Abstract
As widely generated by-products with significant bioactive compound content, sugarcane molasses exhibits high potential for valorization. For the purpose of bioactive compound extraction from sugarcane molasses, ultrasound-assisted extraction with various hydroethanolic solvents (0, 2.5, 5, 7.5, and 10% ethanol) at different pH values (4.11, 5.11, 6.11, and 7.11) was examined. In the obtained sugarcane molasses extracts, the content of total phenolics, monomeric anthocyanins, total flavonoids, total tannins, and antioxidant capacity (DPPH) was estimated alongside the determination of molasses’ major components through GC-MS analysis Based on the GC-MS analysis of molasses, sugars and nitrogenous compounds emerged as the most abundant compounds classes. Hydroethanolic solvent of 2.5% at pH 6.11 exhibited the most prominent extraction power regarding total phenolics (22074.98 µg GAE mL−1) and total flavonoids (245.42 µg QE mL−1). Furthermore, extraction with 2.5% hydroethanolic solvent at pH 5.11 displayed the highest total tannins (1177.85 µg CE mL−1). The behavior of monomeric anthocyanins in ultrasound-assisted extraction with hydroethanolic solvent was slightly different, where 2.5% hydroethanolic solvent extracted the highest amount at pH 4.11 (11.1 µg CGE mL−1) and 7.11 (10.68 µg CGE mL−1). The results of the DPPH assay indicated that extracts obtained using 2.5% hydroethanolic solvent at pH 4.11 (68.35%) and pH 5.11 (68.10%) evinced the highest neutralization power against DPPH free radicals. In conclusion, 2.5% ultrasound-assisted hydroethanolic solvent and pH 4.11-6.11 were the most suitable for extracting bioactive compounds from sugarcane molasses.
Keywords:
molasses; sonication; total phenolics; flavonoids; anthocyanins; tannins; side-stream valorization 1. Introduction
The emerging interest of consumers in products with health-promoting properties has reinforced a strong tendency toward the valorization of bioactive compounds originating from natural sources [1]. It is advocated that the corresponding compound addition can improve the quality and nutritional value of processed food by inhibiting the destructive oxidative reaction during storage [2]. Bioactive compounds are natural or synthetic compounds that are also capable of exerting a wide range of biological responses and can be extracted from various biological matrices of animal, plant, and marine origin, as well as their by-products [3]. Thus, sugarcane, as well as its products (juices) and by-products (bagasse, molasses) generated during processing, represent a recognized source of natural-origin bioactive compounds and Maillard reaction products [4].
Sugarcane molasses, as a by-product from the sugar industry, besides its sucrose, mineral, low fat, and crude protein content, is rich in bioactive compounds (phenolic acids, flavonoids, and tannins), which have strong antioxidant effects and high biological performance [5,6]. Nevertheless, diverse types and amounts of bioactive compounds can be present in molasses due to the extraction conditions applied in sugarcane processing [7,8]. This mainly refers to melanoidins, bioactive compounds present in molasses that can demonstrate diverse bioactivities, including antioxidant, antiallergenic, antimicrobial, and cytotoxic properties, considering their roles as reducing agents, metal chelators, and radical scavengers [9]. Additionally, phenolic acids, such as ferulic acid, p-coumaric acid, p-hydroxybenzaldehyde [10], chlorogenic, and syringic acid [8], as well as flavonoids, namely diosmin and swertisin [8], are some of the major identified phenolic compounds with antioxidant effects in molasses. Correspondingly, sugarcane molasses and its extract can serve as a novel source for the recovery and usage of bioactive compounds, representing a natural alternative rich in antioxidant and antibacterial compounds suitable for the replacement of chemical preservatives in the food industry [11].
However, the industry faces challenges in transforming the corresponding substantial residues and by-products into economically profitable food-based ingredients, and green extraction techniques with the usage of eco-friendly solvents represent a promising approach. Among these, ultrasound-assisted extraction (UAE) is recognized as a green extraction technique that results in high extraction efficiency of bioactive compounds at mild temperatures due to the formation of cavitation bubbles generated by acoustic waves. Subsequent burst of the cavitation bubbles in contact with the material surface can increase the mass transfer process of the targeted compound into solvent and effectively prevent the degradation of active compounds at high temperatures [12,13]. Moreover, the usage of UAE results in improved extraction yield of total polyphenol content, total monomeric anthocyanin content, and total antioxidant capacity [14].
Scientific sources have identified the use of a suitable solvent as one of the paramount aspects of recovering phenolic and other bioactive compounds from sugar beet molasses [12]. Accordingly, the use of eco-friendly solvent, such as ethanol, which is reusable, non-toxic, applicable in the food industry, and found to preserve bioactive compounds during storage [15,16], is another way to enhance bioactive compounds’ extractability and combat the food sustainability challenge. A step forward in eco-friendly solvent application is certainly hydroethanolic solvent usage, which, as the main benefit, provides the most complete recovery of an individual as well as a mixture of hydrophilic and lipophilic bioactive compounds [16]. This approach, however, remains rather underexplored when it comes to molasses, especially considering hydroethanolic solvents with low ethanol share (up to 10%).
Sugar beet molasses subjected to UAE with acidic ethanol as the solvent resulted in promising results regarding total phenolic content (173 µg GAE g−1), total anthocyanins (318 µg g−1), as well as total antioxidant capacity (43%) [12]. A literature review reveals that the use of water to prepare an aqueous extract of sugarcane molasses [17] and ethanol to prepare an alcoholic extract [8] containing bioactive compounds exerting biological activity is expanding, but hydroethanolic solvent usage for these purposes is scarce. Accordingly, coupling green extraction techniques, such as UAE, with a hydroethanolic solvent with low ethanol share as an extraction medium in the recovery of bioactive compounds from sugarcane molasses can bring improvements regarding their preservation, reduce the environmental burden due to reduced ethanol consumption, thus aligning with the Sustainable Development Goals (SDGs), and lower the high cost of the ethanol recovery after extraction. Simultaneously, a high amount of phenolic compounds can be extracted with shortened time and low energy consumption.
Considering the presented research gap, the present study aims to investigate the synergistic effect of UAE and hydroethanolic solvent (share 2.5 to 10%) as extraction media on various bioactive compounds’ content (phenols, flavonoids, tannins, anthocyanins) and their antioxidant activity (DPPH) in sugarcane molasses extract. Simultaneously, the impact of different pH values on the extraction efficiency of individual bioactive compound types was estimated, and sugarcane molasses was characterized in terms of sugars, organic acids, and nitrogenous compound profiles. The purpose of the research was to foster the application of sugarcane molasses extract rich in bioactive compounds in formulating new health-promoting food products.
2. Materials and Methods
2.1. Materials
Sugarcane molasses samples (80° Brix) were obtained from sugarcane factories (Ahvaz, Iran) with approximate sucrose and ash content of 60 and 11%, respectively, and manually homogenized before use.
Analytical-grade chemicals, such as Folin–Ciocalteu reagent (2 N), sodium carbonate, sodium nitrite, aluminum chloride hexahydrate, sodium hydroxide, potassium chloride, potassium metabisulfite, hydrochloric acid, sodium acetate trihydrate, ferric chloride, and potassium ferrocyanide, as well as standards of gallic acid, quercetin, cyanidin-3-glucoside, catechin, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), were purchased from Merck Company (Rahway, NJ, USA). Food-grade ethanol was used for the preparation of hydroethanolic solvents.
2.2. Methods
2.2.1. GC-MS Analysis of Sugarcane Molasses
The analysis of compounds (primary metabolites) in freeze-dried (0.05 mbar, overnight) and silylated sugarcane molasses was carried out using the GC-MS technique according to the method of Ali et al. [18].
The GC analysis of samples was performed using the Agilent 7890A Network GC system (Agilent Technologies, Santa Clara, CA, USA) with a flame ionization detector (310 °C) and a split mode injector (with 1.0 µL volume and 250 °C temperature). Separation was performed on an Agilent HP 5MS column (Agilent Technologies, Santa Clara, CA, USA) (30 m length × 0.25 mm inner diameter, film thickness of 0.25 µm) under the following conditions: helium gas at 1.1 mL min−1 and column pressure fixed at 8.13 PSI. The oven temperature was initially kept at 50 °C for 2 min after injection and then increased to 310 °C for 8 °C min−1 and kept constant at 310 °C for 12 min. Compounds were identified using the Wiley library and the literature (NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standardisation and Technology, Gaithersburg, MD, USA) by matching their retention indices and mass spectra patterns with available reference spectra.
2.2.2. Preparation of Samples
A defined quantity of sugarcane molasses (2 g) was diluted with 60 mL of hydroethanolic solvents containing various shares of ethanol (0, 2.5, 5, 7.5, and 10% ethanol) with subsequent adjustments to 4 pH levels (4.11, 5.11 (the original pH of sugarcane molasses), 6.11, and 7.11). Sodium carbonate (20% v/v) and citric acid (20% v/v) were used to adjust the pH of molasses solutions. Prepared samples were subjected to UAE in an ultrasonic bath at the following conditions: 45 °C for 30 min at 35 kHz and 450 W (Bandelin Sonorex, Berlin, Germany) [11]. The molasses extracts released from ethanol through rotary vacuum evaporation at 55 °C and 100 mbar (Rotavapor® R-100, BUCHI, Berlin, Germany) were used for subsequent analysis.
2.2.3. Determination of Total Phenolic Content
The Folin–Ciocalteu method [19] was employed (50 µL extract) for total phenolic content (TPC) determination with absorption detection at 765 nm (Jenway 6405 UV/Vis, Staffordshire, UK). The TPC was expressed as gallic acid equivalent (µg GAE mL−1 extract).
2.2.4. Determination of Total Flavonoid Content
The aluminum chloride colorimetric method described by Chang et al. [20] with absorbance detection at 510 nm (Jenway 6405 UV/Vis, Staffordshire, UK) was applied (50 µL extract) for total flavonoid content estimation, expressed as the quercetin equivalent (µg QE mL−1 extract).
2.2.5. Determination of Total Tannin Content
The total tannin content in samples (50 µL extract) was measured at 395 nm (Jenway 6405 UV/Vis, Staffordshire, UK) and reported as the catechin equivalent (µg CE mL−1 extract) [21].
2.2.6. Determination of Monomeric Anthocyanin Content
The pH differential method was used to evaluate (300 µL extract) the monomeric anthocyanin content following the procedure of Lao and Giusti [20] by measuring absorbance at 520 and 700 nm (Jenway 6405 UV/Vis, Staffordshire, UK). Calculations were performed according to Lao and Giusti [22], and the anthocyanin content was expressed as cyanidin 3-glucoside equivalent (µg C3G mL−1 extract).
2.2.7. Determination of Total Antioxidant Capacity by DPPH Free Radicals
The absorption value of molasses extracts was measured at 517 nm (Jenway 6405 UV/Vis, Staffordshire, UK) after one hour of standing of the reaction mixture containing the sample (150 µL extract) and the 0.1 mM DPPH methanolic solution (3 mL). Trolox was used as the standard, and the inhibition power of extracts was expressed as % of DPPH inhibition [17]. Additionally, IC50 (mg/mL), a concentration of test solution required to inhibit 50% of available DPPH free radicals, was calculated following the function, relating percentages of DPPH inhibition reached by the samples at different concentrations [17].
2.3. Statistical Analysis
The experiments were performed using a completely randomized design method with two variables, pH (at 4 levels) and ethanol (at 5 levels) in 3 replications. Duncan’s test was used to compare the different averages at the level of significance of 5% (p < 0.05). The data analysis was carried out using SAS (version 9.1) statistical software (Institute Inc., Cary, NC, USA), while Excel (2013) was used for graphical representation of the results. Additionally, the Principal Component Analysis (PCA) was performed using Statistica software (Tibco Inc., Palo Alto, CA, USA, 2020, version 14.0.0.15).
3. Results and Discussion
3.1. Sugarcane Molasses Compounds Identified Through GC-MS Analysis
A total of 17 compounds were identified in examined sugarcane molasses through GS-MC analysis, and they are presented in Table 1. Detected classes of compounds were alcohol, inorganic and organic acids, nitrogenous compounds, as well as sugars.

Table 1.
Identified compounds and their content alongside associated retention times (Rt) obtained through GC-MS analysis of sugarcane molasses.
As anticipated, compounds belonging to the sugars class were the most abundant, with eight compounds identified, from which sucrose accounts for 59.74%, followed by talose (9.94%) and mannopyranose (9.19%) (Table 1). When compared with a previous study on sugarcane molasses originating from Egypt [18], higher amounts were obtained for mannopyranose 6-deoxy, fructose, and galactofuranose, as well as sucrose and rare sugars, such as picose and talose.
The presence of picose (D-allulose), as sugar with numerous positive physiological effects, such as lipid metabolism regulation, neuroprotection, as well as anti-obesity effects [23] at higher levels, is highly desirable, especially when taking into account that it is generally recognized as safe (GRAS, GRN No. 1057), thus concurring with the clean label criteria of food products. Similarly, talose can serve as a precursor for antibiotic synthesis, while its methylated forms can serve as inhibitors in inflammation processes [24].
Nevertheless, the absence of D-tagatose and D-allose was noticed for the examined molasses, although the corresponding sugars were previously detected in sugarcane molasses at ~7 and ~5%, respectively [18]. Observed discrepancies in the obtained results and the literature data can be attributed to applied agro-technical measures during sugarcane growth as well as conditions during sugarcane processing. Considering organic acids, malic acid, which is naturally occurring in sugarcane, was the most abundant in the examined molasses, which is in line with the results of Ali et al. [18]. Furthermore, lactic acid’s presence as a compound generated during sugarcane processing was also confirmed (Table 1). Additionally, N,O-hydroxylamine was detected, as well as minor amounts (<0.8%) of pipecolic acid, ethylene glycol, and phosphate (Table 1), corresponding to the results of Ali et al. [18].
3.2. Total Phenolic Content of Sugarcane Molasses Extracts
The impacts of the used hydroethanolic solvent and the applied pH during UAE on bioactive phenolic compounds from sugarcane molasses are shown in Figure 1a. The results indicate that the combination of applied pH and ethanol in the solvent exhibited a significant effect on the content of extracted phenolic compounds (p ˂ 0.05). The TPC ranged from 14,925 to 22,075 µg GAE mL−1 depending on the experimental conditions (Figure 1a).
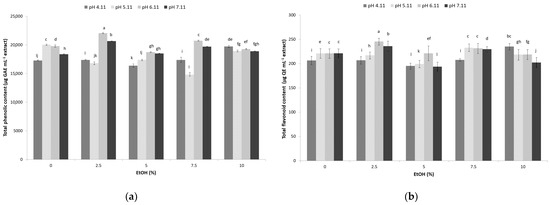
Figure 1.
Effect of ultrasound-assisted hydroethanolic (EtOH-ethanol share 0, 5, 7.5, and 10%) extraction and pH (4.11, 5.11, 6.11, 7.11) on sugarcane molasses: (a) total phenolic content; (b) total flavonoid content. Bars with different letters indicate a statistically significant difference at p < 0.05.
The maximal extraction ability regarding phenolic compounds was obtained with the use of hydroethanolic solvent containing 2.5% ethanol at pH 6.11 (22,074.98 µg GAE mL−1) and 7.11 (20,675.03 µg GAE mL−1) (Figure 1a). High TPC content in sugarcane molasses extracts under the mentioned extraction conditions could be a consequence of the better suitability of solvent mixtures (ethanol and water) when compared with individual solvents and better matching of the polarity of sugarcane molasses phenolic compounds and hydroethanolic solvent mixture, which is crucial for the extraction process. The obtained TPC was higher than the TPC of sugarcane molasses ethanol extract with 75% ethanol share (17,400 µg GAE g−1) [8], as well as 50% (v/v) ethanol extract (2795.60 µg GAE g−1) [25] and aqueous extracts of sugarcane molasses [19,20]. Furthermore, the obtained results of TPC were higher compared to UAE extracts of sugar beet molasses obtained under optimized conditions with 57–63% ethanol [12]. Higher ethanol share in the hydroethanolic solvent combined with UAE at the same pH (6.11 and 7.11) was less efficient in phenolic compound extraction from sugarcane molasses, which is in contrast to sugar beet molasses, where an increase in TPC was achieved with an increase in ethanol concentrations at a fixed HCl concentration (1.55–1.72 mol L−1) [12]. Additionally, acidic conditions (higher HCl concentrations) and lower ethanol content (~60%) resulted in the highest TPC of sugar beet molasses extract, whilst highly acidic conditions in this study (pH 4.11 and 5.11) induced a decline in the TPC of the extracts except in those containing 10% ethanol (Figure 1). It is assumed that the possible destruction of phenolic compounds sensitive to changes in pH [26] can occur, lowering their extraction effectiveness.
Accordingly, for the corresponding hydroethanolic solvent containing 2.5% ethanol, ethanol consumption can be reduced, thus contributing to a decrease in the environmental burden and lowering the high cost of recovering the ethanol used during extraction, while a high amount of phenolic compounds can be extracted in a shortened time and using a low amount of energy by UAE. Due to the proposed health-promoting effect related to the reduction of oxidative stress and conditions connected to it, the attractiveness of phenolic compounds for use in the food industry beyond their preservative role is increasing.
3.3. Total Flavonoid Content of Sugarcane Molasses Extracts
The significant effect (p < 0.05) of the hydroethanolic solvent share used and the pH during UAE on flavonoid’s extractability from sugarcane molasses was also confirmed (Figure 1b). The TFC ranged from 193 to 246 µg mL−1 depending on the applied conditions.
The combination of hydroethanolic solvent with 2.5% ethanol and UAE at pH 6.11 (245.42 µg QE mL−1) and 7.11 (236.33 µg QE mL−1) expressed the highest affinity towards flavonoid compound extraction (Figure 1b). Accordingly, wide usage of organic solvents mixed with various water proportions has been successfully applied in flavonoid extraction from plant sources using UAE [27]. Interestingly, lower percentages of ethanol (2.5%) displayed the highest effect on the extraction of important bioactive flavonoid compounds. This is probably related to the type of flavonoids present in molasses, and it is also a consequence of the presence of a sugar moiety found in molasses, which usually increases the solubility of free flavonoids in water. Additionally, the presence of flavonoid glycosides, which are generally soluble in highly polar solvents, such as water, methanol, and ethanol, enhanced flavonoid extraction [28]. Considering that flavonoid compounds present in sugarcane are mainly in the form of C-glycosides [18], less polar portions of flavonoid glycoside components are soluble in ethanol, and those that have higher polarity are soluble in water; thus, the share of 2.5% ethanol in the solvent was the correct proportion to reach the highest TFC [29]. The obtained results were higher compared to the TFC of sugarcane molasses 50% (v/v) ethanolic extracts (101.8 µg QE g−1) [25] but significantly lower compared to the results of UAE of sugarcane molasses with 70% ethanol (79,500 µg RE g−1) [5], as well as methanol extracts (2970 µg QE g−1) [18]. The observed discrepancies in the reported results are consequences of multiple factors (raw material origin, production process variations) as well as the absence of standardized methodological protocols for this kind of analytical determination [3,17].
Although greater flavonoid yields are usually reported through extraction in an acidic medium, because most of the flavonoids possess phenolic hydroxyl groups, whose number and position determine their acidity [28], in the present study, greater flavonoid content was obtained in weakly acidic and neutral conditions (pH 6.11 and 7.11). Similarly, Ghosh, Chakraborty, and Raychaudhuri [30] stated that with rising pH values from 3.5 to 6.5, the TFC increased in fermented palm juices, as well. It is assumed that the higher extraction efficiency of flavonoids at the mentioned pH could be a consequence of the extraction parameters (power, temperature, extraction duration) and their independent or interactive effects with other molasses constituents, such as sugars, i.e., different pertinent sugar moieties [31].
This demonstrates that hydroethanolic solvent with a small share of ethanol can serve as a solvent with better extracting ability for flavonoids in combination with UAE than classic ethanol extraction, which requires higher ethanol consumption, without significant alterations in the original sugarcane molasses pH value.
3.4. Total Tannin Content of Sugarcane Molasses Extracts
Tannins are polymeric compounds with high nutritional importance that can be extracted from sugarcane molasses using UAE and hydroethanolic solvents. The results presented in Figure 2a show that the combination of pH and percentages of ethanol in the UAE has a significant effect on the extraction rate of tannins (range of 260–1178 µg CE mL−1, p < 0.05). The results indicate that the use of UAE with a hydroethanolic solvent containing 2.5% ethanol at pH 5.11 (1177.85 µg CE mL−1) and 7.5% ethanol at pH 6.11 (1009.45 µg CE mL−1) had the greatest capacity for tannin extraction from sugarcane molasses (Figure 2a). Although there is a paucity of results regarding tannin content in sugarcane molasses, functional drinks prepared from sugarcane molasses have been reported to contain 93.6 µg CE mL−1 of tannins [32], which was less than the amount reported herein. Furthermore, approximately two times higher total tannin content was found in ethanol extract of sugar beet flesh (2200 µg GAE g−1 dry basis) without a specified ethanol share [33].
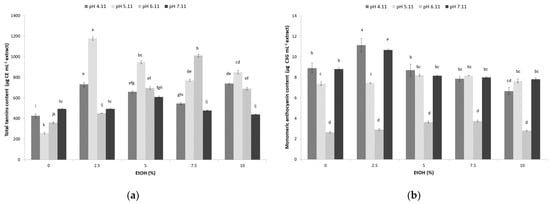
Figure 2.
Effect of ultrasound-assisted hydroethanolic (EtOH–ethanol share 0, 5, 7.5, and 10%) extraction and pH (4.11, 5.11, 6.11, 7.11) on sugarcane molasses: (a) total tannin content; (b) monomeric anthocyanin content. Bars with different letters indicate a statistically significant difference at p < 0.05.
Water represents a preferred solvent for tannins extraction at an industrial scale, while ethanol’s addition contributes to the extraction efficiency as well as ultrasound [34]. Moreover, an acidic environment proves to be beneficial in terms of tannin extraction, probably as a consequence of enhanced tannin release from sugarcane molasses, as previously suggested for vine must [35]. The contribution of UAE as an extraction technique to tannin’s extractability is anticipated to also be significant based on previous findings [36]. Unfortunately, not enough data relevant for comparison are available concerning sugar industry products and by-products.
3.5. Monomeric Anthocyanin Content of Sugarcane Molasses Extracts
Anthocyanins are polymeric compounds with sugar molecules in their structure, and they are nutritionally valuable. The application of UAE with hydroethanolic solvent had a positive effect on anthocyanin extraction from sugarcane molasses, with content in the range of 2.67–11.1 µg C3G mL−1 (p < 0.05). It is assumed that mass transfer is intensified, enabling easier access of solvent to the anthocyanin molecules, under the action of ultrasonic waves and the cavitation effect produced [37].
Furthermore, the results presented in Figure 2b show that the combination of pH and the ethanol percentage for UAE have a significant effect on the extraction of anthocyanins. The use of 2.5% ethanol at pH 4.11 (11.1 µg C3G mL−1) and 7.11 (10.68 µg C3G mL−1) was the most efficient regarding anthocyanin extraction. Higher percentages of ethanol did not show positive effects on the extraction efficacy of these important bioactive compounds (Figure 2b). Similarly, Liu et al. [38] reported a decrease in anthocyanin yield extracted from Malus ‘Royalty’ fruits using UAE when the ethanol content increased up to 100%.
This could be explained by the fact that anthocyanins are classified into the group of water-soluble phenolic pigments [22,39], enabling their better extraction in hydroethanolic solvents with higher water content. This was also previously noticed during the UAE of sugar beet molasses, where the solvent with the lowest ethanol share (60%) gave the highest anthocyanin content under a similar treatment temperature (41 °C) [12].
Other factors affecting anthocyanins’ stability and, consequently, their content are certainly the pH and the time of the ultrasound treatment. In line with the results obtained herein, an acidic environment was the most favorable for anthocyanin extraction from sugar beet molasses through UAE [12]. Prolonged ultrasound treatment (70 min) also had a positive effect on anthocyanins concerning sugar beet molasses [12]. However, the obtained results herein are significantly lower compared to those reported for anthocyanin content of sugar beet molasses UAE extract, which was 318.3 µg g−1 [12], as well as diluted sugar beet molasses, at 14.7 µg g−1 [40]. The concentration of chemical compounds in sugarcane is influenced by various factors, including geographical location, which affect the content of flavonoids and anthocyanins in sugarcane and, consequently, molasses [41]. Additionally, noted discrepancies can be explained by the variability of the anthocyanin content between different sugarcane varieties [39], similarly to the solvent, but also the potential hydrolysis of anthocyanin induced by applied ultrasound [42].
3.6. Total Antioxidant Capacity of Sugarcane Molasses Extracts Determined Using DPPH Free Radicals
Bioactive compounds, including phenols, flavonoids, tannins, and anthocyanins, present in sugarcane molasses strongly remove free radicals (DPPH free radicals) and have high nutritional value [18]. The extraction of these bioactive compounds from sugarcane molasses by combining hydroethanolic solvent and UAE at different pH values was performed, and the results of the antioxidant capacity, expressed as % of inhibition towards DPPH free radicals, are presented in Figure 3. The antioxidant capacity of sugarcane molasses extracts ranged from 56.05 to 68.35% (p < 0.05) depending on the applied conditions, implying a significant effect of the pH and ethanol share.
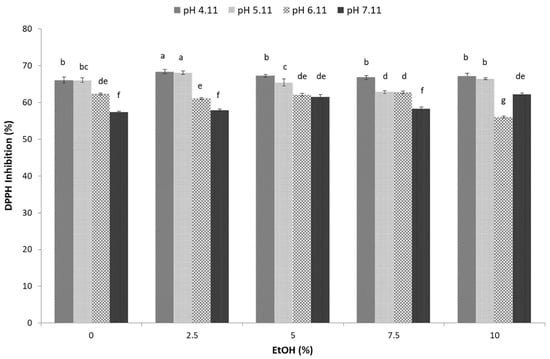
Figure 3.
Effect of ultrasonic-assisted hydroethanolic (EtOH–ethanol share 0, 5, 7.5, and 10%) extraction and pH (4.11, 5.11, 6.11, 7.11) on sugarcane molasses’ total antioxidant capacity. Bars with different letters indicate a statistically significant difference at p < 0.05.
The use of hydroethanolic solvent with 2.5% ethanol at pH 4.11 (IC50 2.53 mg mL−1 (68.35%)) and 5.11 (IC50 2.53 mg mL−1 (68.1%)) during UAE showed the highest efficiency in extracting bioactive compounds with an inhibitory ability towards DPPH free radicals (Figure 3). Higher percentages of ethanol did not show positive effects on the antioxidant capacity of sugarcane molasses extracts, in agreement with previous findings regarding hydroethanolic extracts of plant materials (aromatic plants, olive and orange leaves) [15,16]. This observation could be attributed to the fact that sugarcane molasses bioactive compounds expressing antioxidant activity have a more polar nature, enabling their better extraction in hydroethanolic mixtures containing a lower share of ethanol than in absolute ethanol [16]. Indeed, sugarcane molasses phenolic acids (ferulic acid, p-coumaric acid, p-hydroxybenzaldehyde, chlorogenic and syringic acid) containing the carboxylic group and flavonoids (glycosides) considered to be covalent polar molecules express a higher preference to solvents with a lower ethanol share [43].
Although previous studies performed measurements of antioxidant activity in sugarcane molasses employing DPPH, a comparison between the results is hampered by the ability to express the results in multiple ways. In the studies of Valli et al. [44] and Molina-Cortés et al. [17] on sugarcane molasses, the results were expressed as IC50, referring to the concentration of sample required to achieve a 50% inhibition of the DPPH radicals. The IC50 of 2.53 mg mL−1 obtained herein was higher compared to the 1.47 mg mL−1 reported by Valli et al. [44], but it was also much lower considering the results obtained by Molina-Cortés et al. [17] for C molasses (6.77 mg mL−1), implying both lower and higher antioxidant activity of the sugarcane molasses hydroethanolic extract obtained in the present study, respectively.
Sugarcane and molasses are rich sources of bioactive compounds, such as phenols, terpenoids, phytosterols, and various phenolic acids (p-coumaric, gallic, chlorogenic, and ferulic acid) [4]. These compounds have been found to have biological activity and a positive correlation with the inhibition of free radicals in laboratory conditions [45]. DPPH reacts with phenolic compounds via two mechanisms: rapid sequential proton loss electron transfer (SPLET) in ionizing solvents (such as ethanol) and slower hydrogen atom transfer (HAT) in nonionizing solvents or in the presence of acid [46]. Moreover, the suggestion is that the re-distribution of –OH radicals on the rings of the phenolic molecules can occur at different pH values during simulated digestion [47] and consequently alter the bioactive compounds’ stability and antioxidant activity. In this regard, pH’s role in the human body is multiple; it is the driving force of various vital reactions (intracellular adenosine triphosphate (ATP) synthesis and oxygen transfer in muscle tissues), while in the intestinal tract it sets up the optimal environment for digestive and metabolic enzymes’ action and the bactericidal effects of acids [48]. Considering that the highest antioxidant capacity obtained herein is reached at pH 4.11 and 5.11, it could be said that phenolic compounds in sugarcane molasses will behave as very good antioxidants under acidic conditions (in gastric lumen), whilst they will not be so efficient at pH 7−8, which is characteristic of the intestinal tract, blood, extracellular fluid, and within cells [49]. Furthermore, possible practical applications of extracted sugarcane molasses’ phenolic compounds might include non-alcoholic beverages, edible films, and active packaging materials, thus improving the stability of oxidation-sensitive food products.
3.7. Principal Component Analysis
The Principal Component Analysis (PCA) was used as a valuable tool for grouping similar responses as well as the differentiation of opposite ones. The variability of the applied pH and ethanol share during UAE was explained by two main components by as much as 69.15% (Figure 4).
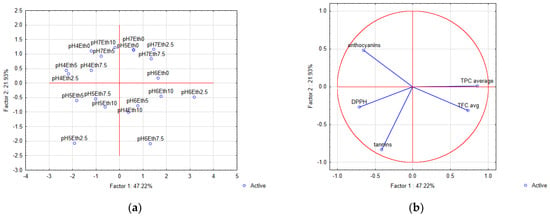
Figure 4.
Principal Component Analysis (PCA) showing (a) the loading plot and (b) and score plot, describing the relationship between the determined bioactive compounds and different ultrasound-assisted hydroethanolic extraction conditions (ethanol share and pH). Eth, ethanol.
Principal component 1 (PC1) explained 47.22 of the total variance, while principal component 2 (PC2) explained 21.93% of the variance (Figure 4). It was demonstrated that sugarcane molasses hydroethanolic extracts with ethanol shares of 2.5, 5, 7.5, and 10% at pH 5.11 (the negative left quadrant, Figure 4a) exhibited a negative influence on the PC1 and PC2 calculations, but the corresponding extraction conditions provided greater antioxidant activity by DPPH and tannin content. The extracts obtained at pH 6.11 and ethanol shares of 2.5, 5, 7.5, and 10% displayed a positive effect on PC1 but a negative effect on PC2 (Figure 4a) and were associated with TFC content (Figure 4b). Furthermore, the smallest share of ethanol in the hydroethanolic solvent used for UAE of sugarcane molasses at pH 5.11, 6.11, and 7.11 (positive right quadrant, Figure 4a) positively influenced PC1 and PC2 coordinate calculations and was related to greater TPC (Figure 4b). In the remaining quadrant, pH 4.11 and pH 7.11, alongside ethanol shares of 0-10%, positively affected PC2 and negatively affected PC1 calculations (Figure 4a) and were associated with anthocyanins content (Figure 4b). Additionally, the DPPH values are in the same quadrant as the total tannins (Figure 4b), indicating that the corresponding conditions are favorable for the extraction of these bioactive compounds, which may be associated with their hydroxyl groups, in line with previous studies [16,34]. Meanwhile, for the most efficient extraction of total phenolics, flavonoids, and anthocyanins, different conditions should be applied (Figure 4b). Accordingly, when using PCA for sugarcane molasses extract samples, the most suitable extraction conditions regarding pH as well as ethanol shares in solvent, which provide greater bioactive compounds content and antioxidant activity, were identified.
4. Conclusions
Sugarcane molasses extract contains bioactive compounds, such as phenols, flavonoids, tannins, and anthocyanins, that have high nutritional value and antioxidant capacity with exploitation potential in the food industry. The extraction of these compounds can be improved by using ultrasound-assisted hydroethanolic extraction. The results indicate that using 2.5% ethanol at pH 6.11 with ultrasonication was the most effective in the extraction of phenolic and flavonoid compounds compared to higher percentages of ethanol. The maximal extraction of tannins was reached using 2.5% ethanol and pH 5.11. Anthocyanins as polymeric compounds were positively affected by ultrasound-assisted hydroethanolic extraction and showed the highest extractable amount in 2.5% ethanol at pH 4.11. Accordingly, the expressed antioxidant capacity of sugarcane molasses hydroethanolic extract, including the set of bioactive compounds (phenols, flavonoids, tannins, and anthocyanins), was highest when 2.5% hydroethanolic solvent was used at pH 4.11.
This study concluded that the UAE could be a promising selective extracting technique for the efficient extraction of bioactive compounds from sugarcane molasses using hydroethanolic solvent with minimal ethanol share under acidic conditions, delivering extracts rich in bioactive compounds. Corresponding extracts can be further applied as constituents of non-alcoholic and milk beverages characterized by a slightly sour taste or as constituents with a preservative role in edible and active packaging materials for diverse food products. This approach can offer a new perspective on sugarcane molasses’ valorization while scaling up the extraction process, and the estimation of sugarcane molasses’ bioactive compounds’ bioavailability upon digestion can guide further research.
Author Contributions
Conceptualization, B.F.; formal analysis, B.F., M.D. (Miljana Djordjević) and M.D. (Marijana Djordjević); investigation, B.F. and S.M.; resources, B.F.; writing—original draft preparation, B.F. and M.D.; writing—review and editing, M.D. (Miljana Djordjević) and M.D. (Marijana Djordjević); visualization, B.F., M.D. (Miljana Djordjević) and M.D. (Marijana Djordjević). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 14830. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Djordjević, M.; Djordjević, M.; Starowicz, M.; Krupa-Kozak, U. Plant-Based Antioxidants in Gluten-Free Bread Production: Sources, Technological and Sensory Aspects, Enhancing Strategies and Constraints. Antioxidants 2024, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cortés, A.; Quimbaya, M.; Toro-Gomez, A.; Tobar-Tosse, F. Bioactive compounds as an alternative for the sugarcane industry: Towards an integrative approach. Heliyon 2023, 9, e13276. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Wang, C.; Zhu, Z.M. Inhibitory effect of sugarcane (Saccharum officinarum L.) molasses extract on the formation of heterocyclic amines in deep-fried chicken wings. Food Control 2021, 119, 107490. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Ighalo, J.O.; Eshiemogie, S.; Omuku, P.E.; Adeniyi, A.G. Valorization of Sugar Industry’s By-products: A Perspective. Sugar Tech 2022, 24, 1052–1078. [Google Scholar] [CrossRef]
- Ali, S.E.; Yuan, Q.; Wang, S.; Farag, M.A. More than sweet: A phytochemical and pharmacological review of sugarcane (Saccharum officinarum L.). Food Biosci. 2021, 44, 101431. [Google Scholar] [CrossRef]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Chandra, R.; Naresh Bharagava, R.; Rai, V. Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Bioresour. Technol. 2008, 99, 4648–4660. [Google Scholar] [CrossRef]
- Asikin, Y.; Takahashi, M.; Mishima, T.; Mizu, M.; Takara, K.; Wada, K. Antioxidant activity of sugarcane molasses against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chem. 2013, 141, 466–472. [Google Scholar] [CrossRef]
- Shafiqa-Atikah, M.; Nor-Khaizura, M.; Mahyudin, N.; Abas, F.; Nur-Syifa, J.; Ummul-Izzatul, Y. Evaluation of phenolic constituent, antioxidant and antibacterial activities of sugarcane molasses towards foodborne pathogens. Food Res. 2020, 4, 40–47. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Bibalan, S. High Value-added Products Recovery from Sugar Processing By-products and Residuals by Green Technologies: Opportunities, Challenges, and Prospects. Food Eng. Rev. 2018, 10, 95–111. [Google Scholar] [CrossRef]
- Demesa, A.G.; Saavala, S.; Pöysä, M.; Koiranen, T. Overview and Toxicity Assessment of Ultrasound-Assisted Extraction of Natural Ingredients from Plants. Foods 2024, 13, 3066. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-Ethanolic Mixtures for the Recovery of Phenols from Mediterranean Plant Materials. Food Bioprocess Technol. 2012, 5, 1384–1393. [Google Scholar] [CrossRef]
- Molina-Cortés, A.; Sánchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric Estimation of Total Phenolic Content and Antioxidant Capacity of Molasses and Vinasses Generated from the Sugarcane Industry. Waste Biomass Valorization 2020, 11, 3453–3463. [Google Scholar] [CrossRef]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Devi, T.; Bono, A.; Sarbatly, R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plant Res. 2009, 3, 67–72. [Google Scholar]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, Q.; Mu, W.; Hu, X.; Sun, Z.; Qiu, Y.; Liu, X.; Wang, Z. Research Advances of d-allulose: An Overview of Physiological Functions, Enzymatic Biotransformation Technologies, and Production Processes. Foods 2021, 10, 2186. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.T.; Blanchard, H.; Leffler, H.; Nilsson, U.J. Protein subtype-targeting through ligand epimerization: Talose-selectivity of galectin-4 and galectin-8. Bioorg. Med. Chem. Lett. 2008, 18, 3691–3694. [Google Scholar] [CrossRef]
- Sampaio, M.R.F.; Machado, M.C.; Lisboa, M.T.; Vieira, M.A.; Zimmer, T.B.R.; Otero, D.M.; Zambiazi, R.C. Physicochemical Characterization and Antioxidant Activity of Refined and Unrefined Sugarcane Products from Southern Brazil. Sugar Tech 2023, 25, 295–307. [Google Scholar] [CrossRef]
- Settharaksa, S.; Jongjareonrak, A.; Hmadhlu, P.; Chansuwan, W.; Siripongvutikorn, S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int. Food Res. J. 2012, 19, 1581–1587. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, H.; Yang, L.; Chen, L.; Xie, Y.; Xiao, J. Chapter 4.8—Flavonoids. In Antioxidants Effects in Health the Bright and the Dark Side, 1st ed.; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 353–374. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, X.; Si, J.; Gong, X.; Wang, S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016, 10, 56. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, R.; Raychaudhuri, U. Determination of pH-dependent antioxidant activity of palm (Borassus flabellifer) polyphenol compounds by photoluminol and DPPH methods: A comparison of redox reaction sensitivity. 3 Biotech 2015, 5, 633–640. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Gadhoumi, H.; Gullo, M.; De Vero, L.; Martinez-Rojas, E.; Saidani Tounsi, M.; Hayouni, E.A. Design of a New Fermented Beverage from Medicinal Plants and Organic Sugarcane Molasses via Lactic Fermentation. Appl. Sci. 2021, 11, 6089. [Google Scholar] [CrossRef]
- Arjeh, E.; Khodaei, S.M.; Barzegar, M.; Pirsa, S.; Sani, I.K.; Rahati, S.; Mohammadi, F. Phenolic compounds of sugar beet (Beta vulgaris L.): Separation method, chemical characterization, and biological properties. Food Sci. Nutr. 2022, 10, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Forino, M.; Picariello, L.; Rinaldi, A.; Moio, L.; Gambuti, A. How must pH affects the level of red wine phenols. LWT-Food Sci. Technol. 2020, 129, 109546. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Dumitrash, P.G.; Bologa, M.K.; Shemyakova, T.D. Ultrasound-assisted extraction of biologically active substances from tomato seeds. Surf. Eng. Appl. Electrochem. 2016, 52, 270–275. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhuo, Y.; Li, Y.; Meng, J.; Wang, Y.; Li, H. Ultrasound-Assisted Extraction of Anthocyanins from Malus ‘Royalty’ Fruits: Optimization, Separation, and Antitumor Activity. Molecules 2022, 27, 4299. [Google Scholar] [CrossRef]
- Farmani, B.; Djordjević, M.; Bodbodak, S.; Alirezalu, K.; Ghanbarpour, A. Powdered Activated Carbon Treatment of Sugar Beet Molasses for Liquid Invert Sugar Production: Effects of Storage Time and Temperatures. Sugar Tech 2022, 24, 522–531. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, H.; Zheng, R.; Khan, M.S.; Fu, X.; Tao, Z.; Zhang, Z. Anthocyanins characterization and antioxidant activities of sugarcane (Saccharum officinarum L.) rind extracts. Ind. Crops Prod. 2018, 113, 38–45. [Google Scholar] [CrossRef]
- Wani, A.K.; Rahayu, F.; Fauziah, L.; Suhara, C. Advances in safe processing of sugarcane and bagasse for the generation of biofuels and bioactive compounds. J. Agric. Food Res. 2023, 12, 100549. [Google Scholar] [CrossRef]
- Chua, L.S.; Wahab, N.S.A.; Soo, J. Water soluble phenolics, flavonoids and anthocyanins extracted from jaboticaba berries using maceration with ultrasonic pretreatment. Food Chem. Adv. 2023, 3, 100387. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P.; Kefalas, P. An Investigation on the Recovery of Antioxidant Phenolics from Onion Solid Wastes Employing Water/Ethanol-Based Solvent Systems. Food Bioprocess Technol. 2009, 2, 337–343. [Google Scholar] [CrossRef]
- Valli, V.; Gómez-Caravaca, A.; Di Nunzio, M.; Danesi, F.; Caboni, M.; Bordoni, A. Sugar Cane and Sugar Beet Molasses, Antioxidant-rich Alternatives to Refined Sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Luo, Z.; Zhong, Z.; Jiang, L.; Tang, K. Extraction optimization by response surface methodology: Purification and characterization of phytosterol from sugarcane (Saccharum officinarum L.) rind. J. Sep. Sci. 2014, 37, 1308–1314. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Yamamura, R.; Inoue, Y.K.; Nishino, K.; Yamasaki, S. Intestinal and fecal pH in human health. Front. Microbiomes 2023, 2. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and pH Effects on the Antioxidant Activity of Caffeic and Other Phenolic Acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).