Synergistic Effects of Phosphorus and EDDS on Enhancing Phytoremediation Efficiency of Ricinus communis L. in Cu and Cd Co-Contaminated Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Materials
2.2. Pot Experimental Design
2.3. Soil Solution Analysis
2.4. Cu, Cd, N, and P in Castor
2.5. Chlorophyll, Lipid Peroxidation, and Antioxidative Enzymes in Castor
2.6. Statistical Analysis
3. Results
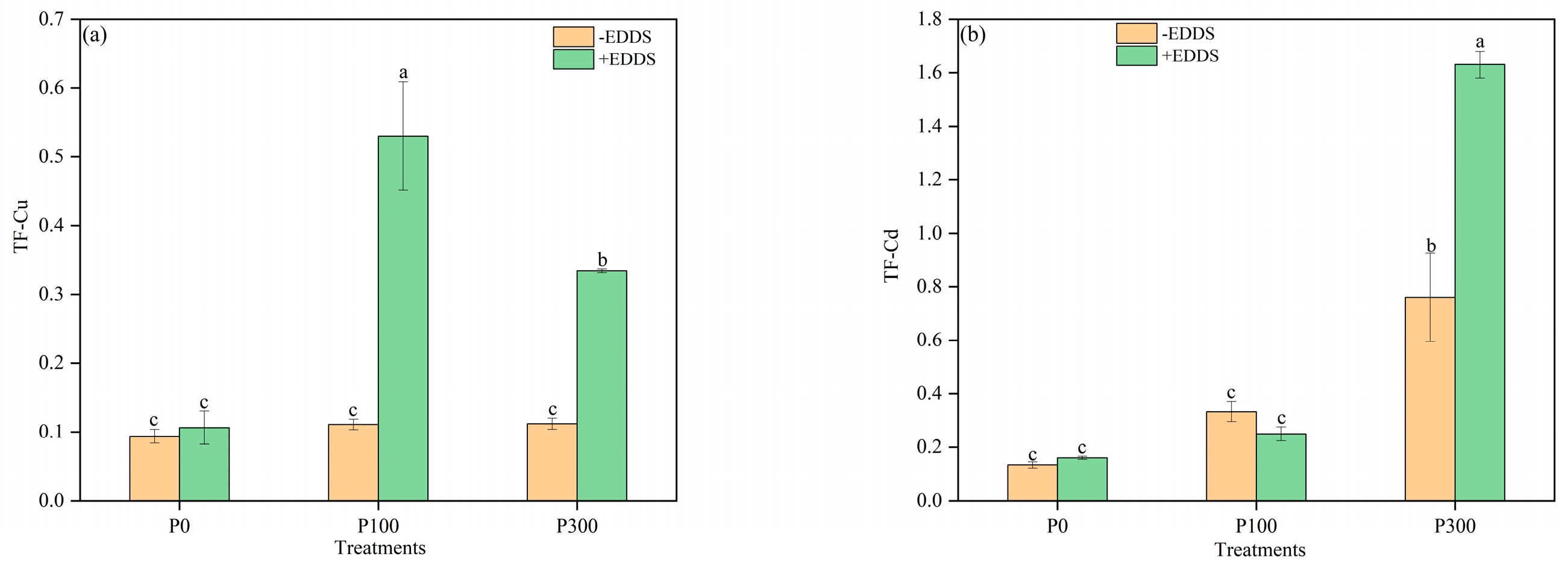
3.1. Uptake and Translocation of Cu and Cd by Castor
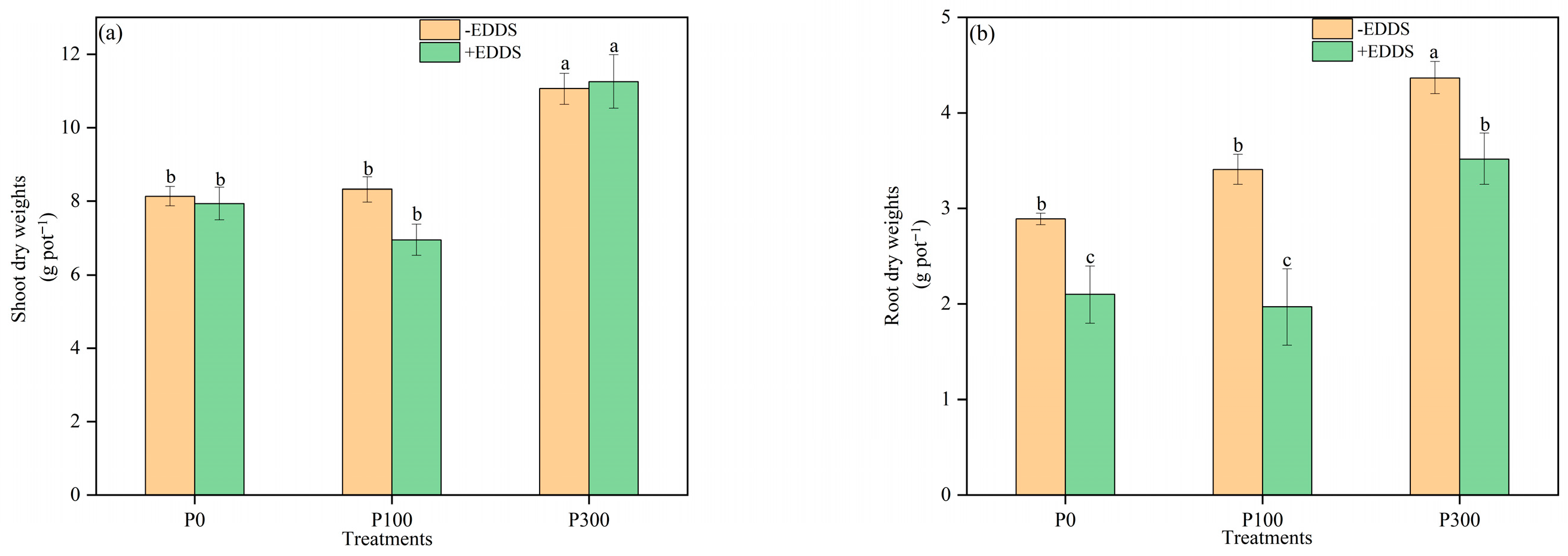
3.2. The Growth of Castor Plants Under P and EDDS Treatments
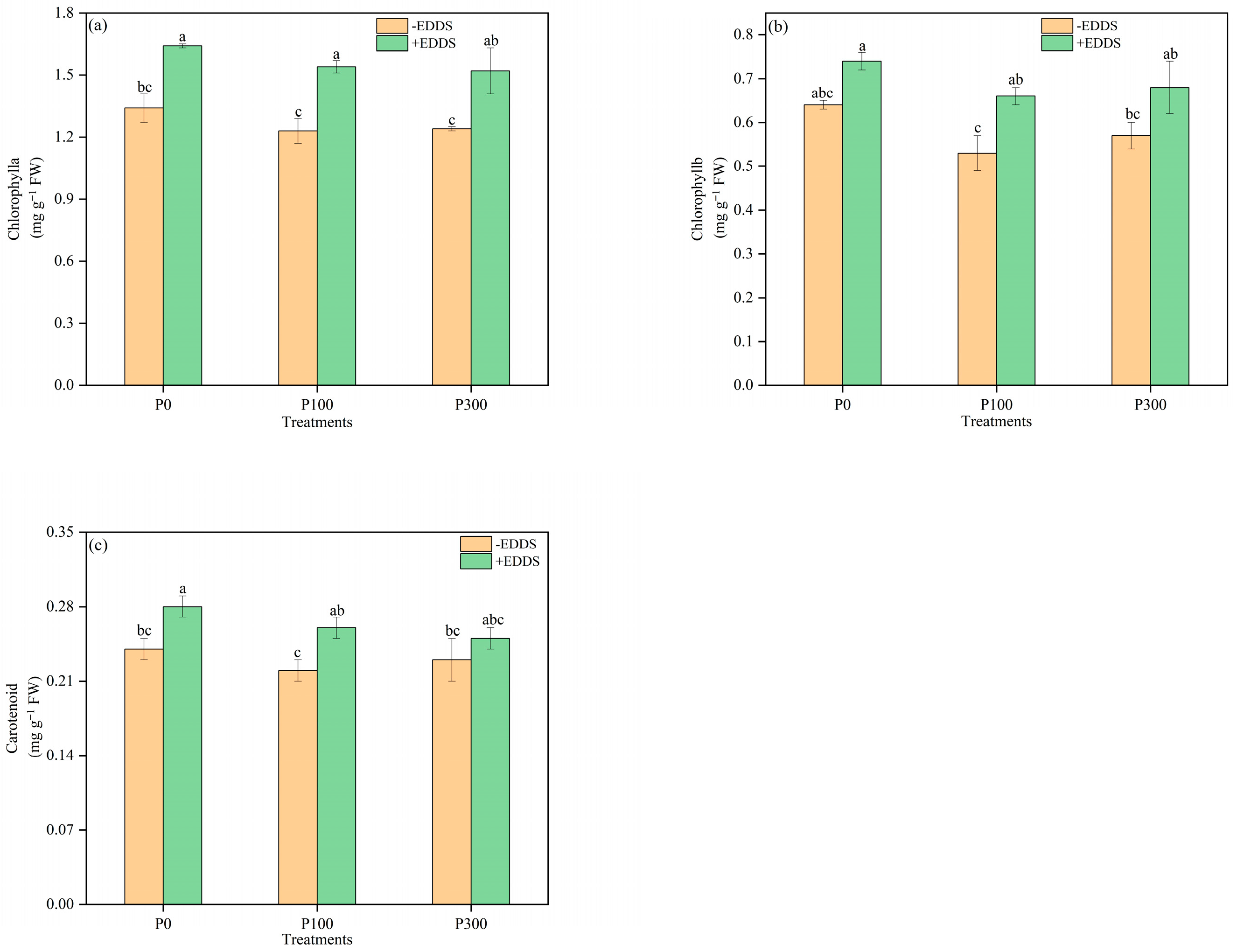
3.3. Chlorophyll and Carotenoid Content in Leaves of Castor Plants Under Various Treatments
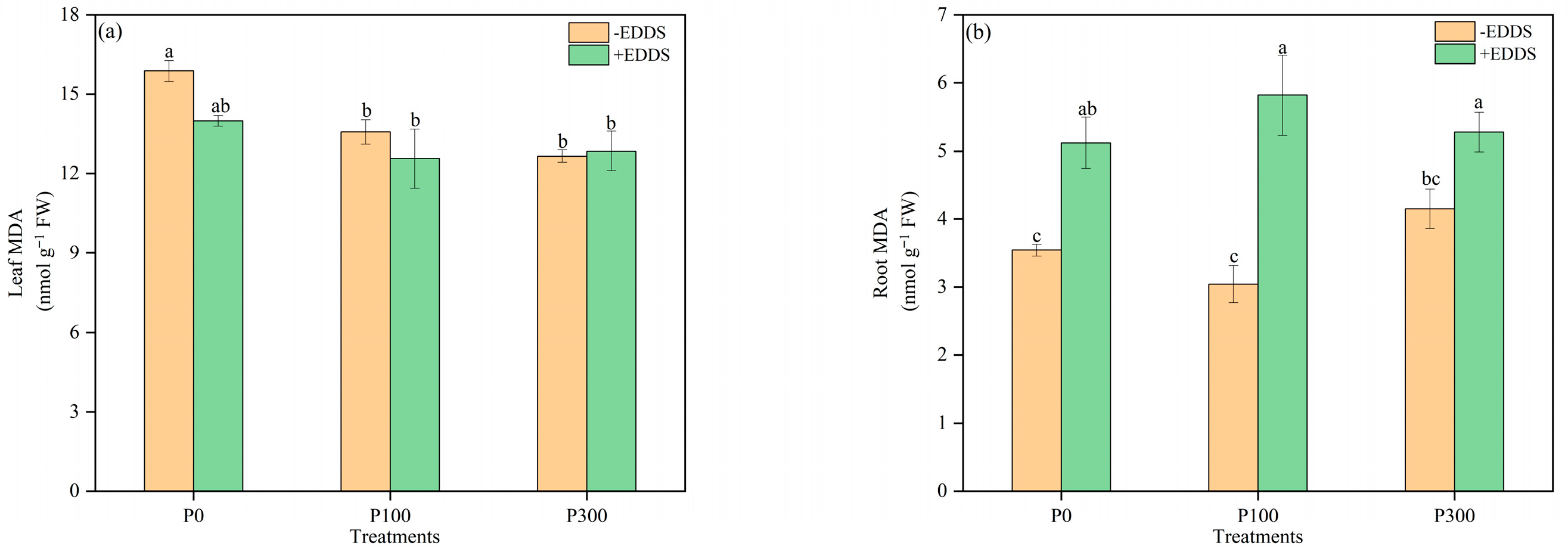
3.4. The Effect of MDA Content of Castor Plants Treated with P and EDDS
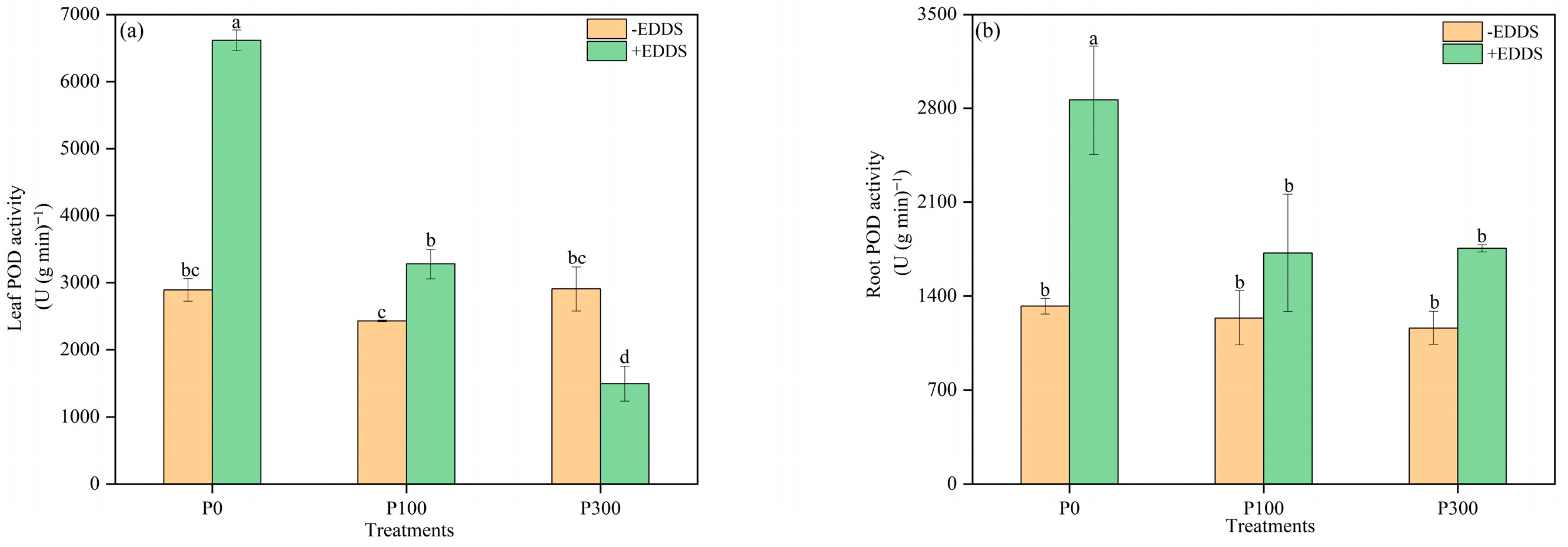
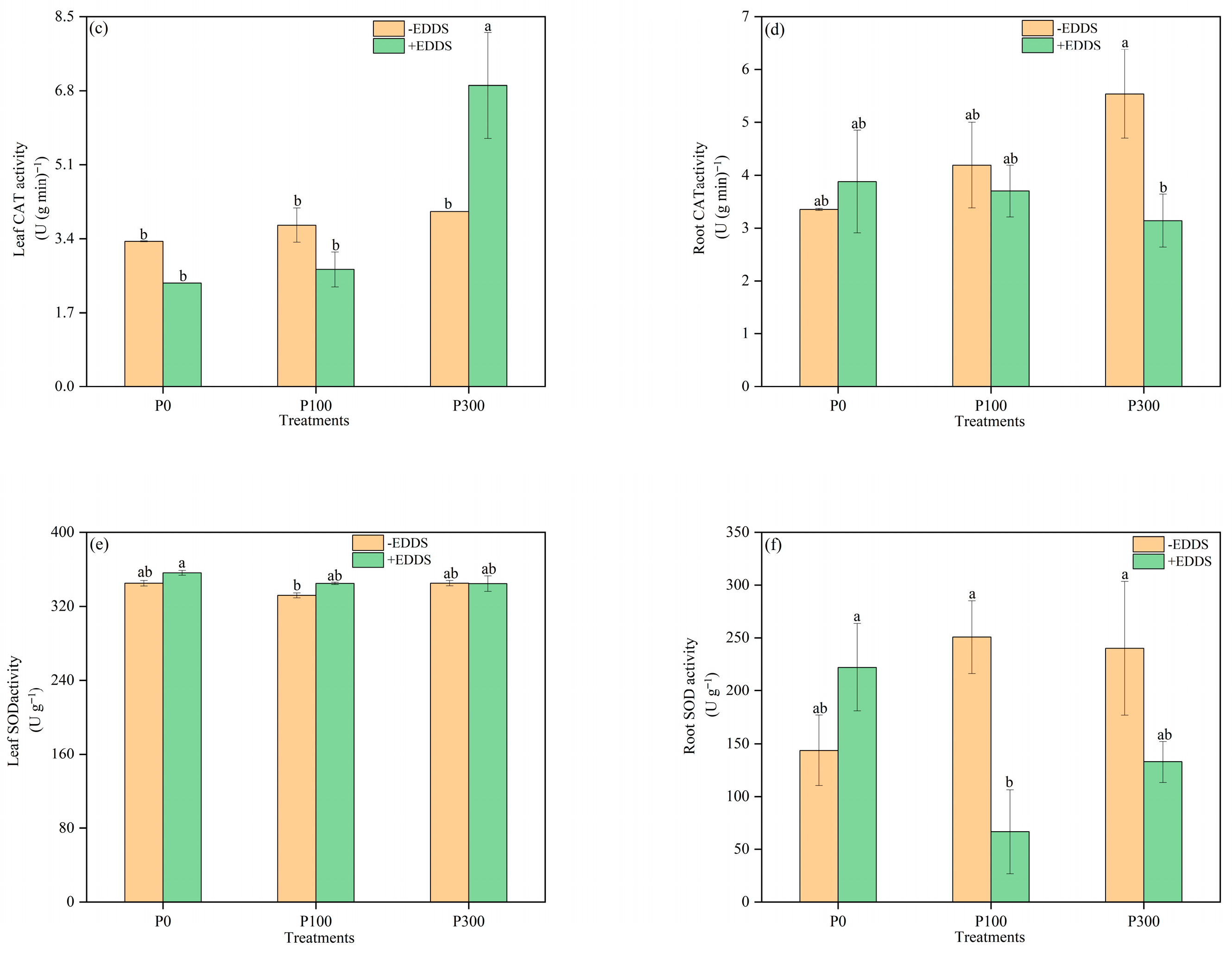
3.5. Antioxidant Enzyme Activities of Leaves and Roots of Castor Plants Under Various Treatments
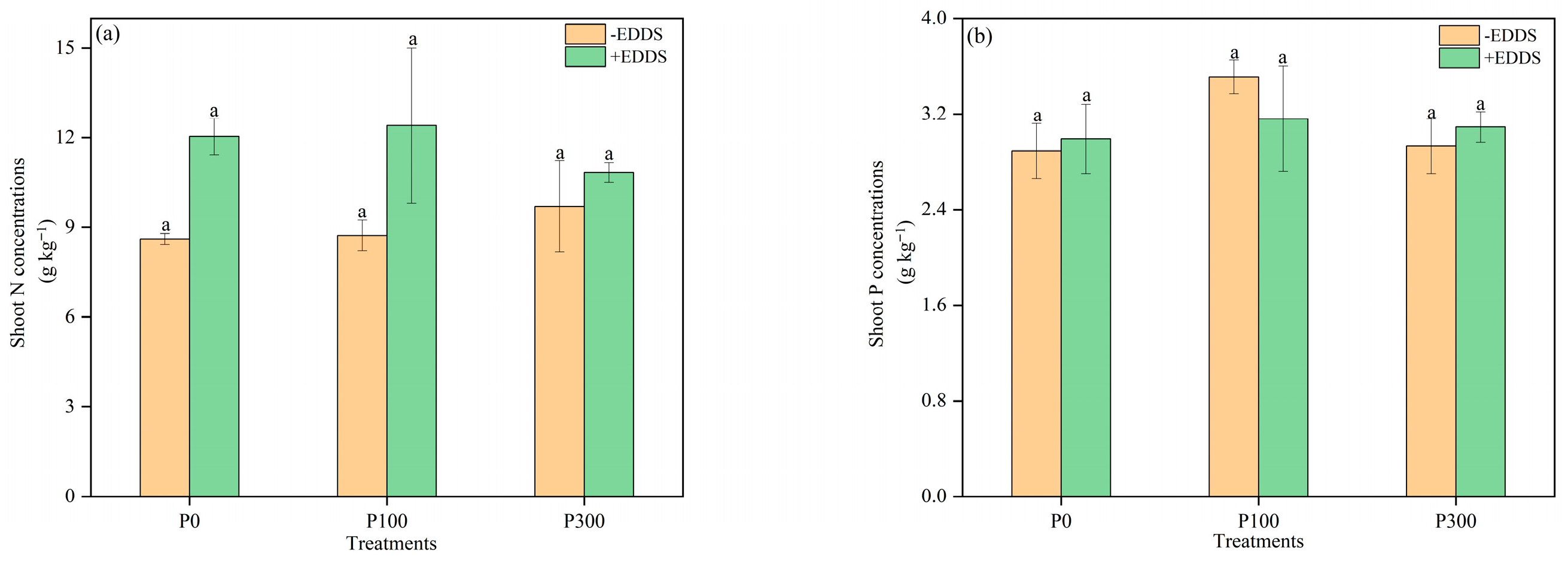
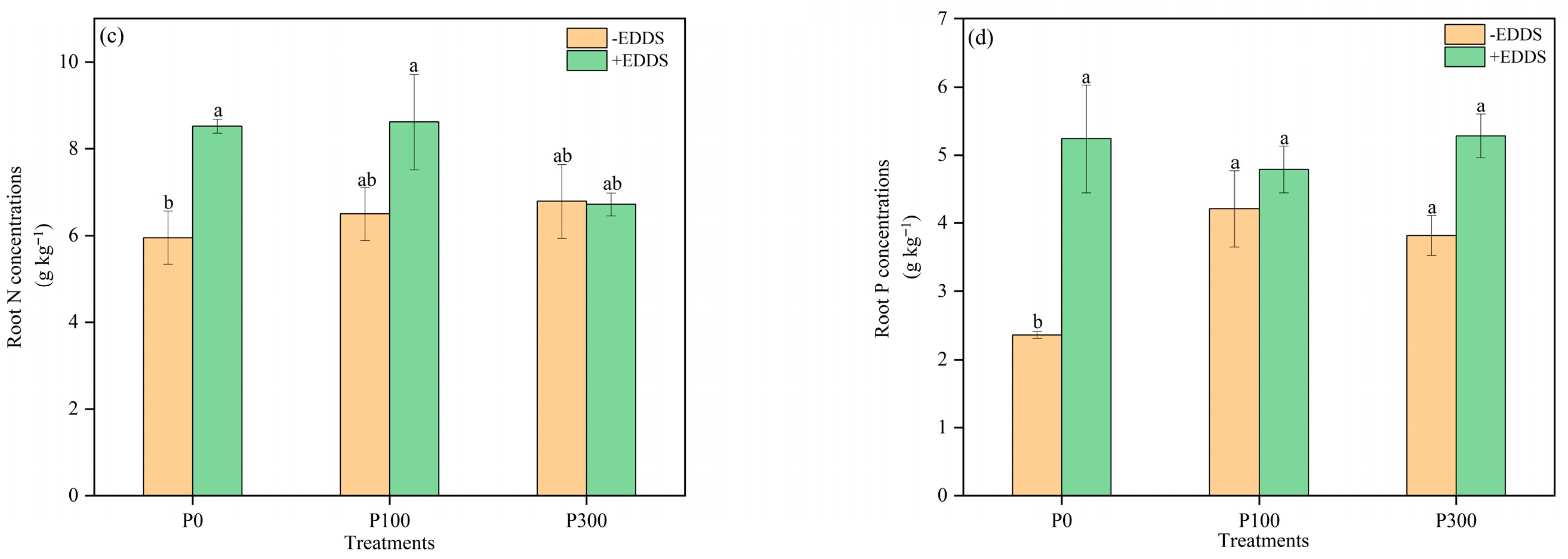
3.6. N and P Concentrations in Leaves and Roots of Castor Plants
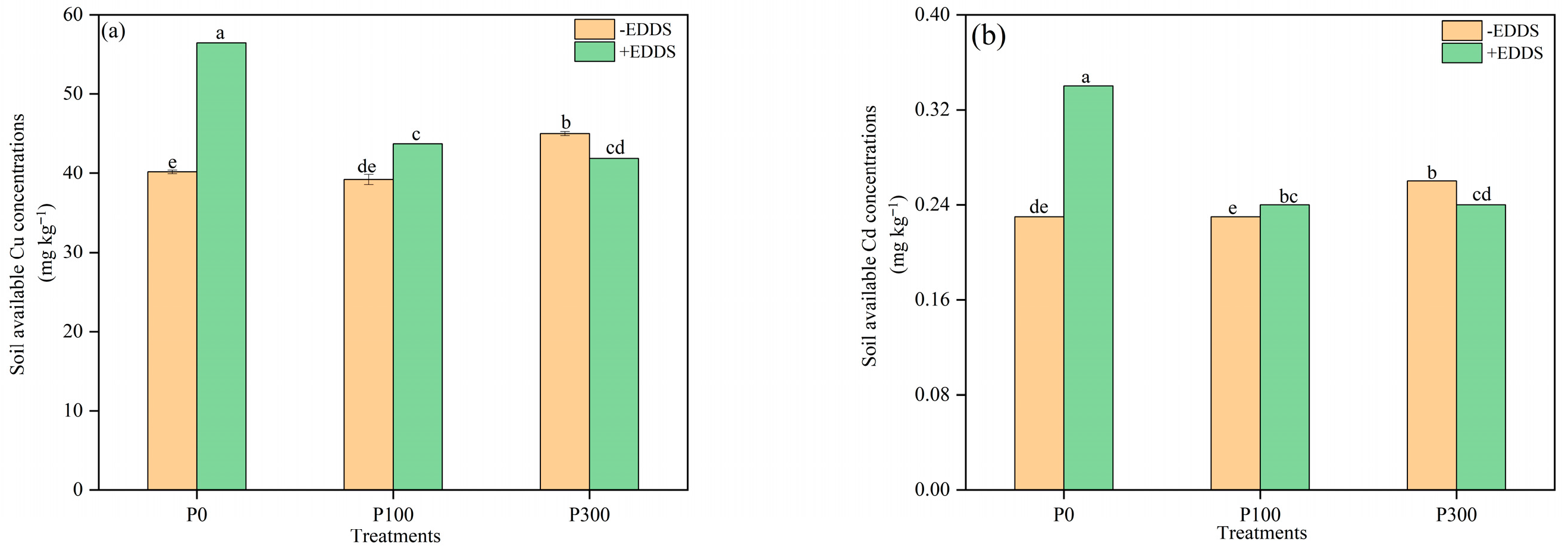
3.7. The Changes in Soil Properties
4. Discussion
4.1. Castor Growth in Cd/Cu Co-Contaminated Soil Under P and EDDS Application
4.2. Plant Uptake of Cu and Cd Under Different Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, S.; Song, C.; Ye, S.; Cheng, C.; Gao, P. The spatiotemporal variation in heavy metals in China’s farmland soil over the past 20 years: A meta-analysis. Sci. Total Environ. 2022, 806, 150322. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Ali, M.; Xiao, P.; Zhao, P.; Wang, H.; Bibo, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Awan, S.; Khan, I.; Rizwan, M.; Irshad, M.; Wang, X.; Zhang, X.; Huang, L. Reduction in the cadmium (Cd) accumulation and toxicity in pearl millet (Pennisetum glaucum L.) by regulating physio-biochemical and antioxidant defense system via soil and foliar application of melatonin. Environ. Pollut. 2023, 328, 121658. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Qin, Y.; Deng, X.; Zhang, Q.; Zou, D.; Zeng, Q. Cichorium intybus L. is a potential Cd-accumulator for phytoremediation of agricultural soil with strong tolerance and detoxification to Cd. J. Hazard. Mater. 2023, 451, 131182. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Qin, X.; Liang, X.; Huang, Q.; Peng, Y. Effects of EDDS on the Cd uptake and growth of Tagetes patula L. and Phytolacca americana L. in Cd-contaminated alkaline soil in northern China. Environ. Sci. Pollut. Res. 2020, 27, 25248–25260. [Google Scholar] [CrossRef]
- Yu, L.; Chen, S.; Wang, J.; Qin, L.; Sun, X.; Zhang, X.; Wang, M. Environmental risk thresholds and prediction models of Cd in Chinese agricultural soils. Sci. Total Environ. 2024, 906, 167773. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Najeeb, U.; Li, X.; Pan, J.; Huang, Q.; Zhou, W.; Liang, Z. Synergistic effects of EDDS and ALA on phytoextraction of cadmium as revealed by biochemical and ultrastructural changes in sunflower (Helianthus annuus L.) tissues. J. Hazard. Mater. 2020, 407, 124764. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Liu, Y.; Huang, G.; Yao, S.; Hu, H. Coupling phytoremediation efficiency and detoxification to assess the role of P in the Cu tolerant Ricinus communis L. Chemosphere 2020, 247, 125965. [Google Scholar] [CrossRef]
- Sheng, P.; Ting, Y.; Chen, J. Biosorption of heavy metal ions (Pb, Cu and Cd) from aqueous solutions by the marine alga Sargassum sp. in single-and multiple-metal systems. Ind. Eng. Chem. Res. 2007, 46, 2438–2444. [Google Scholar] [CrossRef]
- Cerqueira, B.; Covelo, E.; Andrade, L.; Vega, F. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma 2011, 162, 20–26. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Liu, J.; Wu, H.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.; Luo, Y.; Chen, K. Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 2022, 809, 151099. [Google Scholar] [CrossRef]
- Zulkernain, N.; Uvarajan, T.; Ng, C. Roles and significance of chelating agents for potentially toxic elements (PTEs) phytoremediation in soil: A review. J. Environ. Manag. 2023, 341, 117926. [Google Scholar] [CrossRef]
- Han, M.; Ullah, H.; Huan, Y.; Yu, G.; You, S.; Liu, J.; Chen, B.; Shahab, A.; Antoniadis, V.; Shaheen, S.; et al. Cadmium uptake and membrane transport in roots of hyperaccumulator Amaranthus hypochondriacus L. Environ. Pollut. 2023, 331, 121846. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, Y.; Sheng, M.; Peng, H.; Gu, Y.; Xu, H.; Zhao, Y. 24-Epibrassinolide combined with heavy metal resistant bacteria enhancing phytoextraction of Amaranthus hypochondriacus L. in Cd-contaminated soil. J. Hazard. Mater. 2020, 399, 123031. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Liu, X.; Huang, X.; Wang, J.; Zhang, S.; Ji, J. Effect of crop straw biochars on the remediation of Cd-contaminated farmland soil by hyperaccumulator Bidens pilosa L. Ecotoxicol. Environ. Saf. 2021, 219, 112332. [Google Scholar] [CrossRef]
- Chen, L.; Beiyuan, J.; Hu, W.; Zhang, Z.; Duan, C.; Cui, Q.; Zhu, X.; He, H.; Huang, X.; Fang, L. Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): A comprehensive review. Chemosphere 2022, 293, 133577. [Google Scholar] [CrossRef]
- Al-Huqail, A. Stimulating the efficiency of Cd-phytoremediation from contaminated soils by Solanum nigrum L.: Effect of foliar and soil application of yeast extract. S. Afr. J. Bot. 2023, 161, 512–518. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Guo, J.; Zhou, L. Effects of Cd, Cu and Zn on Ricinus communis L. growth in single element or co-contaminated soils: Pot experiments. Ecol. Eng. 2016, 90, 347–352. [Google Scholar] [CrossRef]
- Song, J.; Zhao, F.; Luo, Y.; McGrath, S.; Zhang, H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ. Pollut. 2004, 128, 307–315. [Google Scholar] [CrossRef]
- Yao, S.; Zhou, B. Enhancing phytoremediation of cadmium and arsenic in alkaline soil by Miscanthus sinensis: A study on the synergistic effect of endophytic fungi and biochar. Sci. Total Environ. 2024, 923, 171458. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lei, M.; Yang, J.; Yang, J.; Wan, M.; Chen, T.; Zhou, X.; Gu, S.; Guo, G. Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol. Eng. 2017, 106, 409–414. [Google Scholar] [CrossRef]
- Huang, G.; Rizwan, M.; Ren, C.; Guo, G.; Fu, Q.; Zhu, J.; Hu, H. Influence of phosphorous fertilization on copper phytoextraction and antioxidant defenses in castor bean (Ricinus communis L.). Environ. Sci. Pollut. Res. 2018, 25, 115–123. [Google Scholar] [CrossRef]
- Liu, W.; Gao, J.; Wan, X.; Li, Q.; Fu, Q.; Zhu, J.; Hu, H. Effect of phosphorus fertilizer on phytoextraction using Ricinus communis L. in Cu and Cd co-contaminated soil. Int. J. Phytorem. 2023, 25, 822–831. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Wang, D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L.) enhanced with biodegradable chelating agents. J. Clean. Prod. 2020, 263, 121491. [Google Scholar] [CrossRef]
- Epelde, L.; Hernández-Allica, J.; Becerri, J.M.; Blanco, F.; Garbisu, C. Effects of chelates on plants and soil microbial community: Comparison of EDTA and EDDS for lead phytoextraction. Sci. Total Environ. 2008, 401, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Ahmad, I. Involvement of phosphate supplies in different transcriptional regulation pathway of Oryza sativa L.’s antioxidative system in response to arsenite and cadmium stress. Ecotoxicology 2015, 24, 1259–1268. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Song, Z.; Wang, D.; Qiu, W. Chelator complexes enhanced Amaranthus hypochondriacus L. phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2019, 237, 124480–124488. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Qin, X.; Zhao, L.; Liang, X. Effects of s,s-ethylenediamine disuccinic acid on the phytoextraction efficiency of Solanum nigrum L. and soil quality in cd-contaminated alkaline wheat soil. Environ. Sci. Pollut. Res. 2021, 28, 42959–42974. [Google Scholar] [CrossRef]
- Bao, S. Agro-Chemical Analysis for Soil; Chinese Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron mitigates cadmium toxicity to rapeseed (Brassica napus) shoots by relieving oxidative stress and enhancing cadmium chelation onto cell walls. Environ. Pollut. 2020, 263, 114546. [Google Scholar] [CrossRef] [PubMed]
- Mesnoua, M.; Mateos-Naranjo, E.; Barcia-Piedras, J.; Perez-Romero, J.; Lotmani, B.; Redondo-Gomez, S. Physiological and biochemical mechanisms preventing Cd-toxicity in the hyperaccumulator Atriplex halimus L. Plant Physiol. Biochem. 2016, 106, 30–38. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wang, P.; Li, W.; Lu, J. Effects of ammonium on the antioxidative response in Hydrilla verticillata (L.f.) Royle plants. Ecotoxicol. Environ. Saf. 2010, 73, 189–195. [Google Scholar] [CrossRef]
- Cao, X.; Cui, X.; Xie, M.; Zhao, R.; Xu, L.; Ni, S.; Cui, Z. Amendments and bioaugmentation enhanced phytoremediation and micro-ecology for PAHs and heavy metals co-contaminated soils. J. Hazard. Mater. 2022, 426, 128096. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Liu, W.; Wu, G.; Hu, H. Effect of EDDS application on soil Cu/Cd availability and uptake/transport by castor. Environ. Sci. 2024, 45, 1803–1811. [Google Scholar] [CrossRef]
- Janssen, J.; Weyens, N.; Croes, S.; Beckers, B.; Meiresonme, L.; Peteghem, P.; Carleer, R.; Vangronsveld, J. Phytoremediation of metal contaminated soil using willow: Exploiting plant-associated bacteria to improve biomass production and metal uptake. Int. J. Phytorem. 2015, 17, 1123–1136. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Zha, Y.; Du, W.; Yin, Y.; Guo, H. Urea-enhanced phytoremediation of cadmium with willow in pyrene and cadmium contaminated soil. J. Hazard. Mater. 2020, 405, 124257. [Google Scholar] [CrossRef]
- Gupta, D.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, Z.; Chen, J.; Jiang, J.; Su, Q. Effects of field application of phosphate fertilizers on the availability and uptake of lead, zinc and cadmium by cabbage (Brassica chinensis L.) in a mining tailing contaminated soil. J. Environ. Sci. 2008, 20, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, C.; Dai, C.; Liu, K.; Li, Y. Phosphate: Coupling the functions of fertilization and passivation in phytoremediation of manganese-contaminated soil by Polygonum pubescens blume. Chemosphere 2020, 260, 127651. [Google Scholar] [CrossRef]
- Ju, W.; Duan, C.; Liu, L.; Jin, X.; Bravo-Ruiseco, G.; Mei, Y.; Fang, L. Reduction of Cu and nitrate leaching risk associated with EDDS-enhanced phytoextraction process by exogenous inoculation of plant growth promoting rhizobacteria. Chemosphere 2022, 287, 132288. [Google Scholar] [CrossRef] [PubMed]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, J.; Chan, T.; Chen, Y.; Li, X. Mechanistic insight into the interactions of EDDS with copper in the rhizosphere of polluted soils. Environ. Pollut. 2020, 267, 115453. [Google Scholar] [CrossRef]
- Khilji, S.; Waseem, M.; Tariq, S.; Jabeen, S.; Jamal, A.; Alomranil, S.; Javed, T.; Riaz, A. Microbe assisted phytoremediation of heavy mental contaminated soil by using African marigold (Tagetes erecta L.). Plant Stress 2024, 11, 100369. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Q.; Shan, Y.; Wang, Y.; Song, X.; Lei, X. A comparative study on the efficiency of biodegradable EDDS and micro-electric field on the promotion of the phytoextraction by Commelina communis L. in Cu-contaminated soils. Geoderma 2018, 314, 1–7. [Google Scholar] [CrossRef]
- Jalali, M.; Khanlari, Z. Redistribution of fractions of zinc, cadmium, nickel, copper, and lead in contaminated calcareous soils treated with EDTA. Arch. Environ. Contam. Toxicol. 2007, 53, 519–532. [Google Scholar] [CrossRef]
- Sarkar, D.; Andra, S.; Saminathan, S.; Datta, R. Chelant-aided enhancement of lead mobilization in residential soils. Environ. Pollut. 2008, 156, 1139–1148. [Google Scholar] [CrossRef]
- Yin, F.; Li, J.; Wang, Y.; Yang, Z. Biodegradable chelating agents for enhancing phytoremediation: Mechanism, market feasibility, and future studies. Ecotoxicol. Environ. Saf. 2024, 272, 116113. [Google Scholar] [CrossRef]
- Zhao, L.; Li, T.; Yu, H.; Zhang, X.; Zheng, Z. Effects of [S, S]-ethylenediaminedisuccinic acid and nitrilotriacetic acid on the efficiency of Pb phytostabilization by Athyrium wardii (Hook.) grown in Pb-contaminated soils. J. Environ. Manag. 2016, 182, 94–100. [Google Scholar] [CrossRef]
| Treatments | Cu (mg kg−1) | Cd (mg kg−1) | ||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| P0-EDDS | 7.35 ± 0.01 c | 81.21 ± 9.20 c | 0.91 ± 0.10 d | 6.89 ± 0.39 a |
| P0+EDDS | 32.55 ± 1.63 b | 346.91 ± 85.26 a | 0.93 ± 0.03 cd | 5.82 ± 0.19 ab |
| P100-EDDS | 6.33 ± 0.14 c | 57.39 ± 2.86 c | 1.07 ± 0.02 bc | 3.33 ± 0.48 c |
| P100+EDDS | 105.44 ± 8.91 a | 206.73 ± 30.39 b | 1.34 ± 0.01 a | 5.25 ± 0.58 b |
| P300-EDDS | 6.58 ± 0.67 c | 59.10 ± 5.46 c | 1.10 ± 0.05 b | 1.61 ± 0.32 d |
| P300+EDDS | 34.05 ± 0.15 b | 101.10 ± 0.98 bc | 1.25 ± 0.00 a | 0.79 ± 0.00 d |
| Treatments | Soil pH | Soil-Available P mg kg−1 | Soil NH4+-N mg kg−1 | Soil NO3−-N mg kg−1 | Soil DOC Concentrations mg kg−1 |
|---|---|---|---|---|---|
| P0-EDDS | 7.75 + 0.04 a | 9.09 + 0.28 d | 6.46 + 0.28 abc | 4.29 ± 0.48 b | 100.55 ± 3.91 c |
| P0+EDDS | 7.85 ± 0.02 a | 9.09 ± 0.45 d | 7.37 ± 0.22 a | 22.54 ± 0.60 a | 138.89 ± 9.92 b |
| P100-EDDS | 7.95 ± 0.02 a | 13.19 ± 0.50 cd | 5.54 ± 0.12 c | 2.56 ± 0.50 b | 99.28 ± 0.95 c |
| P100+EDDS | 7.88 ± 0.05 a | 18.47 ± 3.15 bc | 6.72 ± 0.16 ab | 21.51 ± 2.12 a | 162.96 ± 9.16 a |
| P300-EDDS | 7.80 ± 0.00 a | 23.00 ± 0.93 ab | 5.55 ± 0.36 c | 1.59 ± 0.27 b | 99.95 ± 3.41 c |
| P300+EDDS | 7.83 ± 0.03 a | 27.81 ± 0.54 a | 6.00 ± 0.31 bc | 24.11 ± 3.45 a | 146.80 ± 6.31 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Tang, R.; Peng, X.; Yang, X.; Wang, Y.; Hu, H. Synergistic Effects of Phosphorus and EDDS on Enhancing Phytoremediation Efficiency of Ricinus communis L. in Cu and Cd Co-Contaminated Soils. Agriculture 2025, 15, 2153. https://doi.org/10.3390/agriculture15202153
Liu W, Tang R, Peng X, Yang X, Wang Y, Hu H. Synergistic Effects of Phosphorus and EDDS on Enhancing Phytoremediation Efficiency of Ricinus communis L. in Cu and Cd Co-Contaminated Soils. Agriculture. 2025; 15(20):2153. https://doi.org/10.3390/agriculture15202153
Chicago/Turabian StyleLiu, Wenying, Rongli Tang, Xinlei Peng, Xueting Yang, Yi Wang, and Hongqing Hu. 2025. "Synergistic Effects of Phosphorus and EDDS on Enhancing Phytoremediation Efficiency of Ricinus communis L. in Cu and Cd Co-Contaminated Soils" Agriculture 15, no. 20: 2153. https://doi.org/10.3390/agriculture15202153
APA StyleLiu, W., Tang, R., Peng, X., Yang, X., Wang, Y., & Hu, H. (2025). Synergistic Effects of Phosphorus and EDDS on Enhancing Phytoremediation Efficiency of Ricinus communis L. in Cu and Cd Co-Contaminated Soils. Agriculture, 15(20), 2153. https://doi.org/10.3390/agriculture15202153





