Changes in Camelina sativa Yield Based on Temperature and Precipitation Using FDA
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Experiment
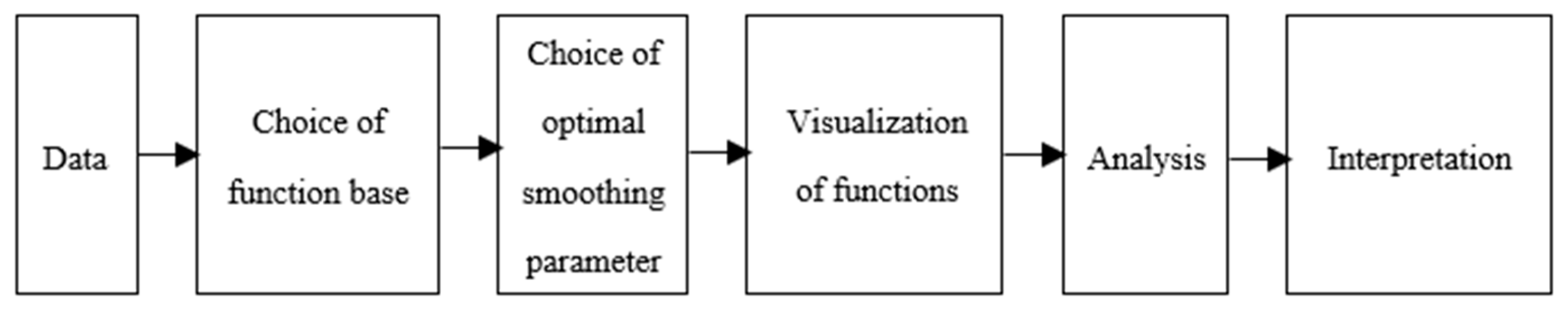
2.3. Statistical Analysis
3. Results
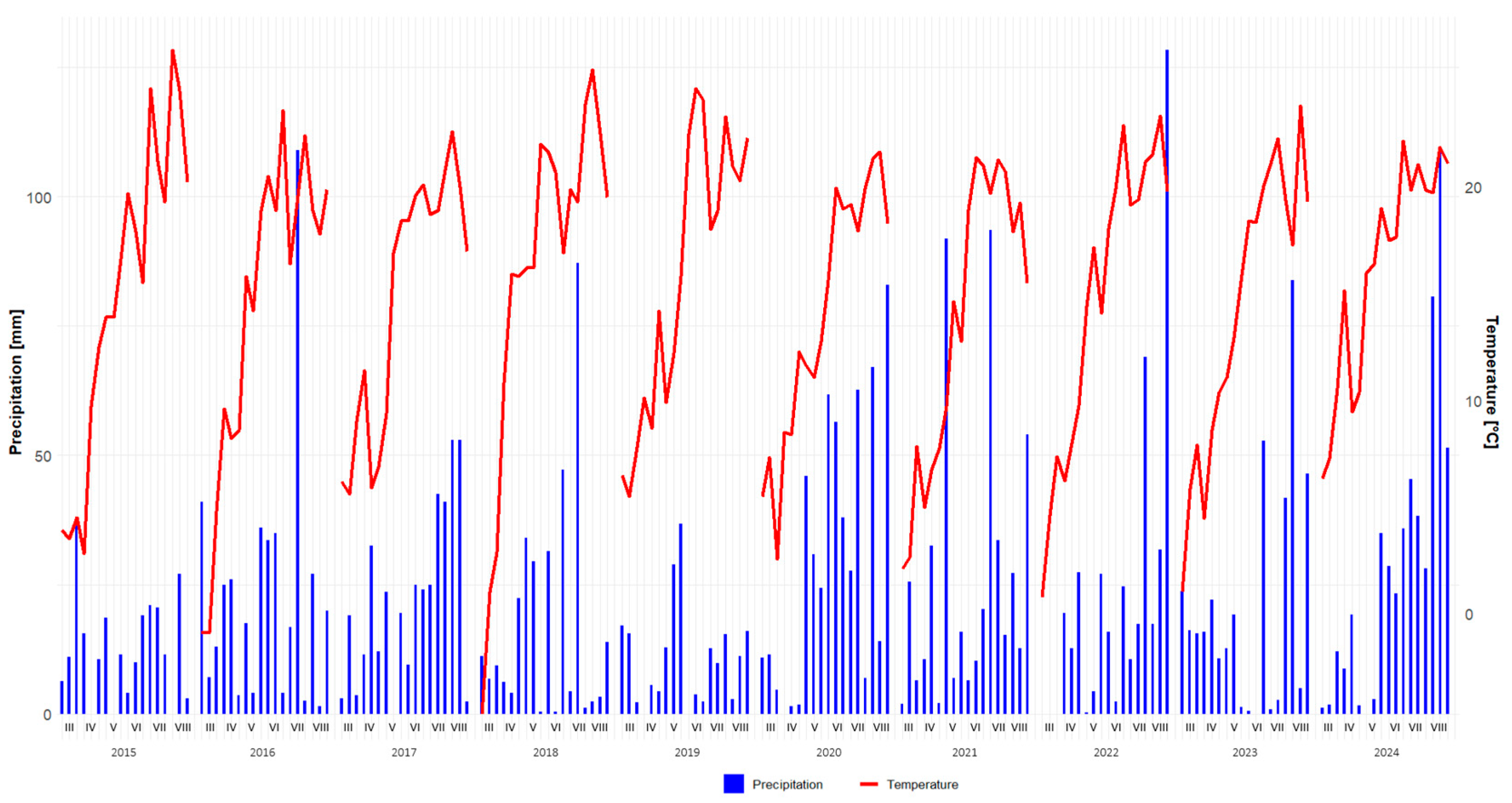
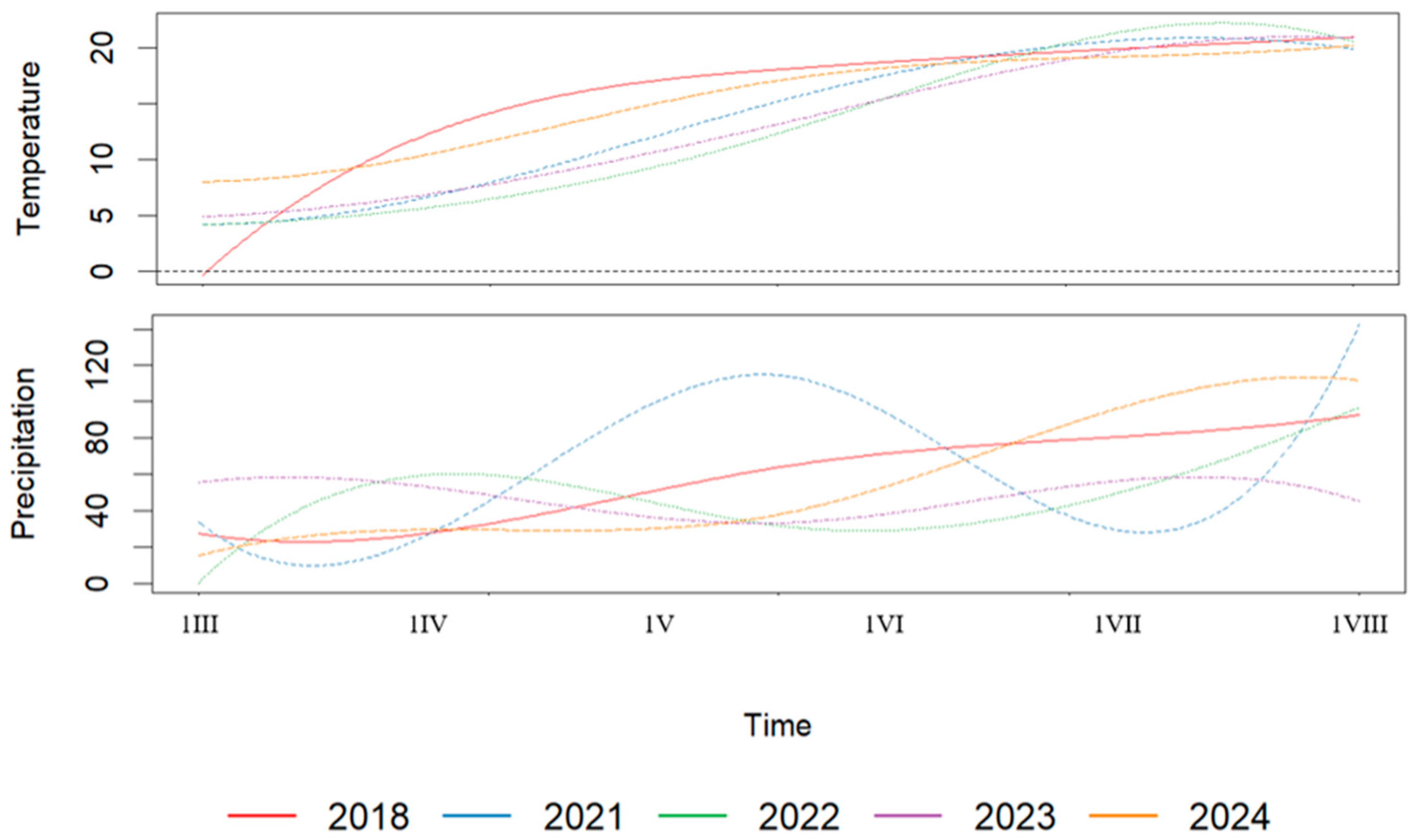
3.1. Weather Variability and Phenological Development
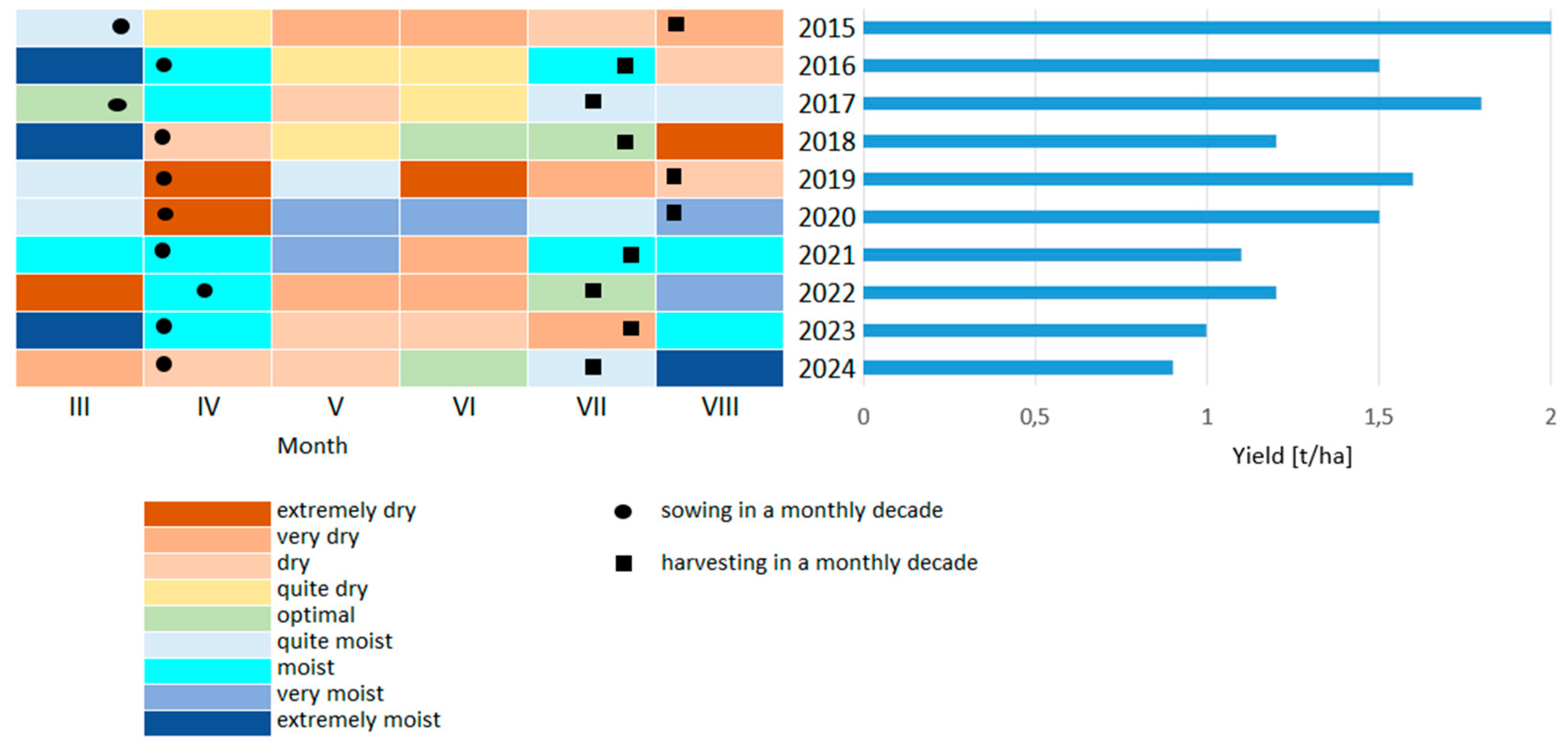
3.2. Hydrothermal Conditions and Selyaninov Coefficient
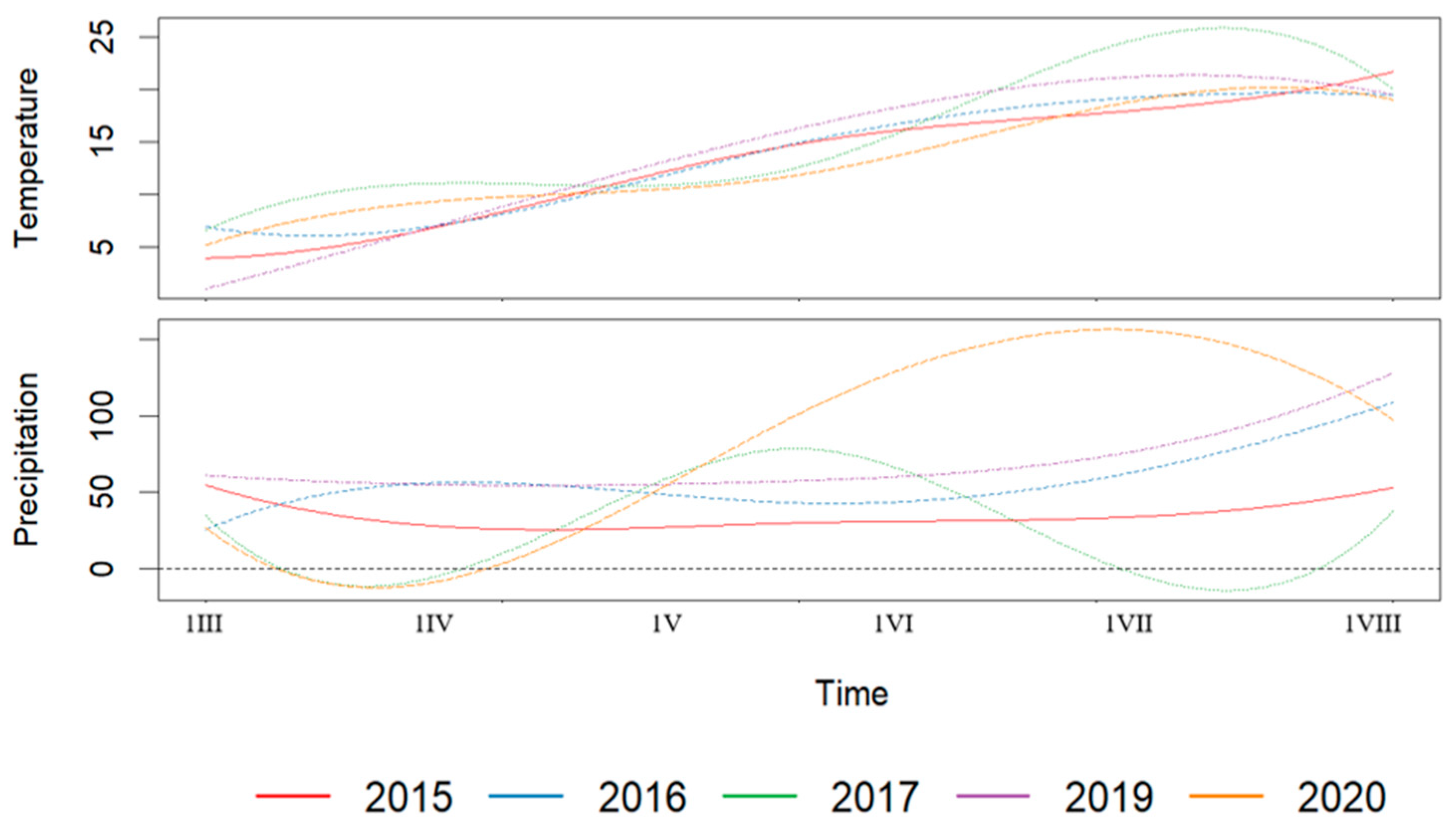
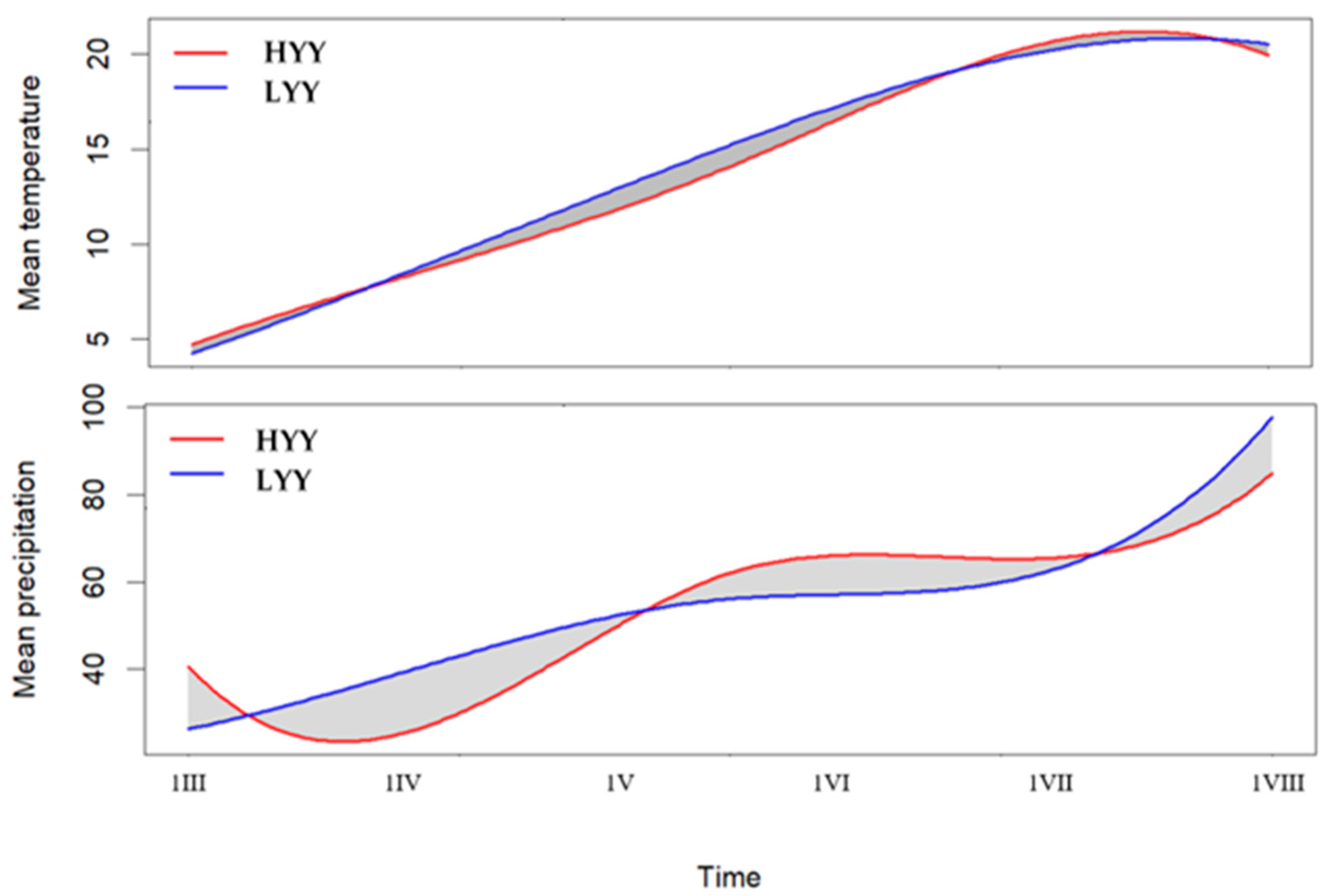
3.3. Cluster-Based Functional Analysis of Weather Patterns and Yield
3.4. Environmental Stress Factors and Functional Modelling of Yield Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Möhring, N.; Muller, A.; Schaub, S. Farmers’ Adoption of Organic Agriculture—A Systematic Global Literature Review. Eur. Rev. Agric. Econ. 2024, 51, 1012–1044. [Google Scholar] [CrossRef]
- Zanetti, F.; Monti, A.; Berti, M. Camelina: An Ancient Oilseed Crop Actively Contributing to the Rural Renaissance in Europe. Agron. Sustain. Dev. 2021, 41, 2. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Graczyk, M.; Stuper-Szablewska, K. Winter camelina seeds as a raw material for the production of erucic acid-free oil. Food Chem. 2020, 330, 127265. [Google Scholar] [CrossRef]
- American Society of Agronomy. Camelina: Where You Grow What You Grow. ScienceDaily. 2017. Available online: https://www.sciencedaily.com/releases/2017/05/170524131115.htm (accessed on 6 August 2025).
- Martinez, S.; Gabriel, J.L.; Alvarez, S.; Capuano, A.; Delgado, M.d.M. Integral Assessment of Organic Fertilization on a Camelina sativa Rotation under Mediterranean Conditions. Agriculture 2019, 11, 355. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J.; Tworkowski, J.; Puttick, D.; Eynck, C.; Załuski, D.; Kwiatkowski, J. Yield and Seed Composition of 10 Spring Camelina Genotypes Cultivated in the Temperate Climate of Central Europe. Ind. Crops Prod. 2019, 138, 111443. [Google Scholar] [CrossRef]
- Juodka, R.; Nainienė, R.; Juškienė, V.; Juška, R.; Leikus, R.; Kadžienė, G.; Stankevičienė, D. Camelina sativa L. Crantz as Feedstuffs in Meat Type Poultry Diet: A Source of Protein and n-3 Fatty Acids. Animals 2022, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Turina, E.L.; Pashtetsky, V.S.; Efimenko, S.G.; Prakhova, T.Y.; Kornev, A.Y.; Liksutina, A.P. Quality of Camelina Oil Cultivated in Black Sea Region. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022015. [Google Scholar] [CrossRef]
- Schuster, A.; Friedt, W. Glucosinolate Content and Composition as Parameters of Quality of Camelina Seed. Ind. Crops Prod. 1998, 7, 297–302. [Google Scholar] [CrossRef]
- Matthäus, B.; Zubr, J. Variability of Specific Components in Camelina sativa Oilseed Cakes. Ind. Crops Prod. 2000, 12, 9–18. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compounds in Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Graczyk, M.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Influence of Variety and Weather Conditions on Fatty Acid Composition of Winter and Spring Camelina sativa Varieties in Poland. Eur. Food Res. Technol. 2021, 247, 465–473. [Google Scholar] [CrossRef]
- Zanetti, F.; Peroni, P.; Pagani, E.; von Cossel, M.; Greiner, B.E.; Krzyżaniak, M.; Monti, A. The Opportunities and Potential of Camelina in Marginal Land in Europe. Ind. Crops Prod. 2024, 211, 118224. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina Uses, Genetics, Genomics, Production, and Management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Hunsaker, D.J.; French, A.N.; Clarke, T.R.; El-Shikha, D.M.; Colaizzi, P.D. Camelina Water Use and Irrigation Response in the Arid Southwestern U.S. Agric. Water Manag. 2013, 118, 92–103. [Google Scholar] [CrossRef]
- Vollmann, J.; Eynck, C. Camelina as a Sustainable Oilseed Crop: Contributions of Plant Breeding and Genetic Engineering. Biotechnol. J. 2015, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Walia, M.K.; Zanetti, F.; Gesch, R.W.; Krzyżaniak, M.; Eynck, C.; Puttick, D.; Alexopoulou, E.; Royo-Esnal, A.; Stolarski, M.J.; Isbell, T.; et al. Winter Camelina Seed Quality in Different Growing Environments across Northern America and Europe. Ind. Crops Prod. 2021, 169, 113639. [Google Scholar] [CrossRef]
- Angelini, L.G.; Abou Chehade, L.; Foschi, L.; Tavarini, S. Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy 2020, 10, 1929. [Google Scholar] [CrossRef]
- Zubr, J. Dietary Fatty Acids and Amino Acids of Camelina sativa Seed. J. Food Qual. 2003, 26, 451–462. [Google Scholar] [CrossRef]
- Moser, B.R. Camelina (Camelina sativa L.) Oil as a Biofuel Feedstock: Golden Opportunity or False Hope? Lipids 2010, 22, 270–273. [Google Scholar] [CrossRef]
- Kokoszka, P.; Reimherr, M. Introduction to Functional Data Analysis, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Kayano, M.; Matsui, H.; Yamaguchi, R.; Imoto, S.; Miyano, S. Gene Set Differential Analysis of Time Course Expression Profiles via Sparse Estimation in Functional Logistic Model with Application to Time-Dependent Biomarker Detection. Biostatistics 2016, 17, 235–248. [Google Scholar] [CrossRef]
- Sartore, L.; Rosales, A.N.; Johnson, D.M.; Spiegelman, C.H. Assessing machine leaning algorithms on crop yield forecasts using functional covariates derived from remotely sensed data. Comput. Electron. Agric. 2022, 194, 106704. [Google Scholar] [CrossRef]
- Matsui, H.; Mochida, K. Functional Data Analysis-Based Yield Modelling in Year-Round Crop Cultivation. Hortic. Res. 2024, 11, 7. [Google Scholar] [CrossRef]
- Bonneu, F.; Makowski, D.; Joly, J.; Allard, D. Machine Learning Based on Functional Principal Component Analysis to Quantify the Effects of the Main Drivers of Wheat Yields. Eur. J. Agron. 2024, 159, 127254. [Google Scholar] [CrossRef]
- Ullah, S.; Finch, C.F. Applications of Functional Data Analysis: A Systematic Review. BMC Med. Res. Methodol. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Selyaninov, G.T. About Climate Agricultural Estimation. Proc. Agric. Meteorol. 1928, 20, 165–177. [Google Scholar]
- Chmist-Sikorska, J.; Kępińska-Kasprzak, M.; Struzik, P. Agricultural Drought Assessment on the Base of Hydro-Thermal Coefficient of Selyaninov in Poland. Ital. J. Agrometeorol. 2022, 1, 3–12. [Google Scholar] [CrossRef]
- Samborski, A.S. Agroclimatic Characterization of Zamosc, Poland Using Hydrothermal Coefficient (HTC). J. Agrometeorol. 2024, 26, 473–476. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Cluster Analysis in R: Unsupervised Machine Learning; STHDA: North Charleston, SC, USA, 2017; Volume 1, Available online: https://books.google.pl/books/about/Practical_Guide_to_Cluster_Analysis_in_R.html?id=plEyDwAAQBAJ&redir_esc=y (accessed on 6 August 2025).
- Ramsay, J.O.; Kokoszka, P. Functional Data Analysis; Springer: New York, NY, USA, 2005. [Google Scholar]
- Ramsay, J.O.; Dalzell, C.J. Some Tools for Functional Data Analysis. J. R. Stat. Soc. Ser. B 1991, 53, 539–572. [Google Scholar] [CrossRef]
- Kokoszka, P.S. Functional Data Analysis with R: Ciprian M. Crainiceanu, Jeff Goldsmith, Andrew Leroux, and Erjia Cui, Boca Raton. J. Am. Stat. Assoc. 2024, 120, 588–590. [Google Scholar] [CrossRef]
- Reshef, D.N.; Reshef, Y.A.; Finucane, H.K.; Grossman, S.R.; McVean, G.; Turnbaugh, P.J.; Lander, E.S.; Mitzenmacher, M.; Sabeti, P.C. Detecting Novel Associations in Large Data Sets. Science 2011, 334, 1518–1524. [Google Scholar] [CrossRef]
- Reshef, Y.A.; Reshef, D.N.; Sabeti, P.C.; Mitzenmacher, M. Equitability, Interval Estimation, and Statistical Power. arXiv 2015, arXiv:1505.02212. [Google Scholar] [CrossRef]
- Reshef, Y.A.; Reshef, D.N.; Finucane, H.K.; Sabeti, P.C.; Mitzenmacher, M. Measuring Dependence Powerfully and Equitably. arXiv 2015, arXiv:1505.02213. [Google Scholar]
- Lin, C.; Canhao, H.; Miller, T.; Dligach, D.; Plenge, R.; Karlson, E.; Savova, G. Maximal Information Coefficient for Feature Selection for Clinical Document Classification. In Proceedings of the ICML Workshop on Machine Learning for Clinical Data, International Conference on Machine Learning, Edinburgh, UK, 26 June–1 July 2012. [Google Scholar]
- Kraskov, A.; Stögbauer, H.; Grassberger, P. Estimating mutual information. Phys. Rev. E 2004, 69. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Pang, N.-N.; Tzeng, W.-J. Resolution dependence of the maximal information coefficient for noiseless relationship. Stat. Comput. 2014, 24, 845–852. [Google Scholar] [CrossRef]
- Robidoux, B. Maximal Information Coefficient: An Introduction to Information Theory. Predictive Analytics and Futurism. Soc. Actuar. 2017. Available online: https://share.google/tZIjxunvYRbfMFayP (accessed on 6 August 2025).
- Albanese, D.; Filosi, M.; Visintainer, R.; Riccadonna, S.; Jurman, G.; Furlanello, C. Minerva and minepy: A C engine for the MINE suite and its R, Python and MATLAB wrappers. Bioinformatics 2012, 29, 407–408. [Google Scholar] [CrossRef]
- Kinney, J.B.; Atwal, G.S. Equitability, mutual information, and the maximal information coefficient. Proc. Natl. Acad. Sci. USA 2014, 111, 3354–3359. [Google Scholar] [CrossRef]
- Meyer, P.E. infotheo: Information-Theoretic Measures. R Package Version 1.2.0. Available online: https://cran.r-project.org/package=infotheo (accessed on 9 September 2025).
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Shekari, F.; Soltaniband, V.; Javanmard, A.; Abbasi, A. The Impact of Drought Stress at Different Stages of Development on Water Relations, Stomatal Density and Quality Changes of Rapeseed (Brassica napus L.). Iran Agric. Res. 2016, 34, 81–90. [Google Scholar]
- Ahmadi, M.; Bahrani, M.J. Yield and Yield Components of Rapeseed as Influenced by Water Stress at Different Growth Stages and Nitrogen Levels. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 755–761. [Google Scholar]
- Ma, Q.; Niknam, S.R.; Turner, D.W. Responses of Osmotic Adjustment and Seed Yield of Brassica napus and B. juncea to Soil Water Deficit at Different Growth Stages. Aust. J. Agric. Res. 2006, 57, 221–226. [Google Scholar] [CrossRef]
- Montesinos-López, A.; Montesinos-López, O.A.; Cuevas, J.; Mata-López, W.A.; Burgueño, J.; Mondal, S.; Huerta, J.; Singh, R.; Autrique, E.; González-Pérez, L.; et al. Genomic Bayesian functional regression models with interactions for predicting wheat grain yield using hyper-spectral image data. Plant Methods 2017, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, N.; Li, Y.; Lin, H.; Yao, N.; Chen, X.; Liu, D.L.; Yu, Q.; Feng, H. Impact of climate variability on grain yields of spring and summer maize. Comput. Electron. Agric. 2022, 199, 107101. [Google Scholar] [CrossRef]
- Pavlov, A.V.; Porokhovinova, E.A.; Slobodkina, A.A.; Matvienko, I.I.; Kishlyan, N.V.; Brutch, N.B. Influence of Weather Conditions in the Northwestern Russian Federation on Flax Fiber Characters According to the Results of a 30-Year Study. Plants 2024, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Krzyśko, M.; Derejko, A.; Górecki, T.; Gacek, E. Principal component analysis for functional data on grain yield of winter wheat cultivars. Biom. Lett. 2013, 50, 81–94. [Google Scholar] [CrossRef]

| HYY | LYY | |
|---|---|---|
| 2.212 1.635 | 9.703 1.366 | |
| 11.964 5.809 | 4.923 6.586 |
| Yield | NDV | 0.6099865 | |
|---|---|---|---|
| Yield | Month | Temperature | Precipitation |
| March | 0.2364528 | 0.2364528 | |
| April | 0.3958156 | 0.3958156 | |
| May | 0.2364528 | 0.2364528 | |
| June | 0.2364528 | 0.2364528 | |
| July | 0.3958156 | 0.1080315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, M.; Kurasiak-Popowska, D.; Niedziela, G. Changes in Camelina sativa Yield Based on Temperature and Precipitation Using FDA. Agriculture 2025, 15, 2051. https://doi.org/10.3390/agriculture15192051
Graczyk M, Kurasiak-Popowska D, Niedziela G. Changes in Camelina sativa Yield Based on Temperature and Precipitation Using FDA. Agriculture. 2025; 15(19):2051. https://doi.org/10.3390/agriculture15192051
Chicago/Turabian StyleGraczyk, Małgorzata, Danuta Kurasiak-Popowska, and Grażyna Niedziela. 2025. "Changes in Camelina sativa Yield Based on Temperature and Precipitation Using FDA" Agriculture 15, no. 19: 2051. https://doi.org/10.3390/agriculture15192051
APA StyleGraczyk, M., Kurasiak-Popowska, D., & Niedziela, G. (2025). Changes in Camelina sativa Yield Based on Temperature and Precipitation Using FDA. Agriculture, 15(19), 2051. https://doi.org/10.3390/agriculture15192051







