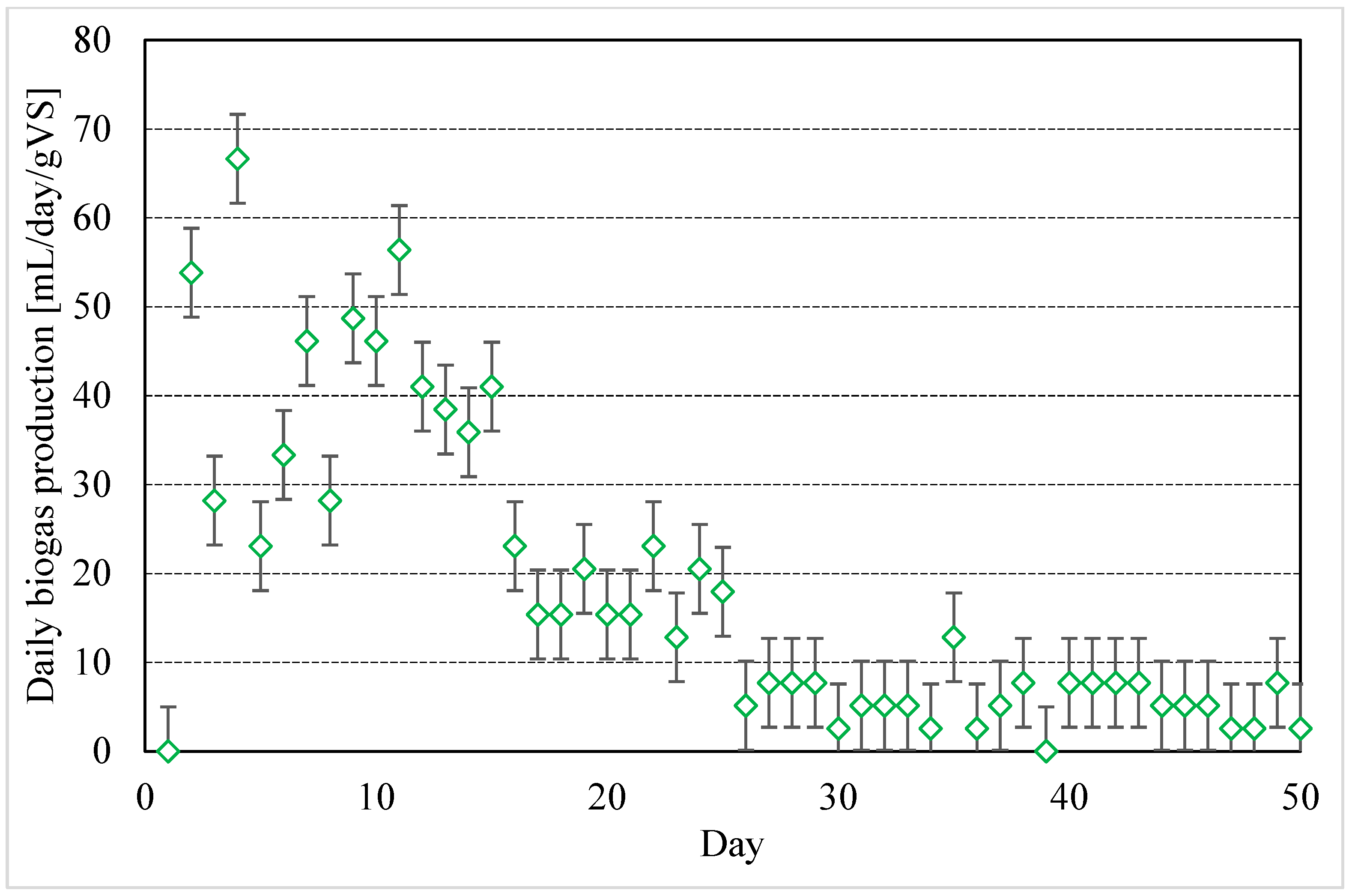
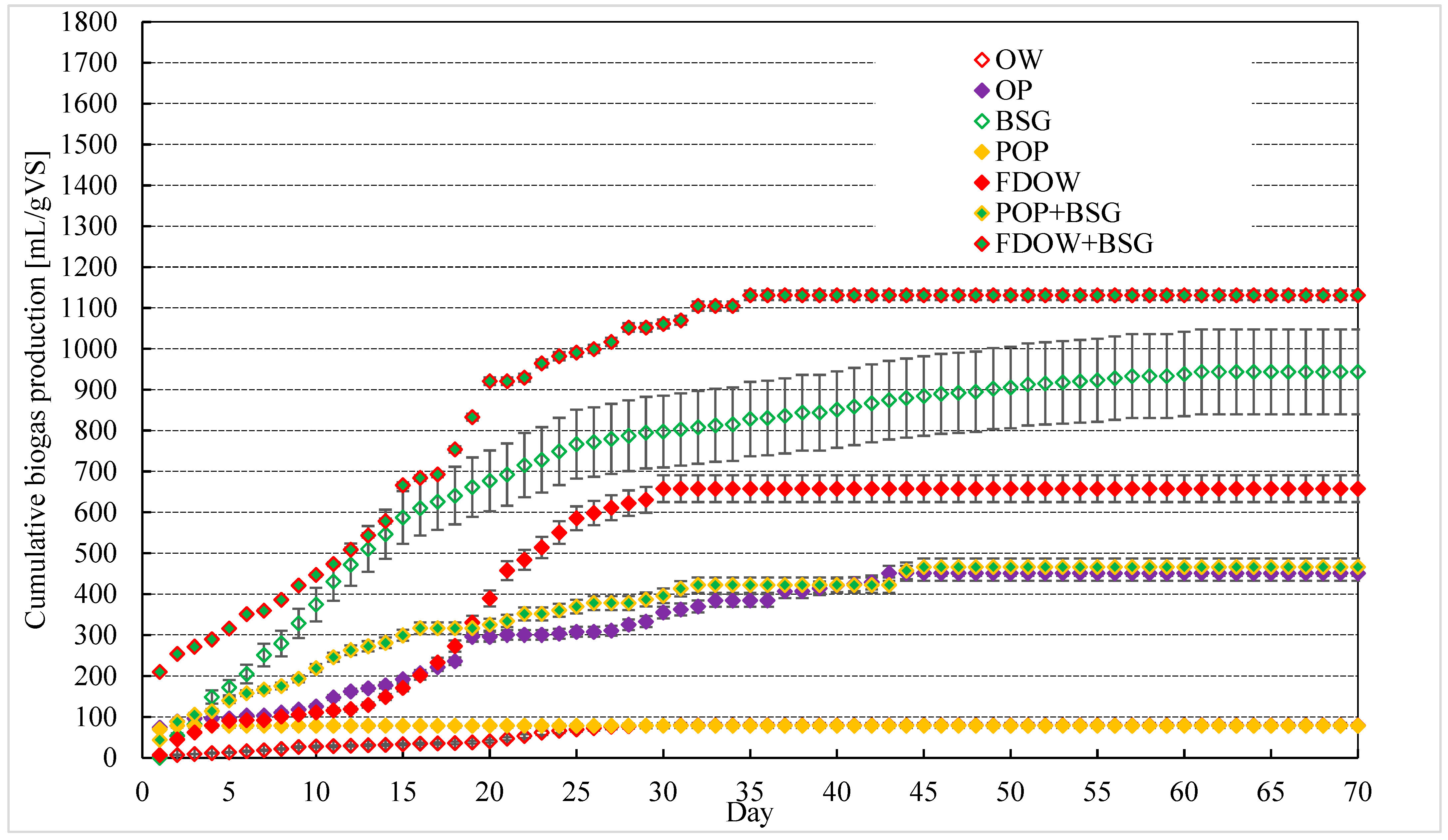
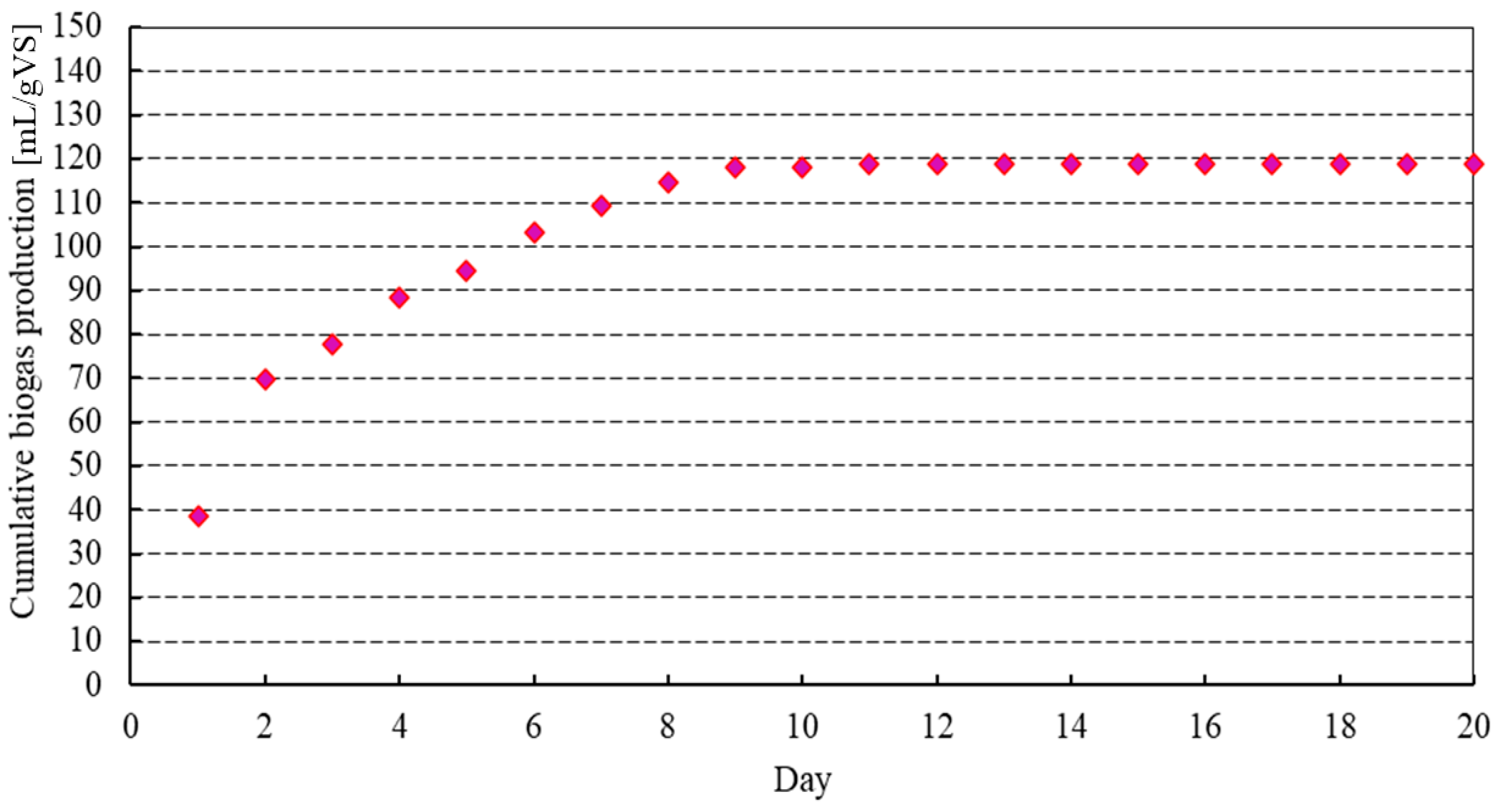
3.2. Biogas Yield for the Different Biomasses
- (1)
- OP: 66.30 vol%;
- (2)
- POP: 49.75 vol%;
- (3)
- OW: 56.99 vol%;
- (4)
- FDOW: 69.31 vol%.
3.3. Co-Digestion of Biomasses to Improve Nutrient Composition
- (i)
- Untreated olive pomace (OP);
- (ii)
- Untreated olive mill wastewater (OW);
- (iii)
- Untreated brewery’s spent grain (BSG);
- (iv)
- Pulp from olive pomace (POP);
- (v)
- Freeze-dried olive mill wastewater (FDOW);
- (vi)
- POP+BSG;
- (vii)
- FDOW+BSG.
3.4. Biogas Production from OW and OW-Based Matrices
3.5. Biogas Production from OP and OP-Based Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IOC [International Olive Council]. Olive Oil Production by Country. 2019. Available online: https://www.internationaloliveoil.org/olive-oil-estimates-2019-20-crop-year/ (accessed on 20 October 2025).
- Di Giacomo, G.; Romano, P. Evolution of the Olive Oil Industry along the Entire Production Chain and Related Waste Management. Energies 2022, 15, 465. [Google Scholar] [CrossRef]
- Rapa, M.; Ciano, S. A Review on Life Cycle Assessment of the Olive Oil Production. Sustainability 2022, 14, 654. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Al-Natsheh, M.S.; Yaghmoor, R.; Al-Rimawi, F. Enrichment of Phenolic Compounds from Olive Mill Wastewater and In Vitro Evaluation of Their Antimicrobial Activities. J. Evid. Based Complement. Altern. Med. 2017, 2017, 3706915. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.A.; Alajlouni, M.A.; Ghariabeh, M.A.; Rusan, M.J. Short-Term Effects of Olive Mill Wastewater Land Spreading on Soil Physical and Hydraulic Properties. Water Air Soil Pollut. 2019, 230, 208. [Google Scholar] [CrossRef]
- Galloni, M.G.; Ferrara, E.; Falletta, E.; Bianchi, C.L. Olive Mill Wastewater Remediation: From Conventional Approaches to Photocatalytic Processes by Easily Recoverable Materials. Catalysts 2022, 12, 923. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of Olive Pomace by a Green Integrated Approach Applying Sustainable Extraction and Membrane-Assisted Concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Processing Parameters on the Extraction of Olive Pomace Oil and Its Bioactive Compounds: A Kinetic and Thermodynamic Study. J. Am. Oil Chem. Soc. 2018, 95, 371–382. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud Point Extraction of Phenolic Compounds from Pretreated Olive Mill Wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Di Mario, J.; Bertoldi, A.; Priolo, D.; Calzoni, E.; Gambelli, A.M.; Dominici, F.; Rallini, M.; Del Buono, D.; Puglia, D.; Emiliani, C.; et al. Characterization of processes aimed at maximizing the reuse of Brewery’s Spent Grain: Novel biocomposite materials, high-added-value molecule extraction, codigestion and composting. Recycling 2025, 10, 124. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-Derived Biomass as a Renewable Source of Value-Added Products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Poletto, M. Lignin: Trends and Applications; BoD—Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Munk, L.; Sitarz, A.K.; Kalyani, D.C.; Mikkelsen, J.D.; Meyer, A.S. Can Laccases Catalyze Bond Cleavage in Lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Martínez, Á.T. Microbial Degradation of Lignin: How a Bulky Recalcitrant Polymer Is Efficiently Recycled in Nature and How We Can Take Advantage of This. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Fan, Y.; Van Klemeš, J.J.; Perry, S.; Lee, C.T. Anaerobic Digestion of Lignocellulosic Waste: Environmental Impact and Economic Assessment. J. Environ. Manag. 2019, 231, 352–363. [Google Scholar] [CrossRef]
- Di Mario, J.; Gambelli, A.M.; Gigliotti, G. Biomethane production from untreated and treated Brewery’s Spent Grain: Feasibility of anaerobic digestion after pretreatments according to biogas yield and energy efficiency. Agronomy 2024, 14, 2980. [Google Scholar] [CrossRef]
- Amirante, R.; Demastro, G.; Distaso, E.; Hassaan, M.A.; Mormando, A.; Pantaleo, A.M.; Tamburrano, P.; Tedone, L.; Clodoveo, M.L. Effects of Ultrasound and Green Synthesis ZnO Nanoparticles on Biogas Production from Olive Pomace. Energy Procedia 2018, 148, 940–947. [Google Scholar] [CrossRef]
- Sözer, S. A research on biogas production from a mixture of olive pomace and cattle manure. Biomass Convers. Biorefin. 2024, 14, 10651–10659. [Google Scholar] [CrossRef]
- Turano, E.; Curcio, S.; De Paola, M.G.; Calabrò, V.; Iorio, G. An Integrated Centrifugation–Ultrafiltration System in the Treatment of Olive Mill Wastewater. J. Membr. Sci. 2002, 209, 519–531. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pereira, A.P.G.; Ferreira, I.C.F.R.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A. Table Olives from Portugal: Phenolic Compounds, Antioxidant Potential, and Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 8425–8431. [Google Scholar] [CrossRef] [PubMed]
- Didry, N.; Seidel, V.; Dubreuil, L.; Tillequin, F.; Bailleul, F. Isolation and Antibacterial Activity of Phenylpropanoid Derivatives from Ballota Nigra. J. Ethnopharmacol. 1999, 67, 197–202. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the Tolerance to Polyphenols of the Anaerobic Digestion of Olive Wastewater through Microbial Adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Oz, N.A.; Uzun, A.C. Ultrasound pretreatment for enhanced biogas production from olive mill wastewater. Ultrason. Sonochem. 2015, 22, 565–572. [Google Scholar] [CrossRef]
- Llanos-Lizcano, R.; Senila, L.; Modoi, O.C. Evaluation of Biochemical Methane Potential and Kinetics of Organic Waste Streams for Enhanced Biogas Production. Agronomy 2024, 14, 2546. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrì, V.; De la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Porqueddu, I.; Ficara, E.; Alibardi, L.; Bona, D.; Brina, A.; Calabrò, P.S.; Casaletta, E.; Cavinato, C.; Daffonchio, D.; De Gioannis, G.; et al. Results of an Italian inter-laboratory study on biochemical methane potential. In Proceedings of the 13th World Congress on Anaerobic Digestion-Recovering (bio) Resources for the World, Santiago de Compostela, Spain, 25–28 June 2013. [Google Scholar]
- Hafner, S.D.; de Laclos, H.F.; Koch, K.; Hollinger, C. Improving inter-laboratory reproducibility in measurement of Biochemical Methane Potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- Montegiove, N.; Gambelli, A.M.; Calzoni, E.; Bertoldi, A.; Puglia, D.; Zadra, C.; Emiliani, C.; Gigliotti, G. Biogas production with residual deriving from olive mill wastewater and olive pomace wastes: Quantification of produced energy, spent energy, and process efficiency. Agronomy 2024, 14, 531. [Google Scholar] [CrossRef]
- Carter Martin, R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Barili, S.; Bernetti, A.; Sannino, C.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Pinchuk, I.; Pezzolla, D.; Turchetti, B.; Buzzini, P.; et al. Impact of PVC microplastics on soil chemical and microbiological parameters. Environ. Res. 2023, 229, 115891. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; No. 939; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Cequier, E.; Aguilera, J.; Balcells, M.; Canela-Garayoa, R. Extraction and characterization of lignin from olive pomace: A comparison study among ionic liquid, sulfuric acid, and alkaline treatments. Biomass Convers. Biorefinery 2019, 9, 241–252. [Google Scholar] [CrossRef]
- Tolisano, C.; Luzi, F.; Regni, L.; Proietti, P.; Puglia, D.; Gigliotti, G.; Di Michele, A.; Priolo, D.; Del Buono, D. A way to valorize pomace from olive oil production: Lignin nanoparticles to biostimulate maize plants. Environ. Technol. Innov. 2023, 31, 103216. [Google Scholar] [CrossRef]
- Holder, N.; Mota-Meira, M.; Born, J.; Sutrina, S.L. New Small Scale Bioreactor System for the Determination of the Biochemical Methane Potential. Waste Biomass Valoriz. 2019, 10, 1083–1090. [Google Scholar] [CrossRef]
- Paz, L.; Gentil, S.; Fierro, V.; Celzard, A. Activated carbons outperform other sorbents for biogas desulfurization. Chem. Eng. J. 2025, 506, 160304. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, X.; Zeng, G.; Li, W.; Li, J. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour. Technol. 2008, 99, 882–888. [Google Scholar] [CrossRef]
- Ge, S.; Han, J.; Sun, Q.; Zhou, Q.; Ye, Z.; Li, P.; Gu, Q. Research progress on improving the freeze-drying resistance of probiotics. Trends Food Sci. 2024, 147, 104425. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jeguirim, M.; Kinigopoulou, V.; Doulgeris, C.; Goddard, M.L.; Jellali, S.; Ghimbeu, C.M. Olive mill wastewater: From a pollutant to green fuels, agricultural and water source and bio-fertilizer—Hydrotermal carbonization. Sci. Total Environ. 2020, 733, 139314. [Google Scholar] [CrossRef] [PubMed]
- Solomakou, N.; Goula, A.M. Treatment of olive mill wastewater by adsorption of phenolic compounds. Rev. Environ. Sci. BioTechnol. 2021, 20, 839–863. [Google Scholar] [CrossRef]
- Dali, I.; Aydi, A.; Stamenic, M.; Kolsi, L.; Grachem, K.; Zizovic, I.; Manef, A.; Delgado, D.R. Extraction of lyophilized olive mill wastewater using supercritical CO2 processes. Alex. Eng. J. 2022, 61, 237–246. [Google Scholar] [CrossRef]
- Mekersi, N.; Addad, D.; Kadi, K.; Casini, S.; Hackenberger, D.K.; Boumazaa, A.; Lekmine, S. Effects of olive mill wastewater and olive mill pomace on soil physicochemical properties and soil polyphenols. J. Mater. Cycles Waste Manag. 2023, 25, 1404–1416. [Google Scholar] [CrossRef]
- El Hajjouji, H.; Bailly, J.R.; Winterton, P.; Merlina, G.; Revel, J.C.; Hafidi, M. Chemical and spectroscopic analysis of olive mill waste water during a biological treatment. Bioresour. Technol. 2008, 99, 4958–4965. [Google Scholar] [CrossRef]
- Wzorek, M.; Junga, R.; Yilmaz, E.; Bozhenko, B. Thermal decomposition of olive-mill byproducts: A TG-FTIR approach. Energies 2021, 14, 4123. [Google Scholar] [CrossRef]
- Dorado, F.; Sanchez, P.; Alcazar-Ruiz, A.; Sanchez-Silva, L. Fast pyrolysis as an alternative to the valorization of olive mill wastes. J. Sci. Food Agric. 2021, 101, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Nasrullah, A.; Ullah, Z.; Muhammad, N. Dicationic ionic liquids as sustainable approach for direct conversion of cellulose to levulinic acid. J. Clean. Prod. 2018, 170, 591–600. [Google Scholar] [CrossRef]
- Ward, K.R.; Matejtschuk, P. The principles of freeze-drying and application of analytical technologies. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; pp. 99–127. [Google Scholar]
- Liu, Y.W.; Pu, H.B.; Sun, D.W. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Buahom, J.; Siripornadulsil, S.; Sukon, P.; Sooksawat, T.; Siripornadulsil, W. Survivability of freeze- and spray-dried probiotics and their effect on the growth and health performance of broilers. Vet. World 2023, 16, 1849–1865. [Google Scholar] [CrossRef]
- Tian, Y.; He, Z.; He, L.; Li, C.; Qiao, S.; Tao, H.; Wang, X.; Zeng, X.; Tian, Y. Effect of freeze-dried protectants on the survival rate and fermentation performance of fermented milk’s directed vat set starters. Cryobiology 2024, 114, 104811. [Google Scholar] [CrossRef]
- Khanal, S.K.; Nindhia, T.G.T.; Nitayavardhana, S. Chapter 11—Biogas from Wastes: Processes and Applications. In Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: Amsterdam, The Netherlands, 2016; pp. 165–174. [Google Scholar]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar] [CrossRef]
- Orive, M.; Cebrian, M.; Zufia, J. Techno-economic anaerobic co-digestion feasibility study for two-phase olive oil mill pomace and pig slurry. Renew. Energy 2020, 97, 532–540. [Google Scholar] [CrossRef]
- Chapleur, O.; Madigou, C.; Civade, R.; Rodolphe, Y.; Mazeas, L.; Bouchez, T. Increasing concentrations of phenol progressively affect anaerobic digestion of cellulose microbial communities. Biodegradation 2016, 27, 15–27. [Google Scholar] [CrossRef]
- Wang, Y.T.; Gabbard, H.D.; Pai, P.G. Inhibition of acetate methanogenesis by phenols. J. Environ. Eng. 1991, 117, 487–500. [Google Scholar] [CrossRef]
- Field, J.A.; Lettinga, G. The methanogenic toxicity and anaerobic degradability of a hydrolysable tannin. Water Res. 1987, 20, 367–374. [Google Scholar] [CrossRef]
- Sang, Y.; Wang, J.; Zhang, Y.X.; Gao, H.N.; Ge, S.Y.; Feng, H.; Zhang, Y.; Ren, F.; Wen, P.; Wang, R. Influence of temperature during freeze-drying process on the viability of Bifidobacterium Longum BB68S. Microorganisms 2023, 11, 181. [Google Scholar] [CrossRef]
- Borja, R.; Pelillo, M.; Rincon, B.; Raposo, F.; Martin, A. Mathematical modelling of the aerobic degradation of two-phase olive mill effluents in a batch reactor. Biochem. Eng. J. 2006, 30, 308–315. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, A.; Cuadros, F. Effect of aerobic pretreatment on anaerobic digestion of olive mill wastewater (OMWW): An ecoefficient treatment. J. Environ. Sci. Health 1994, 29, 851–865. [Google Scholar] [CrossRef]
- Borja, R.; Banks, J.C.; Albe, J.; Maestro, R. The effect of the most important phenolic constituents of OMW on batch anaerobic methanogenis. Environ. Technol. 1996, 17, 167–174. [Google Scholar] [CrossRef]
- Rasi, S. Biogas Composition and Upgrading to Biomethane. Ph.D. Thesis, University of Jyväskylä, Jyväskylä, Finland, 2009. No. 202. [Google Scholar]
- Tarannum, A.; Rao, J.R.; Fathima, N.N. Insights into protein-ionic liquid interaction: A comprehensive overview on theoretical and experimental approaches. Int. J. Biol. Macromol. 2022, 209, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, J.; Montegiove, N.; Gambelli, A.M.; Brienza, M.; Zadra, C.; Gigliotti, G. Waste biomass pretreatments for biogas yield optimization and for the extraction of valuable high-added-value products: Possible combinations of the two processes toward a biorefinery purpose. Biomass 2024, 4, 865–885. [Google Scholar] [CrossRef]



| Parameter | Digestate |
|---|---|
| Moisture [%] | 88.08 ± 0.05 |
| VS [%] | 72.34 ± 0.05 |
| pH | 8.08 ± 0.05 |
| TOC [% of DM] | 53.05 ± 0.01 |
| TKN [% of DM] | 5.47 ± 0.01 |
| Total P [g kg−1 DM] | 3.11 ± 0.01 |
| Total K [g kg−1 DM] | 75.62 ± 0.01 |
| WEOC [g kg−1 DM] | 110.51 ± 0.01 |
| WEN [g kg−1 DM] | 67.86 ± 0.01 |
| Biomass | Moisture (%) | TS (%) | VS (%) | pH | TKN (% on DM) | TOC (% on DM) |
|---|---|---|---|---|---|---|
| OW | 88.08 ± 0.05 | 11.92 ± 0.05 | 90.80 ± 0.05 | 5.10 ± 0.05 | 0.53 ± 0.01 | 61.20 ± 0.01 |
| FDOW | 8.08 ± 0.05 | 91.92 ± 0.05 | 92.60 ± 0.05 | 5.40 ± 0.05 | 1.22 ± 0.01 | 30.86 ± 0.01 |
| OP | 53.05 ± 0.05 | 46.95 ± 0.05 | 97.00 ± 0.05 | 5.50 ± 0.05 | 0.70 ± 0.01 | 47.70 ± 0.01 |
| POP | 5.47 ± 0.05 | 94.53 ± 0.05 | 95.39 ± 0.05 | 3.01 ± 0.05 | 0.31 ± 0.01 | 31.28 ± 0.01 |
| Biomass Tested | Total Biogas [mL] | Total CH4 [mL] | Production Time [Days] |
|---|---|---|---|
| OP | 451 ± 18 | 299 ± 12 | 43 |
| POP | 79 ± 5 | 39 ± 2 | 2 |
| OW | 79 ± 6 | 45 ± 4 | 32 |
| FDOW | 658 ± 33 | 458 ± 23 | 30 |
| Brewery’s Spent Grain | |
|---|---|
| Moisture [%] | 78.25 ± 0.05 |
| pH | 5.06 ± 0.05 |
| TOC [% on dry matter] | 26.66 ± 0.01 |
| TKN [% on dry matter] | 3.89 ± 0.01 |
| Total biogas [mL/gVS] | 944 ± 156.4 |
| Total CH4 [mL/gVS] | 519 ± 57 |
| Production time [days] | 61 |
| Substrate | Dry Matter [g] | VS [%] | VS [g] | Biogas/VS [mL/g] |
|---|---|---|---|---|
| FDOW+BSG | 1.000 ± 0.005 | 0.950 ± 0.005 | 0.950 ± 0.005 | 1131 ± 14.1 |
| BSG | 1.005 ± 0.005 | 0.975 ± 0.005 | 0.980 ± 0.005 | 944 ± 156.4 |
| FDOW | 2.860 ± 0.005 | 0.926 ± 0.005 | 2.648 ± 0.005 | 658 ± 15.8 |
| POP+BSG | 1.000 ± 0.005 | 0.948 ± 0.005 | 0.948 ± 0.005 | 466 ± 23.9 |
| OP | 1.000 ± 0.005 | 0.970 ± 0.005 | 0.970 ± 0.005 | 451 ± 8.3 |
| OW | 5.720 ± 0.005 | 0.908 ± 0.005 | 5.194 ± 0.005 | 79 ± 2.1 |
| POP | 1.000 ± 0.005 | 0.954 ± 0.005 | 0.954 ± 0.005 | 79 ± 1.5 |
| FDOW-PF | 5.720 ± 0.005 | 0.920 ± 0.005 | 5.262 ± 0.005 | 32 ± 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mario, J.; Ranucci, A.; Gambelli, A.M.; Rallini, M.; Priolo, D.; Brienza, M.; Puglia, D.; Del Buono, D.; Gigliotti, G. Biogas Production from Olive Oil Mill Byproducts: A Comparative Study of Two Treatments for Pursuing a Biorefinery Approach. Agriculture 2025, 15, 2204. https://doi.org/10.3390/agriculture15212204
Di Mario J, Ranucci A, Gambelli AM, Rallini M, Priolo D, Brienza M, Puglia D, Del Buono D, Gigliotti G. Biogas Production from Olive Oil Mill Byproducts: A Comparative Study of Two Treatments for Pursuing a Biorefinery Approach. Agriculture. 2025; 15(21):2204. https://doi.org/10.3390/agriculture15212204
Chicago/Turabian StyleDi Mario, Jessica, Antonella Ranucci, Alberto Maria Gambelli, Marco Rallini, Dario Priolo, Monica Brienza, Debora Puglia, Daniele Del Buono, and Giovanni Gigliotti. 2025. "Biogas Production from Olive Oil Mill Byproducts: A Comparative Study of Two Treatments for Pursuing a Biorefinery Approach" Agriculture 15, no. 21: 2204. https://doi.org/10.3390/agriculture15212204
APA StyleDi Mario, J., Ranucci, A., Gambelli, A. M., Rallini, M., Priolo, D., Brienza, M., Puglia, D., Del Buono, D., & Gigliotti, G. (2025). Biogas Production from Olive Oil Mill Byproducts: A Comparative Study of Two Treatments for Pursuing a Biorefinery Approach. Agriculture, 15(21), 2204. https://doi.org/10.3390/agriculture15212204










