Phenotypic Descriptors and Image-Based Assessment of Viola cornuta L. Quality Under Photoselective Shade Nets Using a Naive Bayes Classifier
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Variables and Number of Reproductive Structures
2.2. Percentage of Plant Ground Cover
2.3. Flower and Leaf Area and Color Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Dilta, B.S.; Baweja, H.S.; Kumari, P.; Thaneshwari, T.; Sahare, H.A. Effect of seed priming and planting date on growth, flowering and seed production of pansy (Viola × wittrockiana Gams.). Ann. Biol. 2018, 34, 176–180. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20183334327 (accessed on 8 October 2025).
- Park, J.W.; Kim, W.S. Effect of UV-B radiation on antioxidation in petals of edible flower pansy. Flower Res. J. 2017, 25, 24–28. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Iwata, H.; Hase, N.; Matsuura, S. Genetic combining ability of petal shape in garden pansy (Viola × wittrockiana Gams) based on image analysis. Euphytica 2006, 151, 311–319. [Google Scholar] [CrossRef]
- Buczacki, S. Plantas de Invierno; Octopus Publishing Group Limited: Madrid, Spain, 1997; pp. 84–85. [Google Scholar]
- Warner, R.M.; Erwin, J.E. Prolonged high-temperature exposure differentially reduces growth and flowering of 12 Viola × wittrockiana Gams. Sci. Hortic. 2006, 108, 295–302. [Google Scholar] [CrossRef]
- Collado, C.E.; Hernández, R. Effects of light intensity, spectral composition, and paclobutrazol on the morphology, physiology, and growth of petunia, geranium, pansy, and dianthus ornamental transplants. J. Plant Growth Regul. 2022, 41, 461–478. [Google Scholar] [CrossRef]
- USDA (United States Department of Agriculture). Floriculture Crops 2019. Summary; USDA: Washington, DC, USA, 2019. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/floran20.pdf (accessed on 8 October 2025).
- Stamps, R.H. Use of colored shade netting in horticulture. HortScience 2009, 44, 239–241. [Google Scholar] [CrossRef]
- Almeida, J.M.; Calaboni, C.; Rodrigues, P.H.V. Pigments in flower stems of lisianthus under different photoselective shade nets. Ornam. Hortic. 2021, 27, 535–543. [Google Scholar] [CrossRef]
- Mills-Ibibofori, T.; Dunn, B.L.; Maness, N.; Payton, M. Effect of LED lighting and gibberellic acid supplementation on potted ornamentals. Horticulturae 2019, 5, 51. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jifon, J.; Soundy, P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest storage. Food Rev. Int. 2018, 34, 290–307. [Google Scholar] [CrossRef]
- Ayala-Tafoya, F.; Yáñez-Juárez, M.G.; López-Orona, C.A.; Medina-López, R.; Velázquez-Alcaraz, T.J.; Díaz-Valdés, T. Sunlight transmitted by colored shade nets on photosynthesis and yield of cucumber. Ciência Rural 2018, 48, e20170829. [Google Scholar] [CrossRef]
- Hou, W.; Luo, Y.; Wang, X.; Chen, Q.; Sun, B.; Wang, Y.; Liu, Z.; Tang, H.; Zhang, Y. Effects of shading on plant growth, flower quality and photosynthetic capacity of Rosa hybrida. AIP Conf. Proc. 2018, 1956, 020005. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kasemsap, P.; Vercambre, G.; Gay, F.; Phattaralerphong, J.; Gautier, H. Impact of red and blue nets on physiological and morphological traits, fruit yield and quality of tomato (Solanum lycopersicum Mill.). Sci. Hortic. 2020, 264, 109185. [Google Scholar] [CrossRef]
- Zhao, D.; Hao, Z.; Tao, J. Effects of shade on plant growth and flower quality in the herbaceous peony (Paeonia lactiflora Pall.). Plant Physiol. Biochem. 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Zlatev, Z.; Stoykova, V.; Shivacheva, G.; Vasilev, M. Design and implementation of a measuring device to determine the content of pigments in plant leaves. Appl. Syst. Innov. 2023, 6, 64. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation mechanism of plant pigments biosynthesis: Anthocyanins, carotenoids, and betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef]
- Peretz, O.; Koren, M.; Koren, O. Naive Bayes classifier—An ensemble procedure for recall and precision enrichment. Eng. Appl. Artif. Intell. 2024, 136, 108972. [Google Scholar] [CrossRef]
- PanAmerican Seed. Guía de Información de Productos 2005; PanAmerican Seed Company: West Chicago, IL, USA, 2005; 96p. [Google Scholar]
- Steiner, A.A. The universal nutrient solution. In Proceedings of the 6th International Congress on Soilless Culture, Lunteren, The Netherlands, 29 April–5 May 1984; International Society for Soilless Culture: Wageningen, The Netherlands, 1984; pp. 633–649. [Google Scholar]
- Krishnamoorthy, N.; Srikrishnah, S.; Sutharsan, S. Influence of different shade levels on the growth and quality of Codiaeum variegatum var. ‘Bush on fire’ in the Batticaloa District. Int. J. Res. Publ. 2020, 6, 1–22. Available online: https://www.ijrp.org/paper-detail/217 (accessed on 8 October 2025).
- Torres, A.P.; Lopez, R.G. Measuring Daily Light Integral (DLI). Purdue Extension Publication HO-238-W. 2009. Available online: https://www.extension.purdue.edu/extmedia/HO/HO-238-B-W.pdf (accessed on 8 October 2025).
- Oliveira, J.E.D.; Sabino, J.H.F.; Sillmann, T.A.; Mattiuz, C.F.M. Cultivation under photoselective shade nets alters the morphology and physiology of Begonia Megawatt varieties. Ciência Agrotecnologia 2024, 48, e015924. [Google Scholar] [CrossRef]
- Xia, J.; Mattson, N. Daily light integral and far-red radiation influence morphology and quality of liners and subsequent flowering and development of petunia in controlled greenhouses. Horticulturae 2024, 10, 1106. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Jeon, J.S.; An, G. Influence of climate change on flowering time. J. Plant Biol. 2021, 64, 193–203. [Google Scholar] [CrossRef]
- Austerman, P.; Dunn, B.L.; Singh, H.; Fontanier, C.; Stanphill, S. Height control of greenhouse-grown pansy using colored shade nets. HortTechnology 2023, 33, 36–43. [Google Scholar] [CrossRef]
- Stagnari, F.; Mattia, C.; Galieni, A.; Santarelli, V.; D’Egidio, S.; Pagnani, G.; Pisante, M. Light quantity and quality supplies sharply affect growth, morphological, physiological and quality traits of basil. Ind. Crops Prod. 2018, 122, 277–289. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Díaz-Pérez, J.C.; Nambeesan, S.U. Effect of shade levels on plant growth, physiology, and fruit yield in bell pepper (Capsicum annuum L.). Acta Hortic. 2020, 1268, 311–318. [Google Scholar] [CrossRef]
- Khalid, M.H.B.; Raza, M.A.; Yu, H.Q.; Sun, F.A.; Zhang, Y.Y.; Lu, F.Z.; Si, L.; Iqbal, N.; Khan, I.; Fu, F.L.; et al. Effect of shade treatments on morphology, photosynthetic and chlorophyll fluorescence characteristics of soybeans (Glycine max L. Merr.). Appl. Ecol. Environ. Res. 2019, 17, 2551–2569. [Google Scholar] [CrossRef]
- Mayoli, R.N.; Isutsa, D.K.; Tunya, G.O. Growth of ranunculus cutflower under tropical high altitude conditions. 1: Effects of GA3 and shade. Afr. J. Hortic. Sci. 2009, 2, 13–28. Available online: https://www.academia.edu/48584182/Growth_of_ranunculus_cutflower_under_tropical_high_altitude_conditions_1_Effects_of_GA3_and_shade (accessed on 8 October 2025).
- Jung, J.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.; Box, M.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors in Arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Stamps, R.H.; Giglia, F.F. Environmental modification inside photoselective shadehouses. HortScience 2013, 48, 975–979. [Google Scholar] [CrossRef]
- Roh, Y.S.; Yoo, Y.K. Light quality of light emitting diodes affects growth, chlorophyll fluorescence and phytohormones of Tulip ‘Lasergame’. Hortic. Environ. Biotechnol. 2023, 64, 245–255. [Google Scholar] [CrossRef]
- Zare, S.K.A.; Sedaghathoor, S.; Dahkaei, M.P.; Hashemabadi, D. The effect of light variations by photoselective shade nets on pigments, antioxidant capacity, and growth of two ornamental plant species: Marigold (Calendula officinalis L.) and violet (Viola tricolor). Cogent Food Agric. 2019, 5, 1650415. [Google Scholar] [CrossRef]
- Singh, H.; Dunn, B.L.; Fontanier, C.; Singh, H. Colored shade nets affect growth but not flowering of four greenhouse-grown potted ornamental species. HortScience 2023, 58, 1075–1076. [Google Scholar] [CrossRef]
- Naveena, N.; Thamaraiselvi, S.P.; Rajadurai, K.R.; Sivakumar, R. Effect of coloured shade nets on physiology and quality of cut foliage plants. J. Pharmacogn. Phytochem. 2019, 8, 1141–1144. Available online: https://www.phytojournal.com/archives/2019/vol8issue4/PartS/8-3-641-857.pdf (accessed on 8 October 2025).
- Antoniazzi, L. El cultivo de la viola. Rev. Hortic. 2007, 199, 44–47. Available online: http://www.horticom.com/revistasonline/horticultura/rh199/44_47.pdf (accessed on 8 October 2025).
- Pearson, S.; Parker, A.; Adams, S.R.; Hadley, P.; May, D.R. The effects of temperature on the flower size of pansy (Viola × wittrockiana Gams.). J. Hortic. Sci. 1995, 70, 183–190. [Google Scholar] [CrossRef]
- Hase, N.; Matsuura, S.; Yamaguchi, M. HPLC evaluation of anthocyanins and flavonols in relation to the flower color of pansy (Viola × wittrockiana Gams). Hortic. Res. 2005, 4, 125–129. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Baranska, M.; Schulz, H.; Heiselmayer, P.; Musso, M. Discrimination of carotenoid and flavonoid content in petals of pansy cultivars (Viola × wittrockiana) by FT-Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 1240–1247. [Google Scholar] [CrossRef]
- Skowyra, M.; Calvo, M.; Gallego, M.; Azman, N.; Almajano, M. Characterization of phytochemicals in petals of different colours from Viola × wittrockiana Gams. and their correlation with antioxidant activity. J. Agric. Sci. 2014, 6, 93–105. [Google Scholar] [CrossRef]
- Farzad, M.; Griesbach, R.; Weiss, M.R. Floral color change in Viola cornuta L. (Violaceae): A model system to study regulation of anthocyanin production. Plant Sci. 2002, 162, 225–231. [Google Scholar] [CrossRef]
- Bastías, R.M.; Corelli-Grappadelli, L. Light quality management in fruit orchards: Physiological and technological aspects. Chil. J. Agric. Res. 2012, 72, 574–581. [Google Scholar] [CrossRef]
- Jenshi-Roobha, J.; Saravanakumar, M.; Aravindhan, K.M.; Suganya-Devi, P. The effect of light, temperature, and pH on stability of anthocyanin pigments in Musa acuminata bract. Res. Plant Biol. 2011, 1, 5–12. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20123239922 (accessed on 8 October 2025).
- Ojha, A.; Kumar, V. Image classification of ornamental plants leaf using machine learning algorithms. In Proceedings of the 2022 4th International Conference on Inventive Research in Computing Applications (ICIRCA), Coimbatore, India, 21–23 September 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 834–840. Available online: https://ieeexplore.ieee.org/abstract/document/9985604 (accessed on 8 October 2025).
- Parsons, N.R.; Edmondson, R.N.; Song, Y. Image analysis and statistical modelling for measurement and quality assessment of ornamental horticulture crops in glasshouses. Biosyst. Eng. 2009, 104, 161–168. [Google Scholar] [CrossRef]
- do Nascimento, A.M.M.; Ruiz-Gonzalez, R.; Martínez-Martínez, V.; Medeiros, A.M.; Santos, F.S.; Rêgo, E.R.; Pimenta, S.; Sudré, C.P.; Bento, C.d.S.; Cambra, C.; et al. Ornamental potential classification and prediction for pepper plants (Capsicum spp.): A comparison using morphological measurements and RGB images as data source. Appl. Sci. 2025, 15, 7801. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yu, J.R.; Deng, H.N. Non-destructive prediction of anthocyanin content of Rosa chinensis petals using digital images and machine learning algorithms. Horticulturae 2024, 10, 503. [Google Scholar] [CrossRef]

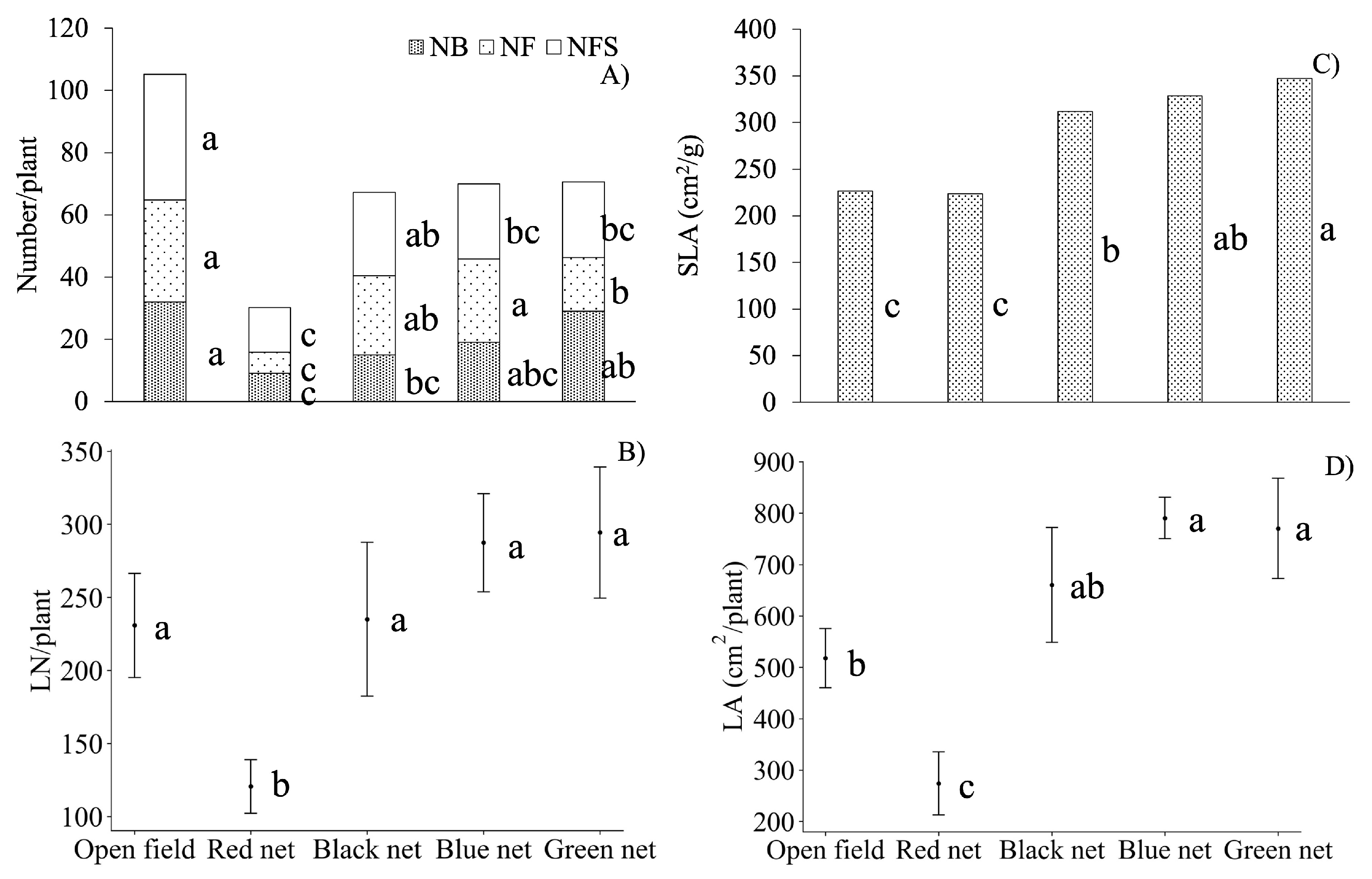
 ), and leaf cover (LC,
), and leaf cover (LC,  ). n = 5. Values with different letters within each cover type indicate significant differences according to Tukey mean test (α = 0.05). DAT = days after transplanting.
). n = 5. Values with different letters within each cover type indicate significant differences according to Tukey mean test (α = 0.05). DAT = days after transplanting.
 ), and leaf cover (LC,
), and leaf cover (LC,  ). n = 5. Values with different letters within each cover type indicate significant differences according to Tukey mean test (α = 0.05). DAT = days after transplanting.
). n = 5. Values with different letters within each cover type indicate significant differences according to Tukey mean test (α = 0.05). DAT = days after transplanting.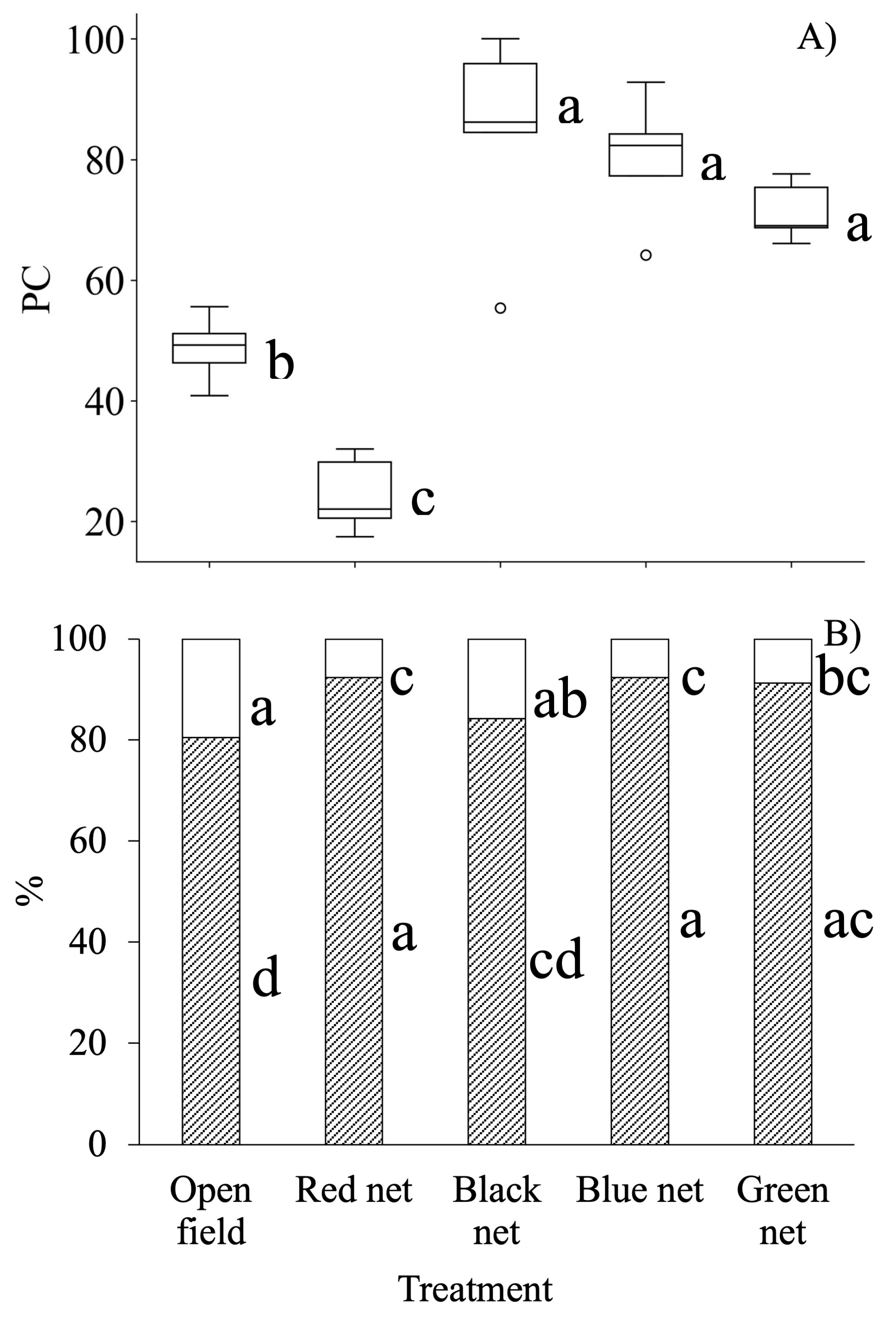
 ), cream (
), cream ( ), and purple (
), and purple ( ) areas at 69 days after transplanting (DAT). Different lowercase letters within each surface type (color class) and different uppercase letters for the total flower surface indicate differences among medians (n = 25) according to the Conover median test with Holm adjustment (α = 0.05).
) areas at 69 days after transplanting (DAT). Different lowercase letters within each surface type (color class) and different uppercase letters for the total flower surface indicate differences among medians (n = 25) according to the Conover median test with Holm adjustment (α = 0.05).
 ), cream (
), cream ( ), and purple (
), and purple ( ) areas at 69 days after transplanting (DAT). Different lowercase letters within each surface type (color class) and different uppercase letters for the total flower surface indicate differences among medians (n = 25) according to the Conover median test with Holm adjustment (α = 0.05).
) areas at 69 days after transplanting (DAT). Different lowercase letters within each surface type (color class) and different uppercase letters for the total flower surface indicate differences among medians (n = 25) according to the Conover median test with Holm adjustment (α = 0.05).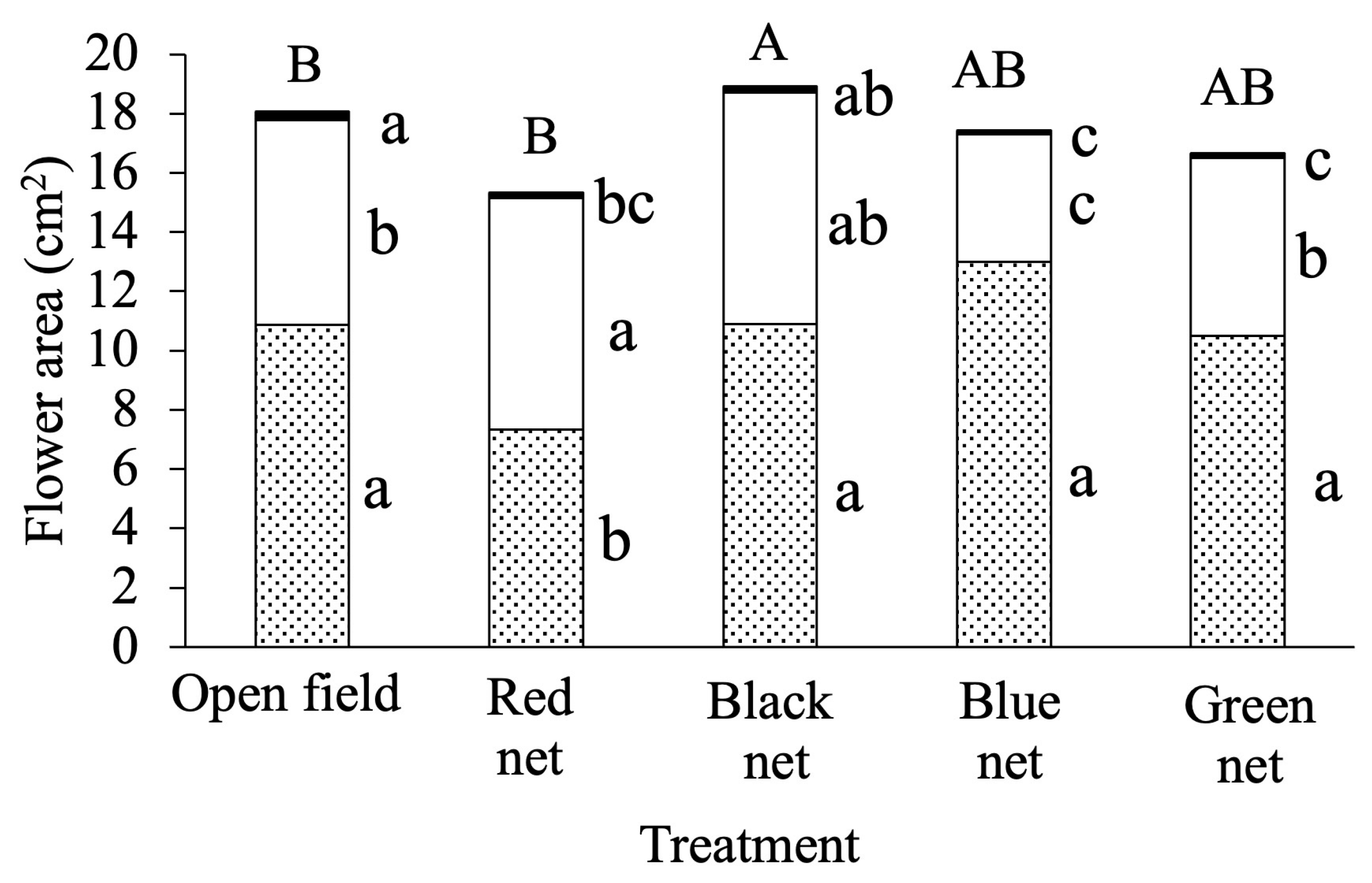
| Treatment | Environment | Air Temperature (°C) | Relative Humidity (%) | Photosynthetic Irradiance (MJ m−2 d−1) |
|---|---|---|---|---|
| T1 | Open field | 19.6 | 42 | 9.69 |
| T2 | Red net | 19.5 | 56 | 5.81 |
| T3 | Black net | 18.6 | 52 | 4.84 |
| T4 | Blue net | 19.3 | 53 | 5.81 |
| T5 | Green net | 18.8 | 58 | 5.81 |
| Treatment | Environment | NS | PH * |
|---|---|---|---|
| T1 | Open field | 22.1 ab | 12.1 |
| T2 | Red net | 13.0 c | 12.1 |
| T3 | Black net | 16.8 bc | 13.6 |
| T4 | Blue net | 26.0 a | 13.3 |
| T5 | Green net | 19.4 b | 14.1 |
| Class | Precision | Recall | F1 | Specificity | FPR | FNR |
|---|---|---|---|---|---|---|
| Purple | 0.982 ± 0.036 | 0.988 ± 0.009 | 0.985 ± 0.019 | 0.990 ± 0.020 | 0.009 ± 0.020 | 0.012 ± 0.009 |
| Cream | 0.988 ± 0.009 | 1.000 ± 0.000 | 0.994 ± 0.004 | 0.994 ± 0.004 | 0.006 ± 0.004 | 0.000 ± 0.000 |
| Yellow | 1.000 ± 0.000 | 0.981 ± 0.040 | 0.990 ± 0.021 | 1.000 ± 0.000 | 0.000 ± 0.000 | 0.018 ± 0.040 |
| Global precision | 0.989 ± 0.014 | |||||
| Treatment | Environment | Area (cm2) | CIE-L | CIE-a | CIE-b |
|---|---|---|---|---|---|
| T1 | Open field | 7.8 b | 49.0 a | −11 a b | 25.4 b |
| T2 | Red net | 5.7 b | 49.4 a | −12 b | 30.5 a |
| T3 | Black net | 11.8 a | 45.4 b | −10 a | 23.5 b |
| T4 | Blue net | 10.8 a | 45.0 b | −11 a b | 24.9 b |
| T5 | Green net | 10.9 a | 45.8 b | −10 a | 24.9 b |
| Environment | Treatment | Class | CIE-L | CIE-a | CIE-b |
|---|---|---|---|---|---|
| Open field | T1 | Purple | 48.3 bc | 27 a | −20 b |
| Red net | T2 | 58.9 a | 21 b | −16 a | |
| Black net | T3 | 49.0 b | 24 a | −20 b | |
| Blue net | T4 | 38.9 c | 29 a | −20 b | |
| Green net | T5 | 48.4 bc | 25 a | −20 b | |
| Open field | T1 | Cream | 83.1 bc | −3 a | 14 a |
| Red net | T2 | 85.4 a | −3 a | 12 a | |
| Black net | T3 | 83.9 ab | −4 a | 12 a | |
| Blue net | T4 | 80.7 d | −2 a | 9 a | |
| Green net | T5 | 82.7 cd | −3 a | 12 a | |
| Open field | T1 | Yellow | 59.8 a | 0.91 a | 59.0 a |
| Red net | T2 | 58.7 a | 0.71 a | 57.9 a | |
| Black net | T3 | 57.9 a | 0.61 a | 57.9 a | |
| Blue net | T4 | 55.8 ab | 0.39 ab | 55.5 ab | |
| Green net | T5 | 54.2 b | −0.58 b | 53.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagg-Torrijos, F.A.; Carrillo-Salazar, J.A.; González-Camacho, J.M.; González-Hernández, V.A. Phenotypic Descriptors and Image-Based Assessment of Viola cornuta L. Quality Under Photoselective Shade Nets Using a Naive Bayes Classifier. Agriculture 2025, 15, 2187. https://doi.org/10.3390/agriculture15212187
Hagg-Torrijos FA, Carrillo-Salazar JA, González-Camacho JM, González-Hernández VA. Phenotypic Descriptors and Image-Based Assessment of Viola cornuta L. Quality Under Photoselective Shade Nets Using a Naive Bayes Classifier. Agriculture. 2025; 15(21):2187. https://doi.org/10.3390/agriculture15212187
Chicago/Turabian StyleHagg-Torrijos, Fátima Alejandrina, José Alfredo Carrillo-Salazar, Juan Manuel González-Camacho, and Víctor Arturo González-Hernández. 2025. "Phenotypic Descriptors and Image-Based Assessment of Viola cornuta L. Quality Under Photoselective Shade Nets Using a Naive Bayes Classifier" Agriculture 15, no. 21: 2187. https://doi.org/10.3390/agriculture15212187
APA StyleHagg-Torrijos, F. A., Carrillo-Salazar, J. A., González-Camacho, J. M., & González-Hernández, V. A. (2025). Phenotypic Descriptors and Image-Based Assessment of Viola cornuta L. Quality Under Photoselective Shade Nets Using a Naive Bayes Classifier. Agriculture, 15(21), 2187. https://doi.org/10.3390/agriculture15212187






