Differential Modulation of Maize Silage Odor: Lactiplantibacillus plantarum vs. Lactiplantibacillus buchneri Drive Volatile Compound Change via Strain-Specific Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Silage Preparation
2.3. Analysis of Silage Chemical Composition and Fermentation Parameters
2.4. Determination of VOCs by HS-SPME-GC-MS
2.4.1. Extraction of VOCs by HS-SPME
2.4.2. GC-MS Analysis
2.4.3. Calculation of rOAV
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of WPMS
3.2. Fermentation Parameters of WPMS
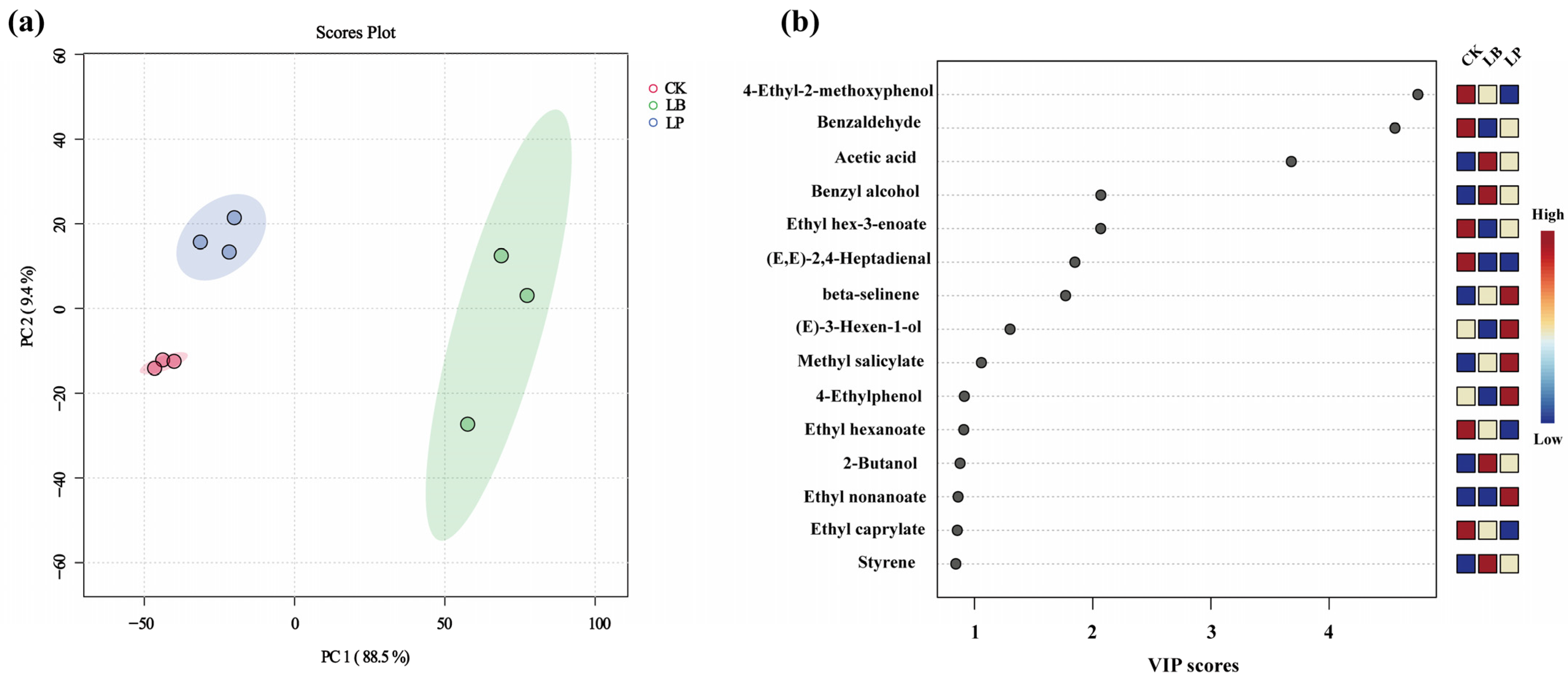
3.3. Analysis of VOCs by HS-SPME-GC-MS
3.4. Analysis of Key VOCs in WPMS from Different Additives Groups
3.5. rOAV Analysis of Volatile Organic Compounds in WPMS from Different Additive Group
4. Discussion
4.1. Effects of Different Additives on the Chemical Composition of WPMS
4.2. Effects of Different Additives on the Fermentation Parameters of WPMS
4.3. Effects of Different Additives on the VOCs of WPMS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Lian, T.J.; Zhang, W.Q.; Cao, Q.T.; Wang, S.L.; Dong, H.M.; Yin, F.B. Improving production of lactic acid and volatile fatty acids from dairy cattle manure and corn straw silage: Effects of mixing ratios and temperature. Bioresour. Technol. 2022, 359, 127449. [Google Scholar] [CrossRef]
- Wang, X.L.; Song, J.M.; Liu, Z.H.; Zhang, G.N.; Zhang, Y.G. Fermentation quality and microbial community of corn stover or rice straw silage mixed with soybean curd residue. Animals 2022, 12, 919. [Google Scholar] [CrossRef]
- Khan, N.A.; Yu, P.Q.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 2015, 95, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, L.F.; Shaver, R.D.; Luck, B.D. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef]
- Tharangani, R.M.H.; Yakun, C.; Zhao, L.S.; Ma, L.; Liu, H.L.; Su, S.L.; Shan, L.; Yang, Z.N.; Kononoff, P.J.; Weiss, W.P.; et al. Corn silage quality index: An index combining milk yield, silage nutritional and fermentation parameters. Anim. Feed Sci. Technol. 2021, 273, 114817. [Google Scholar] [CrossRef]
- Scherer, R.; Gerlach, K.; Südekum, K.H. Biogenic amines and gamma-amino butyric acid in silages: Formation, occurrence and influence on dry matter intake and ruminant production. Anim. Feed Sci. Technol. 2015, 210, 1–16. [Google Scholar] [CrossRef]
- Grant, R.J.; Ferraretto, L.F. Silage review: Silage feeding management: Silage characteristics and dairy cow feeding behavior. J. Dairy Sci. 2018, 101, 4111–4121. [Google Scholar] [CrossRef]
- Hafner, S.D.; Howard, C.; Muck, R.E.; Franco, R.B.; Montes, F.; Green, P.G.; Mitloehner, F.; Trabue, S.L.; Rotz, C.A. Emission of volatile organic compounds from silage: Compounds, sources, and implications. Atmos. Environ. 2013, 77, 827–839. [Google Scholar] [CrossRef]
- Randby, Å. Effect of propanol and dimethylsulphide in grass silage on organoleptic milk quality. J. Anim. Feed Sci. 2007, 16, 102–107. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Khushvakov, J.; Ye, Y.X.; Pedersen, N.C.; Hansen, H.H.; Ahrné, L.; Khakimov, B. Goat milk foodomics: Dietary supplementation of sunflower oil and rapeseed oil modify milk amino acid and organic acid profiles in dairy goats. Front. Vet. Sci. 2022, 9, 837229. [Google Scholar] [CrossRef]
- Ke, Z.; Zhang, T.T.; Guo, R.R.; Yan, Q.; Zhang, H.L.; Hu, X.H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef]
- Xu, D.M.; Wang, N.; Rinne, M.; Ke, W.C.; Weinberg, Z.G.; Da, M.; Bai, J.; Zhang, Y.X.; Li, F.H.; Guo, X.S. The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 2021, 14, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Irawan, A.; Sofyan, A.; Ridwan, R.; Hassim, H.A.; Respati, A.N.; Wardani, W.W.; Sadarman; Astuti, W.D.; Jayanegara, A. Effects of different lactic acid bacteria groups and fibrolytic enzymes as additives on silage quality: A meta-analysis. Bioresour. Technol. Rep. 2021, 14, 100654. [Google Scholar] [CrossRef]
- Wang, L.; Bao, J.Z.; Zhuo, X.L.; Li, Y.Q.; Zhan, W.Y.; Xie, Y.X.; Wu, Z.; Yu, Z. Effects of Lentilactobacillus buchneri and chemical additives on fermentation profile, chemical composition, and nutrient digestibility of high-moisture corn silage. Front. Vet. Sci. 2023, 10, 1296392. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.J.; Wang, H.Y.; Peng, R.; Chen, Z.H.; Xiang, Y.X.; Yan, L. Different commercial microbial additives influence fermentation quality and microbial community of King Grass silage. Fermentation 2025, 11, 264. [Google Scholar] [CrossRef]
- da Silva, N.C.; Nascimento, C.F.; Nascimento, F.A.; de Resende, F.D.; Daniel, J.L.P.; Siqueira, G.R. Fermentation and aerobic stability of rehydrated corn grain silage treated with different doses of Lactobacillus buchneri or a combination of Lactobacillus plantarum and Pediococcus acidilactici. J. Dairy Sci. 2018, 101, 4158–4167. [Google Scholar] [CrossRef]
- Rabelo, C.H.S.; Basso, F.C.; Lara, E.C.; Jorge, L.G.O.; Härter, C.J.; Mesquita, L.G.; Silva, L.F.P.; Reis, R.A. Effects of Lactobacillus buchneri as a silage inoculant and as a probiotic on feed intake, apparent digestibility and ruminal fermentation and microbiology in wethers fed low-dry-matter whole-crop maize silage. Grass Forage Sci. 2018, 73, 67–77. [Google Scholar] [CrossRef]
- Yu, M.G.; Yang, P.; Song, H.L.; Guan, X.S. Research progress in comprehensive two-dimensional gas chromatography–mass spectrometry and its combination with olfactometry systems in the flavor analysis field. J. Food Compos. Anal. 2022, 114, 104790. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Flores, M. Establishment of the contribution of volatile compounds to the aroma of fermented sausages at different stages of processing and storage. Food Chem. 2009, 115, 1464–1472. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.L.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.J.; Zhang, Y.Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef] [PubMed]
- NYT1459-2022; Determination of acid detergent fiber(ADF) in feeds. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2022.
- GB/T 20806-2022; Determination of Neutral Detergent Fiber(NDF) in Feeds. Standardization Administration of China: Beijing, China, 2022.
- GB/T 6438-2007; Animal Feeding Stuffs—Determination of Crude Ash. Standardization Administration of China: Beijing, China, 2007.
- GB/T 42491-2023; Determination of Total Strach Content in Feeds—Enzymatic Method. Standardization Administration of China: Beijing, China, 2023.
- Liu, Y.C.; Du, S.; Sun, L.; Li, Y.Y.; Liu, M.J.; Sun, P.B.; Bai, B.C.; Ge, G.T.; Jia, Y.S.; Wang, Z.J. Volatile metabolomics and metagenomics reveal the effects of lactic acid bacteria on alfalfa silage quality, microbial communities, and volatile organic compounds. Commun. Biol. 2024, 7, 7083. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xu, W.C.; Jiang, G.X.; Sui, M.Y.; Xiao, J.Y.; Ning, Y.Y.; Niaz, R.; Wu, D.W.; Feng, X.G.; Chen, J.H.; et al. Monitoring the dynamic changes in aroma during the whole processing of Qingzhuan tea at an industrial scale: From fresh leaves to finished tea. Food Chem. 2024, 439, 137810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.C.; Niu, Y.W.; Xiao, Z.B. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC×GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Guohe, C.; Guangmei, Z.; He, X.; Jing, Z.; Jianan, H.; Zhonghua, L.; Chao, W. Characterization of the key differential aroma compounds in five dark teas from different geographical regions integrating GC-MS, ROAV and chemometrics approaches. Food Res. Int. 2024, 194, 114928. [Google Scholar] [CrossRef]
- Xie, J.L.; Wang, L.L.; Deng, Y.L.; Yuan, H.B.; Zhu, J.Y.; Jiang, Y.W.; Yang, Y.Q. Characterization of the key odorants in floral aroma green tea based on GC-E-Nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef]
- Nie, T.J.; Gao, Y.; Gao, Y.; Wang, W.Q.; Wang, Z.Q.; Zhang, H.Y.; Chen, H.T.; Wang, S.Q.; Sun, B.G. Potential effect of key aroma-active compounds identified from fried white mushrooms (Agaricus bisporus L.) on saltiness perception. Food Chem. 2025, 490, 145155. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Edition Review; China Science Publishing & Media Ltd.: Beijing, China, 2018. [Google Scholar]
- Liland, K.H. Multivariate methods in metabolomics—From pre-processing to dimension reduction and statistical analysis. TrAC Trends Anal. Chem. 2011, 30, 827–841. [Google Scholar] [CrossRef]
- Oliveira, L.F.C.; Tega, D.U.; Duarte, G.H.B.; Barbosa, L.D.; Ribeiro, H.C.; Castello, A.C.D.; Sawaya, A.; Sussulini, A. Foodomics for agroecology: Differentiation of volatile profile in mint (Mentha × gracilis Sole) from permaculture, organic and conventional agricultural systems using HS-SPME/GC-MS. Food Res. Int. 2022, 155, 111107. [Google Scholar] [CrossRef]
- Nazar, M.; Wang, S.R.; Zhao, J.; Dong, Z.H.; Li, J.F.; Kaka, N.A.; Shao, T. The feasibility and effects of exogenous epiphytic microbiota on the fermentation quality and microbial community dynamics of whole crop corn. Bioresour. Technol. 2020, 306, 123106. [Google Scholar] [CrossRef] [PubMed]
- Elferink, S.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef]
- Lara, E.C.; Basso, F.C.; de Assis, F.B.; Souza, F.A.; Berchielli, T.T.; Reis, R.A. Changes in the nutritive value and aerobic stability of corn silages inoculated with Bacillus subtilis alone or combined with Lactobacillus plantarum. Anim. Prod. Sci. 2016, 56, 1867–1874. [Google Scholar] [CrossRef]
- Kung, L.M.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Ling, W.Q.; Zhang, L.; Feng, Q.X.; Degen, A.A.; Li, J.; Qi, Y.; Li, Y.; Zhou, Y.; Liu, Y.J.; Yang, F.L.; et al. Effects of different additives on fermentation quality, microbial communities, and rumen degradation of alfalfa silage. Fermentation 2022, 8, 660. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zielinska, K.J.; Wróbel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.D.; Choi, K.C. Role of LAB in silage fermentation: Effect on nutritional quality and organic acid production—An overview. AIMS Agric. Food 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.T.; Ke, W.C.; Xu, D.M.; Wang, M.S.; Huang, W.K.; Zhang, Y.X.; Liu, F.; Guo, X.S. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef]
- Ni, K.K.; Yang, H.X.; Hua, W.; Wang, Y.P.; Pang, H.L. Selection and characterisation of lactic acid bacteria isolated from different origins for ensiling Robinia pseudoacacia and Morus alba L. leaves. J. Integr. Agric. 2016, 15, 2353–2362. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Deng, X.C.; Jia, Y.S.; Ge, G.T.; Wang, Z.J.; Liu, M.J.; Bao, J.; Zhao, M.Q.; Si, Q.; Liu, Y.C.; Zhao, W.X. Microbiomics and volatile metabolomics-based investigation of changes in quality and flavor of oat (Avena sativa L.) silage at different stages. Front. Plant Sci. 2023, 14, 1278715. [Google Scholar] [CrossRef] [PubMed]
- Squara, S.; Ferrero, F.; Tabacco, E.; Cordero, C.; Borreani, G. Effect of inoculation with Lentilactobacillus buchneri and Lacticaseibacillus paracasei on the maize silage volatilome: The advantages of advanced 2D-chromatographic fingerprinting approaches. J. Agric. Food Chem. 2022, 70, 12232–12248. [Google Scholar] [CrossRef]
- Chen, Y.G.; Wang, L.L.; Zhang, Y.W.; Zheng, N.; Zhang, Y.Q.; Zhang, Y.D. Investigation of volatile organic compounds of whole-plant corn silage using HS-SPME-GC-MS, HS-GC-IMS and E-Nose. Agriculture 2025, 15, 5. [Google Scholar] [CrossRef]
- Lorenzo, J.M. Influence of the type of fiber coating and extraction time on foal dry-cured loin volatile compounds extracted by solid-phase microextraction (SPME). Meat Sci. 2014, 96, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, K.; Xu, Y.; Thomas-Danguin, T. Perceptual interactions among food odors: Major influences on odor intensity evidenced with a set of 222 binary mixtures of key odorants. Food Chem. 2021, 353, 129483. [Google Scholar] [CrossRef]
- Noci, F.; Monahan, F.J.; Scollan, N.D.; Moloney, A.P. The fatty acid composition of muscle and adipose tissue of steers offered unwilted or wilted grass silage supplemented with sunflower oil and fishoil. Br. J. Nutr. 2007, 97, 502–513. [Google Scholar] [CrossRef][Green Version]
- Mishra, S.; Sachan, A.; Vidyarthi, A.S.; Sachan, S.G. Microbial production of 4-vinylguaiacol from ferulic acid by Bacillus cereus SAS-3006. Biocatal. Biotransform. 2014, 32, 259–266. [Google Scholar] [CrossRef]
- Zhao, K.; Tian, H.Q.; Ren, X.G.; Yu, Y.; Guo, L.N.; Li, Y.; Tao, Y.; Liu, F. Screening of key volatile compounds characterizing the deterioration of maize silage during aerobic exposure. Rev. Bras. Zootec.-Braz. J. Anim. Sci. 2024, 53, e20230042. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Lloyd, S.W.; Preece, J.E.; Moersfelder, J.W.; Stein-Chisholm, R.E.; Obando-Ulloa, J.M. Physicochemical properties and aroma volatile profiles in a diverse collection of California-grown pomegranate (Punica granatum L.) germplasm. Food Chem. 2015, 181, 354–364. [Google Scholar] [CrossRef]



| CAS | Compounds Name | Purity | Supplier | City | Country |
|---|---|---|---|---|---|
| 119-36-8 | Methyl salicylate | ≥99% | Accela | Shanghai | China |
| 4313-03-5 | (E, E)-2,4-Heptadienal | 98% | Adamas | Shanghai | China |
| 1629-58-9 | 1-Penten-3-one | 96% | Adamas | Shanghai | China |
| 90-05-1 | Guaiacol | 99% | Adamas | Shanghai | China |
| 928-97-2 | (E)-3-Hexen-1-ol | 97% | Aladdin | Shanghai | China |
| 2785-87-7 | 2-Methoxy-4-propylphenol | 98% | Aladdin | Shanghai | China |
| 7786-61-0 | 2-Methoxy-4-vinylphenol | 98% | Aladdin | Shanghai | China |
| 2785-89-9 | 4-Ethyl-2-methoxyphenol | 99% | Aladdin | Shanghai | China |
| 93-51-6 | Creosol | 98% | Ark Pharma | Beijing | China |
| 695-06-7 | Gamma-caprolactone | 98% | HWRK | Beijing | China |
| 143-08-8 | 1-Nonanol | 98% | Innochem | Beijing | China |
| 584-02-1 | 3-Pentanol | 99% | Innochem | Beijing | China |
| 64-19-7 | Acetic acid | 99% | Innochem | Beijing | China |
| 98-55-5 | Alpha-terpineol | 98% | Innochem | Beijing | China |
| 140-11-4 | Benzyl acetate | 99% | Innochem | Beijing | China |
| 100-51-6 | Benzyl alcohol | 98% | Innochem | Beijing | China |
| 106-30-9 | Ethyl heptanoate | 99% | Innochem | Beijing | China |
| 123-66-0 | Ethyl hexanoate | 99% | Innochem | Beijing | China |
| 97-64-3 | Ethyl lactate | 98% | Innochem | Beijing | China |
| 106-33-2 | Ethyl laurate | 99% | Innochem | Beijing | China |
| 123-51-3 | Isoamyl alcohol | 99% | Innochem | Beijing | China |
| 103-45-7 | Phenethyl acetate | 99% | Innochem | Beijing | China |
| 60-12-8 | Phenylethyl alcohol | 98% | Innochem | Beijing | China |
| 151-10-0 | 1,3-Dimethoxybenzene | 99% | J&K | Shanghai | China |
| 122-78-1 | Benzeneacetaldehyde | 97.5% | J&K | Shanghai | China |
| 123-92-2 | Isoamyl acetate | 99% | J&K | Shanghai | China |
| 25152-84-5 | (E, E)-2,4-Decadienal | >90% | Macklin | Shanghai | China |
| 2408-37-9 | 2,2,6-Trimethylcyclohexanone | 97% | Macklin | Shanghai | China |
| 78-92-2 | 2-Butanol | ≥99.8% | Macklin | Shanghai | China |
| 565-67-3 | 2-Methyl-3-pentanol | 99% | Macklin | Shanghai | China |
| 6032-29-7 | 2-Pentanol | ≥99.5% | Macklin | Shanghai | China |
| 1604-28-0 | 6-Methyl-3,5-heptadiene-2-one | 98% | Macklin | Shanghai | China |
| 61931-81-5 | Cis-3-hexenyllactate | 98% | Macklin | Shanghai | China |
| 93-89-0 | Ethyl benzoate | >99.5% | Macklin | Shanghai | China |
| 2396-83-0 | Ethyl hex-3-enoate | ≥98% | Macklin | Shanghai | China |
| 123-29-5 | Ethyl nonanoate | 98% | Macklin | Shanghai | China |
| 97-53-0 | Eugenol | 99% | Macklin | Shanghai | China |
| 19329-89-6 | Isoamyl lactate | >99.5% | Macklin | Shanghai | China |
| 91-20-3 | Naphthalene | ≥99.5% | Macklin | Shanghai | China |
| 108-95-2 | Phenol | ≥99.6% | Macklin | Shanghai | China |
| 79-77-6 | Beta-ionone | 95% | Mreda | Beijing | China |
| 138-86-3 | Limonene | 95% | Mreda | Beijing | China |
| 106-24-1 | Geraniol | 98% | Rhawn | Shanghai | China |
| 78-70-6 | Linalool | 98% | Rhawn | Shanghai | China |
| 13019-20-0 | 2-Methyl-3-heptanone | 98% | Sigma-Aldrich | Shanghai | China |
| / | N-alkanes (C7~C40) | 98% | Sigma-Aldrich | Shanghai | China |
| 6728-26-3 | (E)-2-Hexenal | 98% | Sigma-Aldrich | Shanghai | China |
| 2548-87-0 | (E)-2-Octenal | ≥95% | Sigma-Aldrich | Shanghai | China |
| 3777-69-3 | 2-Pentylfuran | ≥98% | Sigma-Aldrich | Shanghai | China |
| 110-93-0 | 6-Methylhept-5-en-2-one | 99% | Sigma-Aldrich | Shanghai | China |
| 142-92-7 | Hexyl acetate | 99% | Sigma-Aldrich | Shanghai | China |
| 97-54-1 | Isoeugenol | 99% | Sigma-Aldrich | Shanghai | China |
| 124-07-2 | Octanoic acid | >99% | Sigma-Aldrich | Shanghai | China |
| 928-95-0 | (E)-2-Hexen-1-ol | >95% | TCI | Shanghai | China |
| 18409-17-1 | (E)-2-octen-1-ol | >92% | TCI | Shanghai | China |
| 111-27-3 | 1-Hexanol | 98% | TCI | Shanghai | China |
| 123-07-9 | 4-Ethylphenol | >97% | TCI | Shanghai | China |
| 100-52-7 | Benzaldehyde | >98% | TCI | Shanghai | China |
| 17283-81-7 | Dihydro-beta-ionone | >90% | TCI | Shanghai | China |
| 2021-28-5 | Ethyl 3-phenylpropanoate | >98% | TCI | Shanghai | China |
| 106-32-1 | Ethyl caprylate | >98% | TCI | Shanghai | China |
| 628-97-7 | Ethyl palmitate | >97% | TCI | Shanghai | China |
| 66-25-1 | Hexanal | >98% | TCI | Shanghai | China |
| 142-62-1 | Hexanoic acid | >98% | TCI | Shanghai | China |
| 108-38-3 | m-Xylene | >99% | TCI | Shanghai | China |
| 124-13-0 | Octanal | >98% | TCI | Shanghai | China |
| 100-42-5 | Styrene | >99% | TCI | Shanghai | China |
| 589-98-0 | 3-Octanol | ≥98% | Yuanye | Shanghai | China |
| 122-97-4 | 3-Phenylpropanol | ≥98% | Yuanye | Shanghai | China |
| 432-25-7 | Beta-cyclocitral | ≥96% | Yuanye | Shanghai | China |
| 17092-92-1 | Dihydroactinidiolide | ≥98% | Yuanye | Shanghai | China |
| 101-97-3 | Ethyl phenylacetate | ≥99.5% | Yuanye | Shanghai | China |
| 104-61-0 | Gamma-nonanolactone | ≥98% | Yuanye | Shanghai | China |
| Items | CK | LP | LB | p-Value |
|---|---|---|---|---|
| DM (% FW) | 28.76 ± 0.78 a | 25.09 ± 0.59 b | 25.95 ± 0.86 b | 0.002 |
| NDF (% DM) | 36.46 ± 2.73 a | 31.54 ± 0.66 b | 32.24 ± 0.60 b | 0.022 |
| ADF (% DM) | 20.07 ± 1.32 a | 17.56 ± 0.25 b | 17.72 ± 0.28 b | 0.014 |
| Ash (% DM) | 3.24 ± 0.06 a | 3.53 ± 0.12 a | 3.22 ± 0.29 a | 0.156 |
| Starch (% DM) | 37.52 ± 1.78 a | 33.33 ± 0.60 b | 31.69 ± 1.27 b | 0.004 |
| Items | CK | LP | LB | p-Value |
|---|---|---|---|---|
| pH | 4.21 ± 0.05 a | 3.81 ± 0.08 c | 3.96 ± 0.05 b | <0.001 |
| LA (% DM) | 5.44 ± 0.16 b | 6.03 ± 0.21 a | 5.69 ± 0.03 b | 0.008 |
| AA (% DM) | 1.60 ± 0.12 b | 1.88 ± 0.03 ab | 2.14 ± 0.25 a | 0.017 |
| PA (% DM) | 0.17 ± 0.02 a | 0.12 ± 0.01 b | 0.14 ± 0.01 b | 0.01 |
| BA (% DM) | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.422 |
| Number | CAS | Compounds | OT A | Odor Descriptions B | rOAV | VIP-Value | ||
|---|---|---|---|---|---|---|---|---|
| CK | LP | LB | ||||||
| Alcohols | ||||||||
| 1 | 123-51-3 | Isoamyl alcohol | 4 | Burnt, cocoa, floral | 13.27 | 12.37 | 18.73 | 0.29 |
| 2 | 111-27-3 | 1-Hexanol | 5.6 | Banana, flower, grass | 44.86 | 39.20 | 29.26 | 0.77 |
| 3 | 928-97-2 | (E)-3-Hexen-1-ol | 110 | Green | 7.33 | 7.87 | 5.78 | 1.30 |
| 4 | 589-98-0 | 3-Octanol | 78 | Citrus, moss, mushroom | 0.26 | 0.21 | 0.29 | 0.11 |
| 5 | 78-70-6 | Linalool | 0.22 | Coriander, floral, lavender | 513.19 | 668.39 | 650.57 | 0.50 |
| 6 | 111-87-5 | 1-Octanol | 125.8 | Bitter almond, fat, floral | 0.33 | 0.40 | 0.39 | 0.17 |
| 7 | 18409-17-1 | (E)-2-Octen-1-ol | 40 | Green, citrus, vegetable | 0.51 | 0.55 | 0.46 | 0.06 |
| 8 | 143-08-8 | 1-Nonanol | 45.5 | Fat, floral, green | 0.72 | 0.79 | 0.71 | 0.03 |
| 9 | 100-51-6 | Benzyl alcohol | 2546.21 | Boiled cherries, moss, roasted bread | 0.11 | 0.11 | 0.13 | 2.07 |
| 10 | 60-12-8 | Phenylethyl Alcohol | 564.23 | Fruit, honey, lilac | 0.16 | 0.15 | 0.13 | 0.19 |
| Aldehydes | ||||||||
| 11 | 66-25-1 | Hexanal | 5 | Apple, fat, green | 2.72 | 3.23 | 2.48 | 0.06 |
| 12 | 6728-26-3 | (E)-2-Hexenal | 88.5 | Green, leafy, fruity | 0.21 | 0.18 | ND | 0.09 |
| 13 | 124-13-0 | Octanal | 0.59 | Citrus, fat, green | 11.78 | 14.81 | 14.09 | 0.04 |
| 14 | 2548-87-0 | (E)-2-Octenal | 3 | Dandelion, fat, fruit | 49.89 | 63.47 | 37.84 | 0.84 |
| 15 | 4313-03-5 | (E,E)-2,4-Heptadienal | 15.4 | Fat, nut | 5.59 | ND | ND | 1.85 |
| 16 | 100-52-7 | Benzaldehyde | 750.89 | Bitter almond, burnt sugar, cherry | 4.13 | 4.11 | 2.54 | 4.56 |
| 17 | 432-25-7 | beta-Cyclocitral | 5 | Tropical, saffron, herbal | 7.32 | 7.48 | 6.32 | 0.02 |
| 18 | 122-78-1 | Benzeneacetaldehyde | 6.3 | Berry, geranium, honey | 43.12 | 45.84 | 34.44 | 0.37 |
| 19 | 25152-84-5 | (E,E)-2,4-Decadienal | 0.03 | Coriander, deep fried, fat | 204.08 | 471.6 | 131.96 | 0.17 |
| Esters | ||||||||
| 20 | 123-92-2 | Isoamyl acetate | 0.15 | Apple, banana, pear | 116.59 | 111.80 | 238.25 | 0.18 |
| 21 | 123-66-0 | Ethyl hexanoate | 5 | Apple peel, brandy, fruit gum | 35.67 | 29.15 | 27.00 | 0.91 |
| 22 | 142-92-7 | Hexyl acetate | 115 | Apple, banana, grass | 1.20 | 1.09 | 1.19 | 0.53 |
| 23 | 106-30-9 | Ethyl heptanoate | 1.9 | Brandy, fruit, wine | 5.44 | 4.99 | ND | 0.05 |
| 24 | 106-32-1 | Ethyl caprylate | 19.3 | Apricot, brandy, fat | 4.63 | 3.15 | 3.53 | 0.85 |
| 25 | 93-89-0 | Ethyl benzoate | 55.56 | Camomile, celery, fat | 0.98 | 1.05 | 0.81 | 0.15 |
| 26 | 695-06-7 | gamma-Caprolactone | 260 | Herbal, coconut, sweet | 0.12 | 0.15 | 0.13 | 0.11 |
| 27 | 140-11-4 | Benzyl acetate | 364 | Fruit | 0.20 | 0.22 | 0.19 | 0.11 |
| 28 | 119-36-8 | Methyl salicylate | 40 | Almond, caramel, peppermint | 6.03 | 7.20 | 5.71 | 1.06 |
| 29 | 104-61-0 | gamma-Nonanolactone | 9.7 | Coconut, creamy, waxy | 6.90 | 7.61 | 4.78 | 0.16 |
| Hydrocarbons | ||||||||
| 30 | 3777-69-3 | 2-Pentylfuran | 5.8 | Butter, floral, fruit | 2.76 | 2.40 | 2.38 | 0.05 |
| 31 | 100-42-5 | Styrene | 65 | Sweet, balsam, floral | 4.82 | 4.96 | 5.60 | 0.84 |
| 32 | 91-20-3 | Naphtalene | 6 | Pungent, dry, tarry | ND | 1.49 | ND | 0.22 |
| 33 | 30364-38-6 | Dehydro-ar-ionene | 2.5 | Licorice | 8.25 | 9.58 | 10.19 | 0.04 |
| Ketones | ||||||||
| 34 | 1629-58-9 | 1-Penten-3-one | 23 | Fish, green, mustard | 0.59 | 0.63 | 0.42 | 0.03 |
| 35 | 2408-37-9 | 2,2,6-trimethylcyclohexanone | 100 | Floral | 0.14 | 0.14 | 0.13 | 0.02 |
| 36 | 110-93-0 | 6-Methylhept-5-en-2-one | 68 | Citrus, mushroom, pepper | 0.46 | 0.55 | 0.53 | 0.16 |
| Phenols | ||||||||
| 37 | 90-05-1 | Guaiacol | 0.84 | Burnt, smoke, woody | 73.29 | 75.50 | 58.14 | 0.08 |
| 38 | 93-51-6 | Creosol | 21 | Spicy, clove, vanilla | 9.09 | 10.57 | 7.56 | 0.49 |
| 39 | 2785-89-9 | 4-Ethyl-2-methoxyphenol | 89.25 | Spicy, smoky, bacon | 23.06 | 20.02 | 19.10 | 4.75 |
| 40 | 97-53-0 | Eugenol | 0.71 | Sweet, spicy, clove | 110.75 | 129.32 | 131.95 | 0.36 |
| 41 | 123-07-9 | 4-Ethylphenol | 13 | Leather, spice, stable | 35.81 | 36.76 | 27.68 | 0.91 |
| 42 | 7786-61-0 | 2-Methoxy-4-vinylphenol | 12.02 | Clove, curry, spice | 2.05 | 2.20 | 1.22 | 0.07 |
| 43 | 2628-17-3 | 4-Vinylphenol | 10 | Chemical, sweet | 0.59 | 0.50 | 0.40 | 0.05 |
| Terpenoids | ||||||||
| 44 | 138-86-3 | Limonene | 34 | Citrus, mint, orange | 0.13 | 0.29 | 0.30 | 0.13 |
| 45 | 23726-93-4 | Damascenone | 0.06 | Apple, rose, honey | 229.87 | 316.27 | 251.57 | 0.07 |
| 46 | 17283-81-7 | Dihydro-beta-ionone | 1 | Violet-like | 10.82 | 16.65 | 10.92 | 0.14 |
| 47 | 106-24-1 | Geraniol | 6.6 | Geranium, lemon peel, peach | 0.68 | 0.85 | 0.71 | 0.02 |
| 48 | 79-77-6 | beta-Ionone | 0.01 | Floral, woody | 1009.11 | 1197.32 | 1176.78 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, S.; Wang, J.; Yang, J.; Li, Y.; He, J.; Han, J.; Xu, H.; Zhu, X.; Ai, N. Differential Modulation of Maize Silage Odor: Lactiplantibacillus plantarum vs. Lactiplantibacillus buchneri Drive Volatile Compound Change via Strain-Specific Fermentation. Agriculture 2025, 15, 2109. https://doi.org/10.3390/agriculture15202109
Xue S, Wang J, Yang J, Li Y, He J, Han J, Xu H, Zhu X, Ai N. Differential Modulation of Maize Silage Odor: Lactiplantibacillus plantarum vs. Lactiplantibacillus buchneri Drive Volatile Compound Change via Strain-Specific Fermentation. Agriculture. 2025; 15(20):2109. https://doi.org/10.3390/agriculture15202109
Chicago/Turabian StyleXue, Shuyuan, Jianfeng Wang, Jing Yang, Yunjie Li, Jian He, Jiyu Han, Hongyan Xu, Xun Zhu, and Nasi Ai. 2025. "Differential Modulation of Maize Silage Odor: Lactiplantibacillus plantarum vs. Lactiplantibacillus buchneri Drive Volatile Compound Change via Strain-Specific Fermentation" Agriculture 15, no. 20: 2109. https://doi.org/10.3390/agriculture15202109
APA StyleXue, S., Wang, J., Yang, J., Li, Y., He, J., Han, J., Xu, H., Zhu, X., & Ai, N. (2025). Differential Modulation of Maize Silage Odor: Lactiplantibacillus plantarum vs. Lactiplantibacillus buchneri Drive Volatile Compound Change via Strain-Specific Fermentation. Agriculture, 15(20), 2109. https://doi.org/10.3390/agriculture15202109







