Assessment of Cabbage (Brassica oleracea Linnaeus) Insect Pests and Management Strategies in Eastern Democratic Republic of Congo
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Socioeconomic and Farm Characteristics
3.2. Farmers’ Knowledge and Perception of Cabbage Pests
3.3. Farmers’ Management Strategies of Cabbage Pests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ndjadi, S.S.; Vumilia, R.K.; Ahoton, L.E.; Saidou, A.; Orou, B.D.; Mugumaarhahama, Y.; Kazamwali, L.M.; Mushagalusa, G.N. Typology and prospects for the Improvement of market gardening systems in South-Kivu, Eastern DR Congo. J. Agric. Sci. 2020, 12, 136. [Google Scholar] [CrossRef]
- Francisco, M.; Tortosa, M.; Martínez-Ballesta, M.; del, C.; Velasco, P.; García-Viguera, C.; Moreno, D.A. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 273–285. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, D.; Jaisval, G.; Kumar, A.; Pathania, R.; Hasan, W.; Narain, T. Biology, diversity, distribution, and characterization of Brevicoryne brassicae (L.). Int. J. Plant Soil Sci. 2024, 36, 336–347. [Google Scholar] [CrossRef]
- Mpumi, N.; Machunda, R.S.; Mtei, K.M.; Ndakidemi, P.A. Selected insect pests of economic importance to Brassica oleracea, their control strategies and the potential threat to environmental pollution in Africa. Sustainability 2020, 12, 3824. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Jiang, M.; Li, F.; Yang, T. Status, biology, impact, and management strategies of Phyllotreta striolata (Fabricius) (Coleoptera: Chrysomelidae): A comprehensive review of biocontrol strategies. Egypt. J. Biol. Pest Control 2025, 35, 17. [Google Scholar] [CrossRef]
- Malik, A.; Poveda, J.; Zuluaga, D.; Boccaccio, L.; Hassan, Z.; Akram, M.; Ali, J. Defence of Brassicaceae plants against generalist and specialised insect pests through the development of myrosinase mutants: A review. Ind. Crops Prod. 2025, 228, 120945. [Google Scholar] [CrossRef]
- Embaby, E.-S.M.; Lotfy, D.E.-S. Ecological studies on Cabbage pests. J. Agric. Technol. 2015, 11, 1145–1160. [Google Scholar]
- Badenes-Perez, F.R.; Shelton, A.M. Pest management and other agricultural practices among farmers growing cruciferous vegetables in the Central and Western highlands of Kenya and the Western Himalayas of India. Int. J. Pest Manag. 2006, 52, 303–315. [Google Scholar] [CrossRef]
- Paul, D.; Mayengo, M.M.; Daudi, S. Parameters estimation, global sensitivity analysis and model fitting for the dynamics of Plutella xylostella infestations in a cabbage biomass. Chaos Solitons Fractals X 2024, 12, 100105. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Stevenson, P.C.; Mochiah, M.B.; Asare, K.P.; Gurr, G.M. Economic analysis of habitat manipulation in Brassica pest management: Wild plant species suppress cabbage webworm. Crop Prot. 2021, 150, 105788. [Google Scholar] [CrossRef]
- Deschodt, P.S.; Cory, J.S.; Franklin, M.T.; Labbé, R.; Tracey, A.P. Trichoplusia ni (Hübner), cabbage looper/fausse-arpenteuse du chou (Lepidoptera: Noctuidae). In Biological Control Programmes in Canada, 2013–2023; Vankosky, M.A., Martel, V., Eds.; CAB International: Wallingford, UK, 2024; pp. 404–410. [Google Scholar] [CrossRef]
- Meenakshi; Thakur, S.; Choudhary, K.; Kumar, R.; Kumar, S.; Sharma, D. Ecofriendly Management of Pieris brassicae in Brassica oleracea: A review. Biol. Bull. Rev. 2023, 13, 691–702. [Google Scholar] [CrossRef]
- Mouafo-Tchinda, R.; Plex Sula, A.; Etherton, B.A.; Okonya, J.; Nakato, G.V.; Xing, Y.; Robledo Buritica, J.; Adhikari, A.; Blomme, G.; Kantungeko, D.; et al. Pathogen and pest communities in food security crops across climate gradients: Anticipating future challenges in the Great Lakes region of Africa. bioRxiv 2025, preprint. [Google Scholar] [CrossRef]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests—A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; FAO on Behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Lal, J.; Swaminathan, R.; Meena, A.K.; Nagar, R. Seasonal incidence of major insect pests of cabbage, Brassica oleracea var. capitata L. J. Entomol. Zool. Stud. 2020, 8, 387–391. [Google Scholar]
- Labou, B.; Brévault, T.; Sylla, S.; Diatte, M.; Bordat, D.; Diarra, K. Spatial and temporal incidence of insect pests in farmers’ cabbage fields in Senegal. Int. J. Trop. Insect Sci. 2017, 37, 225–233. [Google Scholar] [CrossRef]
- Rubabura, K.J.A.; Ndatabaye, L.F.; Lina, A.A.; Muhigwa, B.J.B. Assessment of pesticide use against Tephrtidae fruit fly and other pest among small-scale solanaceous vegetable farmers in Bugorhe-Kabare the Democratic Republic of Congo. NASS J. Agric. Sci. 2022, 4, 27–35. [Google Scholar] [CrossRef]
- Balasha, A.M.; Mulume Dominique, A.; Mwisha Sage, W.; Mukonde Shadya, S.; Zirhumana Mugisho, J. Pesticide choice and use patterns among vegetable farmers on Idjwi island, eastern Democratic Republic of Congo. SAGE Open 2023, 13, 21582440231. [Google Scholar] [CrossRef]
- Matubi, E.M.; Kaounga, G.I.; Zanga, J.; Mbuku, G.B.; Maniania, J.N.K.; Mulenda, B.; Sodi, J.N.M.; Tamfum, J.J.M.; Masiangi, P. Insecticide susceptibility of Anopheles gambiae s.l and identification of some resistance mechanisms in Kwilu Province in the Democratic Republic of Congo. Pan Afr. Med. J. 2020, 37, 79. [Google Scholar] [CrossRef] [PubMed]
- Warra, A.A.; Prasad, M.N.V. African perspective of chemical usage in agriculture and horticulture—Their impact on human health and environment. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 401–436. [Google Scholar] [CrossRef]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Kapeleka, J.A.; Sauli, E.; Sadik, O.; Ndakidemi, P.A. Biomonitoring of Acetylcholinesterase (AChE) activity among smallholder horticultural farmers occupationally exposed to mixtures of pesticides in Tanzania. J. Environ. Public Health 2019, 2019, 3084501. [Google Scholar] [CrossRef]
- Muliele, T.M.; Manzenza, C.M.; Ekuke, L.W.; Diaka, C.P.; Ndikubwayo, D.M.; Kapalay, O.M.; Mundele, A.N. Utilisation et gestion des pesticides en cultures maraîchères: Cas de la zone de Nkolo dans la province du Kongo Central, République démocratique du Congo. J. Appl. Biosci. 2018, 119, 11954. [Google Scholar] [CrossRef]
- Ngakiama, G.N.; Mbela, G.K.; Pole, C.S.; Kyela, C.M. Analyse des connaissances, attitudes et pratiques des maraîchers de la ville de Kinshasa en rapport avec l’utilisation des pesticides et l’impact sur la santé humaine et sur l’environnement. Afr. Sci. 2019, 15, 122–133. [Google Scholar]
- Cokola, M.C.; Van Den Bussche, R.; Noël, G.; Kouanda, N.; Sèye, F.; Yarou, B.B.; Caparros Megido, R.; Bayendi Loudit, S.M.; Lonpi Tipi, E.; Michel, B.; et al. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae): Experience from smallholder farmers in central and western Africa. Food Energy Secur. 2023, 12, e491. [Google Scholar] [CrossRef]
- Mangaza, L.; Sonwa, D.J.; Batsi, G.; Ebuy, J.; Kahindo, J.-M. Building a framework towards climate-smart agriculture in the Yangambi landscape, Democratic Republic of Congo (DRC). Int. J. Clim. Chang. Strateg. Manag. 2021, 13, 320–338. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Márquez, B.P.; Petitpierre, E. Can flowering Barbarea spp. (Brassicaceae) be used simultaneously as a trap crop and in conservation biological control? J. Pest Sci. 2017, 90, 623–633. [Google Scholar] [CrossRef]
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and risks of Intercropping for crop resilience and pest management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Parker, J.E.; Snyder, W.E.; Hamilton, G.C.; Rodriguez-Saona, C. Companion planting and insect pest control. In Weed and Pest Control—Conventional and New Challenges; Soloneski, S., Larramendy, M., Eds.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Zuma, M.; Njekete, C.; Konan, K.A.J.; Bearez, P.; Amiens-Desneux, E.; Desneux, N.; Lavoir, A.-V. Companion plants and alternative prey improve biological control by Orius laevigatus on strawberry. J. Pest Sci. 2023, 96, 711–721. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crops Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Badinenganyi, C.; Mukendi, J.T.; Kabeya, J.P.T.; Fuamba, E.M.; Muepu, J.; Malaba, B.N. Effets des extraits aqueux de ricin (Ricinus communis) et de neem (Azadiracta indica) sur la pression des bio-agresseurs de niébé (Vigna unguiculata). Rev. Marocaine Sci. Agron. Vét. 2023, 11, 420–423. [Google Scholar] [CrossRef]
- Nsomue, A.N.; Mulungu, H.B.; Kishiko, G.M.; Kabemba, M. Effet de quelques plantes locales sur les charançons du maïs (Sitophilus zeamais Motsch.) en entrepôt dans la ville de Kabinda en République Démocratique du Congo. Rev. Afr. D’Environ. D’Agric. 2020, 3, 11–16. [Google Scholar]
- Koleramungu, O.C.; Mirindi Cirhuza, T.; Rudahaba, N.; Kayeye, J.L.B.; Amani, Y.M.; Ntamwira, J.; Bukomarhe, B.; Mongana, E.; Tuombemungu, B.; Muhwanjo, M.W.; et al. Use of insecticide plants in the fight against common bean fly (Genus: Ophiomyia) at the East of the Democratic Republic of Congo. Int. J. Innov. Appl. Stud. 2018, 24, 255–268. [Google Scholar]
- Gakuru, P.N.; Somora, P.M.; Rubayi Sanga, P.; Sendihi, T.S. Effets des plantes compagnes (oignon rouge), des extraits du piment et de l’insecticide chimique sur les populations des ravageurs du chou-fleur (Brassica oleracea var botritis) (R.D.Congo). Ann. UNIGOM 2019, 9, 63–71. [Google Scholar]
- Anjarwalla, P.; Belmain, S.; Sola, P.; Jamnadass, R.; Stevenson, P.C. Guide des Plantes Pesticides; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2020; Available online: https://www.cifor-icraf.org/knowledge/publication/27689/ (accessed on 25 July 2025).
- Baributsa, D.; Díaz-Valderrama, J.R.; Mughanda, D.; Lubanzadio, A.; Nshombo, J.P.C.; Sperling, L.; Baoua, I.B. Grain handling and storage in Lubero and Rutshuru territories in the North Kivu Province, the Democratic Republic of Congo. Sustainability 2021, 13, 9580. [Google Scholar] [CrossRef]
- Halilou, M.S.; Ba, M.N.; Karimoune, L.; Doumma, A. Farmers’ knowledge, perceptions and management of the moringa tree defoliator, Noorda blitealis Walker (Lepidoptera: Crambidae), in Niger. Int. J. Trop. Insect Sci. 2022, 42, 905–915. [Google Scholar] [CrossRef]
- Gangopadhyay, D.; Roy, R.; Roy, K. From pen to pixel: Exploring Kobo Toolbox as a modern approach to smart data collection. Food Sci. Rep. 2024, 5, 8–13. [Google Scholar]
- Dinno, A.; Dunn. Test: Dunn’s Test of Multiple Comparisons Using Rank Sums. Available online: https://cran.r-project.org/web/packages/dunn.test/index.html (accessed on 8 August 2025).
- Graves, S.; Piepho, H.-P.; Selze, L.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons. Available online: https://CRAN.R-project.org/package=multcompView (accessed on 17 August 2025).
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification, 2019 ed.; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 31 July 2025).
- Doss, C.; Meinzen-Dick, R.; Quisumbing, A.; Theis, S. Women in agriculture: Four myths. Glob. Food Secur. 2018, 16, 69–74. [Google Scholar] [CrossRef]
- FAO. Women in Agriculture: Closing the Gender Gap for Development; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; Available online: https://www.fao.org/4/i2050e/i2050e.pdf (accessed on 26 July 2025).
- Kassie, M.; Jaleta, M.; Shiferaw, B.; Mmbando, F.; Mekuria, M. Adoption of interrelated sustainable agricultural practices in smallholder systems: Evidence from rural Tanzania. Technol. Forecast. Soc. Chang. 2013, 80, 525–540. [Google Scholar] [CrossRef]
- Wagemakers, I.; Diki, O.M. Governance of urban agricultural space: Struggle for land in Kinshasa (DRC). In Natural Resources and Local Livelihoods in the Great Lakes Region of Africa: A Political Economy Perspective; Ansoms, A., Marysse, S., Eds.; Palgrave Macmillan: London, UK, 2011; pp. 68–82. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Asante, I.S.; Afun, J.V.K.; Osekre, E.A. Farmers’ perceptions of the diamondback moth, Plutella xylostella (L.), and their adoption of various control methods in cabbage production: Insights from smallholder cabbage farmers in Ghana. Int. J. Trop. Insect Sci. 2025, 45, 723–740. [Google Scholar] [CrossRef]
- Balasha, A.M.; Nsele, M.K. Pesticide use practices by Chinese cabbage growers in suburban environment of Lubumbashi (DR Congo): Main pests, costs and risks. J. Appl. Econ. Policy Anal. 2019, 2, 56–64. [Google Scholar] [CrossRef]
- Furlong, M.; Wright, D.; Dosdall, L. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2012, 58, 517–541. [Google Scholar] [CrossRef]
- Iftikhar, A.; Hafeez, F.; Aziz, M.A.; Hashim, M.; Naeem, A.; Yousaf, H.K.; Saleem, M.J.; Hussain, S.; Hafeez, M.; Ali, Q.; et al. Assessment of sublethal and transgenerational effects of spirotetramat, on population growth of cabbage aphid, Brevicoryne brassicae L. (Hemiptera: Aphididae). Front. Physiol. 2022, 13, 1014190. [Google Scholar] [CrossRef]
- Coapio, G.G.; Cruz-López, L.; Guerenstein, P.; Malo, E.A.; Rojas, J.C. Oviposition preference and larval performance and behavior of Trichoplusia ni (Lepidoptera: Noctuidae) on host and nonhost plants. Arthropod-Plant Interact. 2018, 12, 267–276. [Google Scholar] [CrossRef]
- Li, Y.-X.; Liu, T.-X. Oviposition preference, larval performance and adaptation of Trichoplusia ni on cabbage and cotton. Insect Sci. 2015, 22, 273–282. [Google Scholar] [CrossRef]
- Okamura, Y.; Sato, A.; Tsuzuki, N.; Murakami, M.; Heidel-Fischer, H.; Vogel, H. Molecular signatures of selection associated with host plant differences in Pieris butterflies. Mol. Ecol. 2019, 28, 4958–4970. [Google Scholar] [CrossRef]
- Mewis, I.; Ulrich, C.H.; Schnitzler, W.H. The role of glucosinolates and their hydrolysis products in oviposition and host-plant finding by cabbage webworm, Hellula undalis. Entomol. Exp. Appl. 2002, 105, 129–139. [Google Scholar] [CrossRef]
- Ali, J.; Bayram, A.; Mukarram, M.; Zhou, F.; Karim, M.F.; Hafez, M.M.A.; Mahamood, M.; Yusuf, A.A.; King, P.J.H.; Adil, M.F.; et al. Peach–potato aphid Myzus persicae: Current management strategies, challenges, and proposed solutions. Sustainability 2023, 15, 11150. [Google Scholar] [CrossRef]
- Wamonje, F.O.; Donnelly, R.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.; Mutuku, J.M.; Bruce, T.J.A.; Gilligan, C.A.; et al. Different plant viruses induce changes in feeding behavior of specialist and generalist aphids on common bean that are likely to enhance virus transmission. Front. Plant Sci. 2020, 10, 1811. [Google Scholar] [CrossRef]
- Chen, C.; Harvey, J.A.; Biere, A.; Gols, R. Rain downpours affect survival and development of insect herbivores: The specter of climate change? Ecology 2019, 100, e02819. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, P.N.K.; Prasannakumar, N.R.; Mani, M.; Saroja, S.; Ranganath, H.R. Pests and their management in cruciferous vegetables. In Trends in Horticultural Entomology; Mani, M., Ed.; Springer Nature: Singapore, 2022; pp. 997–1011. [Google Scholar] [CrossRef]
- Andersson, E.; Isgren, E. Gambling in the garden: Pesticide use and risk exposure in Ugandan smallholder farming. J. Rural Stud. 2021, 82, 76–86. [Google Scholar] [CrossRef]
- Mengistie, B.T.; Mol, A.P.J.; Oosterveer, P. Pesticide use practices among smallholder vegetable farmers in Ethiopian Central Rift Valley. Environ. Dev. Sustain. 2017, 19, 301–324. [Google Scholar] [CrossRef]
- Hamedani Radja, K.; Mikani, A.; Mosallanejad, H. Biochemical resistance mechanisms to dimethoate in cabbage Aphid Brevicoryne brassicae (L.) (Hom.: Aphididae). J. Agric. Sci. Technol. 2020, 22, 187–196. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- McClure, M.; Herreid, J.; Jabbour, R. Insecticide application timing effects on alfalfa insect communities. J. Econ. Entomol. 2023, 116, 815–822. [Google Scholar] [CrossRef]
- Madaki, M.Y.; Lehberger, M.; Bavorova, M.; Igbasan, B.T.; Kächele, H. Effectiveness of pesticide stakeholders’ information on pesticide handling knowledge and behaviour of smallholder farmers in Ogun State, Nigeria. Environ. Dev. Sustain. 2024, 26, 17185–17204. [Google Scholar] [CrossRef]
- Mergia, M.T.; Weldemariam, E.D.; Eklo, O.M.; Yimer, G.T. Small-scale farmer pesticide knowledge and practice and impacts on the environment and human health in Ethiopia. J. Health Pollut. 2021, 11, 210607. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Scheringer, M.; Schenker, U.; Hungerbühler, K. Assessment of the environmental persistence and long-range transport of endosulfan. Environ. Pollut. 2011, 159, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Chavan, G.; Nagpal, A.K. Navigating the neurological abyss: A comprehensive review of organophosphate poisoning complications. Cureus 2024, 16, e54422. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, J.I.; Flaws, J.A. The impact of neonicotinoid pesticides on reproductive health. Toxicol. Sci. 2025, 203, 131–146. [Google Scholar] [CrossRef]
- Manzer, S.; Thamm, M.; Hilsmann, L.; Krischke, B.; Steffan-Dewenter, I.; Scheiner, R. The neonicotinoid acetamiprid reduces larval and adult survival in honeybees (Apis mellifera) and interacts with a fungicide mixture. Environ. Pollut. 2024, 360, 124643. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Divekar, P.A.; Majumder, S.; Halder, J.; Kedar, S.C.; Singh, V. Sustainable pest management in cabbage using botanicals: Characterization, effectiveness and economic appraisal. J. Plant Dis. Prot. 2024, 131, 113–130. [Google Scholar] [CrossRef]
- Mayanglambam, S.; Singh, K.D.; Rajashekar, Y. Current biological approaches for management of crucifer pests. Sci. Rep. 2021, 11, 11831. [Google Scholar] [CrossRef]
- Daniel, K.A.M.; Muindi, E.M.D.; Muti, S.M.D. Cabbage (Brassica oleracea) production in Kenya: A review of its economic importance, ecological requirement and production constraints. Int. J. Plant Soil Sci. 2023, 35, 245–254. [Google Scholar] [CrossRef]
- Juventia, S.D.; Rossing, W.A.H.; Ditzler, L.; van Apeldoorn, D.F. Spatial and genetic crop diversity support ecosystem service delivery: A case of yield and biocontrol in Dutch organic cabbage production. Field Crops Res. 2021, 261, 108015. [Google Scholar] [CrossRef]
- Yang, L.; Ali, J.; Ahmad, B.; Yang, S.; Huang, J.; Zhao, J.; Alam, A.; Khan, K.A.; Ghramh, H.A.; Rahman, N.; et al. Garlic as a companion plant for suppressing Myzus persicae infestation in Brassica rapa. Crop Prot. 2025, 187, 106970. [Google Scholar] [CrossRef]
- Baidoo, P.K.; Mochiah, M.B.; Apusiga, K. Onion as a pest control Intercrop in organic cabbage (Brassica oleracea) production system in Ghana. Sustain. Agric. Res. 2012, 1, 36–41. [Google Scholar] [CrossRef]
- Yarou, B.B.; Komlan, F.A.; Tossou, E.; Mensah, A.C.; Simon, S.; Verheggen, F.J.; Francis, F. Efficacy of basil-cabbage intercropping to control insect pests in benin, West Africa. Commun. Agric. Appl. Biol. Sci. 2017, 82, 157–166. [Google Scholar]
- Hithesh, G.R.; Suroshe, S.S.; Keerthi, M.C.; Fand, B.B. Companion planting of flowering annuals enhances the colonization of natural enemies in white cabbage fields. J. Plant Dis. Prot. 2024, 132, 49. [Google Scholar] [CrossRef]
- Da Silva, V.F.; dos Santos, A.; Silveira, L.C.P.; Tomazella, V.B.; Ferraz, R.M. Push-pull cropping system reduces pests and promotes the abundance and richness of natural enemies in Brassica vegetable crops. Biol. Control 2022, 166, 104832. [Google Scholar] [CrossRef]
- Grzywacz, D.; Cherry, A.; Gwynn, R. Biological pesticides for Africa: Why has so little of the research undertaken to date resulted in new products to help Africa’s poor? Outlooks Pest Manag. 2009, 20, 77–81. [Google Scholar] [CrossRef]
- Srinivasan, R.; Tamò, M.; Subramanian, S. The case for integrated pest management in Africa: Transition from a pesticide-based approach. Curr. Opin. Insect Sci. 2022, 54, 100970. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.G.; André, T.P.P.; Pontes, A.D.S.; Souza, S.A.; Oliveira, N.R.X.; Pastori, P.L. Insecticide rotation and adaptive fitness cost underlying insecticide resistance management for Spodoptera frugiperda (Lepidoptera: Noctuidae). Neotrop. Entomol. 2020, 49, 882–892. [Google Scholar] [CrossRef]
- Mahanta, D.K.; Komal, J.; Bhoi, T.K.; Samal, I.; Dash, S.; Jangra, S. RNA interference (RNAi) for insect pest management: Understanding mechanisms, strategies, challenges and future prospects. Biol. Futur. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Yang, Q.; Lin, X.; Liu, Y.; Li, Z.; Swevers, L. Successful oral RNA interference efficiency in the silkworm Bombyx mori through nanoparticle-shielded dsRNA delivery. J. Insect Physiol. 2025, 161, 104749. [Google Scholar] [CrossRef]
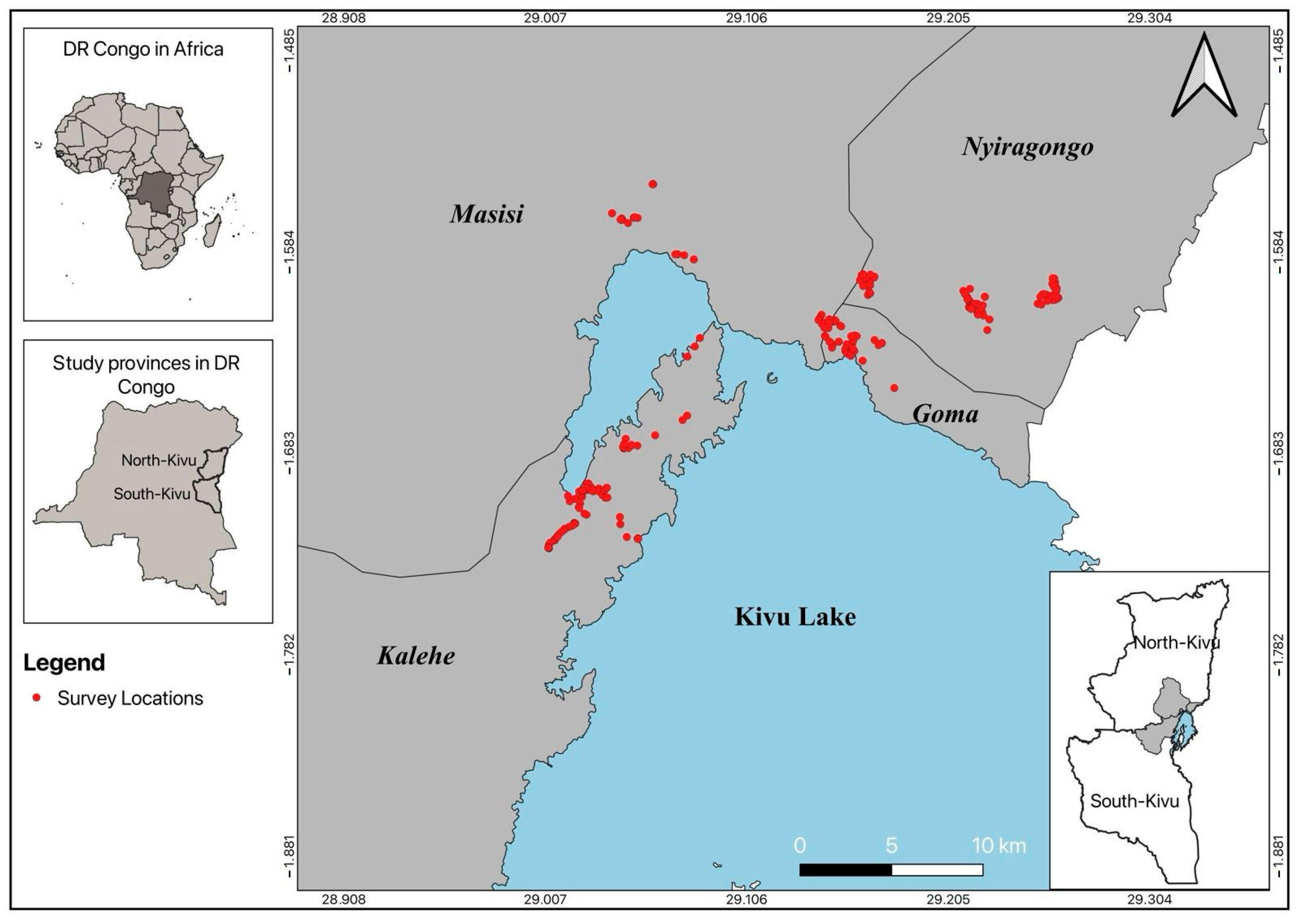
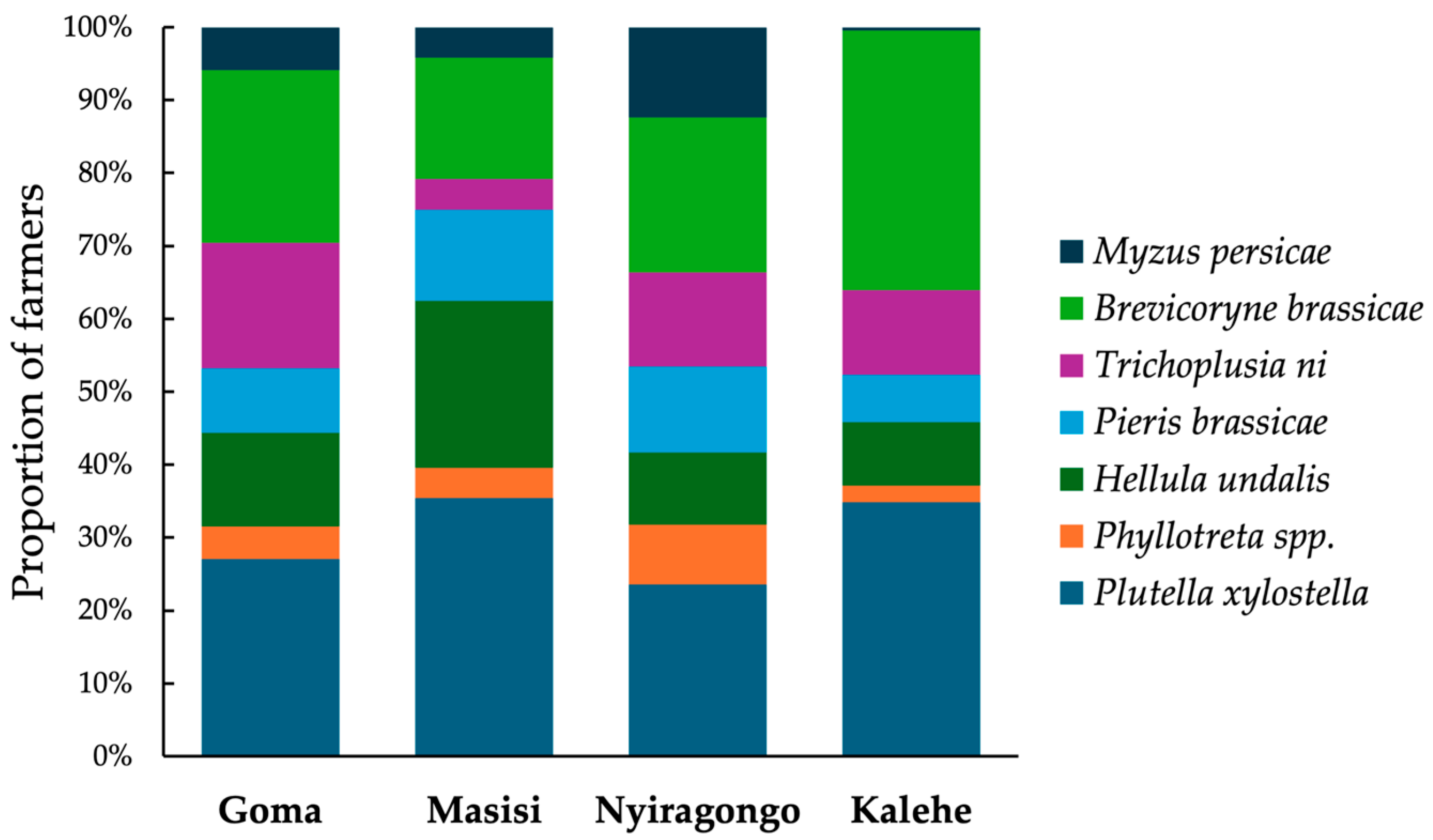
| Survey Areas | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Categories | Goma n = 71 | Nyiragongo n = 145 | Masisi n = 30 | Kalehe n = 184 | Overall n = 430 | χ2 Test | Kruskal–Wallis |
| Gender (%) | Female | 33.8 | 22.8 | 6.7 | 23.4 | 23.7 | 8.89 * | |
| Male | 66.2 | 77.2 | 93.3 | 76.6 | 76.3 | |||
| Level of studies (%) | Uneducated | 8.5 | 19.3 | 26.7 | 34.8 | 24.7 | 39.87 *** | |
| Primary school | 23.9 | 36.6 | 33.3 | 32.6 | 32.6 | |||
| Secondary school | 47.9 | 34.5 | 30.0 | 28.8 | 34.0 | |||
| University level | 19.7 | 9.7 | 10.0 | 3.8 | 8.8 | |||
| Household size | 5.4 ± 2.3 a | 6.5 ± 2.3 c | 8.3 ± 3.1 b | 7.6 ± 2.2 b | 6.9 ± 2.5 | 52.21 *** | ||
| Household active members | 3.8 ± 2.1 a | 5.4 ± 2.3 c | 6.5 ± 2.3 b | 6.4 ± 1.9 b | 5.6 ± 2.3 | 67.31 *** | ||
| Farm size (%) | <0.5 Ha | 77.5 | 29.7 | 23.3 | 10.3 | 28.8 | 136.16 *** | |
| 0.5–1 Ha | 21.1 | 46.9 | 56.7 | 40.2 | 40.5 | |||
| 1–2 Ha | 1.4 | 21.4 | 16.7 | 41.3 | 26.3 | |||
| >2 Ha | 0.0 | 2.1 | 3.3 | 8.2 | 4.4 | |||
| Proportion of land used for cabbage (%) | <25% | 23.9 | 30.3 | 46.7 | 32.1 | 30.7 | 14.55 * | |
| 25–50% | 45.1 | 45.5 | 43.3 | 53.3 | 49.1 | |||
| >50% | 31.0 | 24.1 | 10.0 | 14.7 | 20.2 | |||
| Cropping systems (%) | Monoculture | 47.1 | 61.1 | 42.9 | 46.1 | 51.5 | 57.49 *** | |
| Intercropping | 37.7 | 15.8 | 40.7 | 40.7 | 31.3 | |||
| Companion planting | 14.5 | 15.4 | 5.7 | 5.4 | 11.0 | |||
| Agroforestry | 0.7 | 7.7 | 5.7 | 7.9 | 6.2 | |||
| Survey Areas | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Categories | Goma n = 71 | Nyiragongo n = 145 | Masisi n = 30 | Kalehe n = 184 | Overall n = 430 | χ2 Test |
| Seasonal pest incidence (%) | Long rainy season | 21.1 | 23.4 | 20.0 | 7.6 | 16.0 | 27.52 *** |
| Short rainy season | 33.8 | 26.2 | 23.3 | 20.7 | 24.9 | ||
| Dry mid-season | 45.1 | 50.3 | 56.7 | 71.7 | 59.1 | ||
| Crop damage stage (%) | Nursery stage | 5.8 | 10.8 | 7.7 | 3.6 | 6.9 | 52.58 *** |
| Vegetative growth stage | 21.2 | 21.6 | 36.8 | 17.2 | 22.0 | ||
| Post-transplanting stage | 46.0 | 40.6 | 33.3 | 36.6 | 38.9 | ||
| Cabbage heading | 24.8 | 25.9 | 18.8 | 41.3 | 30.2 | ||
| Post-heading stage | 2.2 | 1.1 | 3.4 | 1.3 | 1.7 | ||
| Information and/or training on pest management (%) | Agriculture public services | 16.8 | 22.6 | 14.3 | 16.7 | 18.3 | 83.18 *** |
| FAO | 8.8 | 25.9 | 4.8 | 17.7 | 17.8 | ||
| NGOs | 32.8 | 33.2 | 19.0 | 15.1 | 24.0 | ||
| Radio/TV | 26.4 | 11.5 | 26.2 | 31.4 | 23.9 | ||
| Other sources | 15.2 | 6.2 | 20.0 | 19.1 | 15.9 | ||
| Survey Areas | ||||||
|---|---|---|---|---|---|---|
| Management Practices (%) | Goma n = 71 | Nyiragongo n = 145 | Masisi n = 30 | Kalehe n = 184 | Overall n = 430 | χ2 Test |
| Use of resistant varieties | 47.9 | 33.1 | 36.7 | 43.5 | 40.2 | 5.76 ns |
| Early planting | 21.1 | 53.8 | 43.3 | 21.7 | 34.0 | 44.08 *** |
| Practice crop rotation | 45.1 | 30.3 | 46.7 | 40.8 | 38.4 | 6.61 ns |
| Intercropping with non-Brassica crops | 22.5 | 15.9 | 3.3 | 9.8 | 13.5 | 10.50 * |
| Intercropping with repellent/trap plants | 4.2 | 12.4 | 3.3 | 7.1 | 8.1 | 6.21 * |
| Practice regular weeding | 62.0 | 60.7 | 43.3 | 47.8 | 54.2 | 8.63 * |
| Practice fertilization | 26.8 | 26.2 | 16.7 | 8.2 | 17.9 | 22.52 *** |
| Application of ash/sawdust | 19.7 | 22.1 | 13.3 | 12.5 | 17.0 | 5.94 ns |
| Hand picking of larvae/egg clusters | 31.0 | 32.4 | 16.7 | 16.3 | 24.2 | 14.30 ** |
| Uproot and burn infected plants | 39.4 | 35.2 | 20.0 | 26.6 | 31.2 | 6.86 ns |
| Destruct crop residues | 23.9 | 36.6 | 30.0 | 16.8 | 25.6 | 16.95 *** |
| Replanting attacked areas | 12.7 | 19.3 | 16.7 | 17.4 | 17.2 | 1.48 ns |
| Use self-prepared local plant extracts | 18.3 | 16.6 | 13.3 | 11.4 | 14.4 | 2.78 ns |
| Scientific Name | Common Name | Family | Plant Parts Used |
|---|---|---|---|
| Allium cepa (Linnaeus) | Onion | Amaryllidaceae | Bulbs |
| Allium sativum (Linnaeus) | Garlic | Amaryllidaceae | Bulbs |
| Azadirachta indica (A.Juss.) | Neem | Meliaceae | Leaves, seeds |
| Cannabis sativa (Linnaeus) | Hemp | Cannabaceae | Leaves |
| Capsicum frutescens (Linnaeus) | Pepper | Solanaceae | Fruits |
| Carica papaya (Linnaeus) | Papaya | Caricaceae | Leaves, seeds |
| Eucalyptus spp. | Eucalyptus | Myrtaceae | Leaves |
| Lantana camara (Linnaeus) | Wild sage | Verbenaceae | Leaves |
| Nicotiana tabacum (Linnaeus) | Tobacco | Solanaceae | Leaves |
| Ricinus communis (Linnaeus) | Castor | Euphorbiaceae | Leaves, seeds |
| Tetradenia riparia (Hochst.) Codd | Ginger bush | Lamiaceae | Leaves |
| Tithonia diversifolia (Hemsl.) A.Gray | Mexican sunflower | Asteraceae | Leaves, seeds |
| Survey Areas | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Categories | Goma n = 71 | Nyiragongo n = 145 | Masisi n = 30 | Kalehe n = 184 | Overall n = 430 | χ2 Test | Kruskal–Wallis |
| Control methods used by farmers (%) | Physical control | 23.7 | 19.1 | 15.6 | 10.4 | 16.3 | 21.44 | |
| Biological control | 21.2 | 12.7 | 6.2 | 13.3 | 14.3 | ** | ||
| Chemical control | 55.1 | 68.1 | 78.1 | 76.2 | 69.4 | |||
| Time of chemical treatment (%) | Morning | 84.5 | 82.1 | 70.0 | 62.5 | 73.3 | 43.50 | |
| Midday | 5.6 | 10.3 | 10.3 | 32.1 | 18.8 | *** | ||
| Evening | 9.9 | 7.6 | 20.0 | 5.4 | 7.9 | |||
| Intervention time after infestation detection (days) | 3.8 ± 2.6 a | 2.4 ± 1.7 b | 4.3 ± 1.9 a | 2.0 ± 1.3 b | 2.6 ± 1.9 | 45.97 *** | ||
| Delay between two successive chemical treatments (days) | 10.6 ± 3.4 a | 12.2 ± 3.4 c | 12.0 ± 2.4 bc | 13.0 ± 2.3 b | 12.2 ± 3.0 | 44.09 *** | ||
| Number of chemical applications | 3.3 ± 1.5 a | 4.4 ± 2.8 c | 3.8 ± 2.0 ac | 2.8 ± 2.2 b | 3.5 ± 2.4 | 60.01 *** | ||
| Survey Areas | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Categories | Goma n = 71 | Nyiragongo n = 145 | Masisi n = 30 | Kalehe n = 184 | Overall n = 430 | χ2 Test |
| Wear PPE (%) | Yes | 39.4 | 35.2 | 36.7 | 45.1 | 41.9 | 3.54 ns |
| No | 60.6 | 64.8 | 63.3 | 54.9 | 58.1 | ||
| Type of PPE used (%) | Face mask | 19.1 | 24.7 | 13.3 | 16.2 | 19.5 | 19.38 ns |
| Long clothes | 25.7 | 17.2 | 24.4 | 24.8 | 22.3 | ||
| Rubber boots | 40.4 | 44.6 | 51.1 | 49.2 | 46.1 | ||
| Gloves | 12.5 | 12.7 | 11.1 | 9.5 | 11.3 | ||
| Glasses | 2.2 | 0.7 | 0.0 | 0.3 | 0.8 | ||
| Information on pesticide use (%) | Previous experience/Reading instructions | 22.7 | 24.8 | 15.6 | 11.9 | 18.4 | 18.17 * |
| Agriculture extension services | 10.9 | 8.8 | 6.2 | 8.5 | 9.0 | ||
| Other farmers | 42.0 | 42.5 | 46.9 | 48.5 | 45.2 | ||
| Agrochemical retailers | 24.4 | 23.9 | 31.2 | 31.1 | 27.5 | ||
| Health problems related to pesticide use (%) | Headache/Coughing | 28.0 | 26.6 | 28.0 | 23.5 | 25.5 | 13.64 ns |
| Stomachache | 13.3 | 6.5 | 6.5 | 9.2 | 9.0 | ||
| Skin irritation | 24.0 | 27.9 | 28.0 | 26.8 | 26.7 | ||
| Eye irritation | 20.4 | 21.1 | 23.7 | 21.9 | 21.6 | ||
| Breathing problems | 14.2 | 17.9 | 14.0 | 18.6 | 17.3 | ||
| Commercial Name | Active Ingredient | Family | WHO Class | Adoption Rate (%) |
|---|---|---|---|---|
| Cyperscope 5EC | Cypermethrin 5% | Pyrethroid | II | 6.8 |
| Dudu Aba+ | Abamectin 2% | Avermectin | Ib | 5.8 |
| Dudu acelamectin 5%EC | Abamectin 2% + acetamiprid 3% | Avermectin + Neonicotinoid | Ib + II | 13.0 |
| Dudu alpha 3EC | Alpha-cypermethrin 3% | Pyrethroid | II | 17.1 |
| Dudu cyper 5% EC | Cypermethrin 5% | Pyrethroid | II | 2.1 |
| Lava 100% EC | Dichlorvos 100% | Organophosphorus | Ib | 2.5 |
| Kuu-Kill | Chlorpyrifos 48% | Organophosphorus | II | 0.5 |
| Roket 44EC | Profenofos 40% + cypermethrin 4% | Organophosphorus + Pyrethroid | II + II | 20.1 |
| Simba + | Cypermethrin 5% | Pyrethroid | II | 2.2 |
| Tafgor 40EC | Dimethoate 40% | Organophosphorus | II | 6.1 |
| Thiodan | Endosulfan 50% | Organochlorine | II | 23.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gakuru, P.N.; Muhashy Habiyaremye, F.; Noël, G.; Caparros Megido, R.; Francis, F. Assessment of Cabbage (Brassica oleracea Linnaeus) Insect Pests and Management Strategies in Eastern Democratic Republic of Congo. Agriculture 2025, 15, 2203. https://doi.org/10.3390/agriculture15212203
Gakuru PN, Muhashy Habiyaremye F, Noël G, Caparros Megido R, Francis F. Assessment of Cabbage (Brassica oleracea Linnaeus) Insect Pests and Management Strategies in Eastern Democratic Republic of Congo. Agriculture. 2025; 15(21):2203. https://doi.org/10.3390/agriculture15212203
Chicago/Turabian StyleGakuru, Patient Niyibizi, François Muhashy Habiyaremye, Grégoire Noël, Rudy Caparros Megido, and Frédéric Francis. 2025. "Assessment of Cabbage (Brassica oleracea Linnaeus) Insect Pests and Management Strategies in Eastern Democratic Republic of Congo" Agriculture 15, no. 21: 2203. https://doi.org/10.3390/agriculture15212203
APA StyleGakuru, P. N., Muhashy Habiyaremye, F., Noël, G., Caparros Megido, R., & Francis, F. (2025). Assessment of Cabbage (Brassica oleracea Linnaeus) Insect Pests and Management Strategies in Eastern Democratic Republic of Congo. Agriculture, 15(21), 2203. https://doi.org/10.3390/agriculture15212203








