Abstract
Strawberry crown and root rot diseases are caused by soil-borne pathogens including Macrophomina phaseolina (Mp) and Verticillium dahliae (Vd). The symptoms caused by these pathogens are very similar and difficult to distinguish, and traditional culture-based detection methods are laborious, time-consuming, and slow in providing results. In this work, we developed a duplex PCR-NALFIA assay using two pairs of species-specific primers labeled at the 5′ end with different molecules for the simultaneous identification of Mp and Vd. For the NALFIA assay, a lateral flow device (LFD) for the detection of two analytes was used. The method was developed by single and duplex PCR (Mp, Vd, Mp + Vd) using increasingly complex biological systems: (i) DNA from pure cultures of the pathogens; (ii) DNA from artificially inoculated cut melon stems; and (iii) DNA from artificially inoculated strawberry plants cv. Aromas. The duplex PCR protocol was effective in detecting the two pathogens within melon tissues and provided good results with strawberry crown tissues only when the DNA samples were purified by removing the PCR inhibitors. The amplicons were used for both agarose gel electrophoresis (AGE) and NALFIA assays and demonstrated the greater sensitivity of the NALFIA assay (10 pg) for simultaneous detection of the two pathogens.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) belongs to the Rosaceae family and is one of the most popular fruits in the world. Strawberries are grown commercially in 76 countries around the world [1], and based on FAO reports, global strawberry production has exceeded 9.56 million tons worth over 25.5 billion dollars in 2022 [2].
The health benefits deriving from the consumption of strawberries are linked to their richness in vitamins, minerals, fiber, and compounds with antioxidant and anti-inflammatory properties [3].
Strawberry cultivation is susceptible to soil-borne pathogens that persist within the soil matrix and in residues on the soil surface. These pathogens are distributed globally, and without adequate disease management, they cause significant losses to the strawberry industry every year [4,5].
Historically, methyl bromide was utilized for pre-plant soil fumigation to manage soil-borne pathogens [6]. However, due to its negative environmental impact, it was phased out worldwide with the Montreal Protocol in 1986 to preserve the ozone layer, which protects the Earth from ultraviolet radiation (UV) [7,8]. As a result, soil-borne pathogens have become a major problem in many strawberry-producing countries [9,10,11].
Two of the most important yield-reducing pathogens in this category are the fungi Macrophomina phaseolina (Tassi) Goid and Verticillium dahliae Kleb., both of which cause crown and root rot diseases [12,13].
M. phaseolina is a soil- and seed-borne generalist necrotrophic fungal pathogen with a wide distribution, capable of infecting over 500 plant species in more than 100 families [14]. Its importance is particularly accentuated in the context of climate change, especially in conditions of increased thermal stress and drought [15,16], making the Mediterranean region a favored environment for its diffusion [17]. The longevity of its microsclerotia, which last from 2 to 15 years, contributes to its ability to survive harsh environmental conditions [18].
V. dahliae is recognized globally for its aggressiveness and ability to cause vascular diseases in plants. It infects over 400 dicotyledonous plant species, including economically important crops [19,20,21]. The fungus produces microsclerotia that can remain in the soil for up to 10 years, allowing it to survive unfavorable conditions [22].
Strawberry diseases, which damage root and crown tissues, can often go unnoticed in the soil, delaying detection until severe symptoms appear in above-ground parts of the plant, such as stunting, wilting, chlorosis, and eventually plant death. This is especially important because the symptoms caused by the two pathogens are similar, which complicates traditional detection methods [23]. Early detection of pathogens in seeds, mother plants, and propagation material is crucial to applying proper control measures at the appropriate time [24,25]. This is why, in the management of strawberry crops, particular attention should be paid to the early diagnosis of pathogens in the propagation plant material [26].
Traditional pathogen detection methods often involve culture-based morphological identification. However, this approach is laborious and time-consuming, making it unsuitable for early diagnosis. Visual recognition of symptoms using traditional disease scales has always been essential for diagnosis; however, this method is overly subjective and can present complications when the disease is caused by soil-borne pathogens that produce similar symptoms. To improve decision-making in disease control, a detection method should be as rapid, sensitive and accurate as possible. Nucleic acid-based and serological methods possess these characteristics and provide essential tools in the diagnosis of plant diseases, in addition to traditional methods [27,28].
Polymerase chain reaction (PCR) is nowadays the most important and widespread nucleic acid-based technique for the detection of plant pathogens. In PCR-based diagnostics, primers are designed to pair with unique DNA sequences of target organisms. This allows the detection of a single target in complex mixtures of DNA without the need to isolate pathogens. Efficient DNA extraction is critical to successful PCR, which can be hindered by inhibitors in sample analysis. PCR inhibitors have represented a major obstacle to the development and application of PCR-based diagnostic methods in diseased tissues of many plants, including strawberry [24,25,29].
Multiplex PCR (M-PCR) is a variant of PCR based on the use of more than one primer pair, allowing the simultaneous detection of different genetic sequences by performing a single reaction [27]. The main advantage of M-PCR is the considerable saving of time and effort by simultaneously amplifying multiple loci; however, this technique presents challenges in optimizing the amplification process. The presence of multiple primer pairs can lead to problems such as poor sensitivity and specificity, preferential amplification of certain loci, or the formation of primer dimers [30,31].
Several studies have focused on the simultaneous detection of strawberry pathogens using M-PCR protocols; some examples are the detection of three species of Pythium [32], Phytophthora nicotianae and P. cactorum [33], Fusarium oxysporum f. sp. fragariae, P. nicotianae and Colletotrichum gloeosporioides [34], and Fusarium oxysporum f. sp. fragariae and C. gloeosporioides [35].
Lateral flow immunoassay (LFIA) is an established technology applied to a wide variety of point-of-care (POC) or field diagnostic applications [36]. It is a test based on the principles of chromatography, used mainly for the qualitative analysis of various types of analytes. Its purpose is the detection of the presence of a target substance in a liquid sample. This type of technology has attracted increasing interest due to the short analysis times, low costs and the fact that even non-specialized personnel can easily use it [37,38].
Nucleic acid lateral flow immunoassay (NALFIA) is a LFIA variant used to test the presence or absence of pathogens’ DNA in biological samples. In NALFIA, nucleic acids are captured on lateral flow test strips in an antibody-dependent format: the biosensor employs an antibody capture line and a labeled amplicon. In this case, an amplified double-stranded nucleic acid sequence specific to a target organism can be detected by using primers with two different tags (e.g., biotin and fluorescein isothiocyanate). The analyte is recognized by binding to a tag-specific antibody (anti-fluorescein antibody) previously sprayed onto a nitrocellulose membrane, and avidin-labeled gold nanoparticles are used as a reporter, enabling the visualization [39,40]. Amplification of the target sequence takes place using primers with two different labels at the 5′ end, e.g., biotin and FITC. If the reaction occurs, all amplicons will be labeled with biotin and FITC. The PCR reaction is then mixed with a buffer and poured onto the sample pad of a lateral flow device (LFD), generating a colored band on the test line that is visible to the naked eye [41,42].
The NALFIA assay can be combined with M-PCR detection methods [43]. This type of analysis involves the use of more than one pair of primers: forward primers are labeled with different molecules, while reverse primers are labeled with the same molecule. Reaction membranes feature more than one test line to recognize and bind the label of forward primers and provide a multi-analyte test [41]. The primers can be labeled at the 5′ position with different molecules to achieve a separation of the respective amplification products in the LFD reaction membrane. The most commonly used labels are Biotin, Fluorescein isothiocyanate (FITC), Carboxyfluorescein (FAM), Dinitrophenol (DNP) and Texas red [44].
The aim of this work was to develop a molecular diagnostic assay, duplex PCR-NALFIA, using species-specific primers labeled at the 5′ end with different molecules, for the simultaneous identification of M. phaseolina (Mp) and V. dahliae (Vd), causal agents of root and crown rot of strawberry. The detection method was developed by single (Mp and Vd) and duplex PCR (Mp + Vd) using increasingly complex biological systems: (i) DNA from pure cultures of the pathogens; (ii) DNA from artificially inoculated cut melon stems; and (iii) DNA from artificially inoculated strawberry plants. The amplicons, obtained with the selected PCR program, were used for both agarose gel electrophoresis (AGE) and NALFIA assays using a commercial lateral flow device (LFD) for the detection of two analytes and comparing the results obtained.
2. Materials and Methods
2.1. Fungal Isolates and Culture Conditions
During the development of the PCR-NALFIA duplex assay, in addition to some isolates of M. phaseolina and V. dahliae, other fungal species were used as outgroups: Diplodia seriata and Neofusicoccum parvum (family Botryosphaeriaceae); Verticillium tricorpus and Verticillium nubilum (Table 1). Fungal cultures were grown on different media to achieve optimal growth. All fungi were incubated at 25 °C with a 12/12 NUV/light cycle unless otherwise indicated.

Table 1.
Fungal isolates used in this study.
M. phaseolina, D. seriata and N. parvum isolates were cultivated on Potato Dextrose Agar (PDA, Biolife Italiana S.r.l., Milano, Italy).
All Verticillium species were grown on Dextrose Yeast Extract Asparagine Agar [DYEAsp agar, g L−1: dextrose 10, yeast extract 1, L-asparagine 0.5, K2HPO4·3H2O 0.5, MgSO4·7H2O 0.25, FeCl3 (10% solution) 0.5 mL, agar 20], with the exception of V. nubilum, which was grown on PDA.
Fungal mycelium for DNA extraction was grown in 25 mL Yeast Malt Broth in 50 mL Falcon tubes (YMB—0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 1% glucose). Each tube was inoculated with five 5 × 5 mm blocks of mycelium from PDA or DYEAsp agar cultures. The tubes were placed in a Multi RS-60 programmable rotator (Biosan, Riga, Latvia) (orbital 60 rpm/20 s; reciprocal 90°/30 s; vibro 3°/5 s) and incubated at room temperature (22 ± 2 °C) for 5–7 days. Mycelium was harvested by filtration through sterile Miracloth (Calbiochem, San Diego, CA, USA), washed thoroughly using sterile distilled water and pressed dry between sterile paper towels. The collected mycelium was used immediately for DNA extraction or stored at −20 °C until use.
2.2. Artificial Inoculations on Cut Melon Stems
With the aim of establishing a biological model system to test the performance of the duplex PCR-NALFIA assays, single (Mp, Vd) or double (Mp + Vd) artificial inoculations with M. phaseolina (Mp) and V. dahliae (Vd) were carried out on cut melon stems (Cucumis melo hybrid F1 ‘Eldorado’) using the method described by Twizeyimana et al. [46], with some modifications. Melon plants were grown in a climate chamber with a 12 h photoperiod at 22 ± 1 °C with a relative humidity of 65%. A representative isolate of each species was chosen for the artificial inoculation experiments (M. phaseolina 10726 and V. dahliae 10361).
Melon stems, 4 cm long, were cut from the plants. The open end of a 100 μL pipette tip was pushed into the margin of actively growing M. phaseolina or V. dahliae agar cultures and a disc of fungal mycelium and agar was cut and removed. The pipette tip containing the colonized agar disc was placed over the cut side and pushed down to embed the stem into the medium and secure the tip onto the stem. Pipette tips were sealed at the pointed end with heat. The inoculated stems were placed on a sterile microscope slide, inside a Petri dish containing a Whatman filter moistened with sterile water, to maintain high humidity levels. The Petri dishes were then sealed with parafilm and incubated at 25 ± 1 °C (Figure 1).
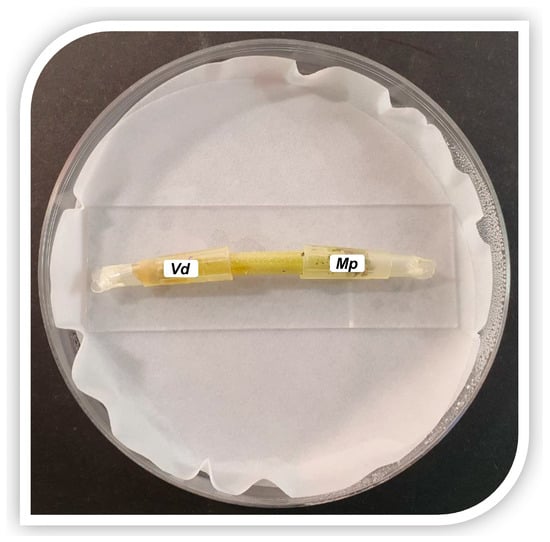
Figure 1.
Artificial inoculation of a cut melon stem with Verticillium dahliae (left side) and Macrophomina phaseolina (right side).
Five days after inoculations, tips were removed and stems were surface sterilized for 2 min in an aqueous solution containing sodium hypochlorite (NaOCl; 1% available chlorine), rinsed twice in sterile distilled water, and air-dried in a laminar flow cabinet. The melon stems were cut into two halves by making a longitudinal cut. Each half was then cut into 3 pieces (central, Vd side and Mp side).
The pieces from each position of each stem were placed in a 1.5 mL Eppendorf tube and stored at −20 °C for subsequent DNA extraction and amplification or plated on DYEAsp agar medium supplemented with streptomycin sulfate (0.3 g L−1, Sigma-Aldrich, Saint Louis, MO, USA) to re-isolate the inoculated fungi, track their stem colonization, and compare the microbiological results with the PCR amplification results (Figure S1). Controls for inoculation experiments consisted of melon stems inoculated with mycelium-free agar discs.
This experiment allowed us to verify the simultaneous presence of M. phaseolina and V. dahliae in the inoculated melon stem portions. By precisely tracing their presence in the tissues with microbiological analyses, it was possible to choose the other half of the samples for molecular analyses with greater accuracy.
2.3. Artificial Inoculations of Strawberry Plants
The detection method was evaluated by single and duplex PCR (Mp, Vd, Mp + Vd) using an increasingly complex biological system consisting of strawberry plants (Fragaria × ananassa Duch.) cv. Aromas grown in pots and maintained in a climate chamber with a 12 h photoperiod at 25 ± 1 °C with 65% relative humidity.
Artificial inoculations were performed using the toothpick method proposed by Pickel et al. [47], with some modifications. Wooden toothpick tips were used and 8–12 tips were placed on 7–14-day old cultures of the two pathogens for several days until they were covered with mycelium and microsclerotia (Figure 2a–d).

Figure 2.
Artificial inoculation method of strawberry crowns with infected wooden toothpick tips. Colonies of Verticillium dahliae (a) and Macrophomina phaseolina (c) used for toothpick tip inoculation. Stereomicroscope images (bars = 750 µm) of a toothpick tip colonized by Verticillium dahliae (b) and Macrophomina phaseolina (d) on which fungal hyphae and the production of microsclerotia are evident. (e) Strawberry crown subjected to double inoculation treatment with toothpick tips colonized by Verticillium dahliae and Macrophomina phaseolina. (f) Strawberry plant inoculated with Verticillium dahliae and Macrophomina phaseolina showing evident symptoms of collapse. (g) Strawberry control plant inoculated with uncolonized toothpick tips.
The same representative isolates of each species selected above were used. Single inoculations were carried out by creating two diametrically opposite wounds on the crown, while for the double inoculations, four wounds were created, inoculating the pathogens in a diametrically opposite way (Figure 2e). For each treatment, twelve strawberry plants were inoculated: single inoculation (Mp, Vd), double inoculation (Mp + Vd) and control.
At the end of the experiments, when disease symptoms became evident in the inoculated plants (Figure 2f), and the non-inoculated plants did not show any symptoms (Figure 2g), the strawberry plants were removed from the pots and were first washed with tap water to remove all attached soil. Crowns and petioles were cut from the plants and surface sterilized for 2.5 min in an aqueous solution containing sodium hypochlorite (NaOCl; 1% available chlorine), rinsed twice in sterile distilled water, and air-dried in a laminar flow cabinet. The sterilized tissues were divided in half: one half was placed on agar media as previously described (see Section 2.2), while the other half was cut into small pieces and stored at −20 °C for DNA extraction (Figure S2).
On the basis of the cultural and morphological characteristics of the colonies originating from the strawberry tissues, different fungal genera were identified. The fungal structures were observed with a Leica MZ FLIII stereomicroscope or with a Leitz Dialux 22 compound microscope. The experiment was carried out in order to verify the presence of the two inoculated pathogens and also the quantity and diversity of the mycoflora present in the strawberry tissues. This information allows us to estimate the qualitative composition of the extracted DNA.
2.4. DNA Extraction from Fungal Mycelium and Inoculated Cut Melon Stems
Total genomic DNA was extracted from fungal mycelium; 0.2–0.5 g of mycelium were placed into 2 mL sterile extraction tubes prefilled with 0.36 g of acid-washed silica glass beads (Sigma-Aldrich, USA). The tubes were filled with 500 µL of sample prep solution (Genesig® Easy DNA/RNA Extraction Kit, Primerdesign, UK). Mycelium was homogenized using the bead-beating method through a BeadBug™ Microtube Homogenizer (Benchmarck Scientific Inc., Sayreville, NJ, USA). Three cycles of 30 s and 4000 rpm each were performed; each cycle was interspersed with a 30 s pause in which the samples were placed on ice to keep the temperature of the homogenate low.
Subsequently, the homogenate was subjected to DNA extraction following the Genesig® Easy DNA/RNA Extraction Kit protocol. The purified DNA was then resuspended in 50 µL nuclease-free water (ThermoFisher Scientific™, Vilnius, Lithuania).
DNA was also extracted from inoculated and non-inoculated melon stems using the same procedure described above with some modifications. Before placing the samples into the 2 mL extraction tubes, they were inserted into 2 mL Eppendorf tubes filled with 500 µL of sample prep solution and pretreated with a G50 tissue grinder with a pestle (Coyote Bioscience, Beijing, China) used to homogenize the tissues. The disrupted tissues in the sample prep solution were then transferred to 2 mL extraction tubes prefilled with 0.36 g of 0.5 mm acid-washed silica glass beads, following the same protocol as described for the extraction of DNA from mycelium.
The DNA, if necessary, was concentrated using the DNA Clean & Concentrator™ kit (Zymo Research, Tustin, CA, USA) according to the manufacturer’s instructions.
2.5. DNA Extraction from Inoculated Strawberry Crowns
DNA extraction from crowns of strawberry plants was performed as previously described (see Section 2.4). Due to the hardness of the crown tissues, the homogenization process consisted of five to six cycles of 120 s each at 4000 rpm using a BeadBug™ Microtube Homogenizer (Benchmarck Scientific Inc., Sayreville, NJ, USA) with silica glass beads and sample prep solution. To obtain DNA suitable for PCR amplification, the extracted samples were purified with the OneStep™ PCR Inhibitor Removal Kit (Zymo Research, Tustin, CA, USA) and, if necessary, concentrated using the DNA Clean & Concentrator™ Kit (Zymo Research, Tustin, CA, USA) according to the manufacturer’s instructions (Figure S3).
2.6. DNA Amplification and Sequencing
The concentration of each DNA sample was measured by a Qubit™ 4 Fluorometer (ThermoFisher Scientific™, Vilnius, Lithuania) while its integrity was visually examined by 1% (w/v) AGE performed in 0.5× TBE buffer (45 mM Trizma base, 44 mM boric acid, 1 mM EDTA, pH 8.4) followed by GelRed™ staining (Biotium Inc., Fremont, CA, USA) according to the manufacturer’s instructions.
The templates included the following: (i) DNA from mycelium of the two pathogens and non-target isolates listed in Table 1 (adjusted to 1 ng µL−1); (ii) DNA from inoculated melon stems; and (iii) DNA from inoculated strawberry crowns both adjusted to 10 ng µL−1.
To determine the amplifiability of the extracted DNAs, the internal transcribed spacer (ITS) region of rDNA was amplified by PCR using the universal primer pairs ITS1/ITS4 and ITS5/ITS4 [48] according to the procedure described by Pecchia et al. [49].
Species-specific primers based on the rDNA intergenic spacer (IGS) of M. phaseolina (Mp102F/Mp102R, amplicon length 102 bp) and V. dahliae (Vd7b/Vd10, amplicon length 139 bp) were used for the development of the duplex PCR-NALFIA assay [42,50]. The reverse primers Mp102R and Vd10 were labeled with fluorescein isothiocyanate (FITC), which interacts with the gold nanoparticles labeled with FITC-specific antibodies, thus enabling the colorimetric reaction. The forward primers Mp102F and Vd7b were labeled at the 5′ end with biotin (Bio) and digoxygenin (Dig), respectively. Each primer was tested against the others for potential dimer formation by the Primer Dimer program (http://www.primer-dimer.com/ accessed on 5 February 2024). The same unlabeled primers were used for sequencing experiments.
PCRs using species-specific primers were performed in a 25 µL reaction volume containing 5 µL of 5× Colorless GoTaq® Flexi Reaction Buffer, 1.5 mM MgCl2, 0.2 mM dNTP mix, 0.2 µM of each M. phaseolina primer, 0.1 µM of each V. dahliae primer, 1.25 U GoTaq® MDx Taq Polymerase, Glycerol-free, 0.25 M betaine and 1 µL of template DNA, with volumes adjusted to 25 µL with nuclease-free water. Single and duplex PCR conditions included an initial denaturation step of 94 °C for 2 min, followed by 25 amplification cycles of a 30 s denaturation step at 94 °C, an annealing step of 30 s at 57 °C and an extension step of 30 s at 72 °C. After 25 cycles, a final extension of 4 min at 72 °C was performed. Negative controls (no DNA) and positive controls (DNA from pure culture of each pathogen) were included for each set of reactions.
The optimization of the duplex PCR-NALFIA protocol described above involved the study of some parameters (primer concentration, number of PCR cycles, type of Taq polymerase, use and concentration of betaine).
The sensitivity of the duplex PCR assay was tested using DNA extracted from the mycelium of M. phaseolina (10726) and V. dahliae (10361) as templates serially diluted from 1 ng to 1 pg μL−1.
Amplification products were analyzed by AGE (1.5% w/v) in 0.5× TBE buffer and detected by UV fluorescence after GelRed™ (Biotium, Inc., Fremont, CA, USA) staining according to the manufacturer’s instructions. The 100 bp DNA ladder (Promega, Madison, WI, USA) was used as the molecular size marker.
The amplicons were then purified using the QIAquick PCR purification Kit (Qiagen Italia, Milano, Italy) and sent to BMR Genomics (Padova, Italy) for sequencing in both directions with the same set of primers used for amplification.
2.7. NALFIA Assay
After the PCR reactions, the resulting double-labeled amplicons were divided into two aliquots: one for the AGE assay, as previously described, and the other for the NALFIA assay.
The NALFIA assay was performed using a commercial lateral flow device (LFD) for the detection of two analytes labeled with biotin and digoxigenin, respectively (Milenia® HybriDetect, MGHD2, Milenia Biotec, Giessen, Germany). LFD was used to verify the presence or the co-presence of M. phaseolina and V. dahliae amplicons. Each NALFIA assay included a negative control (no DNA).
To prepare the NALFIA assay, 10 µL of PCR products were mixed with 70 µL of assay buffer in a 0.2 mL Eppendorf tube. The sample pad side of the LFD was then inserted into the Eppendorf containing the mixture. After a few seconds, the conjugated gold nanoparticles (coupled with polyclonal goat anti-FITC antibodies) migrated along the analytical membrane. It usually takes 2 to 5 min for clear signals to appear. Non-captured gold nanoparticles flow to the control line and are fixed there by specific antibodies (polyclonal anti-goat antibodies) which lead to a coloration of the control line. As the incubation time increases, the formation of an intensely colored control band appears (Figure 3).
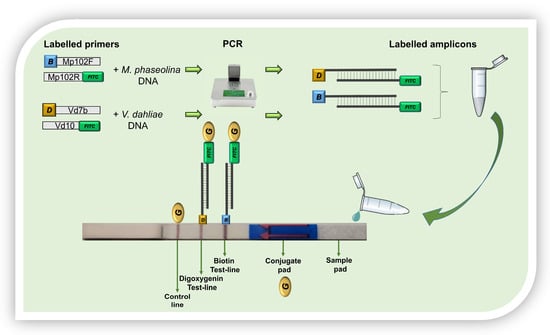
Figure 3.
Lateral flow device for the detection of two analytes used in this study. B = biotin; D = Digoxygenin; FITC = fluorescein isothiocyanate; G = conjugated gold nanoparticles.
The results obtained with the NALFIA assay were then compared with those obtained with AGE.
3. Results
3.1. PCR-NALFIA Assay Using DNA Extracted from Mycelium
Using the chosen PCR program, the correct functioning of species-specific primers was tested with single PCRs, using the DNA extracted from the mycelium of the isolates reported in Table 1 (Figure 4) as a template. PCR amplification resulted in a single band of approximately 100 bp for M. phaseolina isolates and 140 bp for V. dahliae isolates. No amplification was detected when the template was DNA extracted from the mycelium of outgroup isolates or in the negative controls (without DNA). The results obtained by AGE (Figure 4a,b) and NALFIA assays (Figure 4c,d) are consistent with each other.
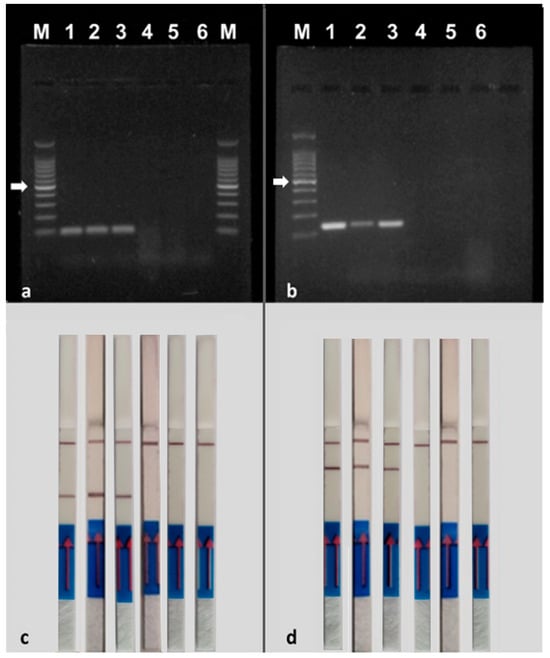
Figure 4.
Specificity of PCR using the primer pair MP102F/MP102R labeled with biotin and FITC for the detection of Macrophomina phaseolina and the primer pair Vd7b/Vd10 labeled with digoxigenin and FITC for the detection of Verticillium dahliae. (a,b) = agarose gel electrophoresis; (c,d) = NALFIA assay. (a,c). 1–3 = Macrophomina phaseolina (10726, PVS-Mp1, DAFE SP19-24); 4 = Diplodia seriata DAFE SP19-25; 5 = Neofusicoccum parvum DAFE SP19-26; 6 = negative control (no DNA); M = 100 bp DNA ladder. (b,d) 1–3 = Verticillium dahliae (10361, 10357, 10355); Verticillium nubilum 10464; Verticillium tricorpus PD593; 6 = negative control (no DNA); M = 100 bp DNA ladder. The NALFIA assay was carried out with the same amplicons used in the agarose gel electrophoresis. The white arrows indicate the 500 bp band of the marker.
Each species-specific primer pair was then used in PCR experiments with the DNA of the other target species to check for any non-specific interactions. No amplification product was detected after observation of AGE, indicating that the species-specific primers only interact with the DNA of the target species (Figure S4). This led to the conclusion that non-specific amplicons should not be obtained in any duplex PCR.
Sequencing of the specific fragments purified from PCR reactions confirmed the expected sequences.
Duplex PCR was performed by inserting primer pairs MP102F/MP102R and Vd7b/Vd10 into the same PCR mix, using DNA extracted from M. phaseolina and V. dahliae mycelium as a template (1 ng each). The PCR products were used to perform AGE and NALFIA assays. Duplex PCR resulted in simultaneous amplification of amplicons of different sizes of approximately 100 bp for M. phaseolina and 140 bp for V. dahliae (Figure 5). Also in this case, the results obtained by AGE (Figure 5, left panel) and NALFIA assays (Figure 5, right panel) are consistent with each other. No amplification was detected in the negative controls (without DNA). No primer dimer production was observed in either the AGE or the NALFIA assay, although primers MP102R and Vd7b obtained the lowest dimer score (−7.85 kcal/mol) after in silico PrimerDimer analysis. The results indicate that the two primer pairs can work well together in the same PCR mix with the PCR program and primer concentrations set in the protocol.
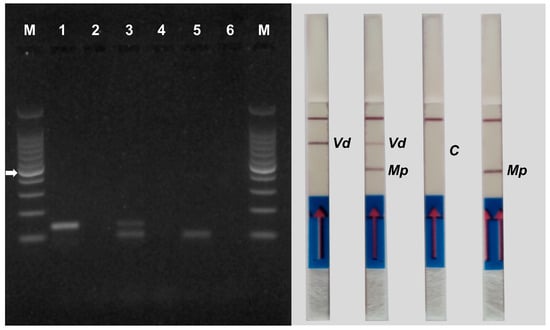
Figure 5.
Gel electrophoresis (left panel) and NALFIA assay (right panel) of single and duplex PCR using DNA extracted from mycelium of Verticillium dahliae and Macrophomina phaseolina. Primer pair Vd7b/Vd10 labeled with digoxigenin and FITC was used for the detection of Verticillium dahliae (lane 1) and primer pair MP102F/MP102R labeled with biotin and FITC was used for the detection of Macrophomina phaseolina (lane 5). Both primer pairs were used for duplex PCR (lane 3). A negative control (no DNA) was performed for each PCR reaction (lanes 2, 4 and 6). M = 100 bp DNA ladder. The white arrow indicates the 500 bp band of the marker. The NALFIA assay was carried out with the same amplicons used in agarose gel electrophoresis. Vd = Verticillium dahliae; Mp = Macrophomina phaseolina; C = control of duplex PCR.
Duplex PCR was also performed using DNA (1 ng) extracted from the mycelium of the outgroup isolates (Diplodia seriata, Neofusicoccum parvum, Verticillium nubilum, and Verticillium tricorpus) as a template. The PCR products were used to perform AGE and NALFIA assays. No amplification product was detected after both assays, indicating that the species-specific primers only interact with the DNA of the target species even in the duplex PCR assay (Figure S5).
Duplex PCR reactions performed using 10-fold serial dilutions of template DNAs (DNA from mycelium of M. phaseolina isolate 10726 and V. dahliae isolate 10361), from 1.0 ng to 1.0 pg, were analyzed by AGE and NALFIA assays. For AGE, 10 μL of PCR products of each reaction were used. V. dahliae amplicons were visible on the gel only at 1 ng of template DNA while M. phaseolina amplicons were visible at up to 100 pg of template DNA. For the NALFIA assay, 10 μL of PCR products from each reaction were also used; in this case, the amplicons from both pathogens were detected up to 10 pg of template DNA, indicating the increased sensitivity of this assay (Figure 6).
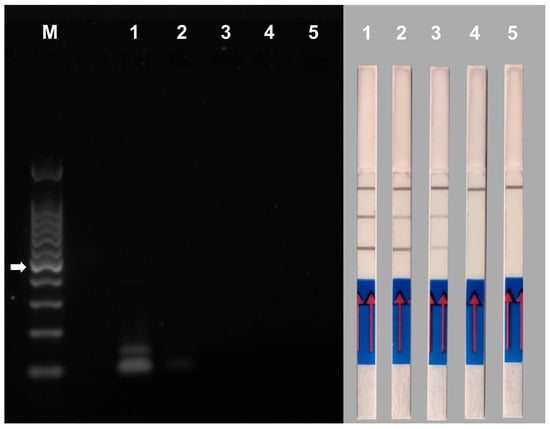
Figure 6.
Sensitivity of duplex PCR using the primer pair MP102F/MP102R labeled with biotin and FITC for the detection of Macrophomina phaseolina and the primer pair Vd7b/Vd10 labeled with digoxigenin and FITC for the detection of Verticillium dahliae. The assay was performed using 10-fold serial dilutions of template DNAs ranging from 1.0 ng and 1.0 pg. Gel electrophoresis (left panel) and NALFIA assay (right panel). 1 = 1 ng of template DNAs; 2 = 100 pg of template DNAs; 3 = 10 pg of template DNAs; 4 = 1 pg of templated DNAs; 5 = negative control (no DNA); M = 100 bp DNA ladder. The white arrow indicates the 500 bp band of the marker.
3.2. Artificial Inoculations on Cut Melon Stems
M. phaseolina and V. dahliae were consistently recovered from half portions of inoculated cut melon stems plated on Dextrose Yeast Extract Asparagine agar plates supplemented with Streptomycin sulfate (DYEAsp+S). The results obtained are summarized in Table 2. Melon stems are a good growth substrate for both pathogens; however, they were not always isolated together from the central portion of the stem in all cases analyzed. On the contrary, the frequency of isolation of the distal portions of the stems was very high for both fungi (92%).

Table 2.
Isolation frequency of Macrophomina phaseolina (Mp) and Verticillium dahliae (Vd) from different portions of inoculated cut melon stems (n = 12). The cut stems were inoculated with Mp on the right side and with Vd on the left side. Portions were plated on Dextrose Yeast Extract Asparagine agar plates supplemented with streptomycin sulfate (DYEAsp+S). Central = central portion of the inoculated stem; Vd side = Verticillium dahliae inoculation side; Mp side = Macrophomina phaseolina inoculation side.
Control stems not inoculated with the two fungi were used as controls. No growth of V. dahliae or M. phaseolina was observed from the control samples, but several saprophytic fungi (e.g., Penicillium sp., Aspergillus sp., Alternaria sp., Trichoderma sp.) were observed. Based on these results, different inoculated and non-inoculated stem portions were selected for DNA extraction.
3.3. Duplex PCR-NALFIA Assay Using DNA Extracted from Cut Melon Stems
Single and duplex PCRs were conducted on samples inoculated with M. phaseolina and V. dahliae in which both pathogens were observed by microbiological analyses. Negative controls were included in each set of PCR reactions (no DNA) (Figure 7).
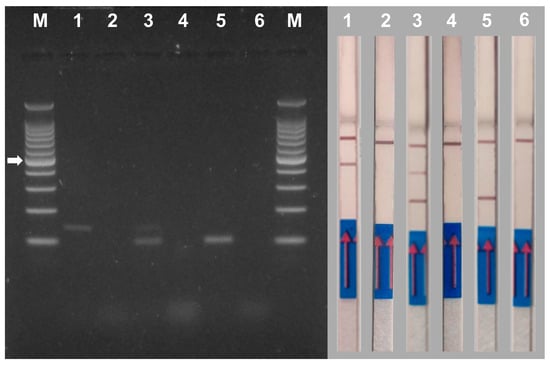
Figure 7.
Gel electrophoresis (left panel) and NALFIA assay (right panel) of single and duplex PCR using DNA extracted from melon stem tissues inoculated with Verticillium dahliae and Macrophomina phaseolina. Primer pair Vd7b/Vd10 labeled with digoxigenin and FITC was used for the detection of Verticillium dahliae (1) and primer pair MP102F/MP102R labeled with biotin and FITC was used for the detection of Macrophomina phaseolina (5). Both primer pairs were used for duplex PCR (3). A negative control (no DNA) was performed for each PCR reaction (2, 4 and 6). M = 100 bp DNA ladder. The white arrow indicates the 500 bp band of the marker. The NALFIA assay was carried out with the same amplicons used in agarose gel electrophoresis.
In the AGE assay samples, 1 and 5 gave rise to the expected single amplicons of V. dahliae (139 bp) and M. phaseolina (102 bp), respectively. Sample 3 produced the expected two bands of both pathogens. The control of each reaction (samples 2, 4 and 6 without DNA) showed no bands. (Figure 7, left panel).
The same PCR reactions used for the AGE analysis were used to perform the NALFIA assay in order to compare their performance (Figure 7, right panel). Samples 1 and 5 tested positive for the presence of V. dahliae and M. phaseolina, respectively. Sample 5 tested positive for the presence of both pathogens. Control samples 2, 4 and 6 did not show the presence of positive signals in the test lines as expected. Analysis of several DNA samples (n = 12) yielded similar results.
3.4. Artificial Inoculations of Strawberry Plants
All inoculated plants showed severe damage resulting from inoculation with M. phaseolina and/or V. dahliae (single or double inoculations). The strawberry plants showed evident symptoms of wilting, chlorosis and/or necrosis of the older lower leaves and the petioles had completely collapsed. Symptoms progressively appeared over the course of approximately two months (12–58 days post inoculation), and as more severe symptoms emerged, plants were sampled. None of the control plants showed such disease symptoms, and the inoculated isolates were not reisolated from those plants.
Culturable fungi were recovered from plated inoculated and non-inoculated strawberry tissues. On the basis of the cultural and morphological characteristics of the colonies, different fungal genera were identified (Figure 8). Out of 24 plants inoculated with M. phaseolina, it was recovered from 12 plants, while V. dahliae was never recovered from the 24 inoculated plants. In total, the dominant phylum was Ascomycota, except for the genus Ceratobasidium, which belongs to the phylum Basidiomycota. Furthermore, we were not able to identify a few taxa at the genus level (Others).
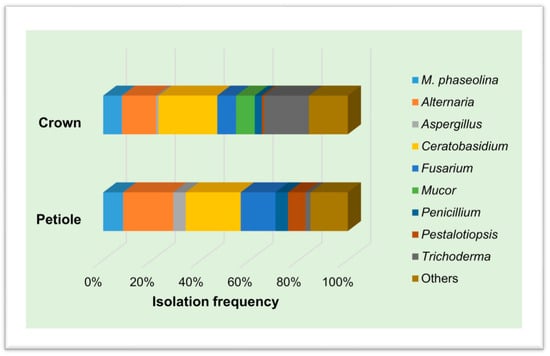
Figure 8.
Isolation frequency (%) of culturable fungal genera recovered from crowns and petioles of strawberry plants.
3.5. Duplex PCR-NALFIA Assay Using DNA Extracted from Strawberry Crowns
The presence of PCR inhibitors in strawberry plant tissues is one of the main issues we faced during the development of the duplex PCR-NALFIA assay.
The DNA extracted from strawberry crowns using a commercial kit based on the use of magnetic particles was tested in PCR experiments with both the ITS universal primer pairs (ITS1/ITS4 and ITS5/ITS4) and the species-specific primer pairs (MP102F/MP102R and Vd7b/Vd10) without obtaining any result.
When the extracted DNA samples were purified by specifically eliminating potential contaminants that could inhibit downstream PCR, positive results were obtained. If necessary, the samples were concentrated to obtain a quantity of DNA equal to approximately 10 ng µL−1.
In the case of DNA samples extracted from strawberry crowns inoculated separately with the two pathogens, no visible bands were observed in the AGE assay. No visible bands were observed even when using DNA samples extracted from control plants inoculated with toothpick tips not colonized by M. phaseolina or V. dahliae. The only visible bands are those of the positive controls of the experiment, consisting of DNA extracted from the mycelium of the fungi (Figure 9a,b, left panel). However, in the NALFIA assay using the same samples analyzed with the AGE assay, the sticks showed that the strawberry samples were positive for the presence of M. phaseolina or V. dahliae, demonstrating the increased sensitivity of this assay. The positive controls showed a very clear band in the sticks, while the samples from the control plants, as expected, showed no band in the sticks (Figure 9a,b, right panel).
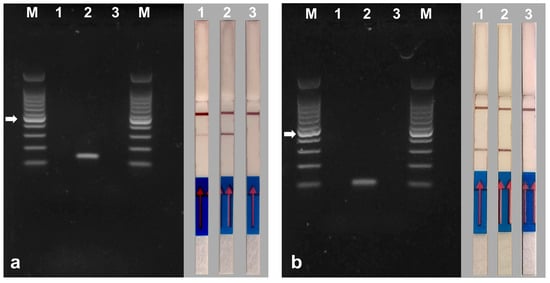
Figure 9.
Gel electrophoresis (left panel) and NALFIA assay (right panel) of PCR using DNA extracted from strawberry crown tissues inoculated with Verticillium dahliae (a) and Macrophomina phaseolina (b). Primer pair Vd7b/Vd10 labeled with digoxigenin and FITC was used for the detection of Verticillium dahliae and primer pair MP102F/MP102R labeled with biotin and FITC was used for the detection of Macrophomina phaseolina. 1 = samples of strawberry crowns inoculated with Verticillium dahliae (a) and Macrophomina phaseolina (b). 2 = DNA extracted from mycelium of Verticillium dahliae (a) and Macrophomina phaseolina (b). 3 = samples of non-inoculated strawberry crown. M = 100 bp DNA ladder. The white arrow indicates the 500 bp band of the marker. The NALFIA assay was carried out with the same amplicons used in agarose gel electrophoresis.
DNA samples from strawberry plants inoculated with M. phaseolina and V. dahliae were subjected to duplex PCR using the species-specific primers. In the AGE assay, sample 1 did not show any visible bands, while sample 2 showed faint bands for both pathogens. The positive control of the experiment showed two very intense bands, and no visible bands were observed even when using DNA extracted from the control plant (Figure 10, left panel). In this case, the NALFIA assay proved to be more sensitive than the AGE assay, identifying both pathogens in the DNA samples of strawberries inoculated with both fungi, as well as in the positive control. The sample from the control plant showed no band on the stick, as expected (Figure 10, right panel). Analysis of several DNA samples (n = 12) yielded similar results.
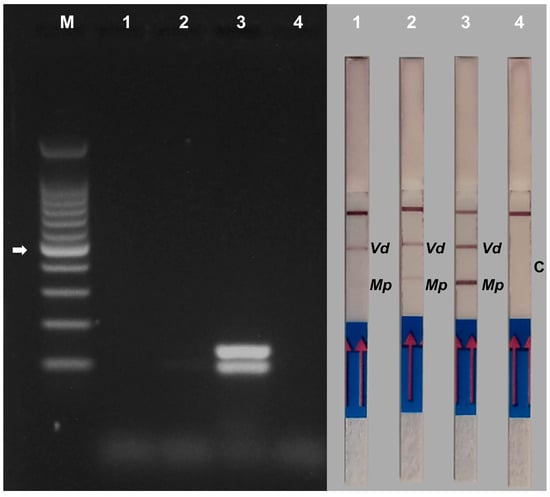
Figure 10.
Gel electrophoresis (left panel) and NALFIA assay (right panel) of duplex PCR using DNA extracted from strawberry crown tissues inoculated with Verticillium dahliae and Macrophomina phaseolina. Primer pair Vd7b/Vd10 labeled with digoxigenin and FITC was used for the detection of Verticillium dahliae and primer pair MP102F/MP102R labeled with biotin and FITC was used for the detection of Macrophomina phaseolina. Both primer pairs were used for duplex PCR. 1 and 2 = samples of strawberry crowns inoculated with both pathogens. 3 = DNA extracted from mycelium of Verticillium dahliae and Macrophomina phaseolina. 4 = sample of non-inoculated strawberry crown. M = 100 bp DNA ladder. The white arrow indicates the 500 bp band of the marker. The NALFIA assay was carried out with the same amplicons used in agarose gel electrophoresis. Vd = Verticillium dahliae; Mp = Macrophomina phaseolina; C = control non-inoculated sample.
4. Discussion
In this study, we successfully developed a new duplex PCR-NALFIA assay, which can simultaneously detect M. phaseolina and V. dahliae, the causal agents of crown and root rot of strawberry, directly from diseased strawberry crowns.
The PCR protocol described in this study represents the first attempt to establish a duplex detection method for both M. phaseolina and V. dahliae. With the PCR protocol developed, the length of each amplicon reflected the expected amplicon length obtained from other studies using the same species-specific primers [42,50].
A critical aspect of the application of multiplex PCR is the primer-to-template ratio. Excessively high ratios can lead to the formation of primer dimers [31]. Therefore, species-specific primer concentrations for this protocol were kept as low as possible: 0.2 µM for MP102F/MP102R and 0.1 µM for Vd7b/Vd10. Furthermore, it has recently been shown that one of the factors influencing the performance of the NALFIA assay is the presence of unreacted PCR primers [51]. These concentrations were sufficient to amplify 1 ng of DNA from pure cultures of the pathogens and 10 ng of DNA extracted from artificially inoculated melon and strawberry crowns.
Primer selection plays a key role in the optimization of multiplex PCR protocols. Primer length should be 18–24 bp or more, with a GC content of 35–60% and an annealing temperature (Ta) of 55–58 °C [30]. These conditions are almost optimal in our case: the MP102R primer is 16 bp in length and the MP102F primer has a GC content of 67% and primers for V. dahliae fall within the parameters described above.
The PCR cycle chosen for this protocol consisted of 25 cycles, a shorter program than usual. This decision was driven by the need to adapt the diagnostic system for the NALFIA assay. Indeed, by reducing the number of PCR cycles, the possibility of forming false positives displayed on the lateral flow device (LFD) is drastically decreased [39].
The duplex PCR protocol developed was first validated on DNA extracted from the mycelium of the two pathogens and subsequently on increasingly complex biological systems: cut melon stems and potted strawberry plants.
Artificial inoculations of cut melon stems made it possible to trace the presence of the two pathogens in plant tissues to more accurately choose samples for molecular analyses. Both pathogens rapidly colonize melon tissues as melon is one of several hosts of both M. phaseolina and V. dahliae, which are associated with melon root rot [17,52]. The lack of a true plant defence system and the absence of other nutrient sources allowed pathogens to grow inside melon stems, which proved to be a good model system for monitoring pathogen growth within plant tissue. The duplex PCR protocol was effective in detecting the two pathogens within melon tissues.
When artificially inoculated pot-grown strawberry plants were used, the developed duplex PCR protocol provided good results only when the DNA samples were purified by removing the PCR inhibitors.
It is well known that the detection of pathogens on strawberry tissues is constantly challenged by the presence of PCR inhibitors such as polysaccharides and polyphenols. These inhibitors can interfere with Taq polymerase by chelating magnesium ions (Mg2+), precipitating the enzyme or inhibiting its activity by forming secondary structures or binding to the target DNA [53]. Furthermore, PCR inhibitors reduce the sensitivity of the reaction, and when the initial sequences of the target DNA are in low copy, they interfere with the first critical cycles of amplification and can cause false negative results [24].
Over time, several solutions have been proposed to solve the problem of the presence of PCR inhibitors in DNA samples extracted from strawberry plant tissues, both with the use of commercial kits and with specific DNA extraction protocols [24,29,53,54].
Another way to overcome the presence of PCR inhibitors is to dilute the DNA extracts to reduce their concentration. Although this method can reduce the presence of inhibitors, it also affects the efficiency and sensitivity of the assay by reducing the number of amplifiable DNA copies [55].
In this study, the presence of PCR inhibitors in DNA samples extracted from strawberry crowns was resolved using a PCR inhibitor removal kit specifically designed to eliminate polyphenolic compounds, humic/fulvic acids, tannins and melanin. After the purification process, the presence of M. phaseolina and V. dahliae was successfully detected in strawberry DNA samples, both individually and simultaneously. The duplex PCR protocol proved effective in the detection of the two pathogens within the strawberry crowns, with greater sensitivity highlighted by the use of the NALFIA assay compared to the AGE assay. Similar results were obtained when the PCR-NALFIA assay was developed for the detection of M. phaseolina [42].
Similarly, some authors, during the development of a diagnostic assay for Xanthomonas fragariae in strawberry crown tissues, clearly highlighted a lack of amplification of the target DNA when performing standard PCR on genomic DNA extracted from the mixture of bacteria and strawberry crown tissue extracts. They tried a number of common DNA extraction kits/methods without positive results and hypothesized that the PCR inhibitors present in crown tissue extracts were not eliminated or reduced satisfactorily to allow standard PCR to occur [56]. Thus, our results help to confirm that the quality of DNA extracted from strawberry plant material (particularly crowns) may represent a bottleneck in disease diagnosis.
We used the toothpick inoculation method to create infected material to evaluate the duplex PCR-NALFIA assay in strawberry tissues. This method has proven to be very effective for both M. phaseolina [47,57] and V. dahliae [58,59], which were inoculated individually. In this study, both pathogens were co-inoculated into strawberry plants, which still obtained good results. Similar results were obtained by Degani et al., who proposed a toothpick-mediated co-inoculation method for M. phaseolina and Magnaporthiopsis maydis in cotton sprouts [60].
The isolation of several culturable fungi from strawberry crowns highlighted that the extracted DNA samples used to test the effectiveness of the developed duplex PCR protocol contained a mixture of DNAs: from the plant, from inoculated pathogenic fungi and from naturally occurring non-inoculated fungi. This evidence made the duplex PCR identification method more robust, even if the presence of the two pathogens was very low. In fact, using 1 ng of DNA extracted from pure cultures of M. phaseolina and V. dahliae as a positive control, it was possible to observe rather intense bands in both the AGE assay and the NALFIA assay. The extraction, amplification, and identification of microbial DNA in different environmental samples is quite difficult, especially when the pathogens are present in a low amount [61].
The sensitivity threshold of the duplex PCR reaction was assessed by testing serial dilutions of DNA extracted from the mycelium of M. phaseolina and V. dahliae as templates. The limit of detection of the NALFIA assay for both pathogens was 10 pg, whereas that of AGE was 1 ng for V. dahliae and 100 pg for M. phaseolina.
The NALFIA assay always provided positive results, but the bands were less intense when composite DNA (10 ng) samples extracted from infected tissues were used. This suggests that the presence of the two pathogens in these samples is certainly in quantities lower than 1 ng. In a previous study using M. phaseolina, the faint bands correspond to approximately 0.017–1.7 pg of target DNA [42].
The sensitivity of the duplex PCR-NALFIA assay developed in this study is slightly lower than that found in single PCR assays. On the other hand, multiplex PCRs are known to use multiple primer pairs that compete for available reagents in the reaction and this reduces overall sensitivity. Furthermore, optimization of the duplex PCR reaction to avoid non-specific PCR products and false positives in the NALFIA assay may have slightly reduced the sensitivity achievable from single PCR reactions [34].
Despite this, the sensitivity of the developed duplex PCR-NALFIA assay remains high thanks also to the multicopy nature of the target DNA used. Indeed, the high copy number of the intergenic spacer (IGS) of nuclear ribosomal DNA (rDNA) increases the sensitivity of PCR-based techniques, and this region has been used to develop specific diagnostic assays for many plant pathogenic fungi [42]. For V. dahliae, which used primer pair Vd7b/Vd10, the detection limit was 1 pg μL−1 with Scorpion-PCR and 1 fg μL−1 with nested Scorpion-PCR [50].
The PCR-NALFIA duplex assay developed in this study could also be applied to other biological matrices such as soil, seeds or water. The assay is qualitative and can become semi-quantitative since it is a colorimetric detection. Quantitative test results are based on reading the color of the bands with a portable strip reader and could be integrated with electronic devices such as smartphones [37].
The need for rapid and highly sensitive pathogen detection methods is becoming increasingly important, particularly as nurseries and strawberry growers rely on diagnostics to ensure pathogen-free stocks and to distinguish between soil-borne diseases with similar symptoms.
The PCR-NALFIA duplex assay described here has proven to be specific, sensitive, rapid and sufficiently practical, and could be a very powerful and simple tool for the precise diagnosis of M. phaseolina and V. dahliae, two of the most important fungal pathogens of cultivated strawberry in the world.
5. Conclusions
In strawberry cultivation, diseases caused by soil-borne pathogens cause significant production losses. It is therefore essential to have diagnostic techniques with high specificity and sensitivity for the correct identification of the various pathogens and for the application of adequate protection strategies. Two of the most important yield-reducing pathogens in strawberry crops are the fungi Macrophomina phaseolina and Verticillium dahliae, both of which cause crown and root rot diseases.
Management of disease caused by the two strawberry pathogens includes cultural (e.g., soil solarization, anaerobic soil disinfestation, soil amendments and steam application), host resistance, chemical and biological strategies. However, their response to these strategies sometimes differs significantly. For example, soil solarization and anaerobic soil disinfestation have shown positive results in several studies against V. dahliae, but in the case of M. phaseolina, they did not provide complete control in the field [62,63].
Genetic resistance to V. dahliae and M. phaseolina is considered one of many components of an integrated disease management system. Many studies have focused on the resistance of strawberry cultivars to these fungal pathogens. Some commercial cultivars have shown partial resistance to V. dahliae, while for M. phaseolina, a wide range of responses have been found, indicating that host resistance can be used to partially control this pathogen. Furthermore, it has been highlighted that each strawberry cultivar shows different levels of resistance/susceptibility to the two pathogens [11,64]. Therefore, knowing which pathogen is present allows for a more refined approach, using both genetic resistance and cultural practices.
Traditional diagnostic methods are often labor-intensive, time-consuming and less accurate than modern molecular techniques, which offer faster, more reliable and highly sensitive results for identifying target microorganisms. Although some molecular methods require specialized equipment, the proposed PCR-NALFIA duplex assay is characterized by its simplicity and accessibility. The protocol for the simultaneous detection of M. phaseolina and V. dahliae, starting from the extraction of a DNA sample from a strawberry crown, requires approximately 2 h of laboratory work to obtain the final result. This method does not require skilled personnel and expensive equipment, and the results can be easily interpreted. This accessibility, combined with the simplicity and speed of the method, makes it a significant improvement over traditional approaches or more specialized molecular techniques.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15020160/s1, Figure S1: Schematic representation of the method of reisolation of Macrophomina phaseolina and Verticillium dahliae and sampling of plant material for DNA extraction from inoculated cut melon stems; Figure S2: Schematic representation of the method of reisolation of Macrophomina phaseolina and Verticillium dahliae and sampling of plant material for DNA extraction from inoculated strawberry crowns; Figure S3: Schematic representation of the method for DNA extraction from inoculated strawberry crowns; Figure S4: Gel electrophoresis of single PCR using DNA extracted from the mycelium of Verticillium dahliae and Macrophomina phaseolina. Each species-specific primer pair was used with the DNA of the other target species, to check for any non-specific interactions; Figure S5: Specificity of duplex PCR using the primer pair MP102F/MP102R labeled with biotin and FITC for the detection of Macrophomina phaseolina and the primer pair Vd7b/Vd10 labeled with digoxigenin and FITC for the detection of Verticillium dahliae. Duplex PCR was performed using DNA (1 ng) extracted from the mycelium of the outgroup isolates (Verticillium tricorpus, Verticillium nubilum, Diplodia seriata, and Neofusicoccum parvum).
Author Contributions
Conceptualization, V.P., A.M. and S.P.; methodology, V.P. and A.M.; validation, V.P. and A.M.; investigation, V.P., A.M. and S.P.; writing—original draft preparation, V.P. and A.M.; writing—review and editing, V.P., A.M. and S.P.; supervision, S.P.; project administration, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by (i) the University of Pisa, Call for Technological Demonstrators (DT), project “MP102-LABINABAG”, and (ii) the Italian Ministry of Economic Development (MISE) and the University of Pisa, Proof of Concept Call 2020 POCARNO, project “Development of a prototype of commercial molecular diagnostic kit for the diagnosis of the phytopathogenic fungus Macrophomina phaseolina” (acronym MP102IDENTI-KIT).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
Grateful thanks are expressed to Grazia Puntoni (University of Pisa, Italy), for the helpful technical and administrative support, and Virgilio Balmas (University of Sassari, Italy), for kindly providing the Macrophomina phaseolina isolate PVS-Mp1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simpson, D. The economic importance of strawberry crops. In The Genomes of Rosaceous Berries and Their Wild Relatives; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–7. [Google Scholar]
- FAO. Agricultural Data/Agricultural Production/Crops Primary [WWW Document]. FAOSTAT 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 June 2024).
- Hernàndez-Martínez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- Steele, M.E.; Hewavitharana, S.S.; Henry, P.; Goldman, P.; Holmes, G.J. Survey of late-season soilborne pathogens infecting strawberry in Watsonville-Salinas, California. Plant Health Prog. 2023, 24, 104–109. [Google Scholar] [CrossRef]
- Katan, J. Diseases caused by soilborne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- de los Santos, B.; Medina, J.J.; Miranda, L.; Gómez, J.A.; Talavera, M. Soil disinfestation efficacy against soil fungal pathogens in strawberry crops in Spain: An overview. Agronomy 2021, 11, 526. [Google Scholar] [CrossRef]
- Pastrana, A.M.; Borrero, C.; Pérez, A.G.; Avilés, M. Soilborne pathogens affect strawberry fruit flavor and quality. Plant Sci. 2023, 326, 111533. [Google Scholar] [CrossRef]
- Chamorro, M.; Seijo, T.E.; Noling, J.C.; de los Santos, B.; Peres, N.A. Efficacy of fumigant treatments and inoculum placement on control of Macrophomina phaseolina in strawberry beds. Crop Prot. 2016, 90, 163–169. [Google Scholar] [CrossRef]
- López-Aranda, J.M.; Domínguez, P.; Miranda, L.; de los Santos, B.; Talavera, M.; Daugovish, O.; Soria, C.; Chamorro, M.; Medina, J.J. Fumigant use for strawberry production in Europe: The current landscape and solutions. Int. J. Fruit Sci. 2016, 16, 1–15. [Google Scholar] [CrossRef]
- Holmes, G.J.; Mansouripour, S.Y.; Hewavitharana, S.S. Strawberries at the crossroads: Management of soil-borne diseases in California without methyl bromide. Phytopathology 2020, 110, 956–968. [Google Scholar] [CrossRef]
- Steele, M.E.; Mendez, M.; Hewavitharana, S.S.; Holmes, G.J. Survey of soilborne pathogens infecting strawberry in Santa Maria, California. Int. J. Fruit Sci. 2023, 23, 256–266. [Google Scholar] [CrossRef]
- Koster, J.T.; Ding, S.; Holmes, G.J.; Robinson, E.A.; Hewavitharana, S.S. Effect of sequential crop termination and bed fumigation on Verticillium dahliae soil and plant density in strawberry. Int. J. Fruit Sci. 2024, 24, 1–17. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Basandrai, A.K. Will Macrophomina phaseolina spread in legumes due to climate change? A critical review of current knowledge. J. Plant Dis. Prot. 2021, 128, 9–18. [Google Scholar] [CrossRef]
- Mathur, M.; Mathur, P. Global distribution modelling of Macrophomina phaseolina (Tassi) Goid: A comparative assessment using ensemble machine learning tools. Australas. Plant Pathol. 2023, 52, 353–371. [Google Scholar] [CrossRef]
- Cohen, R.; Elkabetz, M.; Paris, H.S.; Gur, A.; Dai, N.; Rabinovitz, O.; Freeman, S. Occurrence of Macrophomina phaseolina in Israel: Challenges for disease management and crop germplasm enhancement. Plant Dis. 2022, 106, 15–25. [Google Scholar] [CrossRef]
- Khan, A.N.; Shair, F.; Malik, K.; Hayat, Z.; Khan, M.A.; Hafeez, F.Y.; Hassan, M.N. Molecular identification and genetic characterization of Macrophomina phaseolina strains causing pathogenicity on sunflower and chickpea. Front. Microbiol. 2017, 8, 1309. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef]
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An overview of the molecular genetics of plant resistance to the Verticillium wilt pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef]
- Kowalska, B. Management of the soil-borne fungal pathogen—Verticillium dahliae Kleb. causing vascular wilt diseases. J. Plant Pathol. 2021, 103, 1185–1194. [Google Scholar] [CrossRef]
- Wilhelm, S. Longevity of the Verticillium wilt fungus in the laboratory and the field. Phytopathology 1958, 45, 180–181. [Google Scholar]
- Koike, S.T.; Gordon, T.R. Management of Fusarium wilt of strawberry. Crop Prot. 2015, 73, 67–72. [Google Scholar] [CrossRef]
- Mirmajlessi, S.M.; Destefanis, M.; Gottsberger, R.A.; Mänd, M.; Loit, E. PCR-based specific techniques used for detecting the most important pathogens on strawberry: A systematic review. Syst. Rev. 2015, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Baldi, P.; La Porta, N. Molecular approaches for low-cost point-of-care pathogen detection in agriculture and forestry. Front. Plant Sci. 2020, 11, 570862. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, A.M.; Basallote-Ureba, M.J.; Aguado, A.; Capote, N. Potential inoculum sources and incidence of strawberry soilborne pathogens in Spain. Plant Dis. 2017, 101, 751–760. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Venbrux, M.; Crauwels, S.; Rediers, H. Current and emerging trends in techniques for plant pathogen detection. Front. Plant Sci. 2023, 14, 1120968. [Google Scholar] [CrossRef]
- Bhat, R.G.; Browne, G.T. Specific detection of Phytophthora cactorum in diseased strawberry plants using nested polymerase chain reaction. Plant Pathol. 2010, 59, 121–129. [Google Scholar] [CrossRef]
- Henegariu, O.; Heerema, N.A.; Dlouhy, S.R.; Vance, G.H.; Vogt, P.H. Multiplex PCR: Critical parameters and step-by-step protocol. BioTechniques 1997, 23, 504–511. [Google Scholar] [CrossRef]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex polymerase chain reaction: A practical approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Asano, T.; Otsubo, K.; Suga, H.; Kageyama, K. Simultaneous detection by multiplex PCR of the high-temperature-growing Pythium species: P. aphanidermatum, P. helicoides and P. myriotylum. J. Gen. Plant Pathol. 2013, 79, 350–358. [Google Scholar] [CrossRef]
- Li, M.; Asano, T.; Suga, H.; Kageyama, K. A multiplex PCR for the detection of Phytophthora nicotianae and P. cactorum, and a survey of their occurrence in strawberry production areas of Japan. Plant Dis. 2011, 95, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Liu, J.; Xu, T.; Li, X.; Li, X.; Li, S.; Wang, H. Simultaneous detection of three crown rot pathogens in field-grown strawberry plants using a multiplex PCR assay. Crop Prot. 2022, 156, 105957. [Google Scholar] [CrossRef]
- Nakano, M.; Inaba, M.; Suehiro, J. Selective visual detection of multiplex PCR amplicon using magnetic microbeads. Biosens. Bioelectron. X 2024, 18, 100461. [Google Scholar] [CrossRef]
- O’Farrel, B. Lateral flow immunoassay systems: Evolution from the current state of the art to the next generation of highly sensitive, quantitative rapid assays. In The Immunoassay Handbook Theory and Applications of Ligand Binding, ELISA and Related Techniques, 4th ed.; Wild, D.G., Ed.; Elsevier Ltd: London, UK, 2013; pp. 89–107. [Google Scholar]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Andryukov, B.G. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef]
- Blažková, M.; Koets, M.; Rauch, P.; van Amerongen, A. Development of a nucleic acid lateral flow immunoassay for simultaneous detection of Listeria spp. and Listeria monocytogenes in food. Eur. Food Res. Technol. 2009, 229, 867–874. [Google Scholar] [CrossRef]
- Blažková, M.; Koets, M.; Wichers, J.H.; Van Amerongen, A.; Fukal, L.; Rauch, P. Nucleic acid lateral flow immunoassay for the detection of pathogenic bacteria from food. Czech J. Food Sci. 2009, 27, S350–S353. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Pecchia, S.; Da Lio, D. Development of a rapid PCR Nucleic Acid Lateral Flow Immunoassay (PCR-NALFIA) based on rDNA IGS sequence analysis for the detection of Macrophomina phaseolina in soil. J. Microbiol. Methods 2018, 158, 118–128. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Baggiani, C. Multiplex lateral flow immunoassay: An overview of strategies towards high-throughput point-of-need testing. Biosensors 2019, 9, 2. [Google Scholar] [CrossRef]
- Roth, J.M.; De Bes, L.; Sawa, P.; Omweri, G.; Osoti, V.; Oberheitmann, B.; Schallig, H.D.F.H.; Mens, P.F. Plasmodium detection and differentiation by direct-on-blood PCR nucleic acid lateral flow immunoassay: Development, validation, and evaluation. J. Mol. Diagn. 2018, 20, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Bostock, R.M.; Davis, R.M.; Usami, T.; Platt, H.W.; Subbarao, K.V. Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS ONE 2011, 6, e28341. [Google Scholar] [CrossRef] [PubMed]
- Twizeyimana, M.; Hill, C.B.; Pawlowski, M.; Paul, C.; Hartman, G.L. A cut-stem inoculation technique to evaluate soybean for resistance to Macrophomina phaseolina. Plant Dis. 2012, 96, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Pickel, B.; Dai, N.; Maymon, M.; Elazar, M.; Tanami, Z.; Frenkel, O.; Toamy, M.A.; Mor, N.; Freeman, S. Development of a reliable screening technique for determining tolerance to Macrophomina phaseolina in strawberry. Eur. J. Plant Pathol. 2020, 157, 707–718. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Tailor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Pecchia, S.; Caggiano, B.; Da Lio, D.; Resta, E. Morphological and molecular identification of Dactylonectria macrodidyma as causal agent of a severe Prunus lusitanica dieback in Italy. Horticulturae 2023, 9, 145. [Google Scholar] [CrossRef]
- Schena, L.; Nigro, F.; Ippolito, A. Real-time PCR detection and quantification of soilborne fungal pathogens: The case of Rosellinia necatrix, Phytophthora nicotianae, P. citrophthora, and Verticillium dahliae. Phytopathol. Mediterr. 2004, 43, 273–280. [Google Scholar]
- Agarwal, P.; Toley, B.J. Unreacted labeled PCR primers inhibit the signal in a Nucleic Acid Lateral Flow Assay as elucidated by a transport reaction model. ACS Meas. Sci. Au 2022, 2, 317–324. [Google Scholar] [CrossRef]
- Aegerter, B.J.; Gordon, T.R.; Davis, R.M. Occurrence and pathogenicity of fungi associated with melon root rot and vine decline in California. Plant Dis. 2000, 84, 224–230. [Google Scholar] [CrossRef]
- Wei, T.; Lu, G.; Clover, G. Novel approaches to mitigate primer interaction and eliminate inhibitors in multiplex PCR, demonstrated using an assay for detection of three strawberry viruses. J. Virol. Methods 2008, 151, 132–139. [Google Scholar] [CrossRef]
- Bartsch, C.; Szabo, K.; Dinh-Thanh, M.; Schrader, C.; Trojnar, E.; Johne, R. Comparison and optimization of detection methods for noroviruses in frozen strawberries containing different amounts of RT-PCR inhibitors. Food Microbiol. 2016, 60, 124–130. [Google Scholar] [CrossRef]
- Munawar, M.A.; Martin, F.; Toljamo, A.; Kokko, H.; Oksanen, E. RPA-PCR couple: An approach to expedite plant diagnostics and overcome PCR inhibitors. BioTechniques 2020, 69, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Turechek, W.W.; Hartung, J.S.; McCallister, J. Development and optimization of a real-time detection assay for Xanthomonas fragariae in strawberry crown tissue with receiver operating characteristic curve analysis. Phytopathology 2008, 98, 359–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, X.L.; Phillips, D.; Li, H.; Sivasithamparam, K.; Barbetti, M.J. Comparisons of virulence of pathogens associated with crown and root diseases of strawberry in Western Australia with special reference to the effect of temperature. Sci. Hortic. 2011, 131, 39–48. [Google Scholar] [CrossRef]
- Slattery, R.J. Inoculum potential of verticillium—Infested potato cultivars. Am. Potato J. 1981, 58, 135–142. [Google Scholar] [CrossRef]
- Retief, E.; Lamprecht, S.; McLeod, A. Characterization and pathogenicity of Verticillium dahliae isolates associated with Verticillium wilt of tomato in the Limpopo Province of South Africa. J. Plant Pathol. 2023, 105, 1465–1481. [Google Scholar] [CrossRef]
- Degani, O.; Becher, P.; Gordani, A. Pathogenic interactions between Macrophomina phaseolina and Magnaporthiopsis maydis in mutually infected cotton sprouts. Agriculture 2022, 12, 255. [Google Scholar] [CrossRef]
- Nnachi, R.C.; Sui, N.; Ke, B.; Luo, Z.; Bhalla, N.; He, D.; Yang, Z. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef]
- Kanaan, H.; Medina, S.; Krassnovsky, A.; Raviv, M. Survival of Macrophomina phaseolina s.l. and Verticillium dahliae during solarization as affected by composts of various maturities. Crop Prot. 2015, 76, 108–113. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Baird, G.; Zavatta, M.; Toyama, L.; Mazzola, M.; Koike, S.T. Anaerobic soil disinfestation (ASD): A strategy for control of soil borne diseases in strawberry production. Acta Hortic. 2016, 1137, 113–120. [Google Scholar] [CrossRef]
- Knapp, S.J.; Cole, G.S.; Pincot, D.D.; Lopez, C.M.; Gonzalez-Benitez, O.A.; Famula, R.A. ‘UC Eclipse’, a summer plant-adapted photoperiod-insensitive strawberry cultivar. HortScience 2023, 58, 1568–1572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).