Study on the Identification and Incidence Pattern of the Pathogen Causing Apple Scab in Wild Apple Forests of Ili, Xinjiang
Abstract
1. Introduction
2. Materials and Methods
2.1. Samplings
2.2. Pathogen Identification of Apple Scab in Ili Wild Apple Forests
2.2.1. Experimental Materials
2.2.2. Fungal Isolation
2.2.3. Morphological Observation
2.2.4. Molecular Identification
2.2.5. Pathogenicity Test
2.3. Apple Scab Occurrence Investigation
2.4. Investigation of Overwintering Sites for Apple Scab Pathogen in Wild Fruit Forests
3. Results
3.1. Pathogen Identification
3.1.1. Pathogenicity
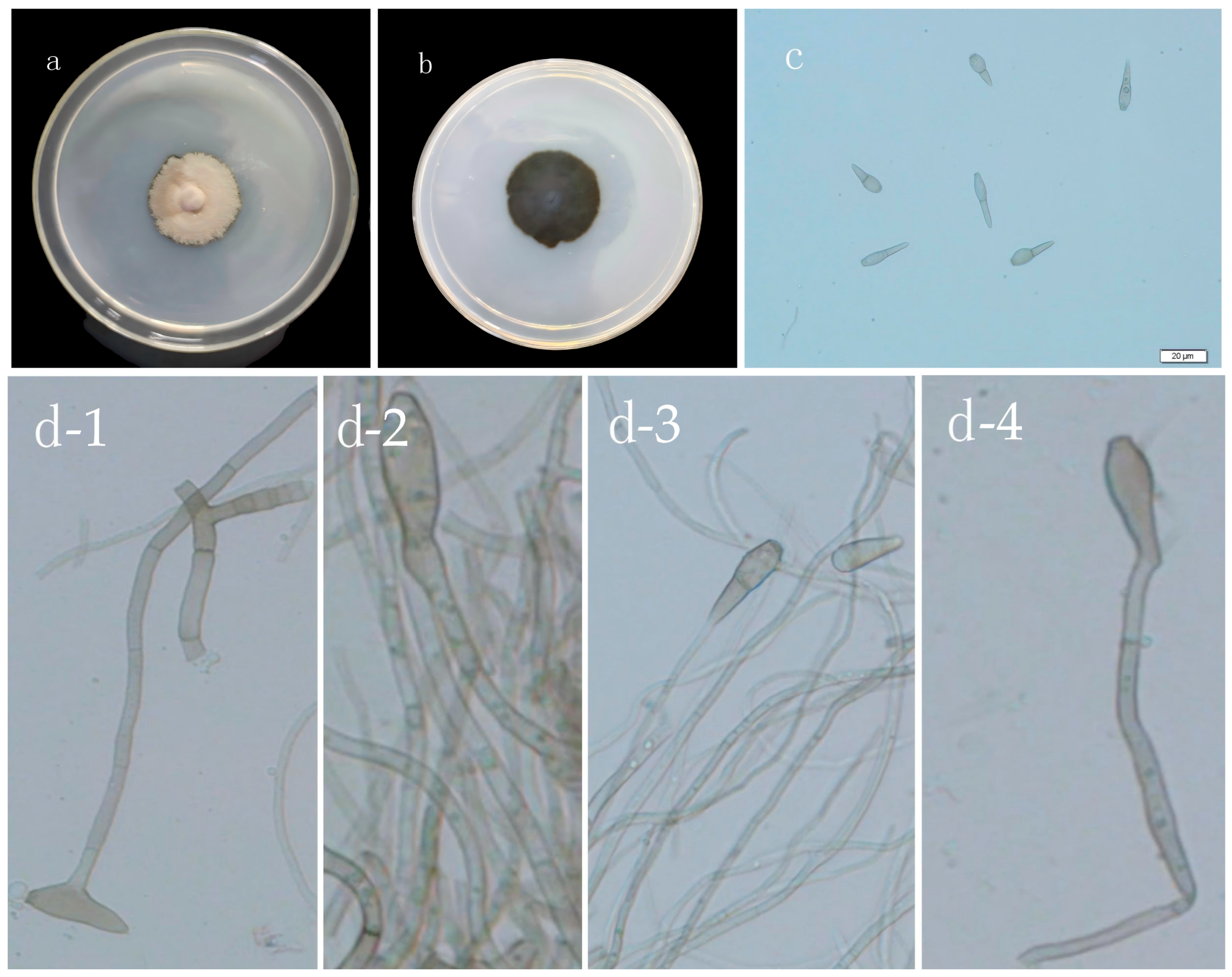
3.1.2. Morphology
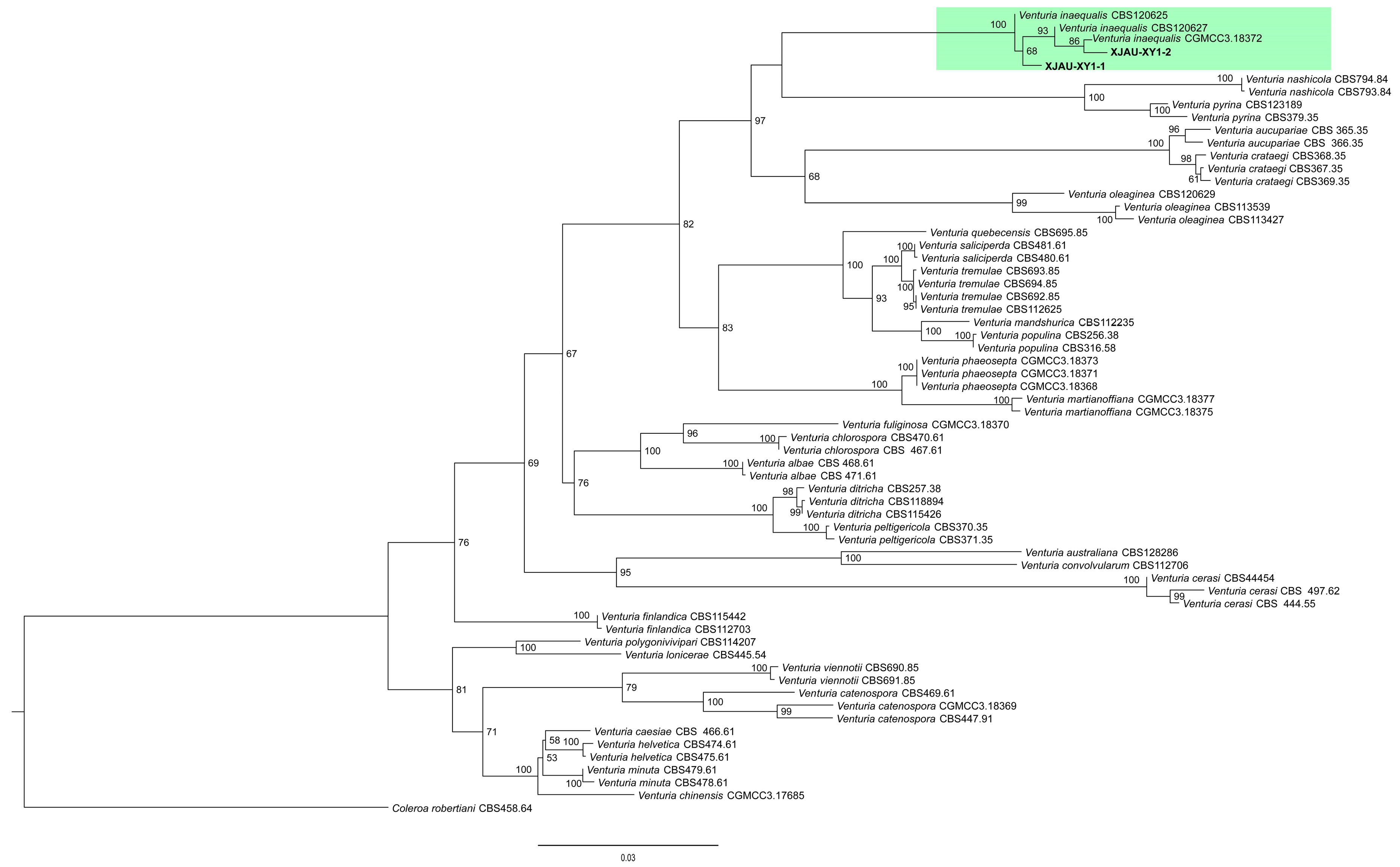
3.1.3. Phylogenetic Analysis
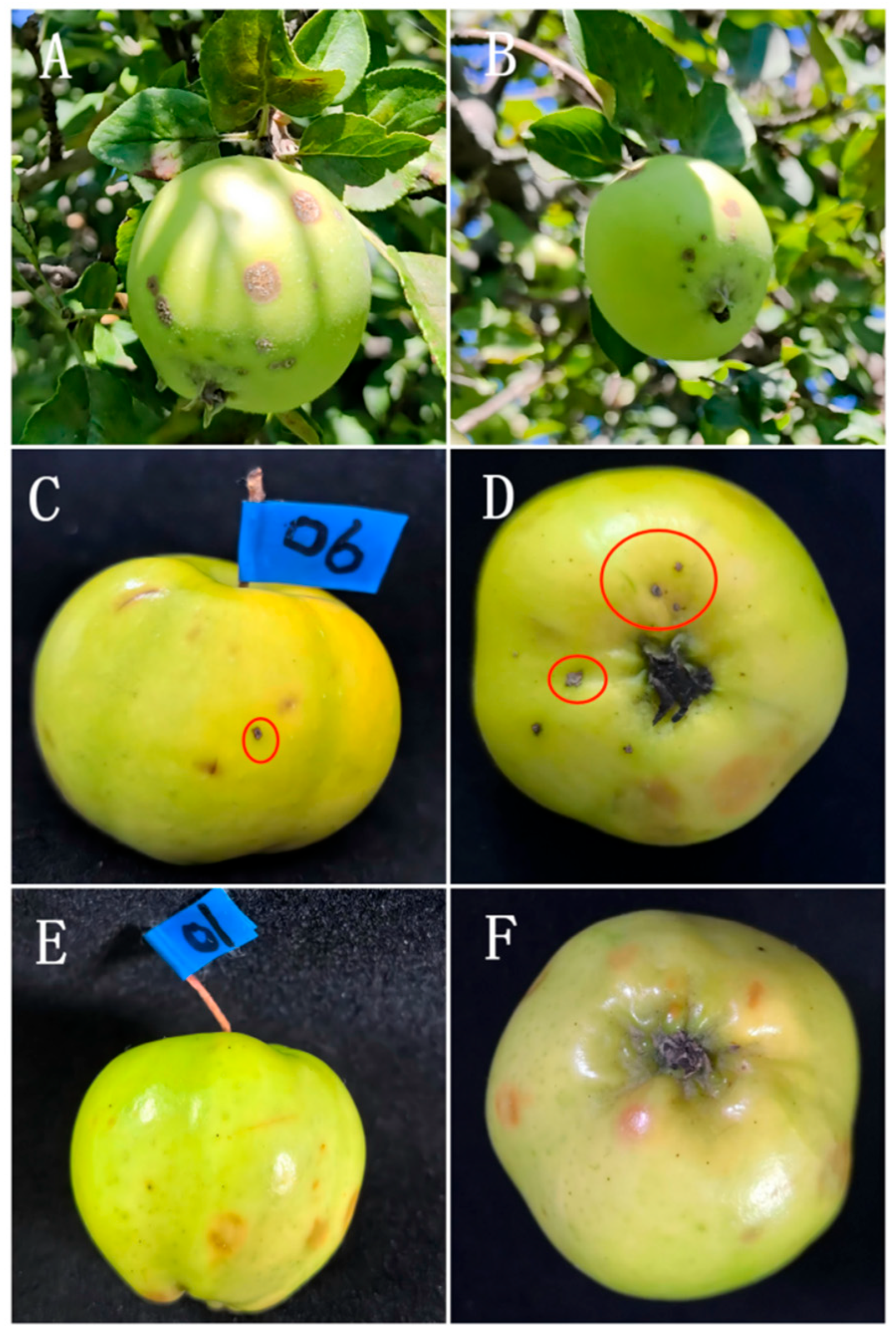
3.2. Disease Symptoms
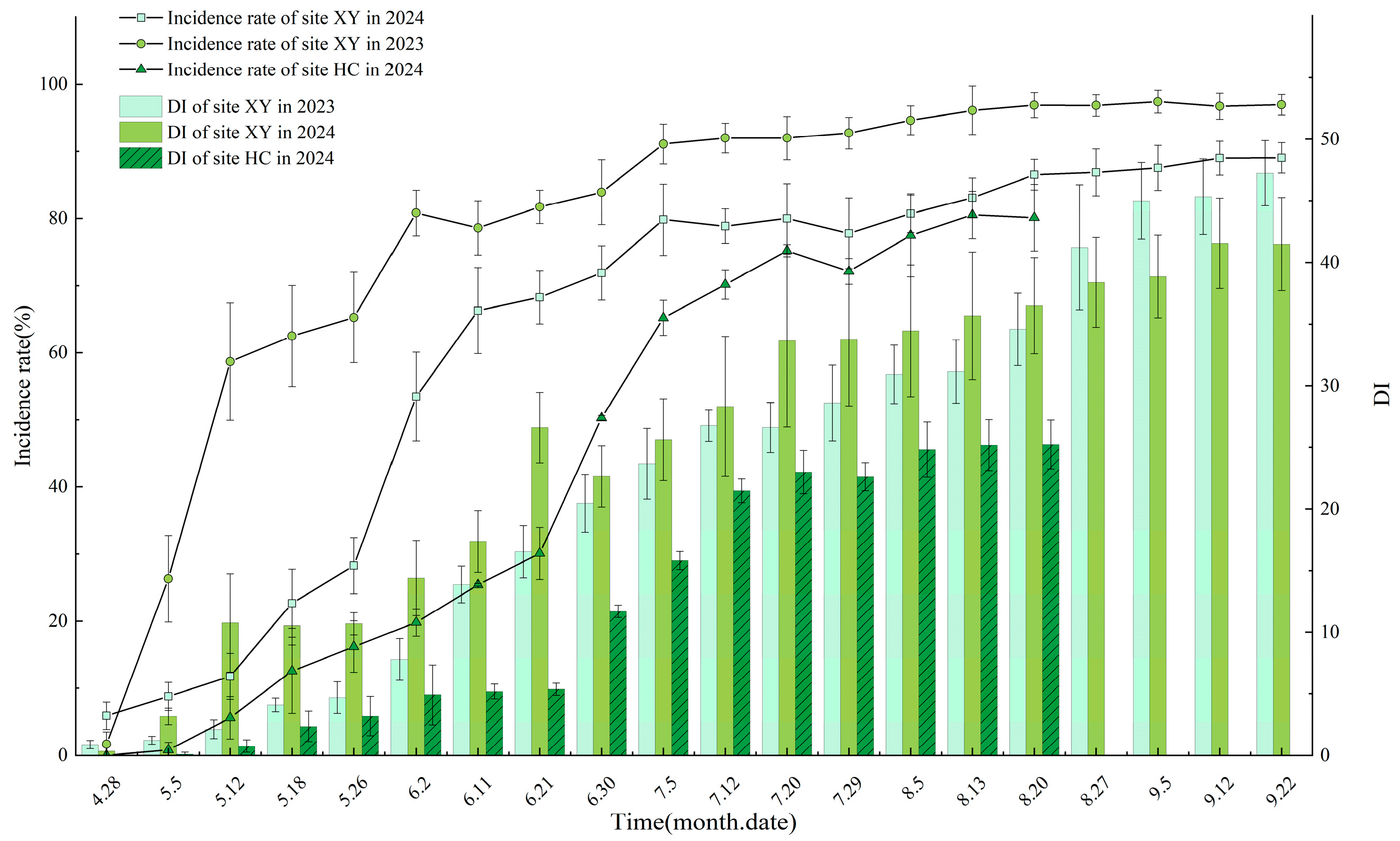
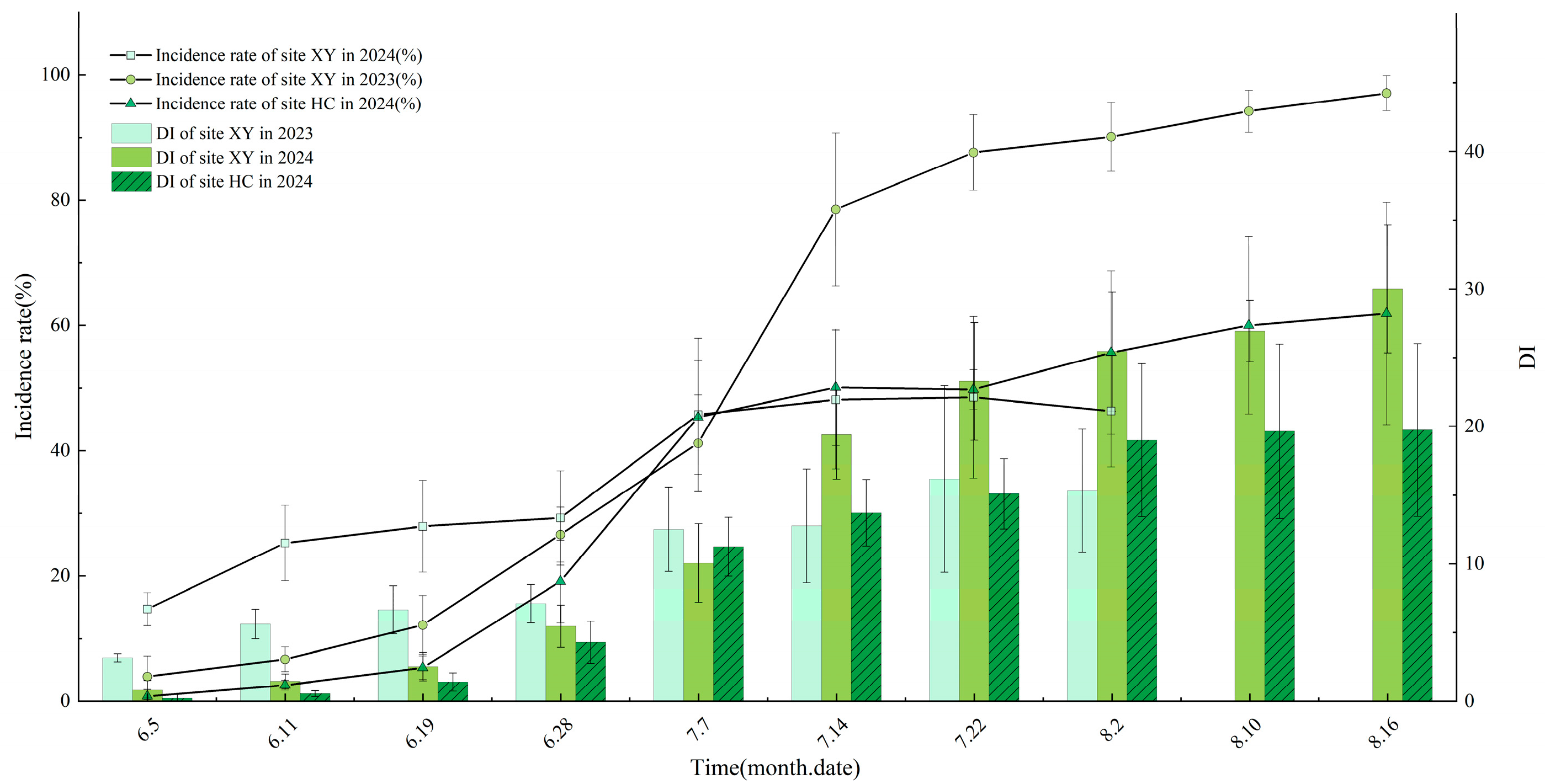
3.3. Leaf Disease Progression
3.4. Fruit Disease Progression
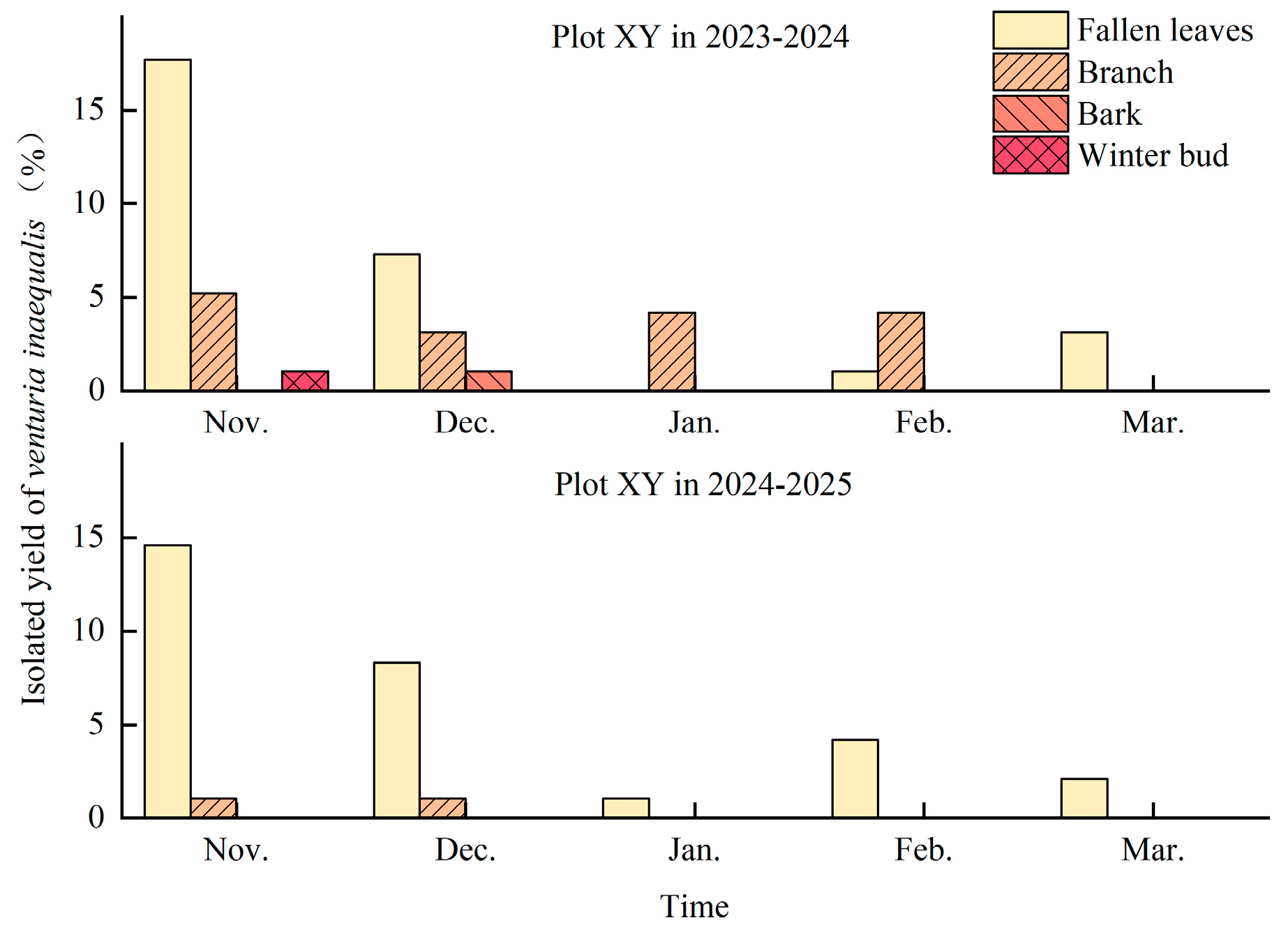
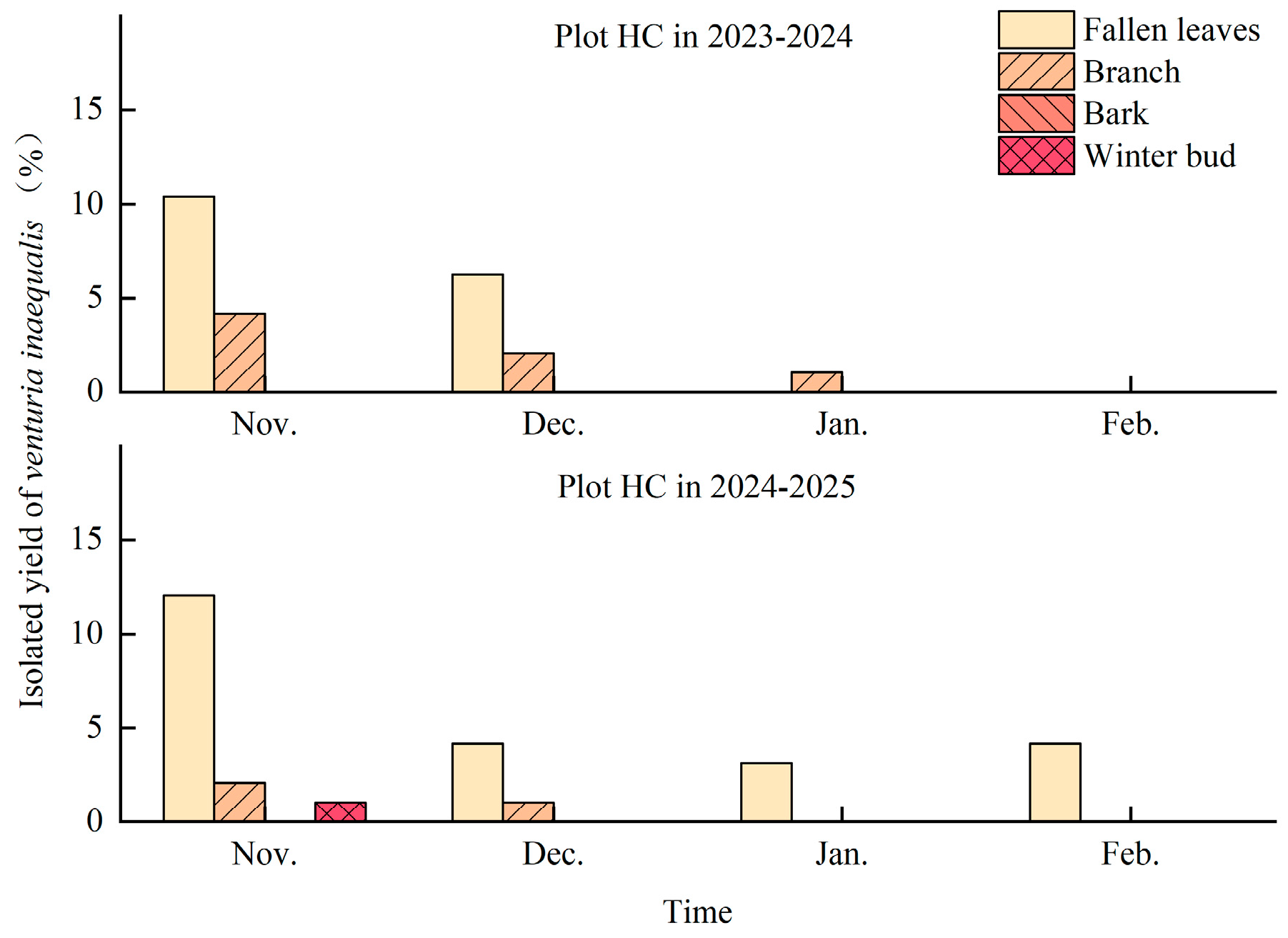
3.5. Overwintering Sites of Venturia inaequalis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Xiao, Y.; Gao, Y.; Sun, S.; Li, L.; Zhang, F.; Wang, K. Current status of research on collection, conservation and utilization of wild Malus resources in China. China Fruits 2021, 10, 6–11. [Google Scholar] [CrossRef]
- Lin, P.; Cui, N. Wild Fruit Forests Resources in Tianshan Mounains; China Forestry Publishing House: Beijing, China, 2000. [Google Scholar]
- Zhang, P.; Lv, Z.; Zhang, X.; Zhao, X.; Zhang, Y.; Tanabekova, G.; Bagila, M.; Zhanera, A.; Cui, Z. Age structure of Malus sieversii population in Ili of Xinjiang and Kazakhstan. Arid. Zone Res. 2019, 36, 844–853. [Google Scholar] [CrossRef]
- Yan, G.; Xu, Z. Study on the wild fruit tree diseases of Tianshan Mountains and their distribution in Xinjiang. Arid. Zone Res. 2001, 18, 47–49. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus×domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhang, H.; Chen, X.S.; Zhang, Y.M.; He, T.M.; Feng, J.R.; Xu, Z. Genetic diversity of fruit morphological traits and content of mineral element in Malus sieversii (Ldb.) Roem. and its elite seedlings. J. Plant Genet. Resour. 2006, 7, 3270–3276. [Google Scholar] [CrossRef]
- Chen, X.; Feng, T.; Zhang, Y.; He, T.; Feng, J.; Zhang, C. Genetic diversity of volatile components in Xinjiang wild apple (Malus sieversii). Genet. Genom. 2007, 34, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Q.; Sun, H.; Qiu, Z.; Wang, Y.; Sun, Z. Study on the phenotype biodiversity of Xinjiang wild apples (Malus sieversii). J. Fruit Sci. 2004, 21, 285–288. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Ren, Y. Identification, biological characteristics and virulence of apple scab in Ili region of Xinjiang. J. Anhui Agric. Sci. 2023, 51, 114–118. [Google Scholar] [CrossRef]
- Dai, P.; Zhang, R.; Sun, G. A checklist of pathogenic fungi on apple in China. Mycosystema 2021, 40, 936–964. [Google Scholar] [CrossRef]
- Li, X. Studies on Genetic Diversity of Venturia inaequalis of Apple, Screening its Resistance to Fungicides and the Mechanism of Qinguan Resistance to Apple Scab in Northwest China. Master’s Thesis, Northwest Agriculture and Forestry University, Shaanxi, China, 2021. [Google Scholar] [CrossRef]
- National Agro-Tech Extension and Service Center. Illustrated Handbook of Plant Quarantine Pests; China Agricuture Press: Beijing, China, 2001; pp. 290–292. [Google Scholar]
- Chen, J. Dissemination Routes and Long Term Prediction Models of Apple Scab. Master’s Thesis, Northwest Agriculture and Forestry University, Shaanxi, China, 2009. [Google Scholar]
- Yuan, F.; Lv, W.; Wang, S. Изучение o первoистoчника вoзбудителя паршияблoни мелкoплoдных сoртoв в зимующей стадиив прoвинции Хэйлунцзян. Acta Phytopathol. Sin. 1965, 1, 23–30. [Google Scholar] [CrossRef]
- Hu, X. Epidemiology of Apple Scab in Shaanxi and Genetic Diversity of Venturia inaequalis with SSR Markers. Ph.D. Thesis, Northwest Agriculture and Forestry University, Shaanxi, China, 2004. [Google Scholar]
- Jia, L.; Yang, J.; Yu, Z. Studies on differentiation of Venturia inaequalis of apple in pathogenicity. J. Northwest AF Univ. (Nat. Sci. Ed.) 2007, 35, 92–96. [Google Scholar] [CrossRef]
- Hu, X.P.; Zhang, J.G.; Chen, J.; Zhou, S.T.; Yang, J.R.; Kang, Z.S. Resistance characteristics of Xinjiang wild apple and Qinguan cultivars to apple scab. Acta Phytopathol. Sin. 2010, 40, 609–614. [Google Scholar] [CrossRef]
- Zhou, Y. Studies on Genetic Diversity Infection Process, Fungicide Resistance and Early Detection of Venturia carpophila in China. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar] [CrossRef]
- Belete, T.; Boyraz, N. Critical review on apple scab (Venturia inaequalis) biology, epidemiology, economic importance, management and defense mechanisms to the causal agent. Plant Physiol. Pathol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, S.; Yang, J. Occurrence and research progress on apple scab in China. Chin. J. Eco-Agric. 2010, 18, 5. [Google Scholar] [CrossRef]
- Pi, S.; Gao, J.; Hou, C.; Ma, A.; Pi, X.; Zhang, S.; Duan, M. Occurrence and control technique of apple Venturia inaequalis in west of Henan province. J. Anhui Agric. Sci. 2007, 35, 1005–1006. [Google Scholar] [CrossRef]
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The causal agent of apple scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yue, D.; Zhang, Y.; Liu, J.; Shi, G.; Li, Q.; Jiang, J.; Li, J. Dynamics of annual growth and decline of apple scab in Longdong and state of an illness differences among different apple varieties. Acta Agric. Boreali-Occident. Sin. 2023, 32, 1495–1501. [Google Scholar]
- Lei, M.; An, X.; Zhou, X.; Luo, L.; Yang, J. Investigation of the annual growth and decline of apple scab and screening of control agents in Tianshui, Gansu Province. S. China Fruits 2024, 53, 182–187+194. [Google Scholar]
- Fang, Z. Research Methods of Plant Diseases, 3rd ed.; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Guerrero, J.C.; Guevara, J.; Gubler, W.D. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in Mexico. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef]
- Adams, G.; Wingfield, M.; Common, R.; Roux, J. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud. Mycol. 2005, 52, 1–144. [Google Scholar] [CrossRef]
- White, J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Glass, L.; Donaldson, C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 3, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.; Whelen, S.; Hall, D. Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Rehner, S. Primers for elongation factor 1-alpha (EF1-alpha), 2001.
- Zhao, P.; Crous, P.W.; Hou, L.W.; Duan, W.J.; Cai, L.; Ma, Z.Y.; Liu, F. Fungi of quarantine concern for China I: Dothideomycetes. Persoonia-Mol. Phylogeny Evol. Fungi 2021, 47, 45–105. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 304, 772–780. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Shi, W.; Wang, A.; Ma, R.; Su, X. Identification and pathogenicity of Dothiorella sarmentorum causing lavender leaf blight disease in Xinjiang, China. Diversity 2024, 16, 148. [Google Scholar] [CrossRef]
- Wang, X. Study on the occurrence regularity of apple scab in Shaanxi and fungicides screening in field. Master’s Thesis, Northwest Agriculture and Forestry University, Shaanxi, China, 2007. [Google Scholar]
- Liu, J.; Dilnur, J.H. Study on overwintering survival of mycelia and conidia of walnut leaf spot pathogens. Xinjiang Agric. Sci. 2018, 55, 1870–1878. [Google Scholar]
- Li, C.; Zhang, D.; Huang, G.; Quan, Y.; Wu, D.; Ma, M.; Cheng, A.; Zhang, X.; Du, N.; Tong, F.; et al. Preliminary study on the epidemic regularity of apple scab in the cool areas of south west China and screening of control fungicides. S. China Fruits 2024, 53, 222–227. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Li, S.; Gao, L.; Gou, J.; Yu, Z.; Yang, J. Prediction of apple scab epidemics in Weibei semi-arid plateau of Shaanxi. J. Northwest AF Univ. (Nat. Sci. Ed.) 2007, 10, 151–154+158. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Li, S.; Gao, L.; Gou, J.; Yu, Z.; Yang, J. Inoculum of primary infection of Venturia inaequalis in arid plateau of Weibei, Shaanxi. Acta Phytopathol. Sin. 2008, 1, 83–87. [Google Scholar] [CrossRef]
- Wrzesień, M.; Treder, W.; Klamkowski, K.; Rudnicki, W.R. Prediction of the apple scab using machine learning and simple weather stations. Comput. Electron. Agric. 2019, 161, 252–259. [Google Scholar] [CrossRef]
- Shi, T.; Li, C.; Cai, J.; Wang, G.; Lu, C.; Han, Q.; Huang, G. Occurrence Pattern Investigation and Fungicides Control Effect in Field for Cassava Brown Leaf Spot Disease in China. Chin. J. Trop. Crops 2019, 40, 2178–2188. [Google Scholar] [CrossRef]
- Shang, H. Apple Scab Pathogen Quarantine. Plant Quar. 2006, 4, 249–252. [Google Scholar] [CrossRef]


| Sample Location | Plot Number | Altitude (m) | Aspect | Gradient (°) | Stand Canopy |
|---|---|---|---|---|---|
| Xinyuan County | XY-1 | 1384.74 | EN40 | 18.0 | 0.75 |
| XY-2 | 1333.37 | WN314 | 8.0 | 0.85 | |
| XY-3 | 1345.86 | - | 0.0 | 0.40 | |
| XY-4 | 1425.34 | WN327 | 23.0 | 0.94 | |
| XY-5 | 1355.24 | EN67 | 5.0 | 0.84 | |
| XY-6 | 1367.32 | ES144 | 14.0 | 0.30 | |
| XY-7 | 1523.13 | S183 | 11.0 | 0.40 | |
| XY-8 | 1274.35 | EN53 | 31.0 | 0.21 | |
| HuoCheng County | HC-1 | 1185.90 | W278 | 3.0 | 0.75 |
| HC-2 | 1130.14 | ES310 | 11.0 | 0.90 |
| Grade | Lesion Coverage (%) | Score |
|---|---|---|
| 0 | 0 | 0 |
| I | 1–10 | 1 |
| II | 11–25 | 3 |
| III | 26–40 | 5 |
| IV | 41–55 | 7 |
| V | >55 | 9 |
| Grade | Disease Severity | Score |
|---|---|---|
| 0 | No lesions on the fruit surface | 0 |
| I | 1–2 lesions per fruit | 1 |
| II | 3–4 lesions per fruit | 3 |
| III | 5–6 lesions per fruit | 5 |
| IV | 7–10 coalescing lesions, covering approximately 1/5 of the fruit surface area | 7 |
| V | >10 coalescing lesions, covering >1/4 of the fruit surface area | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, C.; Shi, W.; Xu, Z.; Li, L.; Ma, R. Study on the Identification and Incidence Pattern of the Pathogen Causing Apple Scab in Wild Apple Forests of Ili, Xinjiang. Agriculture 2025, 15, 2199. https://doi.org/10.3390/agriculture15212199
Li Y, Wang C, Shi W, Xu Z, Li L, Ma R. Study on the Identification and Incidence Pattern of the Pathogen Causing Apple Scab in Wild Apple Forests of Ili, Xinjiang. Agriculture. 2025; 15(21):2199. https://doi.org/10.3390/agriculture15212199
Chicago/Turabian StyleLi, Yaxuan, Caixia Wang, Wanbin Shi, Ziyan Xu, Lan Li, and Rong Ma. 2025. "Study on the Identification and Incidence Pattern of the Pathogen Causing Apple Scab in Wild Apple Forests of Ili, Xinjiang" Agriculture 15, no. 21: 2199. https://doi.org/10.3390/agriculture15212199
APA StyleLi, Y., Wang, C., Shi, W., Xu, Z., Li, L., & Ma, R. (2025). Study on the Identification and Incidence Pattern of the Pathogen Causing Apple Scab in Wild Apple Forests of Ili, Xinjiang. Agriculture, 15(21), 2199. https://doi.org/10.3390/agriculture15212199




