1. Introduction
Tolprocarb (TPC) is a registered fungicide effective against major rice diseases, including rice blast and bacterial leaf blight. TPC exerts a direct antifungal effect on the rice blast fungus
Pyricularia oryzae by inhibiting polyketide synthase, which is essential for melanin biosynthesis. Due to its direct antifungal activity, TPC effectively inhibits rice blast infection [
1]. However, despite this well-defined mode of action against rice blast, TPC shows no direct bactericidal activity against bacteria. Beyond its non-antibacterial nature, TPC treatment significantly reduces lesion size caused by the bacterial leaf blight pathogen
Xanthomonas oryzae pv.
oryzae, which induces wilting and yellowing symptoms in rice leaves [
2]. This effect is attributed to the immunostimulatory action of TPC, and the level of resistance induced is comparable to that of probenazole (PBZ), a well-characterized plant defense activator [
3].
A key aspect of induced plant resistance is the production of phytoalexins, antimicrobial secondary metabolites. In rice, the main phytoalexins are of the diterpene type, which are biosynthesized in response to pathogen infection and environmental stress [
4]. Among these, momilactones A and B, as well as phytocassanes A–G, are strongly accumulated in leaf blades following infection [
5,
6,
7,
8,
9,
10]. These compounds are known to be inducibly synthesized as a part of the direct defense of plants against rice blast and bacterial leaf blight pathogens [
2,
11]. Notably, carpropamid, a melanin biosynthesis inhibitor targeting dehydratase in the melanin biosynthesis pathway (MBI-D), has been reported to enhance momilactone A accumulation only under rice blast inoculation conditions [
12]. On the other hand, probenazole, a plant defense activator, is known to induce the expression of various defense-related proteins, such as flavonoid phytoalexin-related proteins, phenylalanine ammonia-lyase (PAL), and caffeic acid 3-O-methyltransferase (COMT) [
13,
14,
15]. Furthermore, the momilactone-inducing activity of probenazole has also been reported [
16]. To date, aside from these compounds, there have been no reports of a marked enhancement of phytoalexin production in response to a single treatment with a pesticide compound, including fungicides.
Despite evidence that TPC functions as an immunostimulant, it remains unclear whether a single treatment can markedly induce phytoalexin production in rice. In this study, we aimed to determine whether TPC stimulates the biosynthesis and accumulation of diterpene-type phytoalexins, including momilactones and phytocassanes, and whether such induction contributes to disease resistance under both controlled and field conditions. Here, we demonstrate that the fungicide TPC induces immunostimulatory resistance in rice by promoting the expression of genes involved in diterpene-type phytoalexin biosynthesis and the accumulation of these compounds. This response was validated under both laboratory and field conditions. Notably, momilactones, phytoalexins with antimicrobial and allelopathic properties, accumulated in the rhizosphere soil following TPC treatment, and weed growth was suppressed in TPC-treated plots. These findings indicate that TPC enhances rice disease resistance, at least in part, by activating immune responses associated with diterpene phytoalexin production.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Oryza sativa L. cultivars Nipponbare, Kinuhikari, and Koshihikari were used for the following experiments. For gene expression analysis and diterpenoid phytoalexin measurement in leaf blades, Nipponbare seeds were incubated with tap water at 33 °C for 3 days before sowing. Incubated seeds were sown on sterile soil in a 96-well cell tray and grown until they reached the three-leaf stage. Afterward, six seedlings were transplanted into 1/10,000 a Wagner Pot and grown in an unheated greenhouse for one month until chemical treatment. For laboratory trials, measurement of diterpenoid phytoalexin in leaves, roots and rhizosphere soil, Nipponbare seeds were surface-sterilized with 75% ethanol for 1 min and then thoroughly rinsed with tap water. The sterilized seeds were germinated in soil saturated with tap water in a biotron at 28 °C under a 14-h light/10-h dark cycle until they reached the three-leaf stage. Subsequently, the seedlings of 3 plants were transferred to sterile soil and grown under an 8-h light/16-h dark photoperiod at 28 °C for two weeks. For field trials, Kinuhikari or Koshihikari seeds were sown in seedling boxes after a 3-day incubation at 33 °C. Seedlings were grown in an unheated greenhouse until transplanting.
2.2. Chemical Treatment
For gene expression analysis and diterpenoid phytoalexin measurement in leaf blades, rice plants at the 5–6-leaf stage were used for TPC treatment. 416 µL of 10 mg/mL (10,000 ppm) TPC solution in acetone was added to 0.4 L of water in 1/10,000 a Wagner Pot with planted soil (water depth was 4 cm); consequently, the final concentration of TPC in water was 30 µM. For laboratory trials, measurement of diterpenoid phytoalexin in leaves, roots and rhizosphere soil, young rice seedlings with 3–5 expanded leaves were used for the TPC treatment. For the liquid TPC treatment, a 25 mg/mL TPC solution in acetone was added to the growth containers to achieve a final concentration of 30 µM. For the granule TPC treatment, 50 g of TWINKICK™ Boxed Granules—containing 9% tolprocarb and 0.75% cyantraniliprole (Mitsui Chemicals Crop & Life Solutions, Inc., Tokyo, Japan)—was directly applied to the soil in seedling boxes (30 cm × 60 cm), resulting in an estimated final dosage of 12.5 mg per plant. Mock (untreated) control samples were prepared by either adding acetone alone or by not applying the TPC granules. Collected plant materials were either immediately soaked in 80% methanol for metabolite analysis or frozen in liquid nitrogen and stored at −80 °C until used for RNA extraction.
2.3. RNA Preparation and Expression Analysis
Leaf blades were collected from the O. sativa Nipponbare before (0-day control) or after TPC treatment for 1, 3, 5, 7, and 10 days, and total RNAs were extracted from powdered leaf samples using an RNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) in accordance with the manufacturer’s instructions. The purity and quantity of the total RNAs were determined using a Varioskan LUX multimode microplate reader (Roche Diagnostics K.K., Tokyo, Japan). For the RT reaction, a PrimeScript RT reagent Kit (Perfect Real Time) (Takara Bio Inc., Kusatsu, Shiga, Japan) was used. qRT-PCR was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio Inc., Kusatsu, Shiga, Japan).
Gene expression levels were quantified using the method, with normalization to an internal control gene (OsUBQ) and calibration against the untreated control. Primers used for the analysis were as follows: OsKSL4 (Sense: 5′-CGCTTTGTAACTCTAAGGTA-3′, antisense: 5′-ACGTAAAAGGCTTGTATATC-3′), OsGGPS (Sense: 5′-GCTTTCTGATGCAAGGGAACAAC-3′, antisense: 5′-GTACCCATCACCTCAGTTCTG-3′), OsPBZ1 (Sense: 5′-GTGGTTGTGTTTATGTGCCTTTCTATG-3′, antisense: 5′-ACTTGCCTCTCTTTATTCACCCATTG-3′), OsUBQ (Sense: 5′-TCCGAGAGATGGGTTTCATC-3′, antisense: 5′-GCCAAGATTGCCAAGAAGAC-3′).
RNA extracted from leaves three days after TPC treatment was subjected to microarray analysis, which was outsourced to Hokkaido System Science Co., Ltd. (Sapporo, Japan). Based on the resulting transcriptome data, genes exhibiting a greater than twofold change in expression in response to TPC treatment, with a false discovery rate (FDR) < 0.01, were identified as TPC-responsive genes. These genes were selected using a scatter plot.
2.4. Diterpenoid Phytoalexin Measurement
Leaf blades and root tissues from TPC-treated plants were submerged in 2 mL of 80% methanol at 4 °C for 24 h. A 5 μL aliquot of the extract was subjected to LC-MS/MS analysis using multiple reaction monitoring (MRM) transitions as previously described [
17].
Soil samples were prepared by adding 30 mL of 80% methanol to 30 g of soil placed in biocups, followed by thorough mixing. The mixtures were then left to stand at 4 °C for 24 h. After centrifugation to remove the soil residues, the supernatants were collected for analysis.
2.5. Diterpenoid Accumulation After TPC Treatment Under Field Conditions
To investigate the time-course accumulation of diterpenoid-type phytoalexins in rice plants treated with TPC under field conditions, field trials were conducted at two sites: Inashiki, Ibaraki Prefecture, and Yasu, Shiga Prefecture, in the experimental fields of Mitsui Chemicals Crop & Life Solutions (Tokyo, Japan). The rice cultivars used were Kinuhikari (Ibaraki) and Koshihikari (Shiga). Seeds were sown in seedling boxes, and seedlings were grown for three weeks before transplantation. At the time of transplantation, the leaf age was approximately 4.5 leaves.
The TPC treatment was performed using TWINKICK™ box granules, a formulation containing 9% tolprocarb and 0.75% cyantraniliprole (Mitsui Chemicals Crop & Life Solutions, Inc., Tokyo, Japan). On the day of transplantation, 50 g of the granules per seedling box (equivalent to 12.5 mg/plant) was uniformly applied at the base of each rice plant. For the untreated control plots, box granules containing 0.75% cyantraniliprole (FMC Chemicals, Tokyo, Japan) were applied in the same manner. After treatment, seedlings were transplanted into paddy fields using a rice transplanter, with the granules applied together.
Sampling was conducted at the following time points after treatment: 0 days, 1 week, 2 weeks, 1 month, 2 months, and the heading stage. Specifically, the sampling days were as follows: 0, 6, 13, 28, 57, and 74 days at Inashiki in 2020; 0, 6, 14, 32, 61, and 68 days at Yasu in 2020; 0, 7, 14, and 28 days at Inashiki in 2021; and 0, 7, 14, and 30 days at Yasu in 2021. In the 2021 trials, later time points were omitted in order to focus on the early-stage accumulation of momilactones. For each sampling point, tissues from 10 individual plants were pooled and analyzed as a single sample to determine the diterpenoid-type phytoalexin content. For statistical analysis, two or four momilactone values from each location and year were combined to calculate averages and standard deviations. t-tests were performed for each time point to detect significant differences.
2.6. Determination for Exudation Level of Diterpenoid Phytoalexins into the Rhizosphere and Evaluation of Allelopathic Effects on Paddy Weeds
A field experiment for allelopathic activity was conducted at the Yasu site in Shiga Prefecture (Mitsui Chemicals Crop & Life Solutions, Tokyo, Japan) to assess the allelopathic effects of TPC-treated rice plants. The rice cultivar used was Koshihikari. Seedlings at the three-leaf stage (20 days after sowing) were transplanted into 50 cm × 50 cm field plots (n = 1 for 14-day evaluation; n = 3 for 42-day evaluation).
Weed species and the number of individual weeds emerging in each plot were recorded 14 days after transplantation. At 42 days, both the weed species and number were recorded, and the dry weight of each species was measured.
TPC treatment was performed using TWINKICK™ box granules (9% tolprocarb, 0.75% cyantraniliprole; Mitsui Chemicals Crop & Life Solutions, Japan), applied uniformly at the base of each rice plant at a dosage of 12.5 mg/plant on the day of transplantation. In the untreated control plots, cyantraniliprole-only box granules (0.75%, FMC Chemicals, Tokyo, Japan) were applied similarly. Four seedlings were grouped as one clump, and five clumps were transplanted into each plot.
2.7. Quantification of Diterpenoid-Type Phytoalexins from Field-Grown Materials
At 14 and 42 days after the TPC treatment, rice plants and soil samples were collected for diterpenoid-type phytoalexin quantification using LC-MS/MS as described before. For sampling, a cylinder with a 15 cm diameter was used to extract rice plants along with the surrounding soil. Three clumps were sampled per plot. Each sample was separated into the aboveground part (upper three leaves) and the belowground part (soil within 10 cm depth and roots). Each part was analyzed as an independent sample for phytoalexin content.
3. Results & Discussion
3.1. Transcriptomic Changes in Pathogen-Related Genes After TPC Treatment
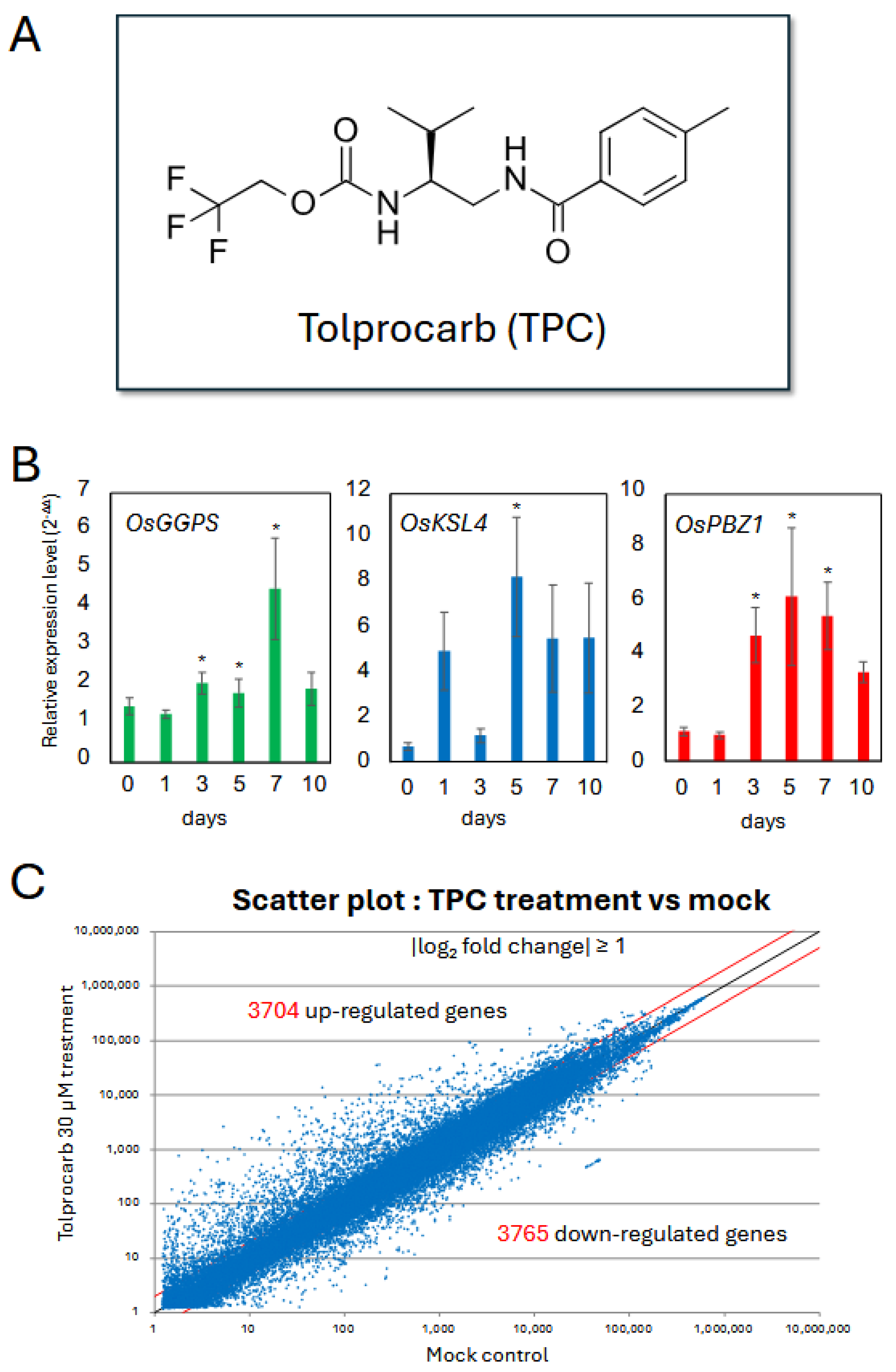
To investigate the mechanism underlying the immunostimulatory effect of TPC in rice, we analyzed gene expression changes following TPC treatment using a microarray approach (
Figure 1A). Fully expanded uppermost leaves were collected at multiple time points after treatment.
Prior to the microarray analysis, we monitored the expression of three key genes to determine the optimal time point for transcriptome profiling of:
OsKSL4, a biosynthetic gene involved in the production of diterpene-type phytoalexins [
18],
OsGGPS, which encodes geranylgeranyl diphosphate synthase functioning in an upstream biosynthetic pathway [
19], and
OsPBZ1, a well-established marker of systemic resistance mediated by salicylic acid (SA) signaling [
20,
21].
Expression profiling revealed that all three genes were significantly upregulated between 3 and 7 days after TPC treatment (
Figure 1B). Based on these results, we selected day 5 after TPC treatment for Agilent 44K rice microarray analysis, using RNA extracted from both treated and untreated samples.
From the microarray data (
n = 3 per group), differentially expressed genes (DEGs) were identified based on a fold change ≥ 2 and a false discovery rate (FDR) < 0.01. As a result, 3704 genes were upregulated and 3765 genes were downregulated by TPC treatment (
Figure 1C).
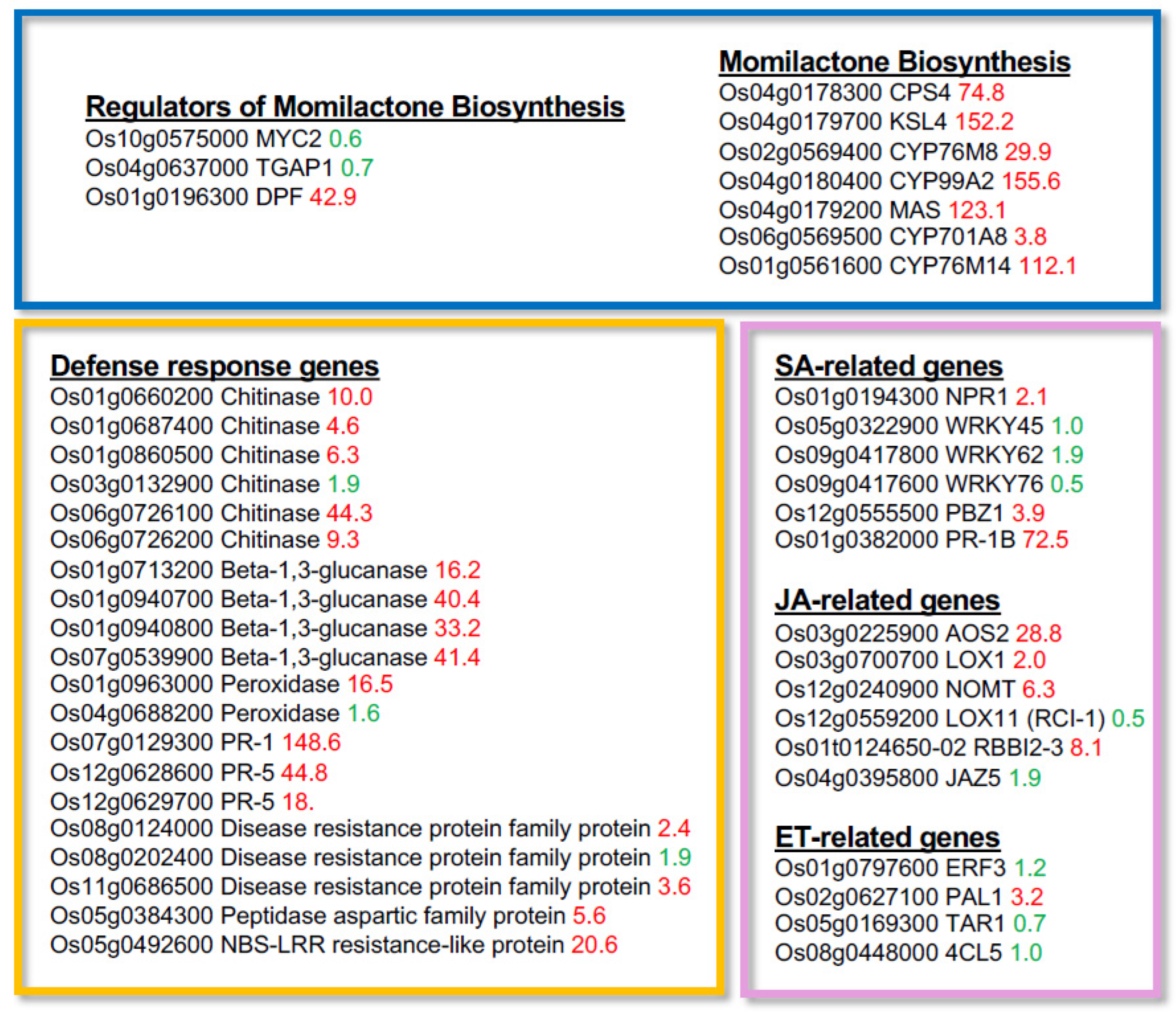
Gene ontology (GO) analysis of the upregulated genes using the PANTHER Overrepresentation Test revealed a significant enrichment of GO terms associated with diterpene-type phytoalexin biosynthesis (
Table 1). In particular, the GO category “phytoalexin metabolic process (accession GO0052314)” included numerous biosynthetic genes for momilactones and phytocassanes, two major classes of diterpene-type phytoalexins in rice (
Table 2). Notably, the transcription factor DPF, a key positive regulator of diterpene phytoalexin biosynthesis, was strongly induced, showing a 42.9-fold increase in expression among upregulated regulators in our transcriptome analysis (
Figure 2). In addition to phytoalexin biosynthetic genes, several pathogen-related genes were also upregulated by TPC treatment, including
Chitinases,
β-1,3-glucanases, and
Peroxidases, as well as genes responsive to SA, jasmonic acid (JA), and ethylene (ET) signaling pathways (
Figure 2) [
22,
23,
24].
Taken together, the data suggest that TPC stimulates rice immune responses at the transcriptomic level. We therefore proceeded to evaluate the corresponding changes in phytoalexin accumulation.
3.2. Inductive Diterpenoid Biosynthesis Under TPC Treatment
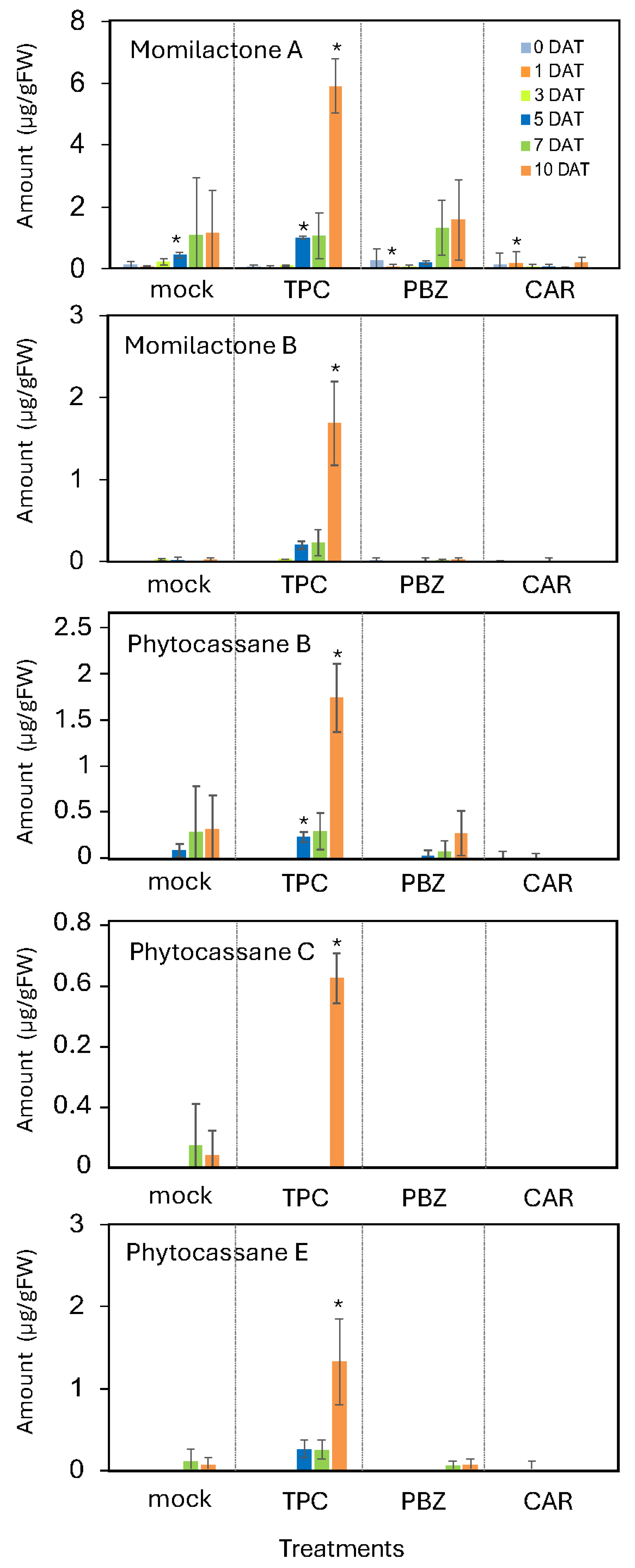
TPC treatment broadly induced the expression of genes involved in phytoalexin biosynthesis, suggesting that it may enhance the production and accumulation of these compounds in rice leaf blades. To investigate this possibility, we quantified the accumulation of diterpene-type phytoalexins under the same pot culture conditions used in the microarray analysis. Phytoalexins were extracted from the leaves with 80% methanol and analyzed by LC-MS/MS.
Compared to the untreated control, treatment with other known resistance inducers—probenazole (PBZ) and carpropamid (CAR)—did not lead to a clear increase in phytoalexin accumulation. In contrast, treatment with 30 μM TPC resulted in a marked accumulation of momilactones A and B, as well as phytocassanes B, C, and E at 10 days post-treatment (
Figure 3).
These results indicate that the accumulation of diterpene-type phytoalexins is a distinctive and robust response to TPC treatment and may contribute to its protective effect against blast fungus infection. In this trial, carpropamid did not induce phytoalexins. According to previous reports, carpropamid induces momilactone A when rice plants are treated together with rice blast fungus. However, when carpropamid is applied alone to rice plants, clear induction of momilactone A has not been observed. Therefore, since this trial was conducted without inoculation of rice blast fungus, the results obtained for carpropamid are consistent with past experiences.
3.3. Accumulation of Diterpenoid Phytoalexins in Roots and Their Exudation into the Rhizosphere Under Experimental Conditions
Momilactone B is known not only for its antimicrobial activity but also for its allelopathic properties, and it has been reported to inhibit the growth of paddy field weeds [
25]. For such allelopathic effects to function effectively in the field, the accumulation of momilactones in rice roots and their subsequent exudation into the rhizosphere are thought to be essential. Therefore, we investigated whether TPC treatment induces the accumulation of diterpene-type phytoalexins in roots and alters their release into the rhizosphere.
Rice seedlings were irrigated with 30 μM TPC, and samples from the aboveground tissues, roots (belowground), and rhizosphere soil were collected and analyzed for momilactones. At 3 days after treatment, momilactone A (MA) showed a clear increase in the aboveground parts, while no significant accumulation was observed in the root tissues or rhizosphere soil compared to the untreated control (
Figure 4).
In the aboveground tissues, the MA:MB (momilactone B) ratio was relatively high, with MB levels approximately one-seventh that of MA. In contrast, in the rhizosphere soil, although the absolute amounts of momilactones were 1/1000 to 1/2000 of those in the shoots, the MB proportion was higher, reaching approximately half that of MA. This shift in the MA:MB ratio in the rhizosphere suggests potential differences in chemical stability, degradation, or selective retention between the two compounds in soil environments.
3.4. Diterpenoid Biosynthesis After TPC Treatment Under Field Conditions
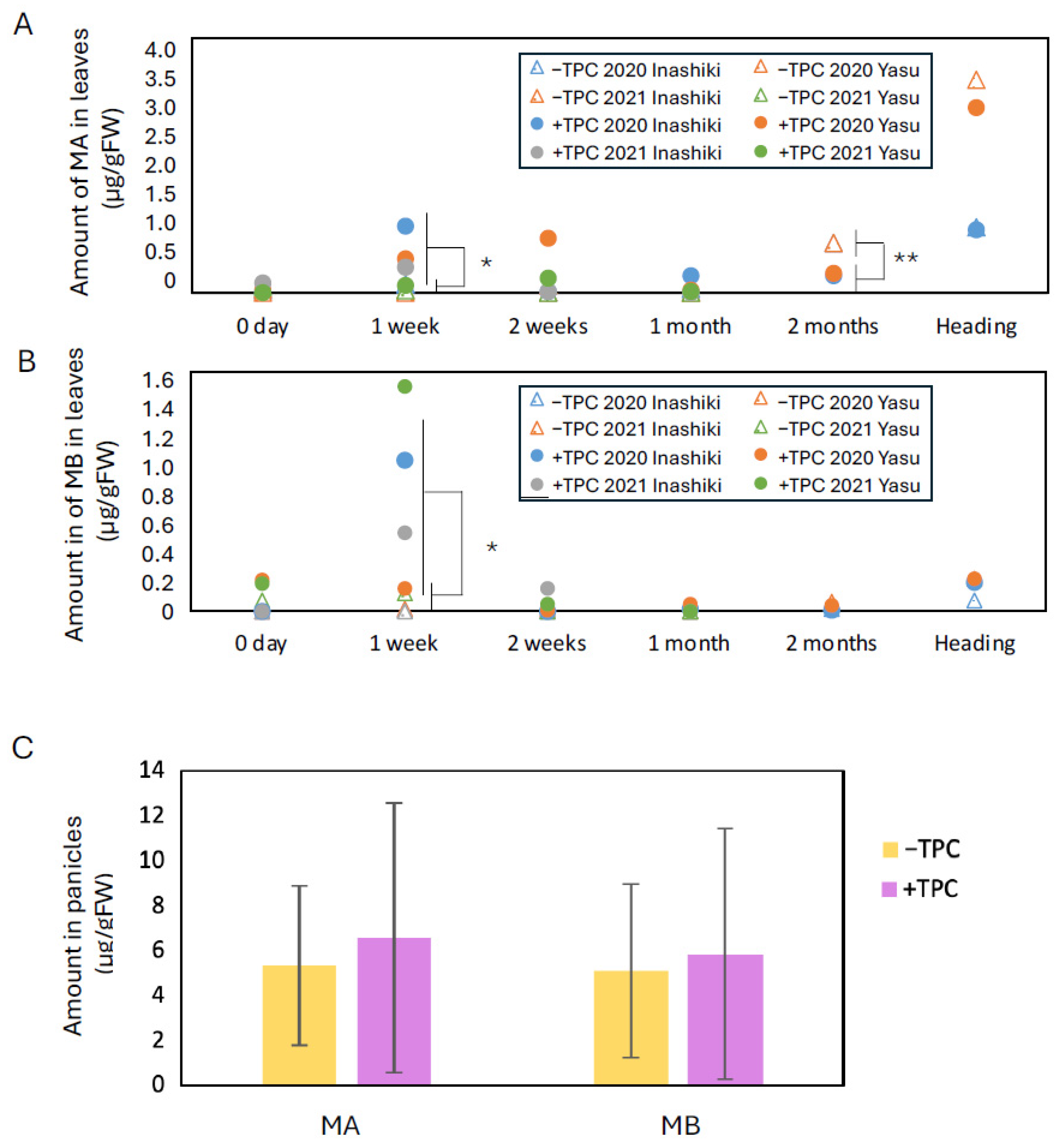
To evaluate the effect of TPC on phytoalexin induction under practical rice cultivation conditions, field experiments were conducted in real paddy fields. For TPC treatment, 9% tolprocarb-containing granules (TWINKICK
TM box granules, containing tolprocarb 9% and cyantraniliprole 0.75%, Mitsui Chemicals Crop & Life Solutions, Japan) were applied to seedling boxes prior to transplanting (50 g/seedling box, approximately 12.5 mg/plant). Leaf samples were collected at multiple time points during plant growth. The experiment was carried out in two separate paddy fields located in different regions and repeated over two consecutive years using two different rice cultivars:
Koshihikari and
Kinuhikari. As shown in
Figure 5, in both field trials, the leaf blade phytoalexin content at 1 week after transplanting was significantly higher in the TPC-treated plots than in the control (
p < 0.1). However, following this peak, the phytoalexin levels per unit leaf blade weight gradually returned to levels similar to those of the control plots within 2 weeks to 1 month after transplanting.
As the plants continued to grow, phytoalexin levels increased again in the leaf blades and were also detectable in reproductive tissues (panicles). However, similar patterns of accumulation were also observed in the control plots, indicating that this late-stage increase was not a specific effect of TPC treatment. Therefore, no phytoalexin-inducing effect of TPC was observed during the maturation phase of rice growth.
3.5. Exudation of Diterpenoid Phytoalexins into the Rhizosphere and Their Allelopathic Effects on Paddy Weeds
In the laboratory-based pot experiments described before, TPC treatment did not significantly alter the amount of momilactones exuded into the rhizosphere. However, to better assess the impact of TPC under conditions closer to actual rice cultivation, we analyzed phytoalexin accumulation and exudation in a field trial that mimicked a real paddy field.
In the test plots, five rice plants were arranged as shown in
Figure 6A. Samples were collected from the aboveground tissues, underground parts (roots), and rhizosphere soil at two time points: 14 days after transplanting, when TPC was previously shown to increase momilactone accumulation in leaves, and 42 days after transplanting, representing a later growth stage (
Figure 5). At day 14, TPC-treated plants showed increase in momilactone accumulation in leaf blades, consistent with earlier findings but it was not significant due to small plot size. Soil momilactone levels remained unchanged at this time point. However, by day 42, no significant difference in leaf momilactone content was observed between treated and control plots, while rhizosphere exudation was significantly increased in TPC-treated plants (
Figure 6B). These findings suggest that TPC treatment under field conditions transiently promotes the accumulation of momilactones in aboveground tissues around 14 days after transplanting, followed by their reduced retention in roots and increased release into the rhizosphere at later stages.
To assess the potential allelopathic effect of momilactone exudation on weed growth, we monitored weed populations in the same test plots. While no significant differences in weed species composition (e.g.,
Monochroria species,
Echinochloa species,
Scirpus species, and other annual weeds) were observed between treated and untreated plots, the total dry biomass of
Monochroria species was significantly reduced in the TPC-treated areas (
Figure 6C). On the other hand, clear reductions in weed biomass were not observed for
Echinochloa and
Scirpus species following TPC treatment. However, the average biomass appeared to be higher compared to the untreated plots. Considering this, it seems that there may be some relationship between TPC treatment and the reduction of weed biomass. This suggests that TPC treatment enhanced momilactone exudation into the rhizosphere, which may have contributed to the suppression of weed growth in the surrounding soil.
4. Conclusions
This study demonstrates that the fungicide tolprocarb (TPC) acts as a potent immunostimulant in rice by inducing the expression of genes related to pathogen defense and stimulating the biosynthesis of diterpenoid phytoalexins, notably momilactones and phytocassanes. Transcriptome analysis revealed the upregulation of key biosynthetic enzymes and defense-related genes, while metabolite profiling confirmed a marked increase in phytoalexin accumulation, particularly in the leaf blade, following TPC treatment.
Unlike conventional resistance inducers such as probenazole or carpropamid, TPC uniquely promoted the accumulation of phytoalexins both under controlled and field conditions. In addition to enhancing disease-related defense, TPC also influenced the distribution of momilactones across plant compartments. Field experiments indicated a transient increase in momilactone levels in aerial tissues, followed by enhanced exudation into the rhizosphere at later stages of growth.
Importantly, this increase in momilactone exudation was associated with reduced weed biomass, suggesting that TPC may contribute not only to disease resistance but also to the suppression of competing weeds through an allelopathic mechanism. Together, these findings highlight TPC as a dual-function agrochemical that reinforces rice immunity and may support weed control via phytoalexin-mediated allelopathy, offering new perspectives for integrated rice crop management.
Our findings reveal that tolprocarb (TPC) enhances disease resistance in rice by activating defense-related genes and promoting the biosynthesis of diterpenoid phytoalexins, including momilactones and phytocassanes. Unlike other known inducers, TPC consistently triggered phytoalexin accumulation in both controlled and field environments. In field-grown rice, TPC treatment led to a temporary increase in leaf momilactones, followed by enhanced exudation into the rhizosphere. This was accompanied by a reduction in weed biomass, suggesting an additional allelopathic effect. These results position TPC as a promising dual-purpose agrochemical with both disease-protective and weed-suppressive capabilities.