Global Potential Distribution of Carpomya vesuviana Costa Under Climate Change and Potential Economic Impacts on Chinese Jujube Industries
Abstract
1. Introduction
2. Materials and Methods
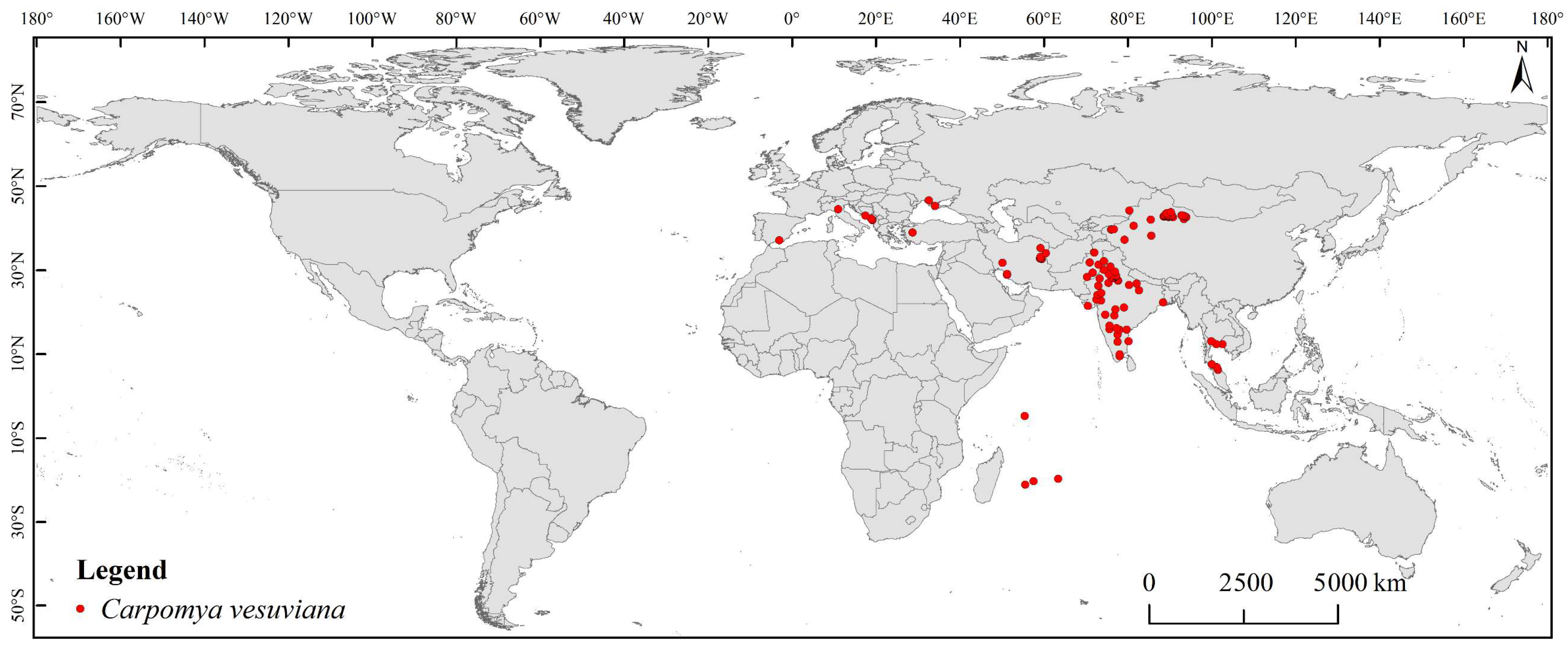
2.1. Occurrence Records
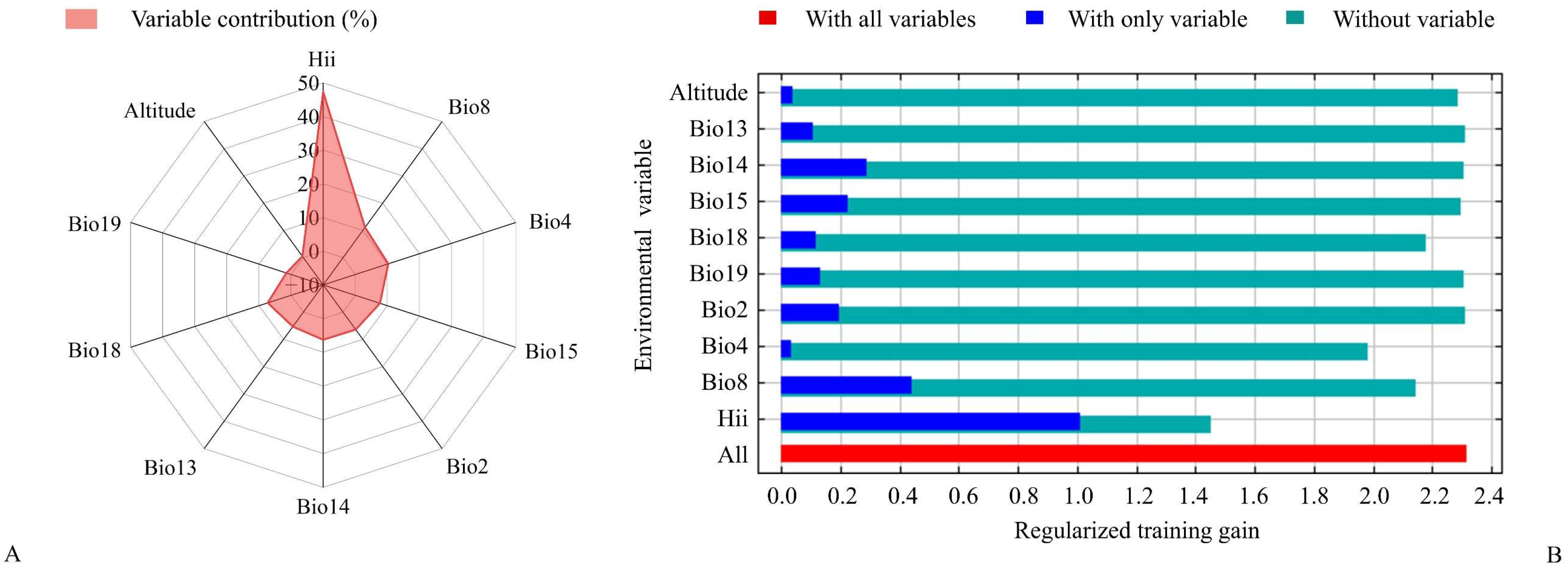
2.2. Selection of Environment Variables
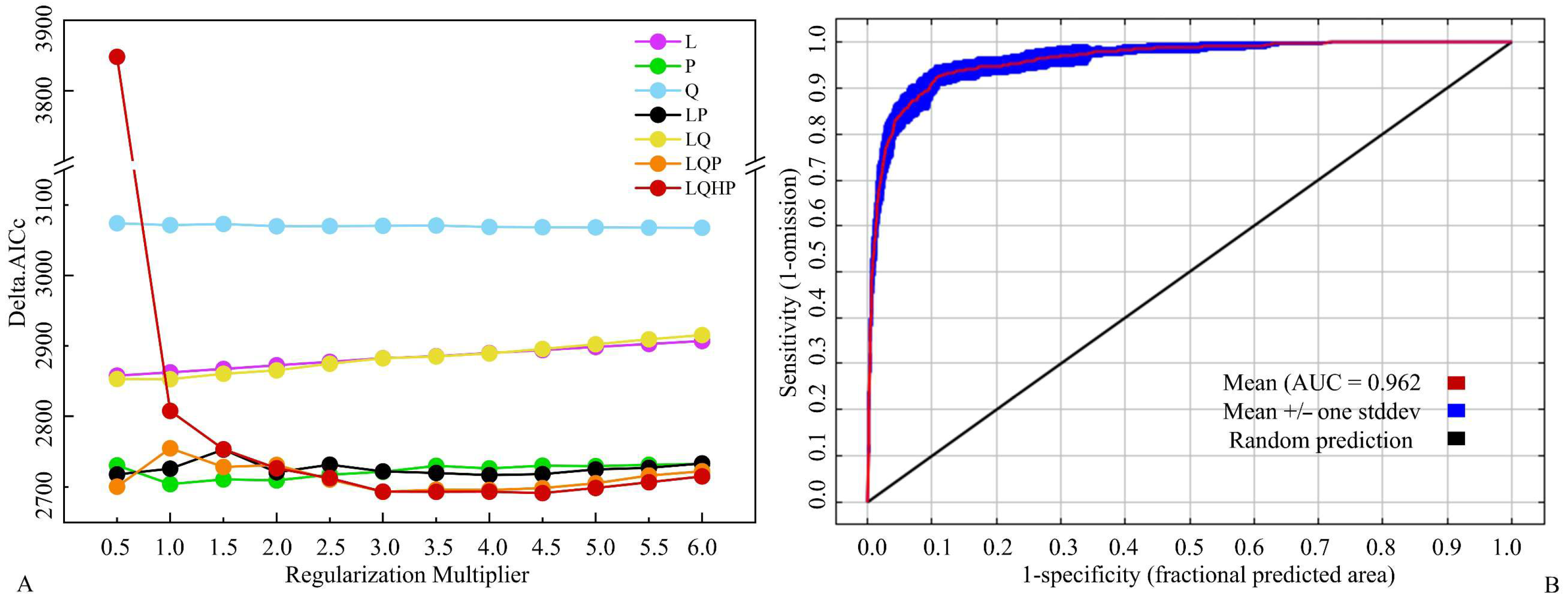
2.3. Model Optimisation, Assessment and Classification of PGDs
2.4. Economic Loss Assessment Model
2.5. Sources of Economic Loss Parameters
3. Results
3.1. Model Optimisation and Accuracy Evaluation
3.2. Significant Environmental Variables
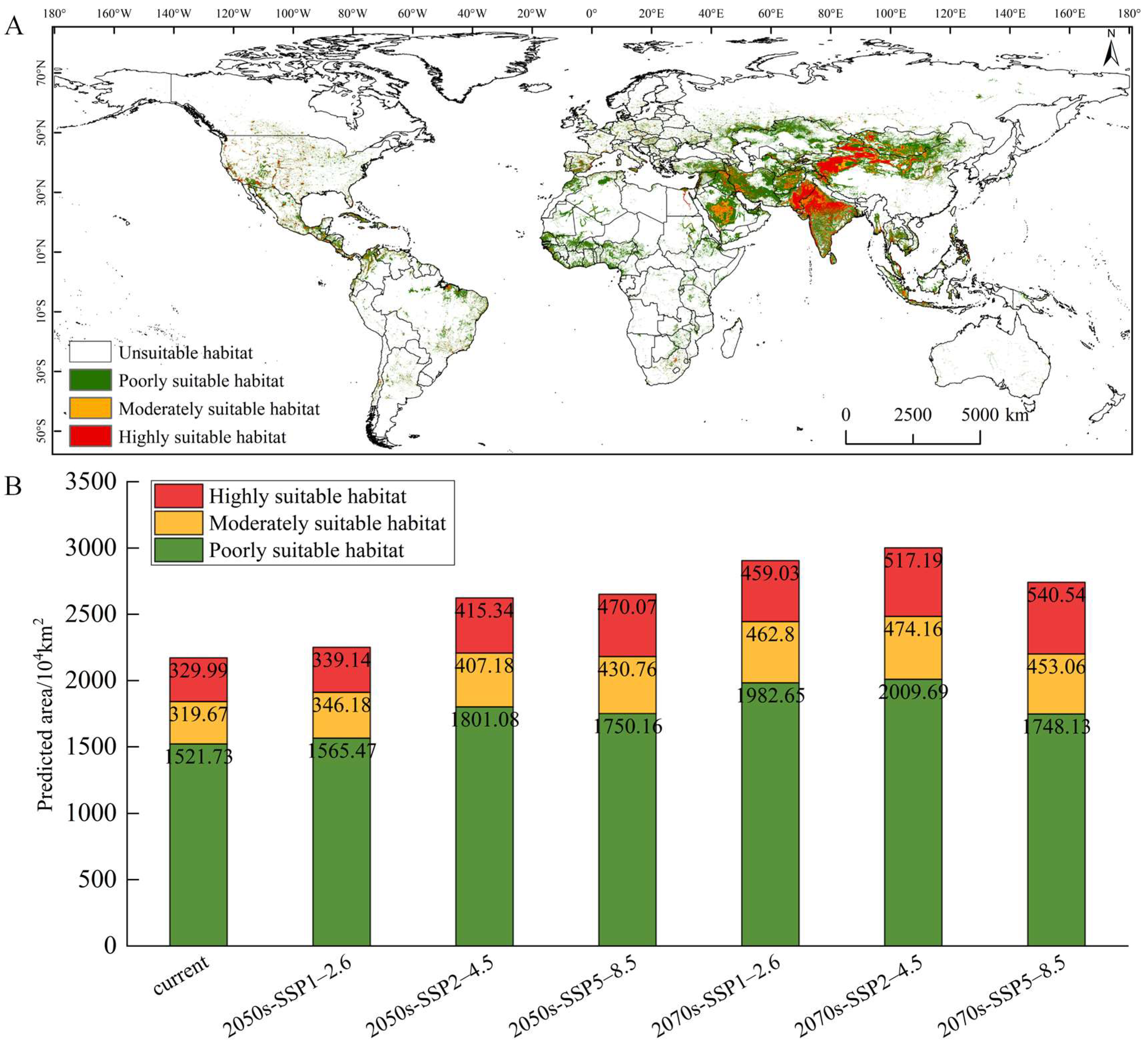
3.3. Current Potential Suitable Habitats
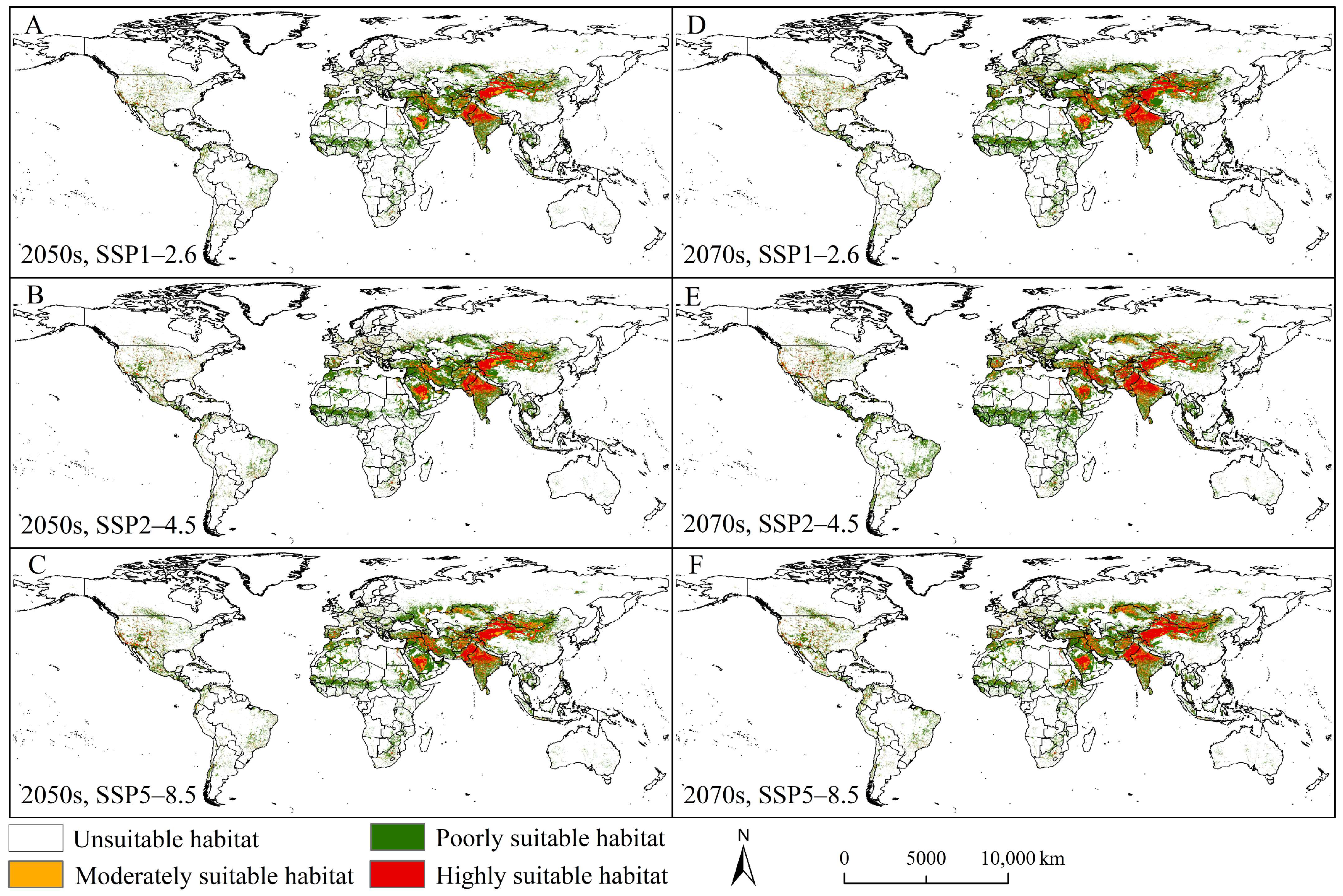
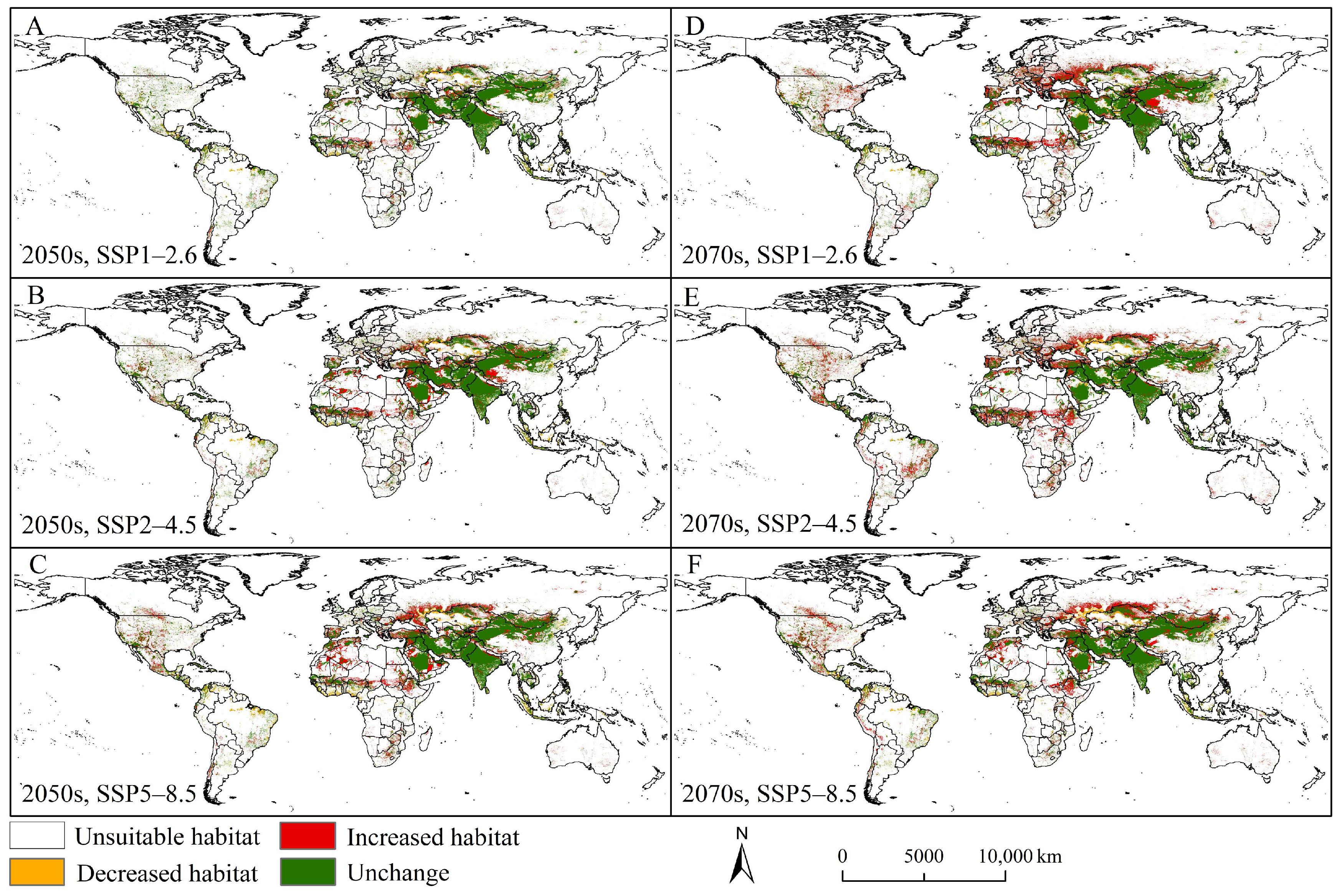
3.4. Future Potential Suitable Habitats and Trends
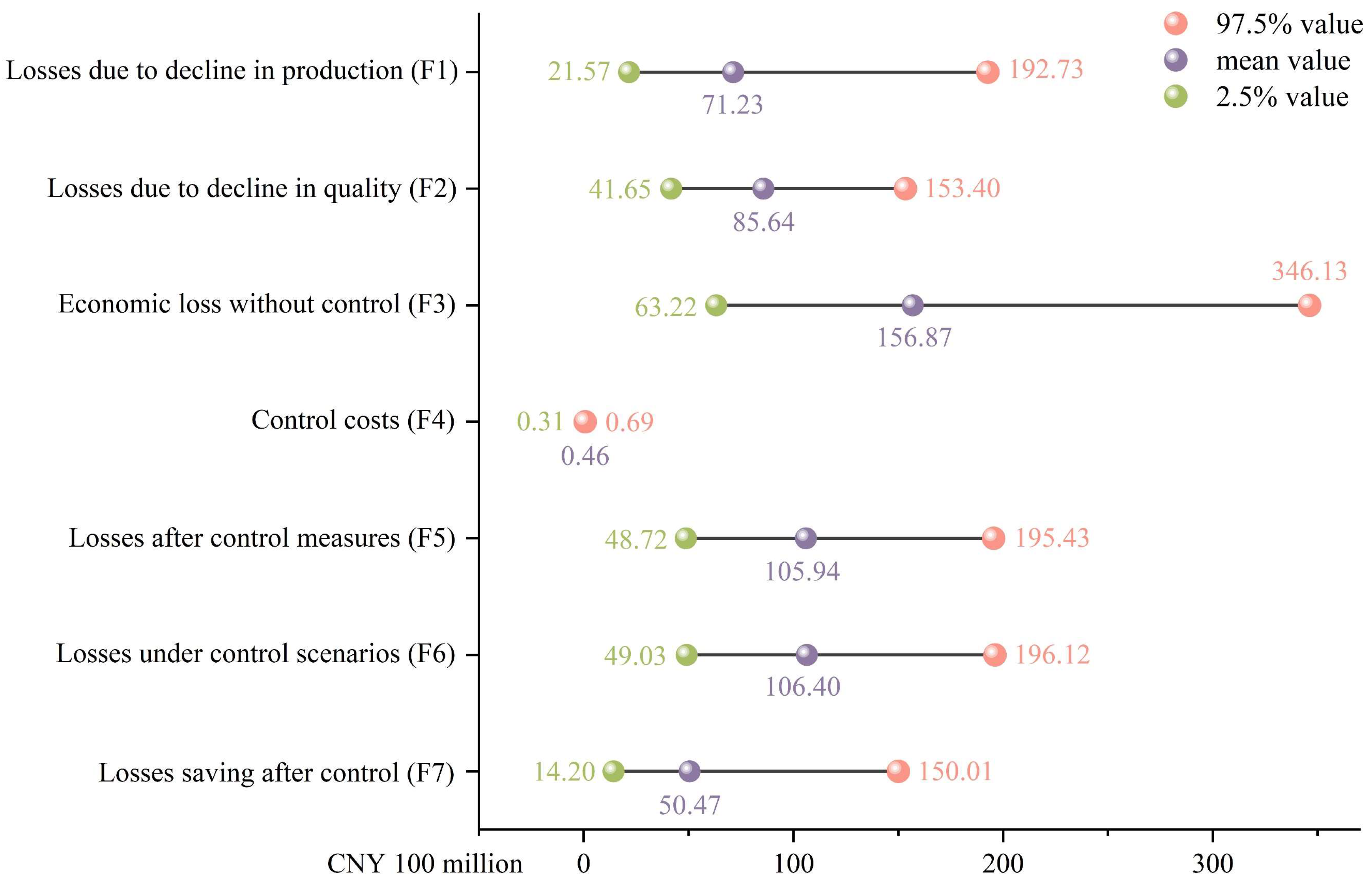
3.5. Potential Economic Losses of C. vesuviana on Chinese Jujube Industry
4. Discussion
4.1. Significant Environmental Variables Influenced Potential Suitable Habitats of C. vesuviana
4.2. Current and Future Distribution Patterns
4.3. Economic Impact on the Jujube Industry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- Chapman, D.; Purse, B.V.; Roy, H.E.; Bullock, J.M. Global trade networks determine the distribution of invasive non-native species. Global. Ecol. Biogeogr. 2017, 26, 907–917. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.B.; Martin, N.; Loureiro, T.G.; Matikinca, P.; Robertson, M.P. Double trouble: The implications of climate change for biological invasions. NeoBiota 2020, 62, 463–487. [Google Scholar] [CrossRef]
- Venette, R.C.; Hutchison, W.D. Invasive insect species: Global challenges, strategies & opportunities. Front. Insect Sci. 2021, 1, 650520. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Zhao, T.Y.; He, R.; Hua, Y.T. Progress and prospects in prevention and control of alien invasive species during the 13th five-year plan period in China. J. Biosaf. 2022, 31, 95–102. [Google Scholar]
- Zhang, X.; Zhao, J.J.; Yan, J.; Fang, G.F.; Huang, J.X. Economic loss assessment of pine wilt disease in mainland China in 2017. J. Beijing For. Univ. 2020, 42, 96–106. [Google Scholar]
- Paul, R.; Subudhi, D.K.; Sahoo, C.K.; Banerjee, K. Invasion of Lantana camara L. and its response to climate change in the mountains of Eastern Ghats. Biologia 2021, 76, 1391–1408. [Google Scholar] [CrossRef]
- Skendžíc, S.; Zovko, M.; Živkovíc, I.P.; Lešíc, V.; Lemíc, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- CABI. Carpomya Vesuviana; CABI Compendium; CAB International: Wallingford, UK, 2024. [Google Scholar]
- Wang, Y.S.; Guan, H.H.; Liu, J.Z.; Ge, Y.C. Control Tchnology for Carpomya vesuviana. Prot. For. Sci. Technol. 2019, 2, 94–95. [Google Scholar]
- Stonehouse, J.; Mahmood, R.; Poswal, A.; Mumford, J.; Baloch, K.N.; Chaudhary, Z.M.; Makhdum, A.H.; Mustafa, G.; Huggett, D. Farm field assessments of fruit flies (Diptera: Tephritidae) in Pakistan: Distribution, damage and control. Crop Prot. 2002, 21, 661–669. [Google Scholar] [CrossRef]
- Azam, K.M.; Al-Ansari, M.S.; Al-Raeesi, A. Fruit flies of Oman with a new record of Carpomya vasuviana Costa (Diptera: Tephritidae). Res. Crops 2004, 5, 274–277. [Google Scholar]
- Farrar, N.; Asadi, G.H.; Golestaneh, S.R. Damage and host ranges of Ber fruit fly Carpomyia vesuviana Costa (Tephritidae) and its rate of parasitism. J. Agric. Sci. 2004, 5, 120–130. [Google Scholar]
- Sookar, P.; Permalloo, S.; Gungah, B.; Alleck, M.; Seewooruthun, S.I.; Soonnoo, A.R. An Area Wide Control of Fruit Flies in Mauritius; Biofabrica Moscamed Brasil: Juazeiro, Brazil, 2006. [Google Scholar]
- Adil, S.; He, S.; Tian, C.; Luo, Y.; Yu, F.; Feng, X. The occurrence of Carpomya vesuviana Costa in Turpan and the distribution of pupae. Plant Quar. 2008, 22, 295–297. [Google Scholar]
- EPPO. EPPO global database for Carpomya vesuviana—Distribution; European and Mediterranean Plant Protection Organization: Paris, France, 2024; Available online: https://gd.eppo.int/taxon/CARYVE (accessed on 28 October 2024).
- Farrar, N.; Mohamadi, M.; Golestaneh, S.R. Biology of the ber fruit fly, Carpomyia vesuviana Costa (Diptera: Tephritidae) and Identification of Natural Enemies in Bushehr Province. Iranian. J. For. Range Protec Res. 2003, 1, 1–23. [Google Scholar]
- He, S.Y.; Wen, J.B.; Satar, A.; Tian, C.M. Research progress of quarantine pest Carpomya vesuviana. Sci. Silvae Sin. 2010, 46, 147–154. [Google Scholar]
- Ding, J.T. Flight Capacity and Environmental Factors Adaptation of Carpomya vesuviana Costa. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2015. [Google Scholar]
- Song, L.H.; Meinhardt, L.W.; Bailey, B.; Zhang, D.P. Genetic Improvement of Chinese jujube for Disease Resistances: Status, knowledge gaps and research needs. Crop Breed. Genet. Genom. 2019, 1, e190015. [Google Scholar]
- Yang, F.; Wang, S.N.; Wang, Z.H.; Yuan, W.Y.; Qin, W.T.; Zhao, J.; Wang, S. Current status of jujube pest, control techniques, and future research prospects. J. Environ. Entomol. 2024, 17, 1–19. [Google Scholar]
- He, S.Y.; Zhu, Y.F.; Adili, S.; Wen, J.B.; Chen, M.; Tian, C.M. Pest risk assessment of Carpomya vesuviana in China. Sci. Silvae Sin. 2011, 47, 107–116. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Mechanistic and correlative models of ecological niches. Eur. J. Ecol. 2015, 1, 28–38. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Tang, C.Q.; Humphreys, A.M.; Fontaneto, D.; Barraclough, T.G.; Paradis, E. Effects of phylogenetic reconstruction method on the robustness of species delimitation using single-locus data. Methods Ecol. Evol. 2014, 5, 1086–1094. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; (MPB-49). [Google Scholar]
- Gengping, Z.; Guoqing, L.; Wenjun, B.; Yubao, G. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar] [CrossRef]
- Kumar, S.; Neven, L.G.; Zhu, H.; Zhang, R. Assessing the global risk of establishment of Cydia pomonella (Lepidoptera: Tortricidae) using CLIMEX and MaxEnt niche models. J. Econ. Entomol. 2015, 108, 1708–1719. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, C.; Zhao, Z.; Pan, X.; Li, Z. Climate change impacts on the global potential geographical distribution of the agricultural invasive pest, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Clim. Change 2019, 155, 145–156. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, Y.J.; Xu, Y.L.; Feng, X.D.; Zhao, S.Q.; Lu, Y.Y.; Li, Z.H. Surveillance and invasive risk of the red imported fire ant, Solenopsis invicta Buren in China. Pest. Manag. Sci. 2023, 79, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Qin, Y.; Fan, Z.; Zhou, T.; Guo, S.K.; Li, Z.H. Assessment of potential economic loss of maize industry in China caused by maize chlorotic mottle virus using @ RISK software. J. Plant Prot. 2022, 49, 1377–1382. [Google Scholar]
- Papadopoulos, C.E.; Yeung, H. Uncertainty estimation and Monte Carlo simulation method. Flow Meas. Instrum. 2001, 12, 291–298. [Google Scholar] [CrossRef]
- Qin, Y.; Ullah, F.; Fang, Y.; Singh, S.; Zhao, Z.; Zhao, Z.; Li, Z. Prediction of potential economic impact of Bactrocera zonata (Diptera: Tephritidae) in China: Peaches as the example hosts. J. Asia Pac. Entomol. 2021, 24, 1101–1106. [Google Scholar] [CrossRef]
- Taylor, A.S.; Cook, D.C. An economic assessment of the impact on the Western Australian viticulture industry from the incursion of grapevine downy mildew. J. Plant Dis. Prot. 2018, 125, 397–403. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.ywze8k (accessed on 15 June 2024).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Reynolds, L.V. Challenges of predicting the potential distribution of a slow-spreading invader: A habitat suitability map for an invasive riparian tree. Biol. Invasions 2011, 13, 153–163. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Altamiranda-Saavedra, M.; Amat, E.; Gómez-P, L.M. Influence of montane altitudinal ranges on species distribution models; evidence in Andean blow flies. PeerJ 2020, 8, e10370. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, W.; Zhao, H.; Xian, X.; Jing, K.; Yang, N.; Lu, X.; Liu, W. Potential global distribution of invasive alien species, Anthonomus grandis Boheman, under current and future climate using optimal MaxEnt model. Agriculture 2022, 12, 1759. [Google Scholar] [CrossRef]
- Zlateva, I.; Raykov, V.; Slabakova, V.; Stefanova, E.; Stefanova, K. Habitat suitability models of five keynote Bulgarian Black Sea fish species relative to specific abiotic and biotic factors. Oceanologia 2022, 64, 665–674. [Google Scholar] [CrossRef]
- Franklin, J.; Miller, J.A. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Gyi, M.M.; Lai, O.P.; Dikshit, A.K.; Sharma, V.P. Eficacy of insecticides for controlling ber fruit fly. Ann. Plant Prot. Sci. 2003, 11, 152–153. [Google Scholar]
- Geng, X.; Zhao, X.Z.; Hou, J.M.; Han, H.Z. Biological characteristics of Carpomya vesuviana and its management. Hebei For. Sci. Technol. 2010, 5, 73–75. (In Chinese) [Google Scholar]
- Xu, H.D.; Lei, H.J.; Li, M.; Wang, J.; Dong, X.B. China’s jujube industry development status. China Rural Sci. Technol. 2022, 4, 49–52. (In Chinese) [Google Scholar]
- Pan, Y.Y. Remote Sensing Monitoring for Leaf Area Index and Yield Assessment of Jujube Orchards; Tarim University: Xinjiang, China, 2024. [Google Scholar]
- Adil, S.; Tian, C.M.; Luo, Y.Q.; Chen, M.; Memet, A. Preliminary report on control test of Carpomya vesuviana Costa. J. Xinjiang Agric. Univ. 2010, 33, 206–209. [Google Scholar]
- Abduwahap, E. Research on Orientation of Adult Carpomyia vesuviana Costa. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2017. [Google Scholar]
- Yu, F.; Kong, W.J.; Ma, J.H.; Memet, N.; Ablik, A. Occurrence of Carpomya vesuviana in Turpan and its prevention and control measures. Plant Prot. 2011, 37, 166–167. (In Chinese) [Google Scholar]
- Pan, F.; Xiao, T.B.; Qin, S.; Chen, H.Y.; Lin, Z.F.; Xie, S.H. Study on yield of bitter gourd damaged by Bactrocera cucurbitae and its economic thresholds. China Plant Prot. 2014, 34, 12–15. (In Chinese) [Google Scholar]
- Vadivelu, K. Biology and Management of ber fruit fly, Carpomyia vesuviana Costa (Diptera: Tephritidae): A review. Afr. J. Agric. Res. 2014, 9, 1310–1317. [Google Scholar]
- Zhang, R.Z.; Wang, X.J.; Adil, S. Identification and precaution of the ber fruit fly, Carpomya vesuviana, a quarantine pest insect in China. Chin. Bull. Entomol. 2007, 44, 928–930. [Google Scholar]
- Schai-Braun, S.C.; Jenny, H.; Ruf, T.; Hackländer, K. Temperature increase and frost decrease driving upslope elevational range shifts in Alpine grouse and hares. Glob. Change Biol. 2021, 27, 6602–6614. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Abarca, M.; Spahn, R. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 2021, 47, 67–74. [Google Scholar] [CrossRef]
- Su, C.X.; Ping, F.; Liu, X.H. Carpomya vesuviana damage and control measures. Friends Fruit Farmers 2020, 12, 36–37. (In Chinese) [Google Scholar]
- Nandihalli, B.S.; Patil, D.R.; Jagginavar, S.B.; Biradar, A.P.; Guled, M.B.; Surkod, V.S. Incidence of fruit borer (Meridarchis scyrodes Meyr.) and fruit fly (Carpomyia vesuviana Costa) on different varieties of ber. Adv. Agric. Res. India 1996, 6, 13–18. [Google Scholar]
- Lv, W.G.; Lin, W.; Li, Z.H.; Geng, J.; Wan, F.H.; Wang, Z.L. Potential geographic distribution of Ber fruit fly, Carpomya vesuviana Costa. Plant Quar. 2008, 22, 343–347. [Google Scholar]
- Lakra, R.K.; Zile, S. Seasonal fluctuations in incidence of ber fruit fly Carpomyia vesuviana Costa (Diptera: Tephritidae) under agro-climatic conditions of Hisar. Haryana Agric. Univ. J. Res. 1985, 15, 42–50. [Google Scholar]
- Gaur, R.K.; Kumar, M.; Sharma, S.; Yadav, B.S. Survey studies on insects and non-insect pest associated with ber crop in South West Haryana. J. Entomol. Zool. Stud. 2020, 8, 856–863. [Google Scholar]
- Zimmermann, H.; Brandt, P.; Fischer, J.; Welk, E.; von Wehrden, H. The Human Release Hypothesis for biological invasions: Human activity as a determinant of the abundance of invasive plant species. F1000Research 2014, 3, 109. [Google Scholar] [CrossRef]
- Ding, J.T.; Adil, S.; Zhu, H.F.; Yu, F.; Alimasi, A.; Luo, L. Flight capacity of adults of the ber fruit fly, Carpomya vesuviana (Diptera: Tephritidae). Acta Entomol. Sin. 2014, 57, 1315–1320. [Google Scholar]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Morris, J.; Sokolov, A.; Reilly, J.; Libardoni, A.; Forest, C.; Paltsev, S.; Schlosser, C.A.; Prinn, R.; Jacoby, H. Quantifying both socioeconomic and climate uncertainty in coupled human–Earth systems analysis. Nat. Commun. 2025, 16, 2703. [Google Scholar] [CrossRef]
- Sharma, H.; Ehlers, T.A.; Glotzbach, C.; Manuel, S.; Katja, T. Effect of rock uplift and Milankovitch timescale variations in precipitation and vegetation cover on catchment erosion rates. Earth Surf. Dynam. 2021, 9, 1045–1072. [Google Scholar] [CrossRef]
- Ahnert, F. Some comments on the quantitative formulation of geomorphological processes in a theoretical model. Earth Surf. Processes. 1977, 2, 191–201. [Google Scholar] [CrossRef]
- Amini, A.; Lotfalizadeh, H.; Peris-Felipo, F.J.; Rasplus, J.-Y. Potential parasitoids for biocontrol of the Ber fruit fly, Carpomya vesuviana Costa (Diptera: Tephritidae). Life 2023, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Basha, J.M.G. Experiments on the control of the fruit borers of jujube (Zizyphus spp.), Carpomyia vesuviana Costa and Meridarchia scyrodes Meyr. in South India. Indian J. Entomol. 1953, 14, 229–238. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Guo, S.; Ge, X.; Zou, Y.; Zhou, Y.; Wang, T.; Zong, S. Projecting the Potential Global Distribution of Carpomya vesuviana (Diptera: Tephritidae), Considering Climate Change and Irrigation Patterns. Forests 2019, 10, 355. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar]
- De Lima, I.S.; Degrande, P.E.; Miranda, J.E.; dos Santos, W.J. Evaluation of the boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae) suppression program in the State of Goiás, Brazil. Neotrop. Entomol. 2013, 42, 82–88. [Google Scholar] [CrossRef]
| Scenario | Evaluation Items | Input Variable | Model and Parameter |
|---|---|---|---|
| Non control scenario | Economic loss caused by production decline (F1) | Production of jujubes in suitable area (Q1) | Pert (23.71, 24.31, 24.91) × 108 kg |
| Damage rate to jujubes (I) | Pert (36.67%, 46.67%, 60.00%) | ||
| Loss rates after damage (R) | Pert (20.00%, 30.00%, 40.00%) | ||
| Market price of jujubes (Pa) | Pert (11.50, 18.00, 24.50) CNY/kg | ||
| Economic loss caused by production decline (F1) | F1 = Q1 × I × R × Pa/(1 − IR) | ||
| Economic loss caused by quality decline (F2) | Production of jujubes in suitable area (Q1) | Pert (23.71, 24.31, 24.91) × 108 kg | |
| Price of jujubes after the quality decline (Pb) | Pert (5.95, 8.73, 11.50) CNY/kg | ||
| Economic loss caused by quality decline (F2) | F2 = Q1 × I × (1 − R) (Pa − Pb)/(1 − IR) | ||
| Economic loss under the no control scenario (F3) | F3 = F1 + F2 | ||
| Control scenario | Control costs (F4) | Planting area of jujubes in suitable area (S) | Pert (48.33, 54.24, 60.15) × 104 hm2 |
| Unit cost of control (C) | Pert (175.21, 182.66, 190.10) CNY/hm2 | ||
| Control costs (F4) | F4 = S × I × C | ||
| Economic loss after control (F5) | Unit yield of jujubes in suitable area (A) | A = Q1/S kg/hm2 | |
| Control effects (M) | Pert (92.37%, 93.69%, 95.00%) | ||
| Efficiency correction coefficient (D) | 2 | ||
| Allowable level of economic damage (E) | E = C/(A × Pa × M) × D × 100% | ||
| Economic loss after control (F5) | F5 = Q1 × I × E × Pa/(1 − IE) + Q1 × I × (1 − E) × (Pa − Pb)/(1 − IE) | ||
| Economic loss under the control scenario (F6) | F6 = F4 + F5 | ||
| Losses saving after control (F7) | F7 = F3 − F6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; Li, M.; Qi, Y.; Zhao, H.; Xian, X.; Guo, J.; Yang, N.; Zhou, H.; Liu, W. Global Potential Distribution of Carpomya vesuviana Costa Under Climate Change and Potential Economic Impacts on Chinese Jujube Industries. Agriculture 2025, 15, 2081. https://doi.org/10.3390/agriculture15192081
Ning J, Li M, Qi Y, Zhao H, Xian X, Guo J, Yang N, Zhou H, Liu W. Global Potential Distribution of Carpomya vesuviana Costa Under Climate Change and Potential Economic Impacts on Chinese Jujube Industries. Agriculture. 2025; 15(19):2081. https://doi.org/10.3390/agriculture15192081
Chicago/Turabian StyleNing, Jingxuan, Ming Li, Yuhan Qi, Haoxiang Zhao, Xiaoqing Xian, Jianyang Guo, Nianwan Yang, Hongxu Zhou, and Wanxue Liu. 2025. "Global Potential Distribution of Carpomya vesuviana Costa Under Climate Change and Potential Economic Impacts on Chinese Jujube Industries" Agriculture 15, no. 19: 2081. https://doi.org/10.3390/agriculture15192081
APA StyleNing, J., Li, M., Qi, Y., Zhao, H., Xian, X., Guo, J., Yang, N., Zhou, H., & Liu, W. (2025). Global Potential Distribution of Carpomya vesuviana Costa Under Climate Change and Potential Economic Impacts on Chinese Jujube Industries. Agriculture, 15(19), 2081. https://doi.org/10.3390/agriculture15192081







