Seed Germination Ecology and Dormancy Release in Some Native and Underutilized Plant Species with Agronomic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Selection and Seed Collection
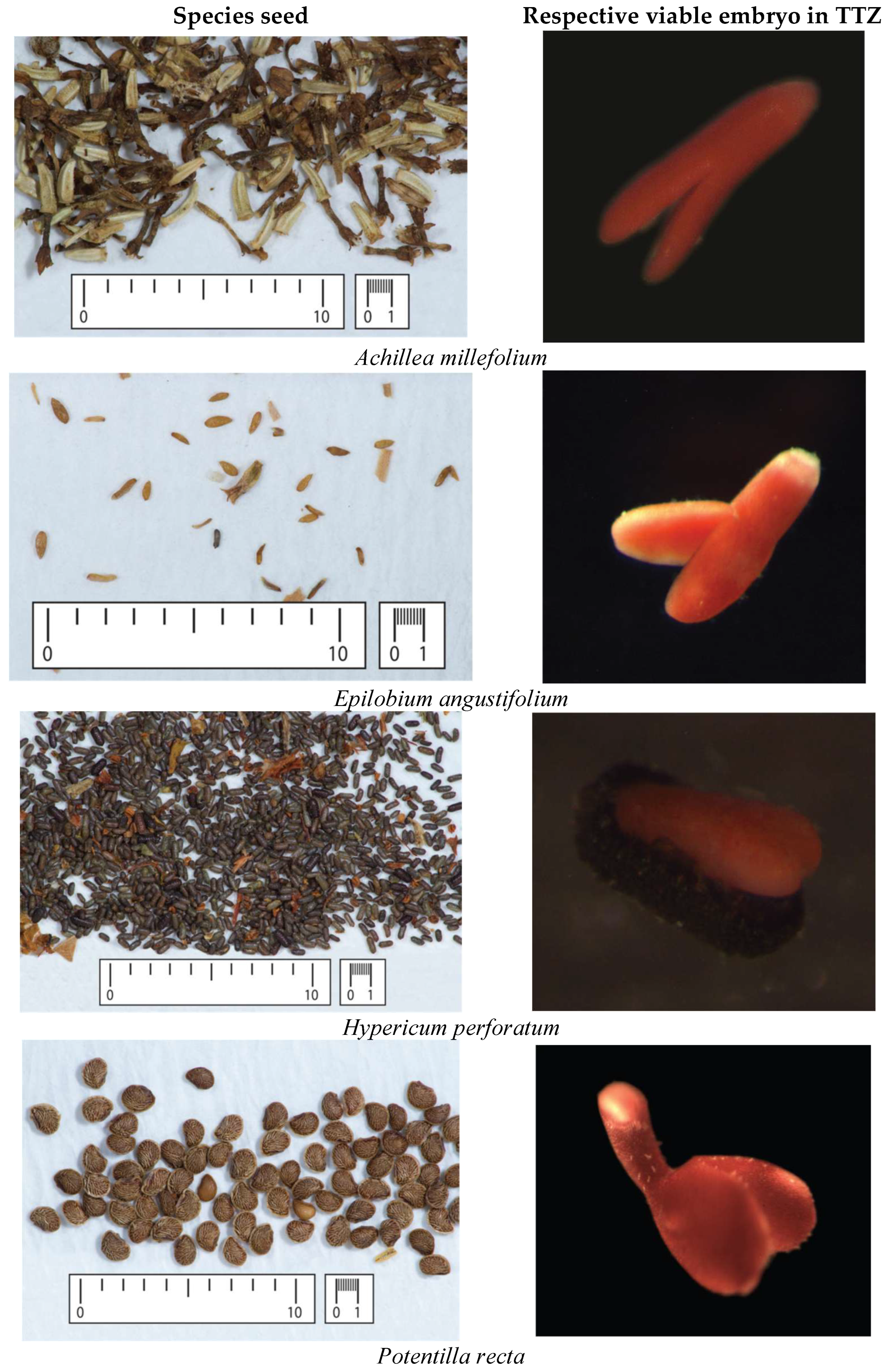
2.2. Seed Viability Testing
2.3. Seed Dormancy and Pre-Treatments
- Cold stratification at 0 ± 1 °C for up to 6 months. CS is a widely used method for species with physiological dormancy. Seeds were put on moist sand and germination was monitored weekly.
- Hormonal treatments: Seeds were soaked for 48 h in either gibberellic acid (GA3) or kinetin (KIN) solutions at concentrations of 500, 1500, or 2500 ppm. Seeds were imbibed in 5 mL of solution, between Whatman filter papers, in a 9 cm Petri dish. Stock solutions were diluted using HPLC-grade water, and pH was adjusted to near-neutral for kinetin treatments, which required NaOH for dissolution.
| Genus | Species | Dormancy Type | Pre-Treatment | References |
|---|---|---|---|---|
| Achillea | A.crithmifolia | Physiological dormancy | Cold stratification | [73] |
| Achillea | A. millefolium | Likely physiological dormancy | Chilling (cold dry storage), light +alternating temperature regimes | [30,74,75] |
| Epilobium | E. angustifolium | Non-deep physiological dormancy | Dry after-ripening | [76,77] |
| Geranium | G. macrorrhizum G. sanguineum | Physical dormancy and physiological dormancy | Dry storage, cold stratification | [78,79] |
| Hypericum | H. perforatum | Non-deep physiological dormancy | Cold stratification, GA3 treatments | [80,81] |
| Potentilla | P. recta | Non-deep physiological dormancy | After-ripening, cold stratification | [82,83] |
| Primula | P.veris | Physiological dormancy | Cold-moist stratification | [84,85] |
| Prunella | P. vulgaris | Physiological dormancy | GA3, cold stratification | [86] |
| Pulsatilla | P. halleri | Morphophysiological dormancy | Warm + cold stratification | [87] |
| Saponaria | S. officinalis | Physiological dormancy | Cold stratification, scarification | [88] |
| Scutellaria | S. altissima | Physiological dormancy | Cold stratification | [89] |
| Stachys | S. germanica | Physiological dormancy | GA3, Kin, cold-moist stratification | [90,91] |
| Teucrium | T. montanum | Physiological dormancy | Cold stratification | [92,93] |
| Thymus | T. praecox T. thracicus | Physiological dormancy | Cold stratification, light | [30] |
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GA3 | Gibberellin |
| TTZ | Tetrazolium chloride |
| KIN | Kinetin/cytokinin |
| CS | Cold stratification |
| ABA | Abscisic acid |
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; AbdullRazis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Vallano, A.; Pontes, C. Escalating costs of innovative medicines: Perspective and proposals. Front. Public Health 2024, 12, 1449707. [Google Scholar] [CrossRef]
- Li, B.; Tang, X.; Le, G. Dietary Habits and Metabolic Health. Nutrients 2023, 15, 3975. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Rao, A. Clean-label alternatives for food preservation: An emerging trend. Heliyon 2024, 10, e35815. [Google Scholar] [CrossRef]
- Kim, M.; Bae, S.M.; Yoo, Y.; Park, J.; Jeong, J.Y. Clean-Label Strategies for the Replacement of Nitrite, Ascorbate, and Phosphate in Meat Products: A Review. Foods 2025, 14, 2442. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on the re-evaluation of butylated hydroxyanisole—BHA (E 320) as a food additive. EFSA J. 2011, 9, 2392. [Google Scholar] [CrossRef]
- Food and Drug Administration. Novel Drug Approvals for 2023. 2023. Available online: https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2023 (accessed on 10 June 2025).
- Sbardelotto, P.R.; Balbinot-Alfaro, E.; da Rocha, M.; Alfaro, A.T. Natural alternatives for processed meat: Legislation, markets, consumers, opportunities and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 10303–10318. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Karapatzak, E.; Krigas, N.; Ganopoulos, I.; Papanastasi, K.; Kyrkas, D.; Yfanti, P.; Nikisianis, N.; Karydas, A.; Manthos, I.; Kosma, I.S.; et al. Documenting Greek Indigenous Germplasm of Cornelian Cherry (Cornus mas L.) for Sustainable Utilization: Molecular Authentication, Asexual Propagation, and Phytochemical Evaluation. Plants 2022, 11, 1345. [Google Scholar] [CrossRef]
- Karapatzak, E.; Dichala, O.; Papanastasi, K.; Manthos, I.; Ganopoulos, I.; Karydas, A.; Badeka, A.V.; Kosma, I.S.; Kyrkas, D.; Yfanti, P.; et al. A Multifaceted Evaluation Approach for Greek Native Neglected and Underutilized Forest Fruit Trees and Shrubs as Natural Sources of Antioxidants: Consolidating the Framework for Their Sustainable Agronomic Exploitation. Plants 2023, 12, 1642. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the Potential of Neglected Local Endemic Plants of Three Mediterranean Regions in the Ornamental Sector: Value Chain Feasibility and Readiness Timescale for Their Sustainable Exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Bastos Lima, M.G.; Palme, U. The Bioeconomy–Biodiversity Nexus: Enhancing or Undermining Nature’s Contributions to People? Conservation 2022, 2, 7–25. [Google Scholar] [CrossRef]
- Díaz, S.; Fargione, J.; Chapin, F.S., III; Tilman, D. Biodiversity Loss Threatens Human Well-Being. PLoS Biol. 2006, 4, e277. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Fraskou, P.; Dimakopoulou, K.; Dariotis, E.; Krigas, N.; Skaltsa, H. Chemometric analysis evidencing the variability in the composition of essential oils in 10 Salvia species from different taxonomic sections or phylogenetic clades. Molecules 2024, 29, 1547. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-Cosmetic Potential of the Local Endemic Plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for Conservation and Sustainable Exploitation of Neglected and Underutilized Phytogenetic Resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- Leonti, M.; Verpoorte, R. Traditional Mediterranean and European herbal medicines. J. Ethnopharmacol. 2017, 199, 161–167. [Google Scholar] [CrossRef]
- Karapatzak, E.; Papagrigoriou, T.; Papanastasi, K.; Dichala, O.; Karydas, A.; Nikisianis, N.; Patakioutas, G.; Lazari, D.; Krigas, N.; Maloupa, E. From the Wild to the Field: Documentation, Propagation, Pilot Cultivation, Fertilization, and Phytochemical Evaluation of the Neglected and Underutilized Amelanchier ovalis Medik. (Rosaceae). Plants 2023, 12, 1142. [Google Scholar] [CrossRef]
- Marcelino, S.; Hamdane, S.; Gaspar, P.D.; Paço, A. Sustainable Agricultural Practices for the Production of Medicinal and Aromatic Plants: Evidence and Recommendations. Sustainability 2023, 15, 14095. [Google Scholar] [CrossRef]
- Papagrigoriou, T.; Iliadi, P.; Mitić, M.N.; Mrmošanin, J.M.; Papanastasi, K.; Karapatzak, E.; Maloupa, E.; Gkourogianni, A.V.; Badeka, A.V.; Krigas, N.; et al. Wild-Growing and Conventionally or Organically Cultivated Sambucus nigra Germplasm: Fruit Phytochemical Profile, Total Phenolic Content, Antioxidant Activity, and Leaf Elements. Plants 2023, 12, 1701. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E.; et al. Influence of Temperature on Seed Germination of Five Wild-Growing Tulipa Species of Greece Associated with Their Ecological Profiles: Implications for Conservation and Cultivation. Plants 2023, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, 256–265. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Lazari, D.; Maloupa, E. Sustainable use of mediterranean medicinal-aromatic plants. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 57–74. [Google Scholar] [CrossRef]
- Zamani, S.; Fathi, M.; Ebadi, M.T.; Máthé, Á. Global Trade of Medicinal and Aromatic Plants. A Review. J. Agric. Food Res. 2025, 21, 101910. [Google Scholar] [CrossRef]
- EEA. Climate Change Adaptation in the Agriculture Sector in Europe. EEA Report No 4/2019. 2019. Available online: https://www.eea.europa.eu/en/analysis/publications/cc-adaptation-agriculture (accessed on 10 July 2025).
- Saadat, S.; Rajabi, M.; Boskabady, M.H. Experimental and clinical studies on pharmacological actions of the genus Achillea: A comprehensive and updated review. Avicenna J. Phytomed. 2024, 14, 530. [Google Scholar] [CrossRef]
- Bouteche, A.; Touil, A.; Narimane, S. Phytochemical composition, ethnomedicinal uses, and pharmacological properties of Achillea ligustica All.: A review. J. Res. Pharm. 2025, 28, 313–325. [Google Scholar] [CrossRef]
- Aćimović, M.; Vujisić, L.; Lončar, B.; Ivanović, S.; Rat, M. Headspace Volatile Profiles of Achillea Species: A. aspleniifolia, A. crithmifolia, A. filipendulina, and A. virescens. Chem. Biodivers. 2025, 22, e202401876. [Google Scholar] [CrossRef]
- Villalva, M.; Silvan, J.M.; Alarcón-Cavero, T.; Villanueva-Bermejo, D.; Jaime, L.; Santoyo, S.; Martinez-Rodriguez, A.J. Antioxidant, Anti-Inflammatory, and Antibacterial Properties of an Achillea millefolium L. Extract and Its Fractions Obtained by Supercritical Anti-Solvent Fractionation against Helicobacter pylori. Antioxidants 2022, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; SredojaTisma, V.; Zarkovic, N. Short Overview of Some Assays for the Measurement of Antioxidant Activity of Natural Products and Their Relevance in Dermatology. Molecules 2021, 26, 5301. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Smirnov, V.; Kvashninova, E.; Khlopin, V.; Vityazev, F.; Golovchenko, V. Isolation, Chemical Characterization and Antioxidant Activity of Pectic Polysaccharides of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 7290. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Jakiw, L.; Khlebnikov, A.I.; Blaskovich, C.L.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of oenothein B isolated from Epilobium angustifolium. J. Immun. 2009, 183, 6754–6766. [Google Scholar] [CrossRef]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef]
- Miliauskas, G.; van Beek, T.A.; Venskutonis, P.R.; Linssen, J.P.H.; de Waard, P. Antioxidative activity of Geranium macrorrhizum. Eur. Food Res. Technol. 2004, 218, 253–261. [Google Scholar] [CrossRef]
- Abarova, S.; Tancheva, L.; Nikolov, R.; Serkedjieva, J.; Pavlova, E.; Bramanti, A.; Nicoletti, F.; Tzvetkov, N.T. Preventive Effect of a Polyphenol-Rich Extract from Geranium sanguineum L. on Hepatic Drug Metabolism in Influenza Infected Mice. Sci. Pharm. 2020, 88, 45. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74. [Google Scholar] [CrossRef]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34. [Google Scholar] [CrossRef]
- Suryawanshi, M.V.; Gujarathi, P.P.; Mulla, T.; Bagban, I. Hypericum perforatum: A comprehensive review on pharmacognosy, preclinical studies, putative molecular mechanism, and clinical studies in neurodegenerative diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3803–3818. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Benzophenones and flavonoids from Hypericum maculatum and their antioxidant activities. Nat. Prod. Res. 2012, 26, 1576–1583. [Google Scholar] [CrossRef]
- Kladar, N.; Božin, B.; Bijelić, K.; Bogavac, M.; Karaman, M.; SrđenovićČonić, B.; Rat, M.; Anačkov, G. Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules 2023, 28, 6218. [Google Scholar] [CrossRef]
- Augustynowicz, D.; Latté, K.P.; Tomczyk, M. Recent phytochemical and pharmacological advances in the genus Potentilla L. sensu lato—An update covering the period from 2009 to 2020. J. Ethnopharmacol. 2021, 266, 113412. [Google Scholar] [CrossRef]
- Bączek, K.; Przybył, J.L.; Mirgos, M.; Kosakowska, O.; Szymborska-Sandhu, I.; Węglarz, Z. Phenolics in Primula veris L. and P. elatior (L.) Hill raw materials. Int. J. Anal. Chem. 2017, 1, 2871579. [Google Scholar] [CrossRef] [PubMed]
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Kapusta, I.; Puchalski, C. Bioactive Phenolic Compounds from Primula veris L.: Influence of the Extraction Conditions and Purification. Molecules 2021, 26, 997. [Google Scholar] [CrossRef]
- Stefanis, I.; Chatzopoulou, P.; Krigas, N.; Karioti, A. Exploring the Chemical Content of Primula veris L. subsp. veris Wild-Growing Populations along a Climate Gradient: An HPLC-PDA-MS Quality Assessment of Flowers, Leaves and Roots for Sustainable Exploitation. Horticulturae 2023, 9, 1120. [Google Scholar] [CrossRef]
- Chintiroglou, P.I.; Krigas, N.; Chatzopoulou, P.; Karioti, A. Development and validation of an HPLC method for the analysis of flowers of wild-growing Primula veris from Epirus, Greece. Planta Med. 2021, 87, 1219–1230. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X.; Zhu, M.-M.; Chen, Y.; Shi, F. Antioxidant Activities of Total Phenols of Prunella vulgaris L. in Vitro and in Tumor-bearing Mice. Molecules 2010, 15, 9145–9156. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.—A Review of its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef]
- Danova, K.; Markovska, Y.; Dimitrov, D.; Kapchina-Toteva, V. In vitro culture of Balkan endemic and rare Pulsatilla species—Secondary metabolite production and phenol & flavonoid determination. Bot. Serb. 2009, 33, 497–506. Available online: https://botanicaserbica.bio.bg.ac.rs/arhiva/pdf/2009_33_2_497_full.pdf (accessed on 3 July 2025).
- Charalambous, D.; Christoforou, M.; Christou, K.; Christou, M.; Ververis, A.; Andreou, M.; Christodoulou, K.; Koutsoulidou, A.; Papachrysostomou, C.; Pantelidou, M. Saponin and Phenolic Composition and Assessment of Biological Activities of Saponaria officinalis L. Root Extracts. Plants 2024, 13, 1982. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. Study on the chemical composition and antioxidant activity of extracts from shoot culture and regenerated plants of Scutellaria altissima L. Acta Physiol. Plant 2015, 37, 1736. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellariaalpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Hanganu, D.; Tiperciuc, B.; Nistor, A.; Vlase, A.-M.; Vlase, L.; Pușcaș, C.; Duma, M.; Login, C.C.; et al. Stachys Species: Comparative Evaluation of Phenolic Profile and Antimicrobial and Antioxidant Potential. Antibiotics 2023, 12, 1644. [Google Scholar] [CrossRef]
- Pashova, S.; Karcheva-Bahchevanska, D.; Ivanov, K.; Ivanova, S. Genus Stachys—Phytochemistry, Traditional Medicinal Uses, and Future Perspectives. Molecules 2024, 29, 5345. [Google Scholar] [CrossRef]
- Bektasevic, M.; Jurin, M.; Roje, M.; Politeo, O. Phytochemical Profile, Antioxidant Activity and Cholinesterase Inhibition Potential of Essential Oil and Extracts of Teucrium montanum from Bosnia and Herzegovina. Separations 2023, 10, 421. [Google Scholar] [CrossRef]
- Kadifkova Panovska, T.; Kulevanova, S.; Stefova, M. In vitro antioxidant activity of some Teucrium species (Lamiaceae). Acta Pharm. 2005, 55, 207–214. Available online: https://hrcak.srce.hr/16758 (accessed on 3 July 2025). [PubMed]
- Dong, Y.; Wei, Z.; Yang, R.; Zhang, Y.; Sun, M.; Bai, H.; Mo, M.; Yao, C.; Li, H.; Shi, L. Chemical Compositions of Essential Oil Extracted from Eight Thyme Species and Potential Biological Functions. Plants 2023, 12, 4164. [Google Scholar] [CrossRef]
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330. [Google Scholar] [CrossRef]
- Petrović, N.V.; Petrović, S.S.; Džamić, A.M.; Ćirić, A.D.; Ristić, M.S.; Milovanović, S.L.; Petrović, S.D. Chemical composition, antioxidant and antimicrobial activity of Thymus praecox supercritical extracts. J. Supercrit. Fluids 2016, 110, 117–125. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology, 4th ed.; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing. Seed Sci. Technol. 1999, 27, 333. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing-1168.html (accessed on 12 September 2024).
- Aosa, S. Tetrazolium Testing Handbook; Association of Official Seed Analysts and Society of Commercial Seed Technologists: Washington, DC, USA, 2010. [Google Scholar]
- Mondoni, A.; Rossi, G.; Orsenigo, S.; Probert, R.J. Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 2012, 110, 155–164. [Google Scholar] [CrossRef]
- Nikolić, B.; Braunović, S.; Jovanović, F.; Eremija, S.; Marković, M.; Rakonjac, L. Seed germination tests in Achillea genus from the Pirot County (Southeastern Serbia). Ethnobotany 2022, 2, 145–170. [Google Scholar] [CrossRef]
- Zarghani, H.; Mijani, S.; Eskandari, S. Temperature Effects on the Seed Germination of Some Perennial and Annual Species of Asteraceae Family. Plant Breed. Seed Sci. 2014, 69, 3–14. [Google Scholar] [CrossRef]
- Bostock, S.J. Seed germination strategies of five perennial weeds. Oecologia 1978, 36, 113–126. [Google Scholar] [CrossRef]
- Myerscough, P.J. Epilobium angustifolium L. J. Ecol. 1980, 68, 169–178. [Google Scholar] [CrossRef]
- Broderick, D.H. The biology of Canadian weeds: 93. Epilobium angustifolium L. (Onagraceae). Can. J. Plant Sci. 1990, 70, 1013–1017. [Google Scholar] [CrossRef]
- Meisert, A. Physical dormancy in Geraniaceae seeds. Seed Sci. Res. 2002, 12, 121–128. [Google Scholar] [CrossRef]
- Vandelook, F.; Van Assche, J.A. A combined physical and physiological dormancy controls seasonal seedling emergence of Geranium robertianum. Plant Biol. 2010, 12, 765–771. [Google Scholar] [CrossRef]
- García, F.; Huertas, M.; Mora, E.; Peña, B.; Varela, F.; González-Benito, M.E. Hypericum perforatum L. Seed Germination: Interpopulation Variationand Effect of Light, Temperature, Presowing Treatments and Seed Desiccation. Genet. Resour. Crop Evol. 2006, 53, 1187–1198. [Google Scholar] [CrossRef]
- Campbell, H.M. Germination, emergence and seedling growth of Hypericum perforatum L. Weed Res. 1985, 25, 259–266. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Role of temperature and light in the germination ecology of buried seeds of Potentilla recta. Ann. Appl. Biol. 1990, 117, 611–616. [Google Scholar] [CrossRef]
- Kołodziejek, J.; Patykowski, J.; Wala, M. Dormancy, germination, and sensitivity to salinity stress in five species of Potentilla (Rosaceae). Botany 2019, 97, 452–462. [Google Scholar] [CrossRef]
- Yankova-Tsvetkova, E.; Petrova, M.; Grigorova, I.; Traykova, B.; Stanilova, M. The Establishment of an Ex Situ Collection of Primula veris in Bulgaria. Plants 2022, 11, 3018. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E. In vitro propagation of Primula veris L. subsp. veris (Primulaceae): A valuable medicinal plant with ornamental potential. Int. J. Bot. St. 2020, 5, 532–539. Available online: https://www.botanyjournals.com/assets/archives/2020/vol5issue5/5-5-43-743.pdf (accessed on 18 May 2025).
- Campbell-Martínez, G.; Olsson, R.; Hersey, A.; Meikle, S.; Freitag, K. Native seed germination trials at the Rae Selling Berry Seed Bank. In Proceedings of the International Plant Propagators Society, Western Region, 2023; Available online: https://archives.pdx.edu/ds/psu/43841 (accessed on 20 July 2025).
- Park, K.; Lee, S.Y.; Ji, B.; Jang, B.K.; Lee, H.; Lee, H.; Song, S.-K.; Cho, J.S. Seed longevity and germinability of Pulsatilla dahurica (Fisch. ex DC.) spreng after storage and accelerated aging test. Hortic. Sci. Technol. 2022, 40, 147–156. [Google Scholar] [CrossRef]
- Fišer Pečnikar, Ž.; Balant, M.; Glasnović, P.; Surina, B. Seed dormancy and germination of the rare, high elevation Balkan endemic Cerastium dinaricum (Caryophyllaceae). Biologia 2018, 73, 937–943. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, H.C.; Lee, S.Y. Seed Dormancy and Germination Characteristics of Scutellaria indica L. var. coccinea ST Kim & ST Lee, an Endemic Species Found on Jeju Island, South Korea. Horticulturae 2025, 11, 1019. [Google Scholar] [CrossRef]
- Güleryüz, G.; Kırmızı, S.; Arslan, H.; Sakar, F.S. Dormancy and germination in Stachys germanica L. subsp. bithynica (Boiss.) Bhattacharjee seeds: Effects of short-time moist chilling and plant growth regulators. Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 943–948. [Google Scholar] [CrossRef]
- Ismaili, S.E.; Maurady, A.; Lachkar, M.; Britel, M.R.; Bakali, A.H. Effect of temperature and different pre-treatments on seed germination of Stachys mouretii Batt. & Pit. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100438. [Google Scholar] [CrossRef]
- Benvenuti, S.; Ceccarini, L.; Macchia, M. Germination ecology of Teucrium marum L.: An endemic species of the Tuscany Arcipelago. Acta Hortic. 2006, 723, 315–320. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Bourgou, S.; Ben Haj Jilani, I.; Ben Othman, W.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; et al. DNA Barcoding, GIS-Facilitated Seed Germination and Pilot Cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian Local Endemic with Potential Medicinal and Ornamental Value. Biology 2022, 11, 462. [Google Scholar] [CrossRef]
- Hereford, J. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 2009, 173, 579–588. [Google Scholar] [CrossRef]
- Joshi, J.; Schmid, B.; Caldeira, M.C.; Dimitrakopoulos, P.G.; Good, J.; Harris, R.; Hector, A.; Huss-Danell, K.; Jumpponen, A.; Minns, A.; et al. Local adaptation enhances performance of common plant species. Ecol. Lett. 2001, 4, 536–544. [Google Scholar] [CrossRef]
- Mortlock, B.W. Local seed for revegetation. Ecol. Manag. Restor. 2000, 1, 93–101. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva–Baskin classification system for primary seed dormancy. Seed Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Rosbakh, S.; Carta, A.; Fernández-Pascual, E.; Phartyal, S.S.C.; Dayrell, R.L.; Mattana, E.; Saatkamp, A.; Vandelook, F.; Baskin, J.; Baskin, C. Global seed dormancy patterns are driven by macroclimate but not fire regime. New Phytol. 2023, 240, 555–564. [Google Scholar] [CrossRef]
- Picciau, R.; Pritchard, H.W.; Mattana, E.; Bacchetta, G. Thermal thresholds for seed germination in Mediterranean species are higher in mountain compared with lowland areas. Seed Sci. Res. 2019, 29, 44–54. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; Pérez-García, F. Seed germination of high mountain Mediterranean species: Altitudinal, interpopulation and interannual variability. Ecol. Res. 2005, 20, 433–444. [Google Scholar] [CrossRef]
- Mattana, E.; Carta, A.; Fernández-Pascual, E.; Keeley, J.E.; Pritchard, H.W. Climate change and plant regeneration from seeds in Mediterranean regions of the Northern Hemisphere. In Plant Regeneration from Seeds; Academic Press: Cambridge, MA, USA, 2021; pp. 101–114. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Guan, C.; Wang, X.; Feng, J.; Hong, S.; Liang, Y.; Ren, B.; Zuo, J. Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of abscisic acid insensitive5 protein in Arabidopsis. Plant Physiol. 2014, 164, 1515–1526. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Roh, Y.S.; Yuan, T.; Roh, M.S. Germination of Pulsatilla cernua var. koreana seeds influenced by temperature, light, and GA3 treatments. Acta Hortic. 2020, 1291, 183–190. [Google Scholar] [CrossRef]
- Yuan, T.; Wei, Q.; Bauchan, G. Germination of Pulsatilla seeds as influenced by seed morphology, moist 5 °C and gibberellin (GA3) treatment, and detection of nickel in seeds. HortScience 2019, 54, 2015–2023. [Google Scholar] [CrossRef]
- Nadjafi, F.; Bannayan, M.; Tabrizi, L.; Rastgoo, M. Seed germination and dormancy breaking techniques for Ferula gummosa and Teucrium polium. J. Arid. Environ. 2006, 64, 542–547. [Google Scholar] [CrossRef]
- Thompson, K.; Ceriani, R.M.; Bakker, J.P.; Bekker, R.M. Are seed dormancy and persistence in soil related? Seed Sci. Res. 2003, 13, 97–100. [Google Scholar] [CrossRef]

| Species | Family | Activity of Interest | Description | References |
|---|---|---|---|---|
| Achillea crithmifoliaWaldst. and Kit. | Asteraceae | Enhancement of CAT, GPx, SOD enzymes | Higher activity of CAT, GPx, and SOD enzymes in leukocytes compared to other Achillea species | [36,37,38] |
| Reduction in lipid peroxidation (LPO) | High levels of phenolics such as caffeoylquinic acids and sesquiterpene lactones reduce lipid peroxidation, protecting cell membranes | [37,38] | ||
| Rich phenolic profile | Contains caffeoylquinic acid derivatives, flavonoids (such as luteolin, apigenin), and sesquiterpene lactones with strong antioxidant activity | [37,38] | ||
| Achillea millefolium L. | Asteraceae | Free radical scavenging, reduces intracellular ROS levels | Strong DPPH/ABTS scavenging, ROS reduction, activation of SOD, CAT, GPx, protection of DNA and membranes | [39,40] |
| Epilobium angustifolium L. | Onagraceae | ROS scavenging, inhibition of enzymatic systems, high ellagitannin content | Oenothein B and flavonoids scavenge ROS, inhibit xanthine oxidase, DPPH/ABTS scavenging, pectin scavenging | [41,42,43] |
| Geranium macrorrhizum L. | Geraniaceae | ROS scavenging | Quercetin glycosides and phenolic acids scavenge free radicals (DPPH, ABTS, superoxide, H2O2) | [44] |
| Geranium sanguineum L. | Geraniaceae | ROS scavenging, cell membrane protection | Standardized Polyphenolic Complex (PC) scavenges ROS (O2−·, H2O2), reduces TBARS, enhances SOD and TAA, and protects cell membranes | [45] |
| Hypericum olympicum L. | Hypericaceae | Acylphloroglucinol metabolites | Strong DPPH/ABTS/FRAP activity from acylphloroglucinols (olympicins), despite low flavonoid content | [46] |
| Hypericum perforatum L. | Hypericaceae | ROS scavenging, inhibition of lipid peroxidation | ROS scavenging, lipid peroxidation inhibition, strong DPPH and TAC inhibition (~0.52 μg/mL, lipid peroxidation IC50 ≈ 0.0079 μg/mL) | [47,48] |
| Hypericum maculatum Crantz | Hypericaceae | ROS scavenging, inhibition of lipid peroxidation | Isoquercitrin and other flavonoid glycosides scavenge free radicals (DPPH, ABTS, FRAP) and inhibit lipid peroxidation (LPO) | [49,50] |
| Potentilla recta L. | Rosaceae | ROS scavenging | Free radical scavenging (DPPH, SO2−, NO) with compounds such as rutin, caffeic acid, ellagic acid | [51] |
| Primula veris L. | Primulaceae | ROS scavenging | Rich in flavonoids and phenolics (quercetin, rutin, isorhamnetin, kaempferol); strong DPPH, ABTS, FRAP activity | [52,53,54,55] |
| Prunella vulgaris L. | Lamiaceae | ROS scavenging | Extract P 60 shows strong scavenging activity (DPPH, ABTS, FRAP), increases SOD, reduces MDA, while polysaccharides scavenge ROS | [56,57] |
| Pulsatilla helleri (All.) Willd. | Ranunculaceae | ROS scavenging | Rich in phenolics, flavonoids and anthocyanidins | [58] |
| Saponaria officinalis L. | Caryophyllaceae | ROS scavenging | Phenolics and flavonoids (protocatechuic acid, rutin, apigenin) scavenge ROS (DPPH, ABTS assays), reduce TBARS | [59] |
| Scutellaria altissima L. | Lamiaceae | ROS scavenging, inhibition of lipid peroxidation | ROS scavenging, AGEs formation inhibition, strong activity in DPPH, FRAP, and lipid peroxidation (LPO) inhibition | [60,61] |
| Stachys germanica L. | Lamiaceae | ROS scavenging | Free radical (ROS) scavenging, very low IC50 in DPPH and FRAP (~688 µM TE/mL). Activity correlates with total phenolic content (TPC) and caffeic acids | [62,63] |
| Teucrium montanum L. | Lamiaceae | ROS scavenging | Strong scavenging activity (DPPH, FRAP); protection of proteins and lipids; rich in phenolic acids (p-coumaric, caffeic, ellagic, chlorogenic) | [64,65] |
| Thymus thracicus Velen. | Lamiaceae | ROS scavenging | Strong scavenging activity; increase CAT and SOD activity and the antioxidant capacity of the THP-1 cells; rich in phenolics, thymol, and linalool | [66,67] |
| Thymus praecox Opiz | Lamiaceae | ROS scavenging | Strong scavenging activity; neutralize DPPH radicals; rich in phenolics, thymol, and linalool | [67,68] |
| Species | GA3500 | GA31500 | GA32500 | Kin500 | Kin1500 | Kin2500 | CS | Control | Viab. |
|---|---|---|---|---|---|---|---|---|---|
| Achillea crithmifolia | 18.50 ± 1.658 f | 25.75 ± 2.287 ef | 38.50 ± 2.398 cd | 32.00 ± 1.155 ed | 62.00 ± 0.707 b | 41.25 ± 1.702 c | 71.50 ± 1.190 c | 41.75 ± 1.109 d | 80 |
| Achillea millefolium | 15.75 ± 1.887 e | 28.75 ± 2.562 d | 41.50 ± 3.797 c | 35.50 ± 2.533 cd | 58.00 ± 2.799 b | 39.00 ± 0.913 bc | 72.25 ± 1.750 a | 42.50 ± 1.848 c | 100 |
| Epilobium angustifolium | 24.00 ± 2.415 c | 43.75 ± 2.213 a | 21.50 ± 2.102 c | 36.00 ± 0.707 ab | 5.25 ± 3.966 d | 2.00 ± 0.408 d | 18.00 ± 2.828 c | 25.25 ± 1.652 bc | 100 |
| Hypericum maculatum | 67.25 ± 0.946 b | 40.25 ± 1.109 b | 3.00 ± 1.080 e | 21.00 ± 2.041 d | 21.75 ± 1.652 d | 14.75 ± 2.955 d | 52.75 ± 1.031 c | 84.50 ± 2.598 a | 100 |
| Hypericum olympicum | 74.75 ± 0.854 b | 38.75 ± 0.479 d | 4.75 ± 0.854 f | 18.75 ± 0.750 e | 21.00 ± 0.707 e | 13.00 ± 4.564 ef | 51.00 ± 1.683 c | 90.00 ± 1.225 a | 100 |
| Hypericum perforatum | 70.00 ± 1.780 b | 42.50 ± 1.708 d | 1.00 ± 0.408 f | 18.75 ± 1.974 e | 23.75 ± 1.652 e | 17.75 ± 2.869 e | 51.50 ± 0.866 c | 78.50 ± 1.848 a | 80 |
| Potentilla recta | 13.25 ± 1.702 bc | 18.75 ± 1.887 bc | 10.50 ± 1.658 c | 22.00 ± 3.162 b | 18.50 ± 1.708 bc | 14.50 ± 1.555 bc | 20.50 ± 2.062 b | 81.50 ± 1.708 a | 100 |
| Primula veris | 85.50 ± 1.708 a | 53.00 ± 1.683 bc | 47.00 ± 1.581 bc | 43.50 ± 1.041 c | 42.00 ± 0.707 b | 19.25 ± 1.315 d | 19.00 ± 2.646 d | 47.00 ± 1.581 bc | 90 |
| Prunella vulgaris | 49.50 ± 0.645 b | 29.75 ± 1.250 c | 32.00 ± 1.414 c | 7.00 ± 1.225 e | 4.50 ± 2.630 e | 9.00 ± 0.707 e | 17.00 ± 1.780 d | 79.25 ± 2.428 a | 90 |
| Pulsatilla halleri | 53.50 ± 1.323 b | 35.50 ± 1.041 c | 34.25 ± 1.109 c | 68.50 ± 2.327 a | 1.50 ± 0.866 e | 8.50 ± 1.500 d | 4.25 ± 1.652 de | 49.25 ± 1.601 b | 80 |
| Saponaria officinalis | 0.00 | 1.75 ± 0.479 c | 1.50 ± 0.289 c | 32.00 ± 3.317 a | 12.00 ± 3.367 b | 12.25 ± 0.250 b | 2.25 ± 1.601 c | 0.00 | 80 |
| Scutellaria altissima | 34.50 ± 1.258 b | 0.00 | 0.00 | 22.50 ± 0.289 c | 11.50 ± 2.533 d | 0.00 | 31.25 ± 1.109 b | 64.75 ± 1.702 a | 70 |
| Stachys germanica | 0.00 | 62.25 ± 1.797 a | 56.25 ± 1.652 ab | 11.75 ± 1.652 c | 46.75 ± 3.425 b | 49.50 ± 1.190 b | 15.00 ± 3.342 c | 63.75 ± 1.601 a | 70 |
| Teucrium montanum | 35.25 ± 2.562 b | 62.75 ± 1.652 a | 60.25 ± 3.092 a | 17.00 ± 2.121 c | 20.25 ± 1.702 c | 34.75 ± 1.377 b | 18.00 ± 3.240 c | 2.50 ± 0.289 d | 100 |
| Thymus thracicus | 16.50 ± 1.555 cd | 14.50 ± 1.708 d | 13.50 ± 2.062 d | 29.75 ± 1.377 b | 22.75 ± 1.493 bc | 29.25 ± 1.250 b | 10.00 ± 1.958 d | 79.25 ± 1.250 a | 100 |
| Thymus praecox | 15.75 ± 0.479 e | 16.25 ± 0.854 de | 11.50 ± 1.756 e | 36.25 ± 1.031 b | 21.75 ± 1.109 d | 30.50 ± 0.957 c | 11.25 ± 1.652 e | 81.75 ± 1.250 a | 100 |
| Species | Dormancy Type | Pretreatment |
|---|---|---|
| Achillea crithmifolia | Physiological | Stratification |
| Achillea millefolium | Physiological | Stratification |
| Epilobium angustifolium | Physiological | GA3_1500 |
| Hypericum maculatum | Physiological | After-ripening |
| Hypericum olympicum | Physiological | After-ripening |
| Hypericum perforatum | Physiological | After-ripening |
| Potentilla recta | Physiological | After-ripening |
| Primula veris | Physiological | GA3_500 |
| Prunella vulgaris | Physiological | After-ripening |
| Pulsatilla halleri | Physiological | GA3_500 |
| Saponaria officinalis | Physiological | Kin_500 |
| Scutellaria altissima | Physiological | After-ripening |
| Stachys germanica | Physiological | After-ripening |
| Teucrium montanum | Physiological | GA3_1500 |
| Thymus thracicus | Physiological | After-ripening |
| Thymus praecox | Physiological | After-ripening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varsamis, G.; Merou, T.; Alexandropoulou, I.; Menti, C.; Karapatzak, E. Seed Germination Ecology and Dormancy Release in Some Native and Underutilized Plant Species with Agronomic Potential. Agriculture 2025, 15, 2139. https://doi.org/10.3390/agriculture15202139
Varsamis G, Merou T, Alexandropoulou I, Menti C, Karapatzak E. Seed Germination Ecology and Dormancy Release in Some Native and Underutilized Plant Species with Agronomic Potential. Agriculture. 2025; 15(20):2139. https://doi.org/10.3390/agriculture15202139
Chicago/Turabian StyleVarsamis, Georgios, Theodora Merou, Ioanna Alexandropoulou, Chrysoula Menti, and Eleftherios Karapatzak. 2025. "Seed Germination Ecology and Dormancy Release in Some Native and Underutilized Plant Species with Agronomic Potential" Agriculture 15, no. 20: 2139. https://doi.org/10.3390/agriculture15202139
APA StyleVarsamis, G., Merou, T., Alexandropoulou, I., Menti, C., & Karapatzak, E. (2025). Seed Germination Ecology and Dormancy Release in Some Native and Underutilized Plant Species with Agronomic Potential. Agriculture, 15(20), 2139. https://doi.org/10.3390/agriculture15202139







