Unveiling the Regulatory Mechanisms of Irradiation Response in Pseudococcus jackbeardsleyi Under Hypoxic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Large-Scale Confirmatory Tests
2.2.1. Modified Atmosphere Treatment
2.2.2. Phytosanitary Irradiation Treatment
2.2.3. Rearing of Mealybugs After Irradiation
2.3. Transcriptional Analysis of P. jackbeardsleyi
2.4. Network Construction and Regulatory Inference
2.5. Reverse Transcription Quantitative PCR (RT-qPCR) Validation
3. Results
3.1. Confirmatory Tests
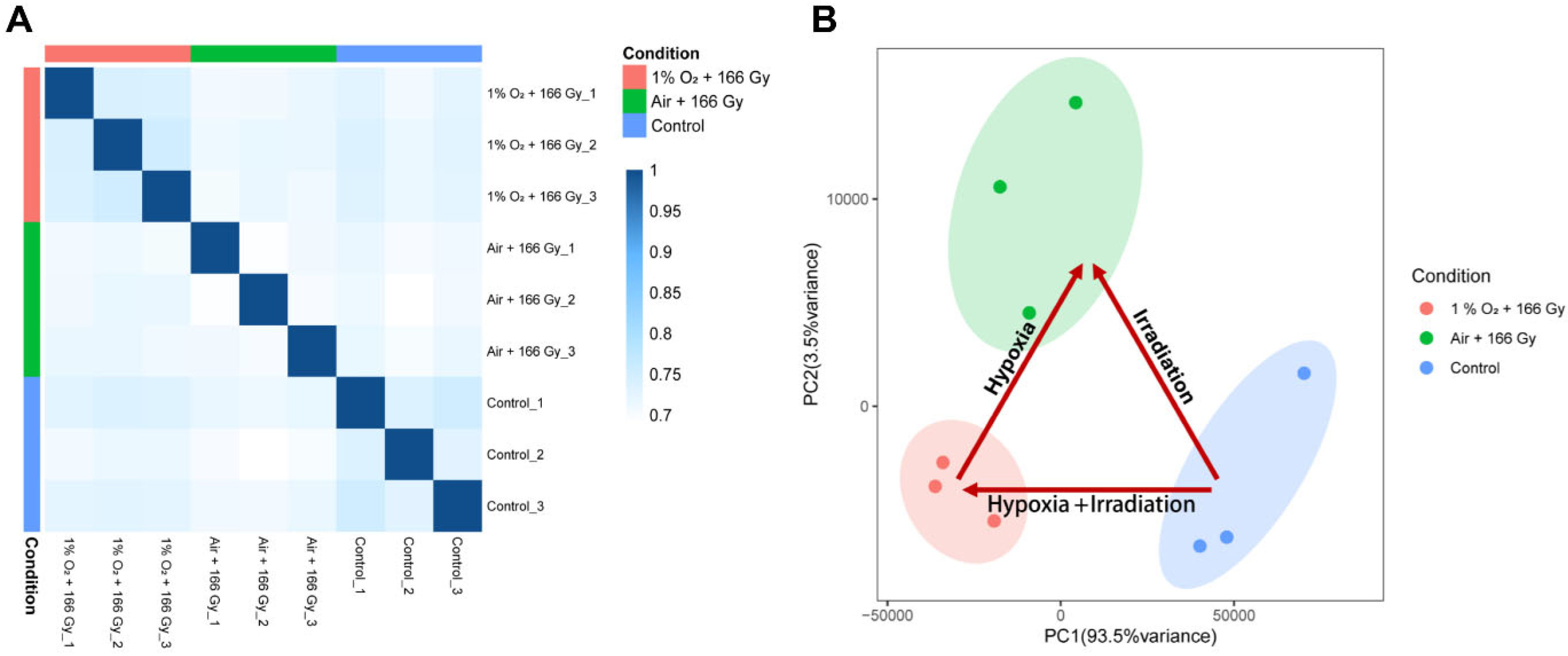
3.2. General Description of RNA-Seq Data
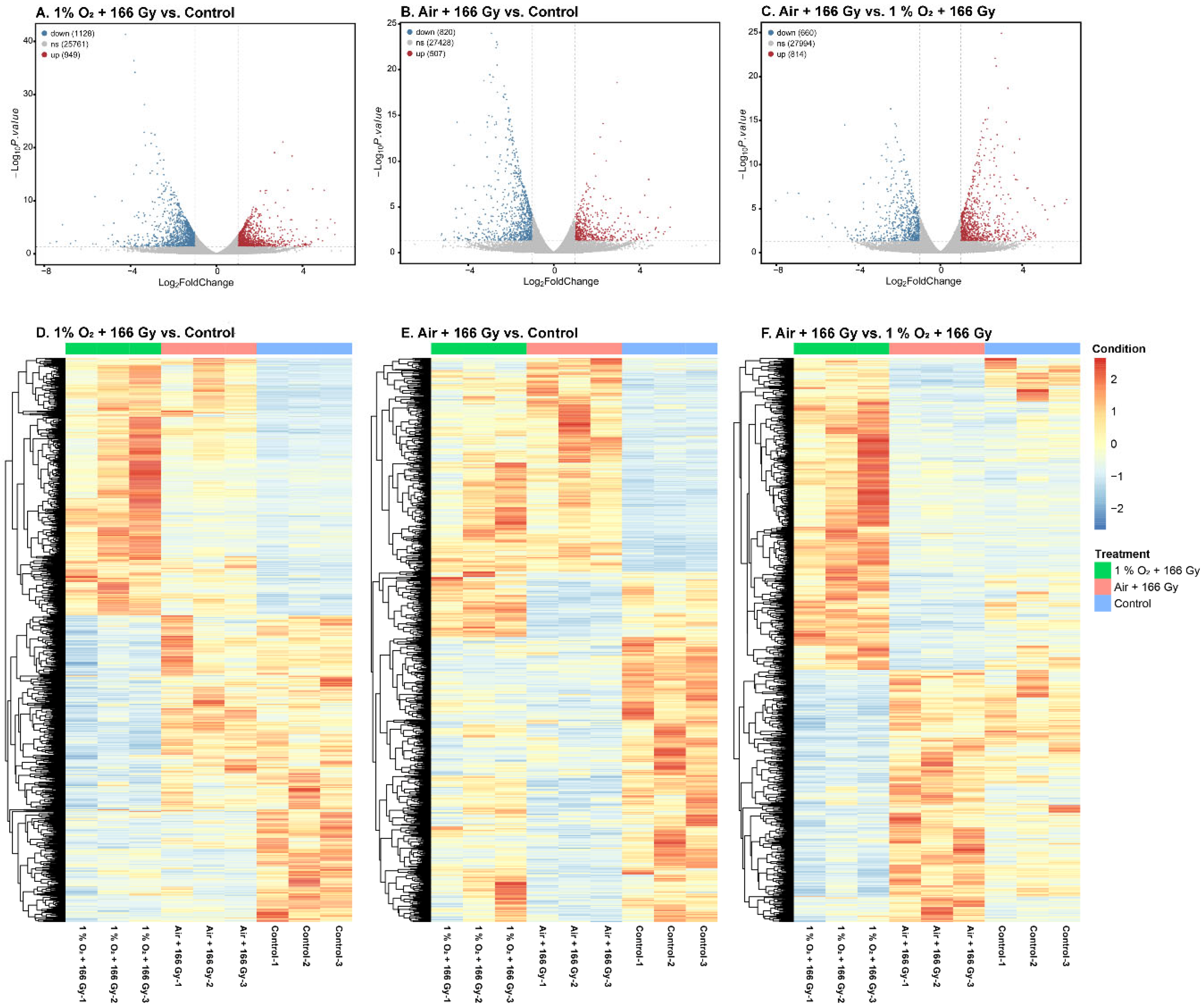
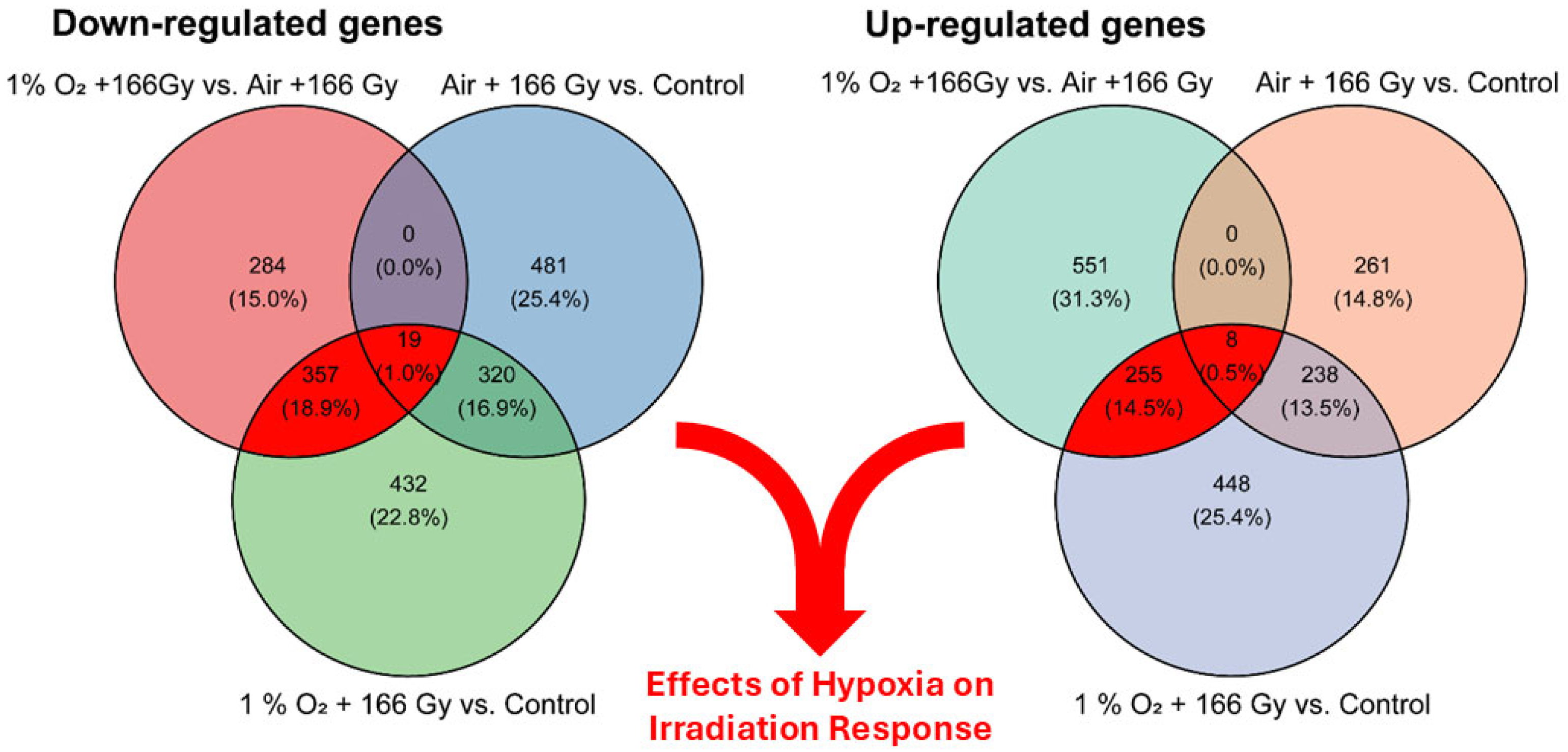
3.3. Analysis of Differentially Expressed Genes (DEGs)
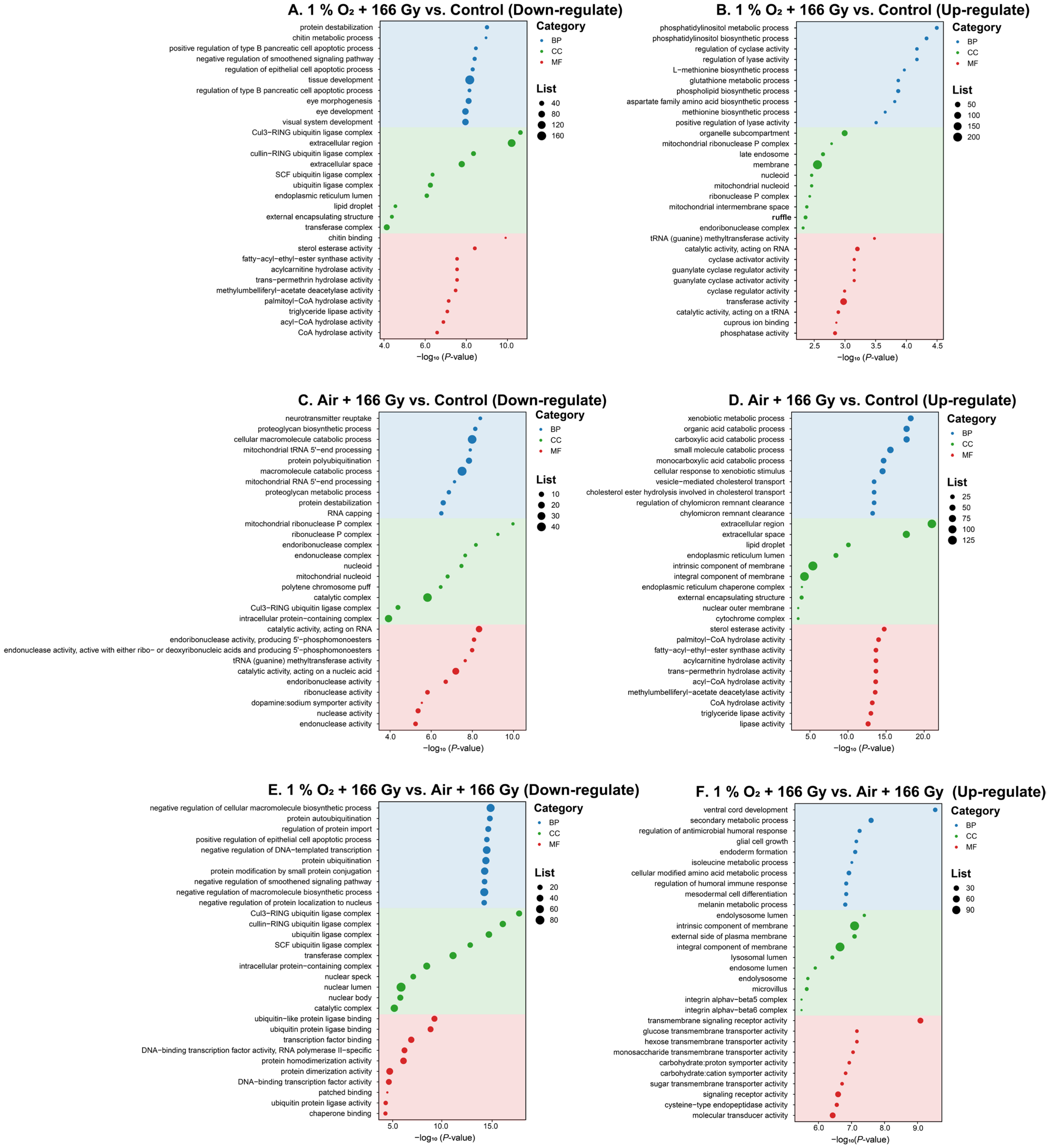
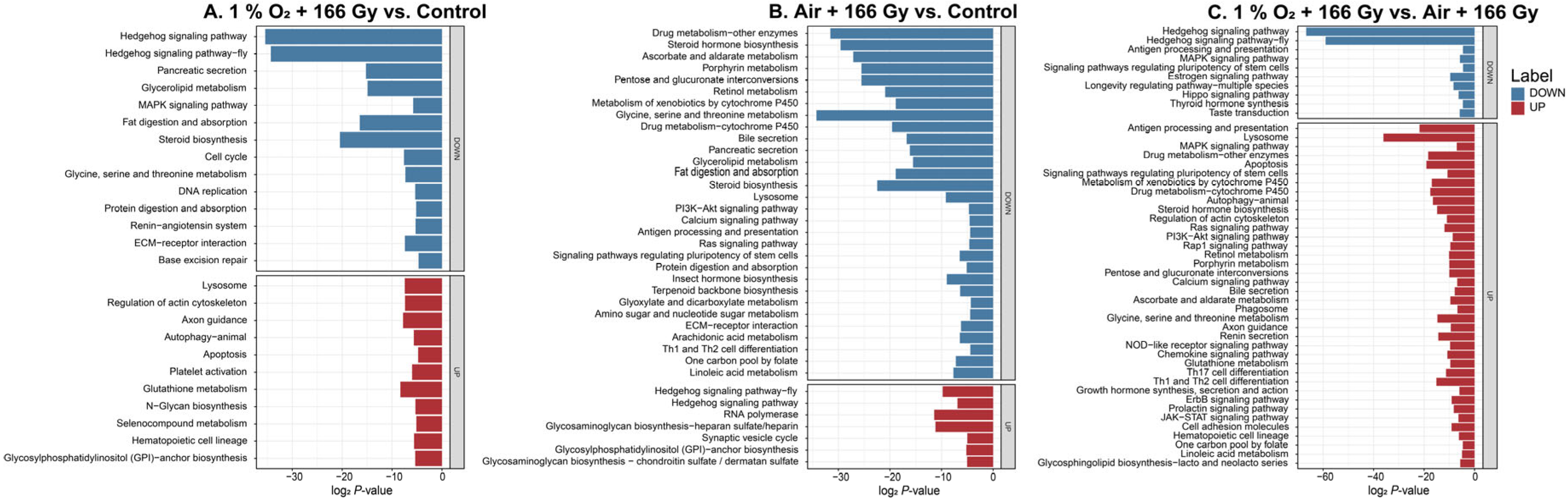
3.4. Functional Annotation and Enrichment Analysis of DEGs
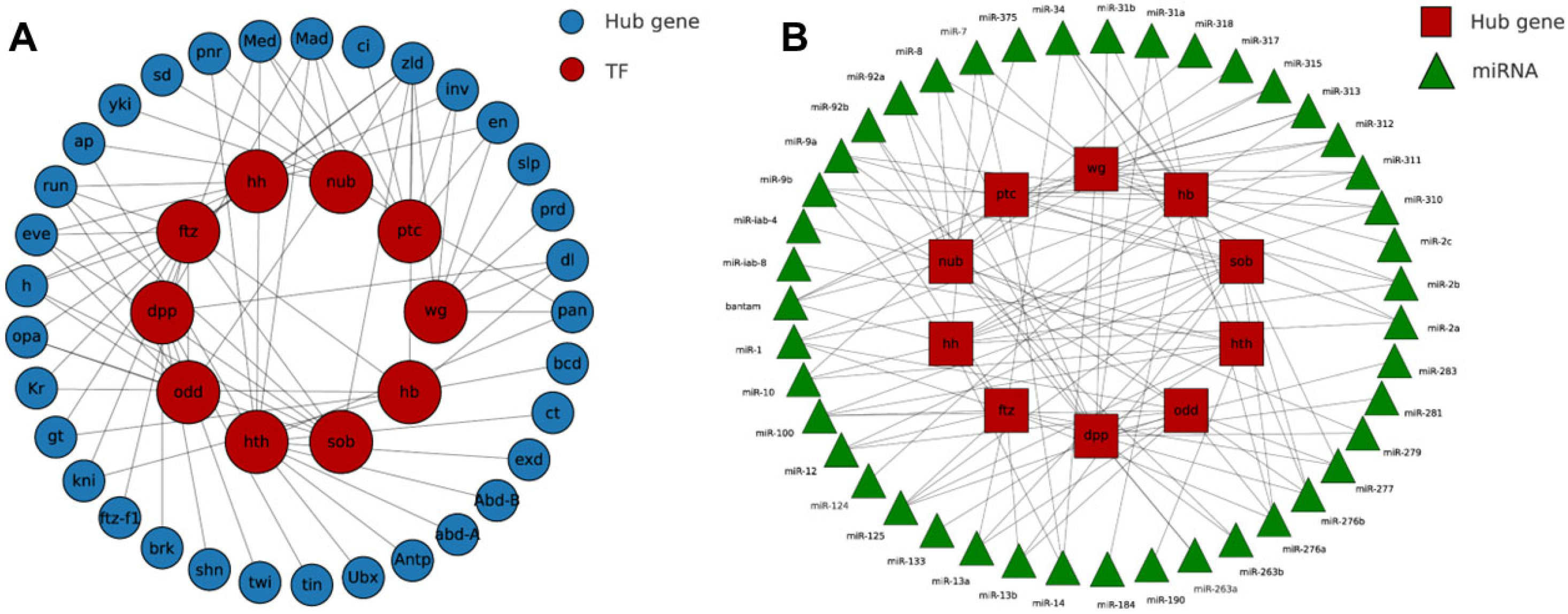
3.5. PPI Network and Hub-Gene Behavior Under Hypoxic Irradiation
3.6. Layered Regulation Under Hypoxic Irradiation Revealed by TF–Hub and miRNA–Hub Networks
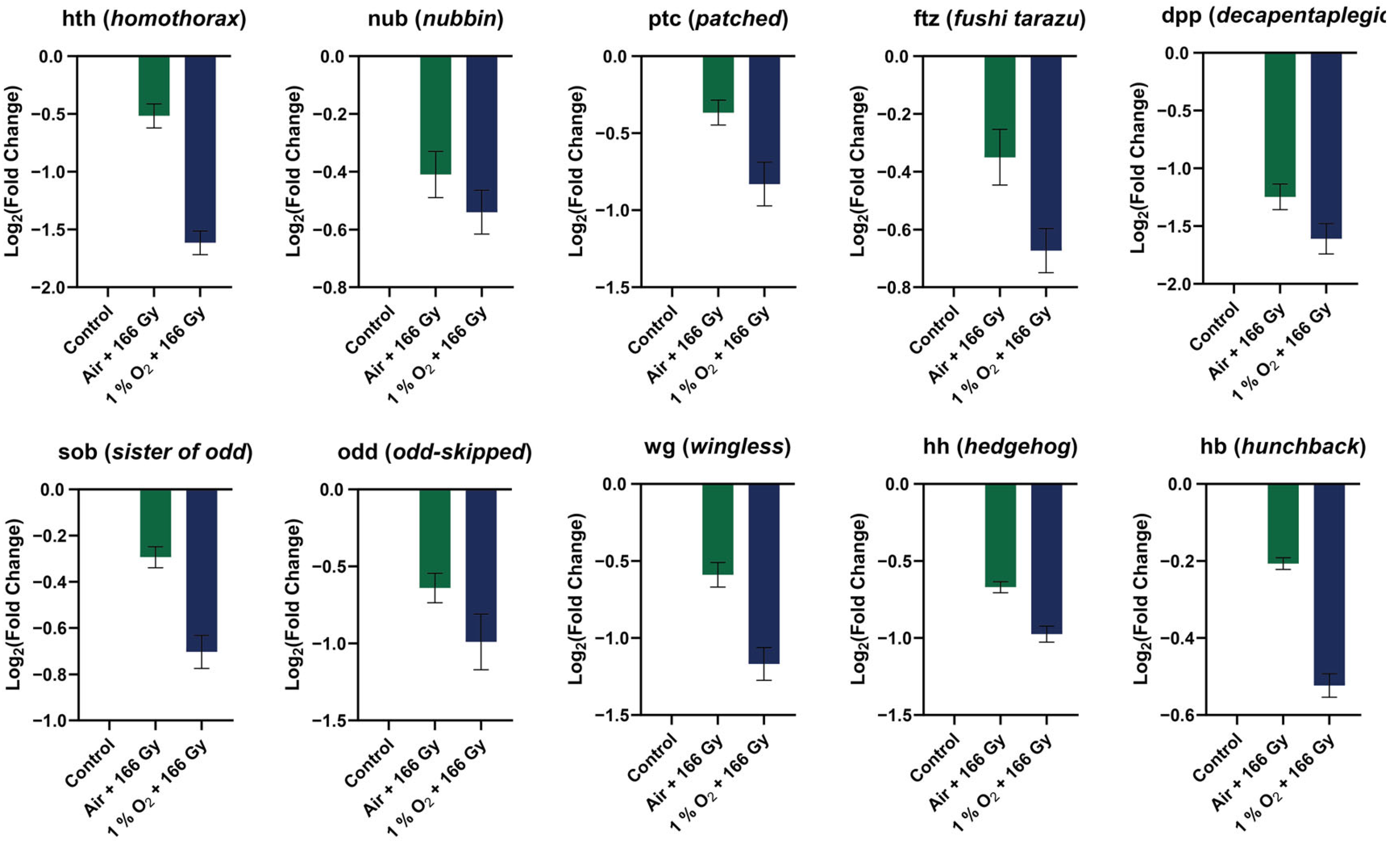
3.7. Validation of RNA-Seq Results by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Follett, P.A. Phytosanitary irradiation for fresh horticultural commodities: Generic treatments, current issues, and next steps. Stewart Postharvest Rev. 2014, 10, 1–7. [Google Scholar]
- Akter, H.; Cunningham, N.; Rempoulakis, P.; Bluml, M. An Overview of Phytosanitary Irradiation Requirements for Australian Pests of Quarantine Concern. Agriculture 2023, 13, 771. [Google Scholar] [CrossRef]
- Karoney, E.M.; Molelekoa, T.; Bill, M.; Siyoum, N.; Korsten, L. Global research network analysis of fresh produce postharvest technology: Innovative trends for loss reduction. Postharvest Biol. Technol. 2024, 208, 112642. [Google Scholar] [CrossRef]
- ISPM 28: PT 7; Phytosanitary Treatments for Regulated Pests—PT 7: Irradiation Treatment for Fruit Flies of the Family Tephritidae. The International Plant Protection Convention (IPPC); Food and Agriculture Organization of the United Nations (FAO): Rome Italy, 2016.
- ISPM 28: PT 19; Phytosanitary Treatments for Regulated Pests—PT 19: Irradiation Treatment for Dysmicoccus neobrevipes, Planococcus lilacinus and Planococcus minor. The International Plant Protection Convention (IPPC); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2016.
- ISPM 28: PT 45; Phytosanitary Treatments for Regulated Pests—PT 45: Irradiation Treatment for Pseudococcus jackbeardsleyi. The International Plant Protection Convention (IPPC); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023.
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetables: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Li, B.; Li, L.; Du, X.; Ren, Y.; McKirdy, S.J.; Liu, T. Comparison of fumigation efficacy of methyl bromide alone and phosphine applied either alone or simultaneously or sequentially against Bactrocera correcta in Selenicereus undatus (red pitaya) fruit. Pest Manag. Sci. 2023, 79, 4942–4951. [Google Scholar] [CrossRef]
- Patil, V.; Baswal, A.K.; Gupta, A.; Mahajan, B.V.C.; Ozturk, B.; Gill, K.S. Impact of Post-harvest Application of Hydroxypropyl Methylcellulose and Methyl Cellulose Based Edible Coatings on Storage Life and Quality of Cold-Stored Strawberry Fruit Cv.‘Winter Dawn’. Appl. Fruit Sci. 2025, 67, 110. [Google Scholar] [CrossRef]
- Hu, A.; Qiu, R.; Wu, Z.; Zhang, H.; Li, W.B.; Li, J. A computational model for oxygen depletion hypothesis in FLASH effect. Radiat. Res. 2022, 197, 175–183. [Google Scholar] [CrossRef]
- Follett, P.A.; Neven, L.G. Current trends in quarantine entomology. Annu. Rev. Entomol. 2006, 51, 359–385. [Google Scholar] [CrossRef]
- Ic, E.; Cetinkaya, N. Food safety and irradiation related sanitary and phytosanitary approaches-Chinese perspective. Radiat. Phys. Chem. 2021, 181, 109324. [Google Scholar] [CrossRef]
- Lengai, G.M.; Fulano, A.M.; Muthomi, J.W. Improving access to export market for fresh vegetables through reduction of phytosanitary and pesticide residue constraints. Sustainability 2022, 14, 8183. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Yang, X.; Wang, D.; Wang, F. Modeling the potential global distribution of the invasive Jack Beardsley mealybug (Hemiptera: Pseudococcidae) under climate change. J. Econ. Entomol. 2025, 118, 589–599. [Google Scholar] [CrossRef] [PubMed]
- More, V.; Hajare, S.N.; Gautam, S. Combination treatment including irradiation improved the keeping quality of bitter melon (Momordica charantia L) with retention of functional bioactives while fulfilling phytosanitary requirement for export. Radiat. Phys. Chem. 2022, 195, 110040. [Google Scholar] [CrossRef]
- Simoneau, J.; Dumontier, S.; Gosselin, R.; Scott, M.S. Current RNA-seq methodology reporting limits reproducibility. Brief. Bioinform. 2021, 22, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.T.; Brustolini, O.J.B.; Martins, Y.C.; Maia, M.A.G.M.; de Vasconcelos, A.T.R. Inference of differentially expressed genes using generalized linear mixed models in a pairwise fashion. PeerJ 2023, 11, e15145. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Zhang, Y.; Beachy, P.A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687. [Google Scholar] [CrossRef]
- da Vinha, A.C.M.F.; Sousa e Silva, C.A.d.A. Overview of irradiation: Advantages to foods of plant origin. S. Fla. J. Health 2022, 3, 248–262. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Li, R.; Wang, G.; Tan, H.; Zhou, Z. Rapid adaptive response of population fitness of Zeugodacus tau during host shifts and implications for integrated pest management strategies. Pest Manag. Sci. 2025, 81, 6525–6537. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, R.; Zhou, W.; Pan, Y.; Tian, H.; Yin, Z.; Chen, W. Fruit fly in a challenging environment: Impact of short-term temperature stress on the survival, development, reproduction, and trehalose metabolism of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2022, 13, 753. [Google Scholar] [CrossRef]
- Couey, H.M.; Chew, V. Confidence limits and sample size in quarantine research. J. Econ. Entomol. 1986, 79, 887–890. [Google Scholar] [CrossRef]
- ISPM 18; Guidelines for the Use of Irradiation as a Phytosanitary measure. The International Plant Protection Convention (IPPC); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2003.
- Koch, C.J. Oxygen Effects in Radiobiology.; Springer: Boston, MA, USA, 1982; pp. 123–144. [Google Scholar]
- Hallman, G.J.; Loaharanu, P. Generic ionizing radiation quarantine treatments against fruit flies (Diptera: Tephritidae) proposed. J. Econ. Entomol. 2002, 95, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Li, B.; Li, L.; Li, B.; Ren, Y.; Liu, T. Correlation between irradiation treatment and metabolite changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae using solid-phase microextraction (SPME) coupled with gas chromatography-mass spectrometry (GC-MS). Molecules 2022, 27, 4641. [Google Scholar] [CrossRef] [PubMed]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with tumor hypoxia for radiotherapy optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Zhang, N.; Yu, Q.; Huang, G. Advances in mitochondrial dysfunction in radiation tissue injury. Front. Physiol. 2025, 16, 1660330. [Google Scholar] [CrossRef]
- Gardner, L.L.; Thompson, S.J.; O’Connor, J.D.; McMahon, S.J. Modelling radiobiology. Phys. Med. Biol. 2024, 69, 18TR01. [Google Scholar] [CrossRef]
- Hallman, G.J.; Loaharanu, P. Phytosanitary irradiation–Development and application. Radiat. Phys. Chem. 2016, 129, 39–45. [Google Scholar] [CrossRef]
- Shan, C.; Li, B.; Li, L.; Liu, Q.; Zou, H.; Liu, T. Development and Metabolomic Profiles of Bactrocera dorsalis (Diptera: Tephritidae) Larvae Exposed to Phytosanitary Irradiation Dose in Hypoxic Environment Using DI-SPME-GC/MS. Insects 2024, 15, 177. [Google Scholar] [CrossRef]
- Suplicy Filho, N.; Calza, R.; Paiva, J.A.A.; Glória, M.B.; Oliveira, D.A.; Raga, A. Evaluation of ionizing radiation in the control of Ceratitis capitata (Wied., 1824) (Diptera-Tephritidae). Arq. Inst. Biol. 2025, 54, 49–55. [Google Scholar] [CrossRef]
- Penca, C.; Beam, A.L.; Bailey, W.D. The applicability of species sensitivity distributions to the development of generic doses for phytosanitary irradiation. Sci. Rep. 2023, 13, 2358. [Google Scholar] [CrossRef]
- Buckingham, G.R. Role of quarantine facilities in biological control. Fla. Entomol. 1992, 75, 414–420. [Google Scholar] [CrossRef]
- Hallman, G.J. Phytosanitary applications of irradiation. Compr. Rev. Food Sci. Food Saf. 2011, 10, 143–151. [Google Scholar] [CrossRef]
- Chen, C.; Condon, C.H.; Boardman, L.; Meagher, R.L.; Jeffers, L.A.; Beam, A.; Bailey, W.D.; Hahn, D.A. Critical PO2 as a diagnostic biomarker for the effects of low-oxygen modified and controlled atmospheres on phytosanitary irradiation treatments in the cabbage looper Trichoplusia ni (Hübner). Pest Manag. Sci. 2022, 76, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R.; et al. Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosoph suzukii. J. Pest Sci. 2018, 91, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyr, H.; Indarwatmi, M.; Seth, R.; Zhan, G. Development of a generic radiation dose for the postharvest phytosanitary treatment of mealybug species (Hemiptera: Pseudococcidae). Fla. Entomol. 2016, 99, 191–196. [Google Scholar]
- Follett, P.; Hamilton, L.; Tagami, Y.; Kaluna, L.; Jarvi, S. Phytosanitary irradiation using X-rays prevents reproduction in the semi-slug Parmarion martensi (Stylommatophora: Ariophantidae), a host of the human pathogenic nematode Angiostrongylus cantonensis (Rhabditida: Angiostrongylidae). Pest Manag. Sci. 2022, 78, 1187–1193. [Google Scholar] [CrossRef]
- Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.; Parker, A.G.; Myers, S.W. Modified atmosphere does not reduce the efficacy of phytosanitary irradiation doses recommended for tephritid fruit flies. Insects 2020, 11, 371. [Google Scholar] [CrossRef]
- Borggrefe, T.; Lauth, M.; Zwijsen, A.; Huylebroeck, D.; Oswald, F.; Giaimo, B.D. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia pathways. Biochim. Biophys. Acta 2016, 1863, 303–313. [Google Scholar] [CrossRef]

| Treatment | Absorbed Dose (Gy) | DUR | No. Potatoes | No. Late Females | Developed for F1 Generation | |
|---|---|---|---|---|---|---|
| No. of Neonates | No. of 2nd Instars | |||||
| Air + 166 Gy | 166–189 | 1.14 | 50 | 3967 | 3710 | 0 |
| Control | 0 | − | 5 | 400 | 3892 | 3764 |
| 1% O2 + 166 Gy | 164.8–185.4 | 1.13 | 50 | 3514 | 3620 | 0 |
| Control | 0 | − | 5 | 380 | 3944 | 3721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Shan, C.; Xu, Q.; Li, B.; Liu, H.; Liu, T. Unveiling the Regulatory Mechanisms of Irradiation Response in Pseudococcus jackbeardsleyi Under Hypoxic Conditions. Agriculture 2025, 15, 2104. https://doi.org/10.3390/agriculture15202104
Li L, Shan C, Xu Q, Li B, Liu H, Liu T. Unveiling the Regulatory Mechanisms of Irradiation Response in Pseudococcus jackbeardsleyi Under Hypoxic Conditions. Agriculture. 2025; 15(20):2104. https://doi.org/10.3390/agriculture15202104
Chicago/Turabian StyleLi, Li, Changyao Shan, Qiang Xu, Baishu Li, Haijun Liu, and Tao Liu. 2025. "Unveiling the Regulatory Mechanisms of Irradiation Response in Pseudococcus jackbeardsleyi Under Hypoxic Conditions" Agriculture 15, no. 20: 2104. https://doi.org/10.3390/agriculture15202104
APA StyleLi, L., Shan, C., Xu, Q., Li, B., Liu, H., & Liu, T. (2025). Unveiling the Regulatory Mechanisms of Irradiation Response in Pseudococcus jackbeardsleyi Under Hypoxic Conditions. Agriculture, 15(20), 2104. https://doi.org/10.3390/agriculture15202104






