Assessing Sugarcane Molasses’ Bioactive Compound Content upon Ultrasound-Assisted Hydroethanolic Extraction at Various pH Values
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. GC-MS Analysis of Sugarcane Molasses
2.2.2. Preparation of Samples
2.2.3. Determination of Total Phenolic Content
2.2.4. Determination of Total Flavonoid Content
2.2.5. Determination of Total Tannin Content
2.2.6. Determination of Monomeric Anthocyanin Content
2.2.7. Determination of Total Antioxidant Capacity by DPPH Free Radicals
2.3. Statistical Analysis
3. Results and Discussion
3.1. Sugarcane Molasses Compounds Identified Through GC-MS Analysis
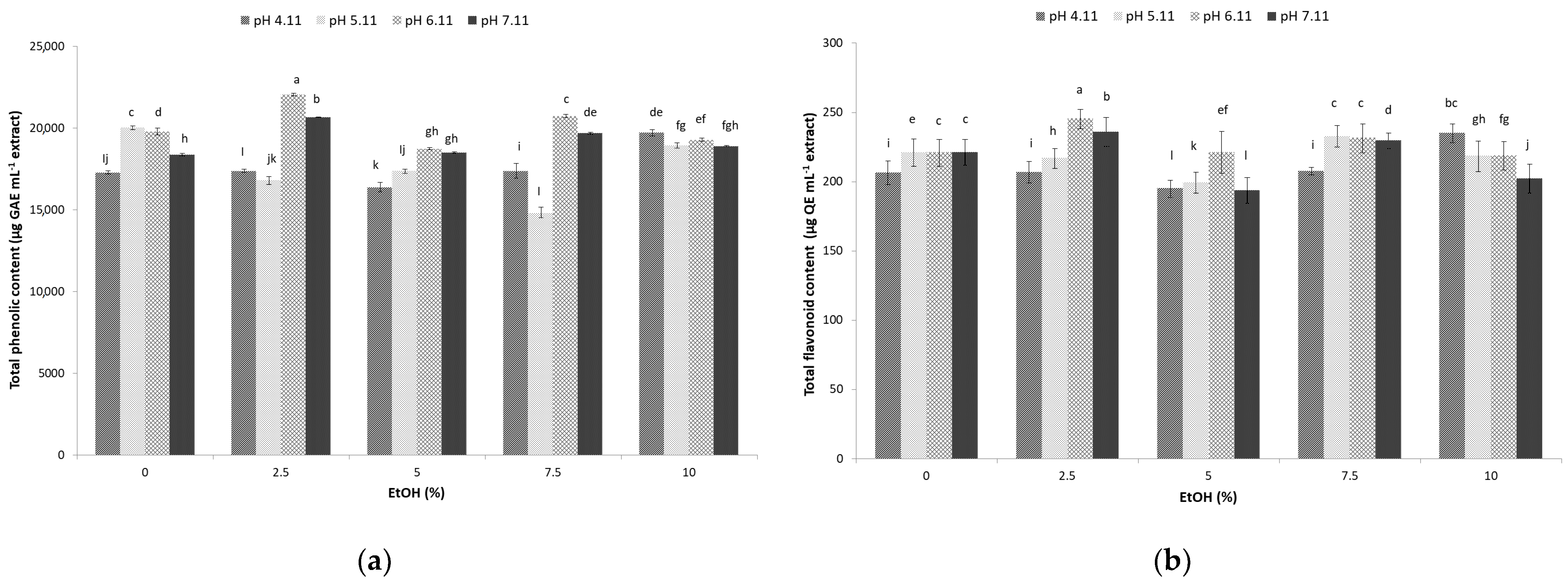
3.2. Total Phenolic Content of Sugarcane Molasses Extracts
3.3. Total Flavonoid Content of Sugarcane Molasses Extracts
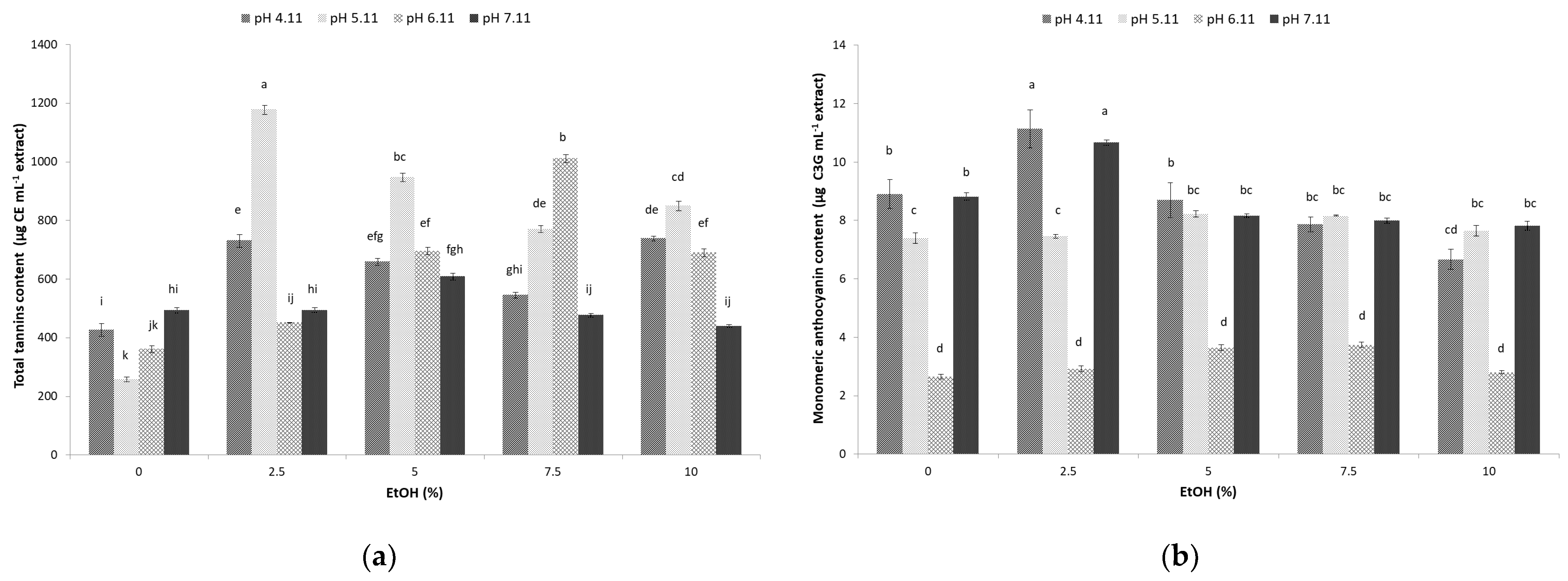
3.4. Total Tannin Content of Sugarcane Molasses Extracts
3.5. Monomeric Anthocyanin Content of Sugarcane Molasses Extracts
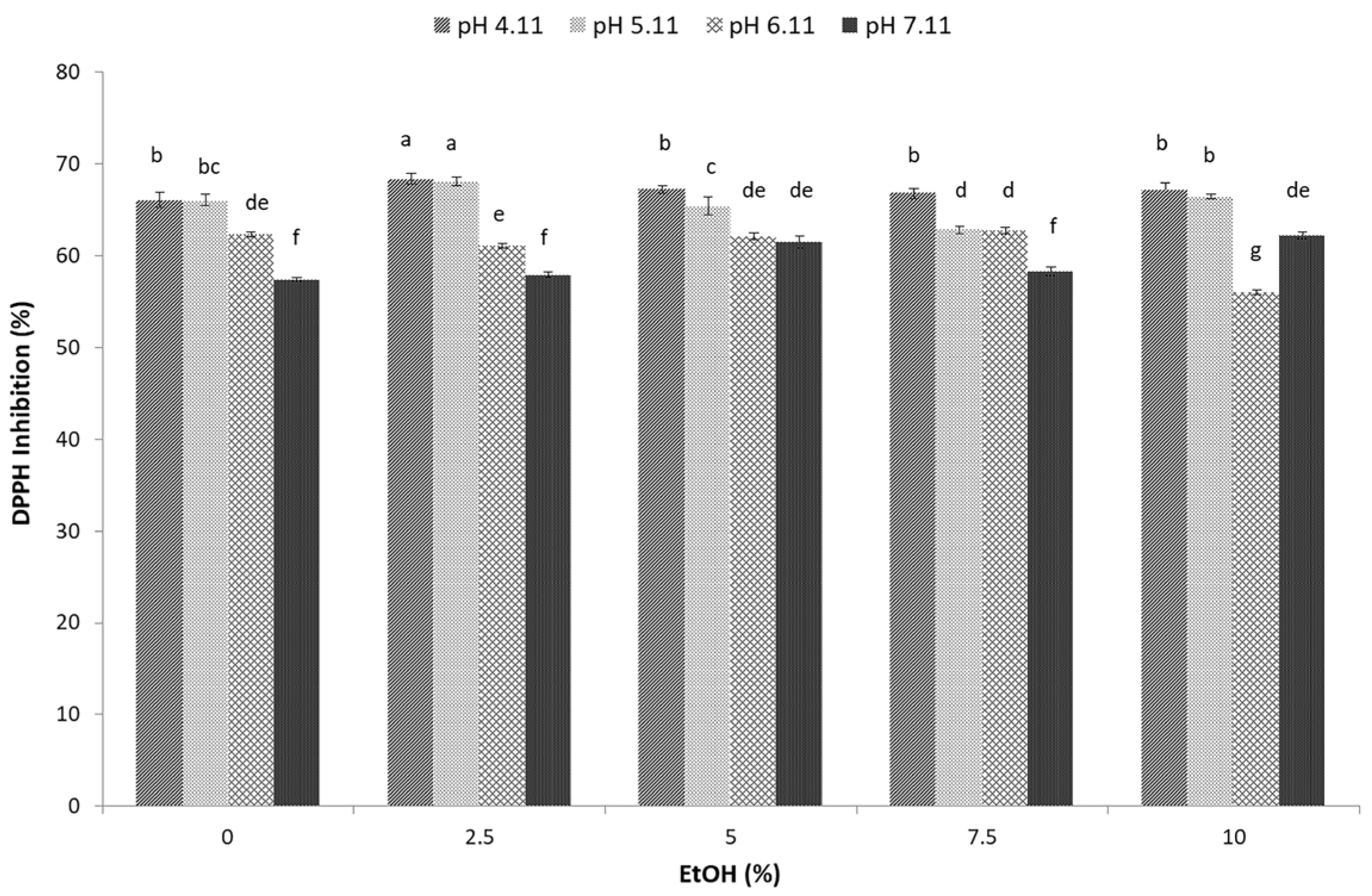
3.6. Total Antioxidant Capacity of Sugarcane Molasses Extracts Determined Using DPPH Free Radicals
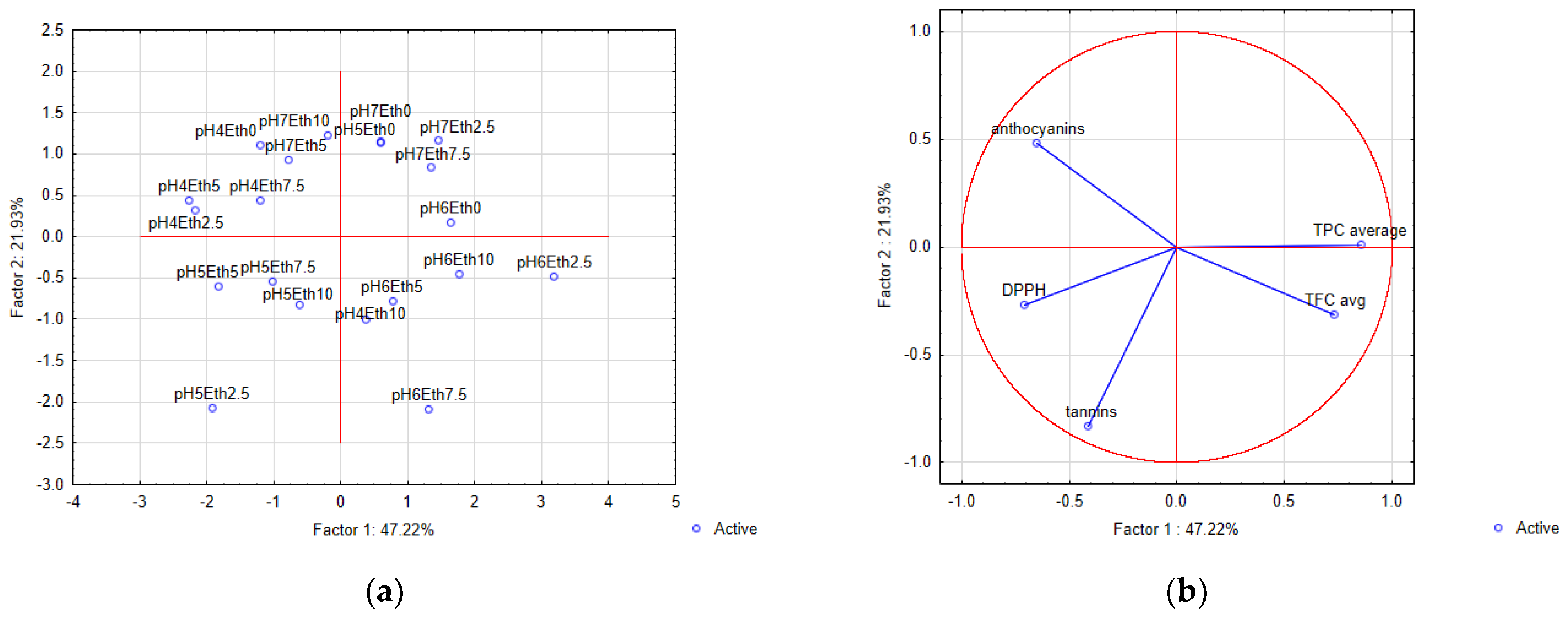
3.7. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 14830. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Djordjević, M.; Djordjević, M.; Starowicz, M.; Krupa-Kozak, U. Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints. Antioxidants 2024, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cortés, A.; Quimbaya, M.; Toro-Gomez, A.; Tobar-Tosse, F. Bioactive compounds as an alternative for the sugarcane industry: Towards an integrative approach. Heliyon 2023, 9, e13276. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Wang, C.; Zhu, Z.M. Inhibitory effect of sugarcane (Saccharum officinarum L.) molasses extract on the formation of heterocyclic amines in deep-fried chicken wings. Food Control 2021, 119, 107490. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Ighalo, J.O.; Eshiemogie, S.; Omuku, P.E.; Adeniyi, A.G. Valorization of Sugar Industry’s By-products: A Perspective. Sugar Tech 2022, 24, 1052–1078. [Google Scholar] [CrossRef]
- Ali, S.E.; Yuan, Q.; Wang, S.; Farag, M.A. More than sweet: A phytochemical and pharmacological review of sugarcane (Saccharum officinarum L.). Food Biosci. 2021, 44, 101431. [Google Scholar] [CrossRef]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Chandra, R.; Naresh Bharagava, R.; Rai, V. Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Bioresour. Technol. 2008, 99, 4648–4660. [Google Scholar] [CrossRef]
- Asikin, Y.; Takahashi, M.; Mishima, T.; Mizu, M.; Takara, K.; Wada, K. Antioxidant activity of sugarcane molasses against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chem. 2013, 141, 466–472. [Google Scholar] [CrossRef]
- Shafiqa-Atikah, M.; Nor-Khaizura, M.; Mahyudin, N.; Abas, F.; Nur-Syifa, J.; Ummul-Izzatul, Y. Evaluation of phenolic constituent, antioxidant and antibacterial activities of sugarcane molasses towards foodborne pathogens. Food Res. 2020, 4, 40–47. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Bibalan, S. High Value-added Products Recovery from Sugar Processing By-products and Residuals by Green Technologies: Opportunities, Challenges, and Prospects. Food Eng. Rev. 2018, 10, 95–111. [Google Scholar] [CrossRef]
- Demesa, A.G.; Saavala, S.; Pöysä, M.; Koiranen, T. Overview and Toxicity Assessment of Ultrasound-Assisted Extraction of Natural Ingredients from Plants. Foods 2024, 13, 3066. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-Ethanolic Mixtures for the Recovery of Phenols from Mediterranean Plant Materials. Food Bioprocess Technol. 2012, 5, 1384–1393. [Google Scholar] [CrossRef]
- Molina-Cortés, A.; Sánchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric Estimation of Total Phenolic Content and Antioxidant Capacity of Molasses and Vinasses Generated from the Sugarcane Industry. Waste Biomass Valorization 2020, 11, 3453–3463. [Google Scholar] [CrossRef]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Devi, T.; Bono, A.; Sarbatly, R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plant Res. 2009, 3, 67–72. [Google Scholar]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, Q.; Mu, W.; Hu, X.; Sun, Z.; Qiu, Y.; Liu, X.; Wang, Z. Research Advances of d-allulose: An Overview of Physiological Functions, Enzymatic Biotransformation Technologies, and Production Processes. Foods 2021, 10, 2186. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.T.; Blanchard, H.; Leffler, H.; Nilsson, U.J. Protein subtype-targeting through ligand epimerization: Talose-selectivity of galectin-4 and galectin-8. Bioorg. Med. Chem. Lett. 2008, 18, 3691–3694. [Google Scholar] [CrossRef]
- Sampaio, M.R.F.; Machado, M.C.; Lisboa, M.T.; Vieira, M.A.; Zimmer, T.B.R.; Otero, D.M.; Zambiazi, R.C. Physicochemical Characterization and Antioxidant Activity of Refined and Unrefined Sugarcane Products from Southern Brazil. Sugar Tech 2023, 25, 295–307. [Google Scholar] [CrossRef]
- Settharaksa, S.; Jongjareonrak, A.; Hmadhlu, P.; Chansuwan, W.; Siripongvutikorn, S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int. Food Res. J. 2012, 19, 1581–1587. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, H.; Yang, L.; Chen, L.; Xie, Y.; Xiao, J. Chapter 4.8—Flavonoids. In Antioxidants Effects in Health the Bright and the Dark Side, 1st ed.; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 353–374. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, X.; Si, J.; Gong, X.; Wang, S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016, 10, 56. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, R.; Raychaudhuri, U. Determination of pH-dependent antioxidant activity of palm (Borassus flabellifer) polyphenol compounds by photoluminol and DPPH methods: A comparison of redox reaction sensitivity. 3 Biotech 2015, 5, 633–640. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Gadhoumi, H.; Gullo, M.; De Vero, L.; Martinez-Rojas, E.; Saidani Tounsi, M.; Hayouni, E.A. Design of a New Fermented Beverage from Medicinal Plants and Organic Sugarcane Molasses via Lactic Fermentation. Appl. Sci. 2021, 11, 6089. [Google Scholar] [CrossRef]
- Arjeh, E.; Khodaei, S.M.; Barzegar, M.; Pirsa, S.; Sani, I.K.; Rahati, S.; Mohammadi, F. Phenolic compounds of sugar beet (Beta vulgaris L.): Separation method, chemical characterization, and biological properties. Food Sci. Nutr. 2022, 10, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Forino, M.; Picariello, L.; Rinaldi, A.; Moio, L.; Gambuti, A. How must pH affects the level of red wine phenols. LWT-Food Sci. Technol. 2020, 129, 109546. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Dumitrash, P.G.; Bologa, M.K.; Shemyakova, T.D. Ultrasound-assisted extraction of biologically active substances from tomato seeds. Surf. Eng. Appl. Electrochem. 2016, 52, 270–275. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhuo, Y.; Li, Y.; Meng, J.; Wang, Y.; Li, H. Ultrasound-Assisted Extraction of Anthocyanins from Malus ‘Royalty’ Fruits: Optimization, Separation, and Antitumor Activity. Molecules 2022, 27, 4299. [Google Scholar] [CrossRef]
- Farmani, B.; Djordjević, M.; Bodbodak, S.; Alirezalu, K.; Ghanbarpour, A. Powdered Activated Carbon Treatment of Sugar Beet Molasses for Liquid Invert Sugar Production: Effects of Storage Time and Temperatures. Sugar Tech 2022, 24, 522–531. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, H.; Zheng, R.; Khan, M.S.; Fu, X.; Tao, Z.; Zhang, Z. Anthocyanins characterization and antioxidant activities of sugarcane (Saccharum officinarum L.) rind extracts. Ind. Crops Prod. 2018, 113, 38–45. [Google Scholar] [CrossRef]
- Wani, A.K.; Rahayu, F.; Fauziah, L.; Suhara, C. Advances in safe processing of sugarcane and bagasse for the generation of biofuels and bioactive compounds. J. Agric. Food Res. 2023, 12, 100549. [Google Scholar] [CrossRef]
- Chua, L.S.; Wahab, N.S.A.; Soo, J. Water soluble phenolics, flavonoids and anthocyanins extracted from jaboticaba berries using maceration with ultrasonic pretreatment. Food Chem. Adv. 2023, 3, 100387. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P.; Kefalas, P. An Investigation on the Recovery of Antioxidant Phenolics from Onion Solid Wastes Employing Water/Ethanol-Based Solvent Systems. Food Bioprocess Technol. 2009, 2, 337–343. [Google Scholar] [CrossRef]
- Valli, V.; Gómez-Caravaca, A.; Di Nunzio, M.; Danesi, F.; Caboni, M.; Bordoni, A. Sugar Cane and Sugar Beet Molasses, Antioxidant-rich Alternatives to Refined Sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Luo, Z.; Zhong, Z.; Jiang, L.; Tang, K. Extraction optimization by response surface methodology: Purification and characterization of phytosterol from sugarcane (Saccharum officinarum L.) rind. J. Sep. Sci. 2014, 37, 1308–1314. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Yamamura, R.; Inoue, Y.K.; Nishino, K.; Yamasaki, S. Intestinal and fecal pH in human health. Front. Microbiomes 2023, 2. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and pH Effects on the Antioxidant Activity of Caffeic and Other Phenolic Acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
| Compound | Class | Rt (min) | Molasses (%) |
|---|---|---|---|
| Ethylene glycol (2TMS) | Alcohol | 7.89 | 0.67 |
| Phosphate (3TMS) | Inorganic acid | 11.57 | 0.51 |
| Pipecolic acid (N,O-2TMS) | Nitrogenous compound | 17.86 | 0.01 |
| N,O-hydroxylamine (2TMS) | Nitrogenous compound | 8.35 | 1.23 |
| Lactic acid (2TMS) | Organic acid | 6.31 | 0.07 |
| Glyceric acid isomer (3TMS) | Organic acid | 13.74 | 0.05 |
| Acetoacetic acid (2TMS) | Organic acid | 16.68 | 0.1 |
| Malic acid (3TMS) | Organic acid | 17.08 | 0.22 |
| Ribitol (5TMS) | Sugars | 22.39 | 0.03 |
| Mannopyranose 6-deoxy-(4TMS) | Sugars | 25.10 | 2.11 |
| Picose (5TMS) | Sugars | 25.93 | 4.14 |
| Mannopyranose (5TMS) | Sugars | 26.07 | 9.19 |
| Fructose (5TMS) | Sugars | 27.13 | 2.26 |
| Talose (5TMS) | Sugars | 27.78 | 9.94 |
| Galactofuranose (5TMS) | Sugars | 27.90 | 3.65 |
| Sucrose | Sugars | 38.87 | 59.74 |
| Unknown | Unknown | 24.41 | 5.12 |
| Total | 99.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farmani, B.; Djordjević, M.; Mohammadkhani, S.; Djordjević, M. Assessing Sugarcane Molasses’ Bioactive Compound Content upon Ultrasound-Assisted Hydroethanolic Extraction at Various pH Values. Agriculture 2025, 15, 158. https://doi.org/10.3390/agriculture15020158
Farmani B, Djordjević M, Mohammadkhani S, Djordjević M. Assessing Sugarcane Molasses’ Bioactive Compound Content upon Ultrasound-Assisted Hydroethanolic Extraction at Various pH Values. Agriculture. 2025; 15(2):158. https://doi.org/10.3390/agriculture15020158
Chicago/Turabian StyleFarmani, Boukaga, Miljana Djordjević, Somayeh Mohammadkhani, and Marijana Djordjević. 2025. "Assessing Sugarcane Molasses’ Bioactive Compound Content upon Ultrasound-Assisted Hydroethanolic Extraction at Various pH Values" Agriculture 15, no. 2: 158. https://doi.org/10.3390/agriculture15020158
APA StyleFarmani, B., Djordjević, M., Mohammadkhani, S., & Djordjević, M. (2025). Assessing Sugarcane Molasses’ Bioactive Compound Content upon Ultrasound-Assisted Hydroethanolic Extraction at Various pH Values. Agriculture, 15(2), 158. https://doi.org/10.3390/agriculture15020158







