Abstract
Global geographical, climatic, and ecological diversity has given rise to a wealth of domestic animals, which are essential for food security and agricultural sustainability. Since the 1960s, these critical genetic resources have declined significantly due to overdevelopment, ecological degradation, and climate change, posing a serious threat to global food security. In the face of these challenges, we emphasize the critical importance of promoting indigenous livestock and poultry germplasm resources in biodiversity conservation to enhance the adaptability and resilience of agricultural systems. To promote the sustainable management and conservation of genetic resources, a multistakeholder international cooperation framework is needed. Globally, many national and international institutions have initiated a variety of conservation measures, legislation, and technical strategies. In particular, genebanks play an indispensable role in the conservation of important livestock and poultry genetic resources. These banks not only aid in maintaining biodiversity but also provide valuable genetic material for future breeding programmes and scientific research. Through systematic collection, conservation and evaluation, genebanks ensure the long-term availability and sustainable use of genetic resources and provide an important foundation for addressing global environmental change and agricultural challenges.
1. Introduction
Livestock and poultry germplasm resources are key to a nation’s basic agricultural strategy, as they effectively act as the ‘chip’ for livestock development. The conservation of livestock and poultry germplasm resources not only ensures food security but also provides the basis for improved national resilience and sustainable agricultural growth. The importance of these germplasm resources goes beyond meeting the demand for safe, high-quality, and diverse animal products. They also make a significant contribution to increasing the incomes of local farmers and herders, promote the sustainable utilization of resources, and conserve the environment and cultural heritage. In addition, livestock and poultry germplasm resources play a crucial role in addressing future climate change and food security challenges as the global population increases and demand for animal products [1]. It enables farmers to select appropriate livestock or develop new breeds in response to changing environmental conditions, such as climate change, emerging or recurring disease threats, new knowledge of human nutritional needs, and changes in market conditions or societal demands [2]. However, a concerning trend has emerged since the 1960s: the accelerating depletion of these vital genetic reserves. This escalating vulnerability underscores a pressing global challenge [3].
To safeguard irreplaceable livestock and poultry genetic resources, a large number of conservation initiatives and legislation have been developed both domestically and internationally. Key among these are the Convention on Biological Diversity [4], the State of the World’s Animal Genetic Resources for Food and Agriculture [5], and some national protection legislation [6,7]. These initiatives and legislation aim to advocate for the conservation and management of livestock and poultry genetic resources to safeguard global food security and biodiversity. In addition, multiple conservation strategies, including in situ and ex situ methods, have been developed to protect these resources. Both methods are effective tools for safeguarding livestock and poultry genetic resources and have been extensively adopted in contemporary practises. The establishment of a germplasm resource genebank can effectively preserve existing genetic resources and provide important raw biological materials for the improvement of breeds and the mechanistic analysis of excellent traits, which is highly valuable for the protection and research of livestock and poultry genetic resources.
In this review, we begin with a detailed account of the quantity, spatial distribution, and advantageous characteristics of livestock and poultry germplasm resources on a global scale. We then focus on the current endangered status of livestock and poultry germplasm resources, revealing the threats to their survival and loss of genetic resources. Based on this background, we systematically discuss the conservation strategies adopted by different nations and regions, including policies and regulations, the construction of protected areas, and the development and management of genebanks. The importance of the construction of genebanks and their role in the conservation of livestock and poultry genetic resources are also discussed.
2. Global Overview of Livestock and Poultry Germplasm Resources
2.1. Present Situation of World Animal Germplasm Resources
2.1.1. Abundant Germplasm Resources of Livestock and Poultry
Livestock and poultry germplasm resources refer to livestock, poultry, special livestock and poultry and all their breeds, strains, types, genetic materials, and other resources, which are direct sources of food and health for human beings and are indispensable and important resources for the development of human society. To conserve global animal germplasm resources more efficiently, the Food and Agriculture Organization (FAO) of the United Nations has established a comprehensive repository, the FAO’s Global Databank for Animal Genetic Resources for Food and Agriculture (Global Databank, https://www.fao.org/home/zh (accessed on 18 February 2023)). The database contains genetic information on various animal species, including livestock, poultry, and wildlife. As of September 2022, the Global Databank boasts contributions from 182 nations (alongside 15 dependent territories), aggregating data for 37 distinct species. The data show that national breed populations have been increasing over the last three decades, gradually increasing from 3019 national breed populations in 1995 to 15,313 in 2022. The number of mammal populations increased from 2719 to 11,555, and the number of avian populations increased from 863 to 3758 (Figure 1).
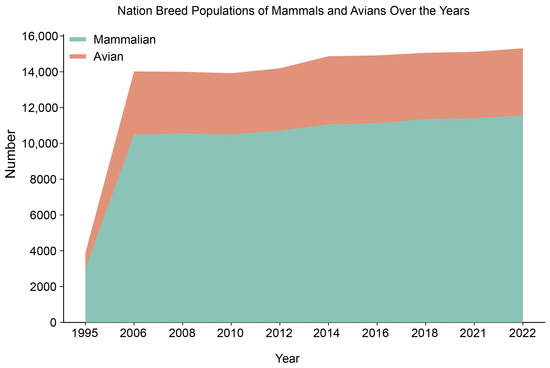
Figure 1.
Status of information held in the Global Animal Genetic Resources Database. (Data from the 12th session of the ITWG on Animal Genetic Resources for Food and Agriculture, 2023) (https://www.fao.org/3/cc3705en/cc3705en.pdf, accessed on 5 January 2025).
To provide real-time insights into global livestock diversity, the FAO initiated the Domestic Animal Diversity Information System (DAD-IS, https://www.fao.org/dad-is (accessed on 20 October 2022)) in 1996. The system is the backbone of the Global Databank and the centre of a scalable global network of information systems. As of September 2022, there are a total of 8859 (including 595 extinct breeds) livestock and poultry genetic resources in the world, of which 7739 (87.3%) are local breeds, 564 (6.4%) are regional cross-border (reported in only one region) breeds, and 556 (6.3%) are international cross-border (reported in more than one region) breeds. Excluding extinct breeds, there were 8264 existing livestock and poultry breeds (Figure 2A), including 7153 local breeds, 555 regional cross-border breeds and 556 international cross-border breeds (Figure 2B), the number of chicken breeds accounted for the largest share (Figure 2C). Regionally, Europe and the Caucasus region account for approximately 44% of these breeds, while Africa, despite its rich genetic potential, accounts for a mere 12% (Figure 2A). For historical and economic reasons, the breeding industry in this region started late and has lagged behind that of developed regions. Such a context has engendered major challenges for African regions, particularly in the identification of breeds, excavation of genes, and exploitation of speciality resources of livestock breeds.
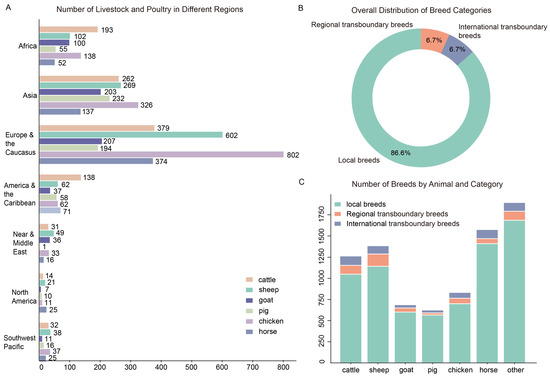
Figure 2.
Overview of global livestock and poultry genetic resources (Source: DAD-IS). (A) Quantitative distribution of livestock and poultry genetic resources in different continents or regions. The first seven rows represent the quantitative distribution of local breeds and regional transboundary breeds in different regions (Africa, Asia, Europe and the Caucasus, Latin America and the Caribbean, Near Middle and East, North America, and Southwest Pacific) for the quantitative distribution of livestock and poultry genetic resources. The eighth row represents the number of international transboundary breeds (not belonging to any of the regions). (B) Percentage of different types of livestock and poultry resources (local breeds, regional transboundary breeds, and international transboundary breeds). (C) Number of three resource types in different livestock or poultry breeds.
To alleviate this gap with developed regions, it is imperative for Africa to strengthen global cooperation and introduce and utilize high-yield breeds while preserving the genetic diversity of local breeds. Popular transboundary breeds that have gained widespread recognition in the global livestock industry include Boer goats [8], Holstein cows [9], and White Leghorn chickens [10]. These breeds have been introduced in several countries due to their high growth rate [8] and high milk [9] or egg production [11]. The widespread distribution of these transboundary breeds emphasizes the strong global collaboration and exchange in the livestock industry. At the country level, the interplay between native resources and superior introduced genetics has profound implications. This symbiotic relationship promotes breed development and enhances the overall productivity of livestock and poultry resources.
2.1.2. Severe Issues Affecting the Variety of Livestock and Poultry Species
Biodiversity is the basis for maintaining ecosystem homeostasis and functioning and is related to human survival and development, with far-reaching implications for human health and well-being [12,13]. However, in recent years, due to a variety of factors such as human overexploitation, ecological damage, and climate change, species diversity has declined sharply. Climate change is currently widely recognized as a major threat to global biodiversity, and it is occurring at a rapid pace. This may prevent some species and populations from adapting in time, thereby accelerating their extinction [14]. Over the past few decades, climate change has caused significant shifts in species distributions and abundances [15], and it is anticipated that thousands of species may face the risk of extinction within the next 100 years [16]. The current extinction rate of species is 1000–10,000 times greater than the natural extinction rate [17], which means that organisms on Earth are disappearing at an alarming rate. Globally, the diversity of many livestock and poultry species has been severely affected. This may be due to insufficient attention given to livestock and poultry germplasm resources and low levels of conservation and exploitation in many countries, which have led to the extinction of animal genetic resources [18].
To increase awareness of endangered livestock and poultry breeds, the FAO has established criteria to classify endangerment levels based on breeding populations [5]. According to the DAD-IS data, the number of extinct breeds increased from 8 (pre-1990) to 131 post-2010, with the extinction times of 274 breeds uncertain (Figure 3A). As of September 2022, 595 out of 8859 breeds are extinct (7%) (Figure 3B,C), and 2360 are at risk (27%). Moreover, 4769 (54%) new additions had unknown population sizes (Figure 3B), increasing their risk status. Efforts to research these unknown breeds should be intensified to develop effective protection measures. Additionally, governmental and relevant entities must enhance the management and conservation of these genetic resources and promote public awareness to safeguard this critical biodiversity.
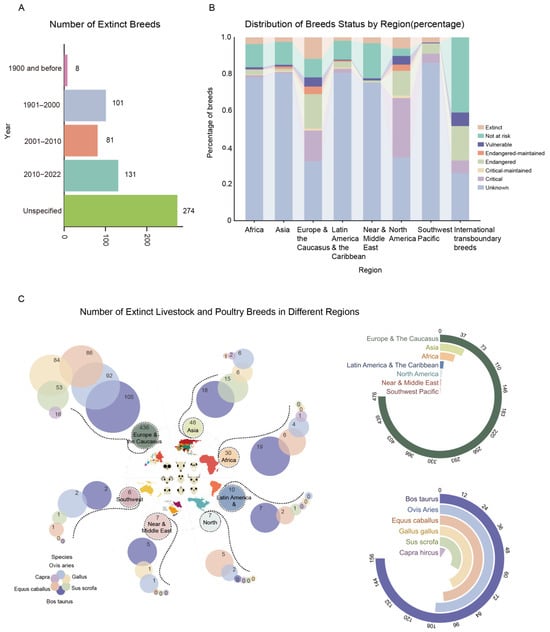
Figure 3.
Endangered status of livestock and poultry germplasm resources in the world. (A) Annual changes in the number of extinctions of livestock and poultry breeds. (B) Proportion of livestock and poultry breeds with different levels of endangerment (secure, extinct) in different continents or regions. (C) Number of extinct livestock and poultry breeds in different continents or regions (the colours of the inner circle represent different regions and the colours of the outer circles stand for the different varieties).
2.2. Protection of Animal Germplasm Resources
2.2.1. Conservation Plans and Policies
In response to the global decline in livestock and poultry diversity, nations and international organizations worldwide have emphasized the paramount importance of conserving, developing, and harnessing animal genetic resources and have proposed a number of programmes and policies, including the Convention on Biological Diversity (CBD) [19], the “State of the World’s Animal Genetic Resources for Food and Agriculture” report [5] and the “Global Plan of Action for Animal Genetic Resources” (GPA) [20]. The Global Plan of Action represents a significant advancement in international efforts to conserve and sustainably use animal genetic resources, focusing on global collaboration [21]. By 2019, the FAO had received 104 country-specific progress reports (http://www.fao.org/animal-genetics/global-policy/reporting-system/countries/en/ (accessed on 14 June 2020)), four regional progress reports (http://www.fao.org/animal-genetics/global-policy/reporting-system/regions/en/ (accessed on 14 June 2020)) and fourteen reports from international organizations (http://www.fao.org/animal-genetics/global-policy/reporting-system/international-organizations/en/ (accessed on 14 June 2020)), and the submission of the reports shows that there are wide variations in the overall level of implementation across countries and regions [22,23]. Concurrently, nations have developed specific policies to support genetic diversity. These initiatives help to understand and preserve the genetic diversity of livestock and poultry globally.
2.2.2. Modalities for the Conservation of Genetic Resources
Up to now, reflection on the management of genetic resources considered a three-step linear model: conservation → evaluation → utilization [24], so the conservation of genetic resources is fundamental. By safeguarding the genetic diversity of livestock and poultry breeds, we bolster their resilience against environmental vicissitudes, diseases, and market demands. Consequently, the conservation, evolution, and harnessing of these genetic resources have become important antidotes to the paucity of genetic material beleaguering global livestock and poultry [25]. Conservation efforts, which are essential for addressing the shortage of genetic materials, are largely categorized into in situ and ex situ strategies. While 82% of countries practice in situ conservation, coverage is limited to an average of 23% of species. Conversely, ex situ conservation, primarily through genebanks, offers broader protection for critical and endangered genetic resources, preserving materials such as frozen sperm, embryos, embryonic cells, gonadal tissues, and DNA. These genebanks not only support in situ conservation but can also be used to form resource populations for genetic studies and the evaluation and identification of germplasm resources.
2.3. Overview of Livestock and Poultry Germplasm Resources in China
2.3.1. China Has Abundant and Diversified Livestock and Poultry Genetic Resources
China has a long history of livestock and poultry farming, dating back thousands of years, and is a vast territory with a complex and varied climate, topography and landscape, with five different climatic zones and three terrain terraces. Recently, to meet various needs, people have selected and matched these breeds and continue to introduce elite foreign lineages, thereby continually enriching China’s already vast reservoir of livestock and poultry genetic wealth.
The genetic diversity of livestock and poultry in China has been increasing over the decades. The 1986 edition of the “Journal of Chinese Livestock and Poultry Breeds” recorded 282 distinct breeds. In 2011, the Journal of Livestock and Poultry Genetic Resources of China recorded 777 germplasm resources of domestic animals (excluding silkworms). By 2021, the “Chinese Livestock and Poultry Genetic Resources Breed List” had registered a total of 948 breeds; poultry dominated this list, contributing 36.5%, followed by sheep (17.6%); and cattle (13.9%) (Figure 4). This highlights the advanced use of poultry breeds in China. Currently, China’s broilers, laying hens, and broiler ducks have a strong market presence, holding approximately half of the market share.
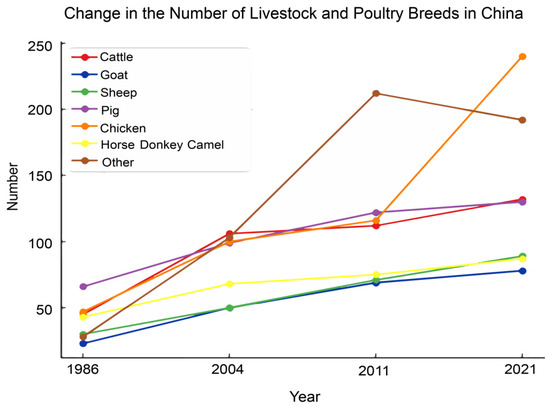
Figure 4.
Annual change in number of different livestock species in China (Data source: China Breeds Journal, Country Reports, China Livestock and Poultry Genetic Resources Journal, China Livestock and Poultry Genetic Resources Breed List).
2.3.2. Distinctive Germplasm Characteristics of Local Livestock and Poultry Germplasm Resources in China
Optimizing the potential of indigenous livestock and poultry germplasm resources is imperative for preserving species diversity and bolstering human health. Local breeds have excellent characteristics, such as high fertility, good meat quality, resilience to adversity, and resistance to disease. For example, the high-fertility Meishan pig has significantly contributed to global pig breeding. Moreover, various cashmere goat breeds known for their superior cashmere quality, such as Liaoning Cashmere goats, can produce up to 2 kg of cashmere, and Inner Mongolia Cashmere goat goats have an average cashmere fineness of 14.5 mm. These resources highlight the importance of preserving and utilizing China’s unique genetic assets.
2.3.3. High Risk of Endangerment for a Few Breeds
Among the rich livestock and poultry breeds in China, some rare breeds are facing serious danger. The second national genetics survey since 2006 revealed that 15 local breeds have gone extinct and that 59 are endangered or near extinction, together accounting for approximately 14% of local breeds. What is more worrying is that some local breeds whose population size has not yet reached the endangered level have experienced a gradual decrease in genetic diversity within the breed due to the decline in the number of males. In addition, insufficient knowledge of the characteristics of some local breeds, coupled with a lack of adaptation to the development needs of the current market and the threat of foreign bloodlines, has led to varying degrees of decline in the population size of some breeds, which further exacerbates the risk of loss of livestock and poultry genetic resources in China.
To better protect these valuable germplasm resources, China conducted the third National Livestock and Poultry Genetic Resources Census in 2021. Initially, 24 endangered livestock and poultry breeds, including Zhangmu cattle and Hetao big-eared pigs, were evaluated and identified, and salvage conservation actions were urgently initiated in 2022. During this census, genetic materials such as sika deer were collected for the first time, the protection of more than 30 local breeds of livestock and poultry, including Huabei donkey, was strengthened through measures such as in vivo protection and collection of genetic materials, and the rescue collection and preservation of more than 200,000 samples of livestock and poultry genetic materials were undertaken.
2.4. Conservation of Livestock Germplasm Resources
2.4.1. Protection Legislation and Systems
Since 2005, China has implemented numerous regulations for the protection and sustainable management of livestock and poultry genetic resources, leading to the development of a comprehensive protection system and modernization of the animal husbandry sector. The country has established a cataloguing system and tiered protection strategy with oversight at both the national and provincial levels to safeguard the economic, ecological, and cultural value of these resources. The National Catalogue of Livestock and Poultry Genetic Resources details 17 traditional and 16 special types, while the National List of Livestock and Poultry Resource Breeds for Protection initiated in 2000 with 78 breeds was expanded to include 159 endangered breeds by 2014. Additionally, 566 breeds, which constitute the “second tier” of breed protection resources and play a pivotal role in China’s agricultural production and ecosystem, have been designated at the provincial level.
2.4.2. Establishment of a Multilevel Mechanism for the Conservation of Genetic Resources
Over the past few years, China’s livestock and poultry genetic resources protection system has been continuously optimized, gradually establishing a multifaceted protection system that combines in situ and ex situ protection, complemented by in vivo protection and preservation of genetic materials, and the construction of three protection barriers: the National Livestock and Poultry Germplasm Resource Bank, the Regional Genebank, and the Breeding Farm Protection Zone.
China has significantly advanced in situ conservation for livestock and poultry genetic resources, establishing 183 national conservation farms and 24 protection zones before 2023 (https://www.moa.gov.cn/). According to the Scientific Report on Biological Germplasm Resources in China, multiple performance evaluation centres, breeding stations, and prime breed farms had been established in 30 provinces, autonomous regions, and municipalities across the nation by December 2019. This has culminated in a robust conservation and breeding system based on conservation and original breed farms, supported by improvement stations and breeding farms and protected by quality inspection centres. The 2014 list shows national protection for a variety of species, with coverage rates of 90% for national-level breeds and 70% for provincial-level breeds [26]. This in situ approach not only aids in the effective management and preservation of the native traits of breeds but also strengthens the breeding industry by ensuring diverse, high-quality livestock products, and a stable supply chain.
Ex situ conservation acts as a vital supplement to China’s strategy for animal germplasm resource protection, proving more cost-effective than in situ methods by reducing conservation costs by more than 90% [27]. To date, China has established 10 national genebanks: nine are living genebanks, while one is dedicated to genetic material storage (https://www.moa.gov.cn/). The preservation of genetic material is based on livestock semen supplemented with embryos, somatic cells, and blood. Given the technological constraints associated with poultry semen freezing, ex situ conservation for poultry mainly focuses on live preservation and somatic cell storage. With the development of ESC and iPSC technologies, Masafumi Katayama established iPSCs in primary fibroblasts from three endangered bird species, providing new preservation tools for ectopic preservation of poultry [28]. In addition, cryopreservation of reproductive organs and tissues can break through the technical limitations of oocyte and embryo cryopreservation, which is of great significance in the conservation of genetic resources in poultry [29]. This study provides new perspectives for preserving the diversity of genetic resources and promotes future research efforts. Furthermore, the National Domesticated Animal Germplasm Resource Bank boasts an extensive collection encompassing genomic libraries, cDNA libraries, functional genes, semen, oocytes, embryos, and other pivotal genetic materials, which effectively promotes the innovative preservation of livestock and poultry germplasm resources in China. Compared to in situ conservation, ex situ genebank preservation is markedly more efficient.
3. Profile of Genebank Construction of Livestock and Poultry Globally
In recent years, with the accelerated decline in genetic diversity due to global urbanization, climate change, land-use change and other threatening factors, countries have paid increasing attention to the conservation and innovative use of germplasm resources. To effectively reduce the loss of agricultural biodiversity, several countries started to establish genebanks in the mid-twentieth century [30]. The establishment of germplasm genebanks is highly valuable for the conservation and restoration of livestock and poultry genetic resources and has been important in conservation of livestock and poultry germplasm resources in various countries [31,32]. In a seminal move, Brazil pioneered the creation of the world’s first national genebank in 1983 [33]. The United States unveiled the National Seed Storage Laboratory (NSSL) in 1999, paralleled by France’s inauguration of its national cryobank [34]. In the late 1990s, some countries embarked on similar paths [35]. By 2015, 64 countries had established genebanks [36]. Currently, approximately 45% of countries globally have instituted national-level genebanks, with another 32% in progress. The increase in the size of genebanks underscores the paramount importance that the international community attaches to the preservation of the Earth’s biological treasures. This phenomenon mirrors the profound importance attributed to the conservation of animal genetic resources. This article primarily focuses on genebanks that are rich in genetic material, equipped with advanced technologies, and have significant influence (Table 1 and Figure 5).

Table 1.
Information on the Main Conservation Institutions of Animal Germplasm Resources in Europe and America.
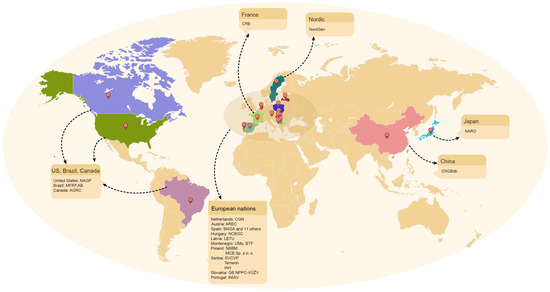
Figure 5.
The global distribution of the largest genebanks for genetic resources related to livestock and poultry (colour designates the genebank in the countries mentioned in this text).
3.1. Developments in the Construction of Important Foreign Germplasm Repositories
3.1.1. NCGRP and AGRIN
To address the decline in livestock and poultry genetic resources, the U.S. Department of Agriculture (USDA) inaugurated the National Animal Germplasm Program (NAGP) in 1999 [37], whose germplasm collection and conservation were conducted at the National Seed Storage Laboratory (NSSL), which applies cryobiological methods for storing semen, ova, oocytes, and other tissues in liquid nitrogen. On 14 January 2002, the United States Agricultural Research Service (ARS) changed the name of the NSSL to the National Center for Genetic Resources Preservation (NCGRP). This facility is committed to all-encompassing preservation of genetic resources spanning animals, plants, and microbes. As of 2022, the NAGP has 389 breeds of animal germplasm resources, 63,968 animals, and 1,263,298 pieces of genetic material. In addition to their ability to be obtained and stored, these invaluable germplasm samples have facilitated critical endeavours such as the reconstruction of requisite populations, the provision of genetic variability for breeders of commercial strains, and the enrichment of breeding schemas, serving as a goldmine for molecular genetics and reproductive physiology research [38,39].
Furthermore, the U.S. has substantially invested in the establishment of an Animal Germplasm Resources Information Network (A-GRIN, https://agrin.ars.usda.gov/ (accessed on 20 May 2024)). The network integrates information on the conservation of animal genetic resources in Brazil and Canada. According to the records from AGRIN, Brazil leads in the preservation of genetic material with 1,635,311 samples, encompassing 90 different animal breeds. The United States followed closely, while Canada had a modest preservation count of 202,109 samples, covering 71 animal breeds. Compared to Canada, the United States and Brazil have richer diversity in the types of genetic material preserved and have initiated the utilization of these valuable genebank resources for genomic analysis. As of 2016, 90% and 20% of animal germplasm samples from the United States and Brazil, respectively, have been employed in genomic research [40], demonstrating the active efforts of these nations in leveraging genetic resources to propel scientific exploration. This revolutionary approach of analyzing the genomes of germplasm samples aids in the rapid extraction of genetic data. Such endeavours enable direct inferences to be made about general patterns in population structure, founding events, introductory history, natural selection and directional selection of genetic traits, thus providing insights into genetic diversity and ancestral relationships and contributing to further advances in the management and conservation of genetic resources.
Using an integrated approach consisting of information systems, genetics, and cryobiology, enriched by collaborations with national and international partners, nations have achieved great strides in germplasm collection, identification, gene discovery, and innovation. Such integrative strategies amplify the capacities of countries to adapt to food insecurity and climate change challenges.
3.1.2. EUGENA
The vast majority of European nations have developed ex situ genebank strategies [41]. The European Regional Focal Point (ERFP) formally established the European Genebank Network for Animal Genetic Resources (EUGENA) (https://eugena-erfp.net/en/ (accessed on 4 January 2025)) in 2013 through a formal agreement between several national genebanks, with the aim of supporting the ex situ conservation and sustainable use of livestock and poultry in Europe [42] and contributing to the implementation of the FAO Global Plan of Action [20] and the Nagoya Protocol [43] in Europe. The EUGENA, which has been operational since 2016, involves 24 national genebanks from 14 countries such as Australia and various European nations. The establishment of the EUGENA provides valuable genetic support for European livestock and poultry germplasm resources, effectively maintaining biodiversity, promoting advanced research related to genetics and breeding, enhancing disaster recovery capabilities, and strengthening cross-border scientific cooperation and cultural heritage.
As of 2024, the EUGENA has collected 4,274,522 genetic specimens, with the Spain contributing more than 2,352,537 materials (55% of the total) and Netherlands contributing more than 547,886 (13%). The collection primarily focuses on semen from livestock such as cattle, sheep, goats, swine, horses, and poultry, while other materials such as oocytes, embryos, embryonic cells, gonadal tissues, and DNA are less common due to cryopreservation limitations. Currently, sample collection combined with the digital organization of genomic and phenotypic information represents a new direction for the utilization of biorepositories, which can significantly improve the utilization rate of genebanks. Through this approach, germplasm and tissue samples are endowed with corresponding genotypic, phenotypic, geographical, environmental, and managerial information [40]. Many countries are beginning efforts to incorporate genomic data into databases, addressing a suite of future challenges that have been articulated by the cognoscenti. This emerging approach not only enhances the tangible value of each conserved sample but also provides a channel for exploring the complex interplay of genetic, environmental, and managerial domains encapsulated within these biological archives.
3.1.3. CRB-Anim
CRB-Anim is a national infrastructure funded by the French National Research Agency (ANR) under the “Investments for the Future” programme [44]. Conceived in 2013, this endeavour sought to unite and strengthen the operations of various biological resource centres (BRCs) to preserve the genetic material of domesticated animal species, mammals, birds, fish, and shellfish. This initiative serves to protect the genetic diversity of domestic animals and supports basic research and medical applications. The main objective of CRB-Anim is to contribute to the scientific and socioeconomic development of preserved samples. CRB-Anim now brings together six biological resource centres that manage reproductive resources (Cryobanque nationale, CERREC) or genomic resources (GADIE, CaniDNA, Labogena, Antagene). Collectively, these BRCs have stored approximately 600,000 biological samples, covering more than 20 species and 260 breeds, primarily preserved as semen, embryos, cells, tissues, DNA, and RNA. The preservation of genetic materials by CRB-Anim has ushered in a new era for combating biodiversity erosion and understanding the relationship between phenotypes and genotypes.
3.1.4. NARO
In 1985, Japan’s Ministry of Agriculture, Fisheries and Food (MAFF) embarked on a Genebank project (National Institute of Agrobiological Sciences, NIAS) [45,46], which was later renamed the NARO Genebank in April 2016. The project is focused on exploring, collecting, identifying, preserving, and distributing services around the genetic resources of animals bred in Japan. The National Agriculture and Food Research Organization (NARO) is a research centre for the preservation of genetic resources, including plants, microbes, and animals [47]. NARO believes that breeding new varieties and pioneering new technologies are crucial to thriving agricultural production, especially the utilization of the results of genomic selection research for breeding work. By 2022, this genebank accumulated a total of 910,000 pieces of genetic material, covering five species, swine, goats, horses, rabbits, and silkworms. Advancements in cryopreservation techniques not only allow for continuous access to samples that were previously frozen but also facilitate cross-time sampling, thereby enhancing the genetic diversity within the genebank. Furthermore, by enhancing precision identification of livestock, gene mining, and innovative germplasm techniques, genes that play a decisive role in key traits such as meat production, egg production, reproduction, quality, stress resistance, and disease resistance can be identified. The establishment of tissue-specific gene expression and regulatory databases offers rich data support for the excavation and functional analysis of key genes related to the germplasm characteristics of livestock and poultry breeds. At the same time, by combining continuously evolving genomic selection and molecular design technologies, technical support can be provided for the creation of new germplasms with superior traits.
3.1.5. NordGen
The Nordic Genetic Resource Center (NordGen, Alnarp, Sweden), located in Sweden, serves as the genebank and knowledge hub for genetic resources in the Nordic countries [48]. It is dedicated to conserving Nordic genetic resources and promoting their sustainable use in agriculture [49]. Currently, there are more than 160 native farm animal breeds in the Nordic region, many of which are at risk of endangerment, only 13% of the Nordic native breeds have viable populations (https://www.nordgen.org/ (accessed on 4 January 2025)). NordGen is actively taking measures to safeguard these endangered breeds. NordGen has therefore developed the EVA v3.0 software programme that monitors inbreeding risks and gives breeding recommendations [50]. However, the emphasis on ex situ conservation in national strategies for protecting animal genetic resources varies among countries [51]. Additionally, differences in conservation strategies exist between Nordic countries, particularly regarding the criteria for classifying breeds as protected. Moreover, at the regional level, there is a lack of established frameworks for sample collection, metadata management, material backup storage, and common strategies and action plans for ex situ conservation [51], further complicating the efforts to protect Nordic animal genetic resources. This should be followed by a continuous strengthening of co-operation between the Nordic countries and the development of a harmonized strategy and action plan for ex situ conservation in order to increase the efficiency of animal genetic resources conservation.
3.2. Construction of China’s National Livestock and Poultry Genebank
3.2.1. National Germplasm Resource Center for Domestic Animals
Following the United States, China launched the “Pilot Construction of Animal Germplasm Resources Description and Sharing” in 2003, leading to the establishment of the National Germplasm Resource Center for Domestic Animals (https://www.cdad-is.org.cn/admin/Login/zzzy_gl (accessed on 20 May 2024)). The centre has been instrumental in collecting, preserving, sharing, and utilizing genetic materials from 43 major animal categories, including livestock and special economic animals. The establishment of the National Germplasm Resource Center for Domestic Animals Information System (http://www.cdad-is.org.cn/ (accessed on 20 May 2024)) marked a pivotal step in integrating resources from domestic animals such as pigs, cattle, sheep, poultry, and deer. By the end of 2022, the centre had accumulated a staggering 1.898 million genetic materials from 1362 breeds, enhancing the breeding industry’s innovation and contributing to food security and sustainable development. The centre supports the creation of high-quality, resilient, and disease-resistant breeds, providing a crucial foundation for preserving and sustainably exploiting livestock and poultry genetic diversity, thus aligning preservation with innovation and societal benefits.
3.2.2. National Livestock and Poultry Breeds Genebank
At present, a total of 10 national livestock and poultry resource genebanks have been constructed, including the National Livestock Genebank, the National Local Chicken Breeds Genebank, the National Waterfowl Genebank, and the National Bee Genebank. The National Livestock Genebank, the largest in Asia, employs modern biotechnology for ex situ gene preservation, holding genetic materials from 390 breeds across various species, totalling 1.27 million entries. Regional genebanks for chickens and waterfowl in Jiangsu, Zhejiang, and Fujian Provinces have preserved genetic material from 86 rare breeds, while the National Bee Genebank stores genetic material for 17 bee breeds. The National Silkworm Genetic Resources Genebanks in Jiangsu and Chongqing have conserved more than 1150 genetic resources, with Chongqing being the largest, covering 90% of known domestic silkworm genetic variations. These efforts mark significant strides in the strategic conservation of agricultural germplasm, supporting the advancement of “molecularly designed breeding” and ensuring the long-term preservation of national agricultural genetic resources.
4. Challenges and Future Directions
The accelerating loss of global biodiversity casts a profound shadow on the genetic resources of livestock and poultry, with numerous breeds now teetering on the brink of extinction. Consequently, there is a pressing imperative at the international level to institute measures such as the establishment of in situ conservation areas and dedicated germplasm banks to ensure the enduring preservation of these genetic resources. The inception of genetic banks is an integral linchpin in safeguarding global livestock genetic diversity and championing the sustainable evolution of the livestock industry. However, there are still a number of problems and challenges in building genebanks.
Foremost, there is a paucity of international collaboration. Currently, interbank communications across nations are marked by pronounced regional differences, but the conservation and utilization of genetic resources is intrinsically a global concern, and regional cooperation and resource sharing are limited geographically, making the preservation of genetic resources insufficient. E. Zonabend [52] also pointed out that, although most countries have undertaken some genetic resources conservation projects, coordination and cooperation at the global level is still insufficient, which affects the conservation and improvement of animal genetic resources, and suggested that the current situation could be improved through enhanced international cooperation.
Second, the glaring disparities in regional development present another issue. Leroy, G point out that developing countries often face dual constraints of technology and funding, which limits their involvement in animal genetic resources management [53]. For example, despite Africa’s treasure trove of genetic resources, many regions are limited by financial constraints and technological lag and thus face the challenge of aptly preserving their genetic assets. In stark contrast, some developed nations, bolstered by abundant resources and cutting-edge technologies, excel in genetic preservation and management, albeit with comparatively constrained genetic resources of their own. This creates palpable differences in the level of preservation across different regions.
Third, there is an imbalance in the type of genetic material preserved. The primary method of preserving genetic resources in genebanks is semen preservation. Brazilian Animal Germplasm Bank (AGB) has almost 60,000 doses of semen in 2009 [33]. However, embryos, cells and tissues, and other genetic material may be extremely important for specific research and breeding projects. To address the above issues, a multifaceted approach is essential. Enhancing global collaboration through platforms such as the FAO is crucial for bridging conservation gaps between nations. In addition, high-throughput sequencing technology allows for rapid and comprehensive sequencing and analysis of breed genomes. This enables the identification of key genes and, with the use of CRISPR technology, facilitates the precise editing or repair of specific genes to maintain or enhance beneficial traits in breeds. These advancements are crucial for the conservation of endangered species and play a significant role in addressing the challenge of declining genetic diversity. The conservation of livestock and poultry genetic resources is crucial for ecological balance and biodiversity, and an understanding of climate change impacts is needed. Increasing public awareness through education and media is vital for community support in conservation efforts. Together, these strategies can address the disparities and challenges in preserving livestock and poultry genetic resources.
Fourth, drastic changes in the global climate pose a threat to the conservation and protection of livestock and poultry genetic resources. However, climate change has not yet been recognized by many countries as an issue for the management and conservation of livestock biodiversity [2]. Climate change poses significant threats to livestock and poultry genetic resources by affecting habitats, causing ecosystem destruction, and leading to feed and water shortages. Such changes can also expand disease transmission, impacting animal health and reproduction. Therefore, urgent measures are required to protect and conserve livestock and poultry genetic resources to address the challenges posed by global climate change.
Fifth, the protection system and policies for livestock and poultry genetic resources are underdeveloped, with unclear roles for various stakeholders beyond primary institutions. Entities such as NGOs, farmer groups, and research and educational institutions have untapped potential for resource protection. Due to inadequate policy support, their contributions are limited. For example, in Africa, many development projects have attempted to alleviate poverty by promoting crossbreeding or modern reproductive technologies, which has led to a threat to the conservation of local farm animal genetic resources [54]. Therefore, strengthening policies, clarifying roles, and providing support and incentives are essential to enhance collaboration among all stakeholders, thereby improving the conservation and sustainable use of livestock and poultry genetic resources.
Author Contributions
Conceptualization, Y.L. and L.J.; methodology, G.L.; software, Q.R.; formal analysis, Q.R.; investigation, Y.G.; resources, L.J.; data curation, Z.W.; writing—original draft preparation, Q.R. and Y.G.; writing—review and editing, L.J. and F.Y.; visualization, Q.R. and P.S.; supervision, Y.M. and Y.P.; project administration, L.J.; funding acquisition, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number No. 32222079 and National Key Research and Development Program of China, grant number 2022YFF1000104.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank all the participants for their comments and help on this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boettcher, P.J.; Hoffmann, I.; Baumung, R.; Drucker, A.G.; McManus, C.; Berg, P.; Stella, A.; Nilsen, L.B.; Moran, D.; Naves, M. Genetic resources and genomics for adaptation of livestock to climate change. Front. Genet. 2015, 5, 461. [Google Scholar] [CrossRef]
- Hoffmann, I. Climate change and the characterization, breeding and conservation of animal genetic resources. Anim. Genet. 2010, 41, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Magoro, A.M.; Mtileni, B.; Hadebe, K.; Zwane, A. Assessment of genetic diversity and conservation in South African indigenous goat ecotypes: A review. Animals 2022, 12, 3353. [Google Scholar] [CrossRef] [PubMed]
- Glowka, L. A Guide to the Convention on Biological Diversity; Union Internationale pour la Conservation de la Nature et de ses Resources: Gland, Switzerland, 1994. [Google Scholar]
- Rischkowsky, B.; Pilling, D. The State of the World’s Animal Genetic Resources for Food and Agriculture; Food & Agriculture Organization: Rome, Italy, 2007. [Google Scholar]
- Wallace, H.; Pollack, M.A.; Roederer-Rynning, C.; Young, A.R. Policy-Making in the European Union; Oxford University Press: New York, NY, USA, 2020. [Google Scholar]
- Wilcove, D.S.; Master, L.L. How many endangered species are there in the United States? Front. Ecol. Environ. 2005, 3, 414–420. [Google Scholar] [CrossRef]
- Malan, S. The improved Boer goat. Small Rumin. Res. 2000, 36, 165–170. [Google Scholar] [CrossRef]
- Knob, D.A.; Alessio, D.R.M.; Thaler Neto, A.; Mozzaquatro, F.D. Reproductive performance and survival of Holstein and Holstein× Simmental crossbred cows. Trop. Anim. Health Prod. 2016, 48, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Farooq, M.; Mian, M.; Durrani, F.; Mussawar, S. Flock size and egg production performance of backyard chicken reared by rural woman in Peshawar, Pakistan. Livest. Res. Rural. Dev. 2003, 15, 80. [Google Scholar]
- Balcha, K.A.; Mengesha, Y.T.; Senbeta, E.K.; Zeleke, N.A. Evaluation of different traits from day-old to age at first eggs of Fayoumi and White leghorn chickens and their reciprocal crossbreeds. J. Adv. Vet. Anim. Res. 2021, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.M.; Candido-Ribeiro, R.; Singh, P.; Lu, M.; Obreht Vidakovic, D.; Booker, T.R.; Whitlock, M.C.; Yeaman, S.; Isabel, N.; Aitken, S.N. How useful is genomic data for predicting maladaptation to future climate? Glob. Chang. Biol. 2024, 30, e17227. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W. Scenarios for global biodiversity in the 21st century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Mariante, A.d.S.; Egito, A.d. Animal genetic resources in Brazil: Result of five centuries of natural selection. Theriogenology 2002, 57, 223–235. [Google Scholar] [CrossRef] [PubMed]
- IRB UN. Convention on Biological Diversity; Treaty Collection; IRB UN: New Delhi, India, 1992. [Google Scholar]
- Declaration, I. Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration. FAO Commission on Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2007. [Google Scholar]
- Hoffmann, I.; Boerma, D.; Scherf, B. The Global Plan of Action for Animal Genetic Resources—The road to common understanding and agreement. Livest. Sci. 2011, 136, 7–14. [Google Scholar] [CrossRef]
- Cao, J.; Baumung, R.; Boettcher, P.; Scherf, B.; Besbes, B.; Leroy, G. Monitoring and progress in the implementation of the global plan of action on animal genetic resources. Sustainability 2021, 13, 775. [Google Scholar] [CrossRef]
- Scherf, B.; Baumung, R. Monitoring the implementation of the global plan of action for animal genetic resources. Biodiversity 2015, 16, 149–156. [Google Scholar] [CrossRef]
- Berthaud, J. Strategies for conservation of genetic resources in relation with their utilization. Euphytica 1997, 96, 1–12. [Google Scholar] [CrossRef]
- Welsh, C.; Stewart, T.; Schwab, C.; Blackburn, H. Pedigree analysis of 5 swine breeds in the United States and the implications for genetic conservation. J. Anim. Sci. 2010, 88, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, L.; Hu, F. Agro-biodiversity conservation and implications in China. In Organic Agriculture and Biodiversity in China; Elsevier: Amsterdam, The Netherlands, 2024; pp. 25–45. [Google Scholar]
- Silversides, F.; Purdy, P.; Blackburn, H. Comparative costs of programmes to conserve chicken genetic variation based on maintaining living populations or storing cryopreserved material. Br. Poult. Sci. 2012, 53, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Hirayama, T.; Tani, T.; Nishimori, K.; Onuma, M.; Fukuda, T. Chick derived induced pluripotent stem cells by the poly-cistronic transposon with enhanced transcriptional activity. J. Cell. Physiol. 2018, 233, 990–1004. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Cheng, K.M.; Silversides, F.G. Production of donor-derived offspring from cryopreserved ovarian tissue in Japanese quail (Coturnix japonica). Biol. Reprod. 2010, 83, 15–19. [Google Scholar] [CrossRef]
- Zomerdijk, F.; Hiemstra, S.-J.; d’Arbaumont, M.; Tixier-Boichard, M.; Boettcher, P. Quality management practices of gene banks for livestock: A global review. Biopreserv. Biobank. 2020, 18, 244–253. [Google Scholar] [CrossRef]
- Blackburn, H. Biobanking genetic material for agricultural animal species. Annu. Rev. Anim. Biosci. 2018, 6, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Leroy, G.; Boettcher, P.; Besbes, B.; Danchin-Burge, C.; Baumung, R.; Hiemstra, S.J. Cryoconservation of animal genetic resources in Europe and two African countries: A gap analysis. Diversity 2019, 11, 240. [Google Scholar] [CrossRef]
- Mariante, A.d.S.; do SMAlbuquerque, M.; Egito Ad McManus, C.; Lopes, M.; Paiva, S. Present status of the conservation of livestock genetic resources in Brazil. Livest. Sci. 2009, 120, 204–212. [Google Scholar] [CrossRef]
- Jacques, A.; Duclos, D.; Danchin-Burge, C.; Mercat, M.-J.; Tixier-Boichard, M.; Restoux, G. Assessing the potential of germplasm collections for the management of genetic diversity: The case of the French National Cryobank. Peer Community J. 2024, 4, e13. [Google Scholar] [CrossRef]
- Blackburn, H. Development of national animal genetic resource programs. Reprod. Fertil. Dev. 2003, 16, 27–32. [Google Scholar] [CrossRef]
- Scherf, B.D.; Pilling, D. The second report on the state of the world’s animal genetic resources for food and agriculture. In FAO Commission on Genetic Resources for Food and Agriculture Assessments; FAO: Rome, Italy, 2015. [Google Scholar]
- Blackburn, H. The national animal germplasm program: Challenges and opportunities for poultry genetic resources. Poult. Sci. 2006, 85, 210–215. [Google Scholar] [CrossRef]
- Blackburn, H. Genetic selection and conservation of genetic diversity. Reprod. Domest. Anim. 2012, 47, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, H.; Plante, Y.; Rohrer, G.; Welch, E.; Paiva, S. Impact of genetic drift on access and benefit sharing under the Nagoya Protocol: The case of the Meishan pig. J. Anim. Sci. 2014, 92, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.R.; McManus, C.M.; Blackburn, H. Conservation of animal genetic resources–A new tact. Livest. Sci. 2016, 193, 32–38. [Google Scholar] [CrossRef]
- Hiemstra, S.J.; de Haas, Y.; Mäki-Tanila, A.; Gandini, G. Local Cattle Breeds in Europe: Development of Policies and Strategies for Self-Sustaining Breeds; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010. [Google Scholar]
- Hiemstra, S.; Martyniuk, E.; Duchev, Z.; Begemann, F. European Gene Bank network for animal genetic resources (EUGENA). Proc. 10th WCGALP (Mackay) 2014, 10, 1. [Google Scholar]
- Buck, M.; Hamilton, C. The Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the Convention on Biological Diversity. Rev. Eur. Community Int. Environ. Law 2011, 20, 47–61. [Google Scholar] [CrossRef]
- Tixier-Boichard, M.; Marthey, S.; Marthey, N. The CRB-Anim web portal: Access to biological resources for animal sciences. In Proceedings of the 69 Annual Meeting of the European Federation of Animal Science (EAAP), Dubrovnik, Croatia, 27–31 August 2018. [Google Scholar]
- Plucknett, D.L.; Smith, N.J. Gene Banks and the World’s Food; Princeton University Press: Princeton, NY, USA, 2014. [Google Scholar]
- Takeya, M.; Yamasaki, F.; Uzuhashi, S.; Aoki, T.; Sawada, H.; Nagai, T.; Tomioka, K.; Tomooka, N.; Sato, T.; Kawase, M. NIASGBdb: NIAS Genebank databases for genetic resources and plant disease information. Nucleic Acids Res. 2010, 39, D1108–D1113. [Google Scholar] [CrossRef][Green Version]
- Okuno, K.; Shirata, K.; Niino, T.; Kawase, M. Plant genetic resources in Japan: Platforms and destinations to conserve and utilize plant genetic diversity. Jpn. Agric. Res. Q. JARQ 2005, 39, 231–237. [Google Scholar] [CrossRef][Green Version]
- Jonatan, J. NordGen Annual Review 2021; NordGen: Alnarp, Sweden, 2022; p. 11. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:norden:org:diva-12489 (accessed on 5 January 2025).
- White, E.-L.F.; Kjetså, M.; Peippo, J. The First Status Report on the Conservation of Farm Animal Genetic Resources (AnGR) in the Nordics: 40 Years of Nordic Collaboration in the Conservation of Animal Genetic Resources; NordGen: Alnarp, Sweden, 2024; p. 7. [Google Scholar]
- Berg, P.; Nielsen, J.; Sørensen, M.K. EVA: Realised and predicted optimal genetic contributions. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Minas Gerais, Brazil, 13–18 August 2006. [Google Scholar]
- Fagerheim White, E.-L.; Peippo, J.; Honkatukia, M. The Nordfrost Project Report: Farm Animal Gene Banks in the Nordic Region–Added Value Through Nordic Cooperation. 2024. Available online: https://discovery.researcher.life/article/the-nordfrost-project-report-farm-animal-gene-banks-in-the-nordic-region-added-value-through-nordic-cooperation/e66d1b94efff3ae4a43962babafe7afe (accessed on 5 January 2025).
- Zonabend, E.; Okeyo, A.; Ojango, J.M.; Hoffmann, I.; Moyo, S.; Philipsson, J. Infrastructure for sustainable use of animal genetic resources in Southern and Eastern Africa. Anim. Genet. Resour./Resour. Génétiques Anim./Recur. Genéticos Anim. 2013, 53, 79–93. [Google Scholar] [CrossRef]
- Leroy, G.; Baumung, R.; Notter, D.; Verrier, E.; Wurzinger, M.; Scherf, B. Stakeholder involvement and the management of animal genetic resources across the world. Livest. Sci. 2017, 198, 120–128. [Google Scholar] [CrossRef]
- Wollny, C.B. The need to conserve farm animal genetic resources in Africa: Should policy makers be concerned? Ecol. Econ. 2003, 45, 341–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).