Abstract
Annual rice growing lands are mainly threatened by soil loss. High-yielding perennial rice cultivars with great socioeconomic values are developed to stabilize fragile rice farms. Nitrogen balance in perennial rice fields can be facilitated by its no-tillage-based management system. However, systematic studies on nitrogen transformation and its distribution pattern are lacking. This study has therefore been conducted to look for the merits of no-tillage-based perennial rice farming on maintaining balanced nitrogen under perennial rice field conditions. From 2021 to 2023, a field experiment was conducted for six successive seasons, and the effect of no-tillage-based perennial rice plantation on apparent nitrogen balance was assessed. Plant nitrogen dry matter production efficiency and nitrogen recovery efficiency under the perennial rice production system were higher than the annual rice farming system by 10.32% (p < 0.05) and 14.17% (p < 0.05) per annum, respectively. Perennial rice systems exhibit higher nitrogen use efficiency and soil nitrogen potential for crops, sustain soil nitrogen balance and enhance soil fertility for long-term rice productivity. Perennial rice farming system is conducive to green and sustainable production in farmland.
1. Introduction
According to Jain et al. [1], the global population has risen to 8 billion in 2022, and the current population is still increasing at a rate of more than 1% per year. It is therefore expected that the global population will exceed 9 billion in 2050 [2]. With the rapid increase in the global population, the demand for food is also rising. Due to annual cropping, marginal lands which currently support 50% of the world population are vulnerable to heavy rainfall, nutrient loss, soil infertility and further risk of degradation [3,4]. Despite annual crops providing high yield, extensive tilling and field preparation are required for an optimal outcome [5].
Rice is an important food crop worldwide, and more than half of the world’s population consumes it as a staple food [6]. For thousands of years, traditional rice production required a huge manpower for plowing, raising seedlings and transplanting. Plowing in paddy fields is beneficial for removing rice stubble, weeds, pests and diseases [7]. But continuous plowing damages field surface, resulting in soil erosion and water runoff [8,9]. In addition, earthworms and other beneficial organisms in the soil are eliminated, resulting in decreased level of organic matter [10].
Researchers develop and advocate green, light and highly productive agricultural systems to ensure sustainable production of food crops and reduce the negative impact on natural ecosystems [11,12]. The conversion of major annual food plants into perennial crops is one effective strategy for solving soil health problems [13,14,15,16]. Compared with annual crops, perennial crops tend to have a longer growing season and deep root to intercept and utilize more precipitation, prevent soil erosion and have a better retention potential of the surface soil towards carbon, nitrogen, phosphorus and microbial populations [9,17,18,19,20,21].
Crossing annual Asian cultivated rice (O. sativa) and perennial male wild rice (O. longistaminata) generated perennial cultivars of rice with an underground vegetative propagation feature. So far, Perennial Rice 23, Yunda 25, Yunda 26 and Yunda 107 have been approved as perennial rice varieties by Yunnan Province of China [22,23]. From a single perennial rice plantation, it is possible to earn a continuous harvest for over four years [24]. Perennial rice production system lacks season-based seed purchase, raising seedlings, plowing, raking, planting and other farming tasks. Therefore, this farming mode is cost-effective and eco-friendly [25,26,27]. Non-tillage plus regeneration-based rice production uses the dormant axillary buds of the above-ground rice stubble to regrow and develop [28]. As compared to the traditional tillage method, a reduced tillage frequency and intensity in no-tilling paddy field condition can improve diverse soil characteristics such as moisture content, pH, bulk density and aggregate stability [29].
Nitrogen is dynamically converted into different nitrogen pools, and its circulation maintains the balance in a soil [30]. In the soil-crop system, mutual conversion and distribution of inorganic/organic nitrogen pools are determined by the input, residual and output nitrogen. Thus, long-term cultivation and fertilization drive the transformation and distribution process of soil nitrogen. Tillage method affects crop nitrogen absorption, soil nitrogen residue and nitrogen loss [31]. Nitrogen evolution mediated by the process of nitrogen conversion and distribution is largely influenced by continuous no-tillage farming [32]. Analyzing nitrogen evolution at various spatial and temporal scales is therefore fundamental for scientific soil nitrogen management and rational fertilization regulation. In the history of crop domestication and improvement, perennial rice breakthrough is the turning point for the shift from annual to perennial mode. The complex root system from multiple and consecutive production seasons of perennial rice production displayed unique patterns of nitrogen assimilation, fixation and loss at different soil layers [27]. In a continuously evolving ecosystem, nitrogen cycle is comprehensively affected by the consistent interaction between plants and soil. Soil and perennial rice roots’ interaction is very complex and tough to analyze. Though Zhang et al. [22] and Huang et al. [33] reported the effect of long-term nitrogen fertilizer application on nitrogen utilization rate and economic earnings from the perennial rice farming, soil nitrogen and nitrogen transformation dynamics derived from the nitrogen utilization potential of perennial rice at different soil layers were not explicitly assessed. Along with the total nitrogen change at different soil layers of perennial rice production system, a systematic and comprehensive analysis of nitrogen mineralization and nitrogen utilization of plants is required for its wide range of cultivation.
At present, perennial rice is researched and cultivated in China and in some other African and Southeast Asian countries [27]. Its recent release and relatively less coverage limited research output on the level, distribution and transformation of soil nitrogen in perennial rice fields. Systematic and in-depth studies on profiling nitrogen balance and dynamics at different soil layers of no-tillage perennial rice farm define the rate of nitrogen fertilizer applied and premises of perennial rice cultivation. This study has therefore been conducted to assess the evolution of organic and inorganic nitrogen forms at three different soil layers of perennial rice fields, identify the magnitude of key transformations in nitrogen cycle and forecast the merits of no-tillage-based perennial rice farming for maintaining balanced nitrogen under perennial rice field conditions.
2. Materials and Methods
2.1. Study Area
The experimental area was located at the Yunnan University Field Test Station (21°57′28″ N, 100°21′20″ E, 1150 m above sea level) in Manla Village, Mengzhe Town, Menghai County, Dai Autonomous Prefecture and Xishuangbanna, Southwest China, in the subtropical plateau monsoon climate zone (Figure 1). The site is a double-cropping rice growing area having abundant rainfall with four seasons (spring, sunny autumn, warm summer and cool winter). The farmland soil type is hydragric anthrosols (arenic, ferric) [34]. Temperature, rainfall, sunshine and relative humidity of the experimental site during the study period (from 2021 to 2023) are indicated in Figure A1.
Figure 1.
Shaded relief map of the experimental site’s geographical location and topography. The basemap image is obtained from the Bigemap® GIS Office satellite map software (version 4.2.0) by Bigemap Data Services Co., Ltd., Chengdu, China. The exact position of the experimental site is indicated by the “☆”.
2.2. Experimental Setup and Fertilization
In this study, field experiment was conducted from 2021 to 2023, with a total of 6 rice production seasons (grouped as early (F) and late (S) rice seasons of each year). The rice type under testing was Perennial Rice 25 (PR25), a perennial rice variety with a strong perennial nature, good wintering ability and stable yield performance. The conventional tillage-based annual farming system (transplanting in each season with plowing after each harvest) and no-tillage (transplanting during the first season and keeping the plants without tillage for five successive regenerating seasons) were the two farming systems set up for this experiment. Unlike the no-tillage-based perennial rice farming mode, the conventional annual-tillage system required seed preparation, seedling rearing, plowing and raking (to a tillage depth of about 20 cm) and rice transplanting at each of the experimental seasons. The experiment was carried out for 3 years of 6 production seasons (Season 1 (2021F), Season 2 (2021S), Season 3 (2022F), Season 4 (2022S), Season 5 (2023F) and Season 6 (2023S)). Annual tillage nitrogen fertilizer (TN1) and perennial no-tillage nitrogen fertilizer (NN1) were the two treatment levels, and each treatment was replicated 4 times with a cell size of 32 m2 in a completely randomized block arrangement. In order to calculate nitrogen recovery efficiency of the plant, both tillage without nitrogen fertilizer (TN0) and no-tillage without nitrogen fertilizer (NN0) treatments were included (Table A3).
For both production methods, 28,486 holes of seedlings were transplanted into each hectare (ha), and 2 rice seedlings were planted in each hole with row spacing of 13 cm × 26 cm. Only the first season of perennial production had similar planting methods as that of the annual production mode. But in the remaining five consecutive seasons, seedlings emerged from the axillary buds of the rice stubble. Urea (N, 46%), superphosphate (P2O5, 12%) and potassium chloride (K2O, 60%) produced by Yuntianhua Co., Ltd., Kunming, China were used as fertilizers. According to local production habits and nature of perennial rice, 360 kg ha−1 of pure N, 180 kg ha−1 of P2O5 and 360 kg ha−1 of K2O were annually applied to the paddy field. Based on the trend for normal agricultural practice, urea was applied with a ratio of 3:4:3:3:4:3 to the respective basal fertilizer: early rice tillering fertilizer: early rice panicle fertilizer: early rice bud-promoting fertilizer: late rice tillering fertilizer: late rice panicle fertilizer. At the beginning, basal fertilizer was applied. At tillering, the onset of panicle differentiation, and 20 days after heading, additional tillering, panicle and bud-promoting fertilizers were applied, respectively. Phosphate fertilizer (calcium superphosphate) was applied in a 1:1 ratio of basal fertilizer to bud-promoting fertilizer. Potash fertilizer (potassium chloride) was applied in a 1:1:1:1 ratio of basal fertilizer: panicle fertilizer: bud-promoting fertilizer: late rice panicle fertilizer. The fertilization time and amount for both annual and perennial production systems of perennial rice paddy field were the same.
Activities during the three years (2021–2023) are indicated in Table A1. Here, sowing and transplanting periods for 2021S, 2022F, 2022S, 2023F and 2023S refer only to the annual production system. At every regenerating season (2021S, 2022F, 2022S, 2023F and 2023S), the perennial production technique retained 10 cm high rice stubble during harvest. Daily field management measures for weed, pest and disease control were carried out according to the high yield management measures of perennial rice [22].
2.3. Dry Matter Nitrogen Determination
During maturity, 6 representative perennial rice plants were sampled from each replicate. Biomass was placed in an oven (Tianjin Taisite Instrument Co., Ltd., Tianjin, China, WGL-230D) at 105 °C for 30 min and stored at 75 °C. A plant sample crusher (Guangzhou Laimei Technology Co., Ltd., Guangzhou, China, FZ102) fitted with 80-mesh was used to crush and screen the dry biomass. According to Kirk [35], the ground and oven-dried dry matter was subsampled and used for the dry matter dissolution. A continuous flow analyzer (SEAL Analytical Co., Ltd., Rotherham, UK, SEAL Analytical AA3) was used to determine the level of dry matter nitrogen content.
2.4. Soil Sample Collection
In the spring of 2021 (before rice planting), a five-point sampling method was employed to assess soil fertility index as fundamentals of the entire study (Table A2). After the harvest of each season, soil samples at a depth of 0–10 cm, 10–20 cm and 20–30 cm were collected from the rows of each plot (Figure 2).

Figure 2.
Soil horizons of the experimental field with a ring knife cross-section after the late season of 2021, where (a1,a2) stand for annual and (b1,b2) for perennial rice cultivation modes.
The soil samples from each soil depth were dried, crushed with a plastic hammer, passed through 40- and 100-mesh screening and packed in plastic sealing bags. The 100-mesh soil samples were used to determine the level of total nitrogen, and the 40-mesh soil samples were used to assess available nitrogen nutrients and other key indicators such as nitrogen mineralization, ammonification intensity and urease activity.
2.5. Soil Total and Available Nitrogen Content Analysis
The H2SO4-semi-micro Kjeldahl method was applied to determine the soil total nitrogen. The protocol developed by Bremner [36] was also used to assess soil nitrogen nutrients (ammonium nitrogen and nitrate nitrogen). Both total and available nitrogen contents were determined by a continuous flow analyzer.
2.6. Analysis of Soil Organic Nitrogen Components
Acid hydrolysis method was used for the separation and characterization of both the total acid hydrolysable organic nitrogen (AHON) and non-hydrolysable nitrogen (NHN) fractions of soil organic nitrogen [37]. Here, air-dried soils were hydrolyzed with 6 mol L−1 of HCl at 110 °C for 12 h. The decomposed forms of AHON called ammonia nitrogen (AMN), amino acid nitrogen (AAN), amino sugar nitrogen (ASN) and hydrolysable unidentified nitrogen (HUN) were determined by the protocol described by Bremner [38].
2.7. Predicting Nitrogen Mineralization from Paddy Soil
Soil mineralized nitrogen in the perennial paddy field was cultured and determined. Soil nitrogen supply capacity was predicted by using the mineralization model developed by the International Rice Research Institute (IRRI).
Based on Waring and Bremner [39], soil nitrogen mineralization culture was prepared. A total of 20.0 g of 40-mesh screened air-dried soil sample was dissolved by 20.0 mL distilled water and incubated for 14 days at either 30 °C (for actual mineralization) or 40 °C (for potential mineralization). A total of 80.0 mL of 2.5 mol L−1 KCl solution was added, followed by shaking at 25 °C for 30 min and filtration. The ammonium nitrogen content of the filtrate was determined by a continuous flow analyzer. The amount of nitrogen mineralization in the paddy soil was calculated using Equation (1), and the IRRI’s paddy field soil nitrogen mineralization dynamic model (2) was employed to determine the nitrogen mineralization rate constant of soil organic nitrogen [40].
Soil mineralized nitrogen content Ya = Ya, t − Y0
- a—culture temperature (°C)
- t—soil submergence culture time (t = 14 days)
- Ya, t—yield of soil ammonium nitrogen (mg kg−1)
- Ya—net yield of ammonium nitrogen (mg kg−1) at a culture temperature “a” = 14 days
- Y0—ammonium nitrogen content in uncultured soil (mg kg−1)
- Y—actual yield of ammonium nitrogen (mg kg−1) at 30 °C after 14 days
- c—mineralization rate constant
- A—nitrogen mineralization potential (mg kg−1) at 40 °C after 14 days.
2.8. Determination of Soil Ammonification Intensity
A sample of 5.00 g air-dried soil was mixed with 2.00 mL sterilized water. A total of 1.00 mL of sterilized 0.2% peptone (20 g L−1) was added into the moistened soil and incubated at 28 °C for 7 days. After the addition of 20.0 mL of 2.00 mol L−1 KCl solution, the ammonium nitrogen content of the filtrate was quantified using a continuous flow analyzer. To identify ammonification intensity, the ammonium nitrogen content of the cultivated soil was deducted from that of the uncultured soil [41].
2.9. Assay of Urease Activity in Paddy Rice Soil
Using 0.1 mg mL−1 nitrogen standard solution, the nitrogen standard curve was established. The measured values of all samples were within the range of the established standard curve. In this study, a 10% urea solution as substrate and citrate phosphate as buffer at pH 6.7 were used. Urease activity, which is defined by the amount of ammonia produced, was determined by the Hoffmann method [42,43]. The sample reaction solution was determined using a microplate reader (Molecular Devices, Co., Ltd., San Jose, CA, USA, SpectraMax i3), with a determination wavelength of 578 nm. Here, the milligrams of NH3-N per gram of soil can be explained:
NH3-N (mg) = (Asample − Ano soil − Ano matrix) × V × (n m−1)
- Asample—soil sample concentration (mg mL−1)
- Ano soil—concentration level without soil (mg mL−1)
- Ano matrix—matter-free concentration (mg mL−1)
- V—volume (50 mL)
- n—proportion
- m—dry soil weight.
2.10. Nitrogen Dry Matter Production Efficiency and Nitrogen Recovery Efficiency of Perennial Rice
Plant system’s nitrogen absorption potential, or Nitrogen Dry Matter Production Efficiency (NDMPE), is defined as the ratio of above-ground dry matter accumulation to above-ground nitrogen accumulation per unit area and characterizes plant roots’ potential on absorbing nitrogen from the soil [44]. Mathematically:
Likewise, Nitrogen Recovery Efficiency (NRE) explained the percentage of nitrogen fertilizer input recovered in the above-ground plant biomass to the amount of fertilizer applied [45]. The computation is:
2.11. Computation of Soil Nitrogen Storage
Soil nitrogen storage is a function of total soil nitrogen content (both organic and mineral), bulk density and sampling depth [46]. i.e.,
Soil weight (kg ha−2) = Soil thickness (cm) × soil bulk density (g cm−3) × 105
Soil nitrogen storage (kg ha−1) = Soil weight (kg ha−2) × soil total nitrogen content (g kg−1) × 103
2.12. Statistical Analysis
Analysis of variance (ANOVA) for each parameter was performed using the Statistical Analysis System computer package v.20.0 (SPSS, Inc., Chicago, IL, USA). Comparison of treatment means was performed by Least Significant Difference (LSD) method. A 2-tailed t-test at p < 0.05 was also used to define statistical differences. All experimental indicators were subjected to statistical analysis using biological replicates, and figures were generated using Origin v2025 (Sys Software, Inc.).
To indicate the influence of the soil’s physicochemical properties on the nitrogen utilization system of perennial rice, Canoco v5.0 (Microcomputer Power, Ithaca, NY, USA) was used. Factors such as TN, NH4+-N, S-UE, NO3−-N, AHON, NHN, AMN, AAN, ASN, HUN, MN, N0, c and AM were standardized using Z-score and taken as environmental variables, and the response variables for redundancy analysis (RDA) of TN1 and NN1 were A, B and NDMPE.
3. Results
3.1. Above-Ground Dry Matter Nitrogen in Perennial Rice
The above-ground dry matter nitrogen content of early rice plants in annual and perennial cultivations systems was in a range from 9.23 to 10.26 g kg−1 (Table 1). During late rice, the above-ground dry matter nitrogen content had a relatively wider range (8.04 to 11.75 g kg−1). In all of the regenerative seasons, above-ground dry matter nitrogen in the annual mode was significantly higher than the perennial mode (p < 0.05). Despite the higher difference between the two modes of production, the trend in the above-ground dry matter nitrogen content of late rice revealed a gradual decrease with an increase in planting years.

Table 1.
Total nitrogen in the above-ground dry matter of perennial rice (g kg−1).
3.2. Dry Matter Nitrogen Output of Perennial Rice Field Under the Two Tillage Practices
The insignificant differences between the annual and perennial systems at the early rice, late rice and whole year of 2021 and 2023 (Figure 3) implicated the limited impact of tillage system on dry matter nitrogen output. At the second production year, dry matter nitrogen output of early rice (2022F) under annual and perennial were 159.13 kg·ha−1 and 180.51 kg·ha−1, respectively, with a significant difference (p < 0.05). However, insignificant differences were reported at late rice (2022S) and the whole year of 2023. Despite perennial system at early season (183.78 kg·ha−1) was insignificantly higher than the annual (176.58 kg·ha−1), the insignificantly higher paddy dry matter nitrogen output under the late season of annual production (179.76 kg·ha−1) compared to perennial cultivation (172.24 kg·ha−1) could compensate and create a comparable result among the two farming systems of the 3rd production year.
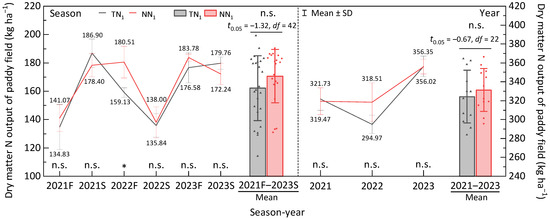
Figure 3.
Dry matter nitrogen output of perennial rice field. F—early season, S—late season, *—significant difference at p < 0.05 between TN1 and NN1 per season under 2-tailed t-test, n.s.—non-significant difference between TN1 and NN1 in a season/year. Data presented in each production season or year is an average value.
As shown in Figure 3, the average dry matter nitrogen output per season in perennial was higher by 8.42 kg ha−1 than the annual. But the difference between the two was only 5.19%, and hence, a non-significant difference existed between the annual and perennial cultivation systems. This observation revealed that dry matter nitrogen output of the paddy field was insignificantly affected by the production mode.
3.3. Nitrogen Dry Matter Production Efficiency and Nitrogen Recovery Efficiency in Perennial Rice Field
In all of the regenerative seasons, perennial rice farming technique was found to have a significantly higher (p < 0.05) plant nitrogen dry matter production efficiency than the annual with 26.06%, 4.83%, 20.38%, 6.96% and 5.62% for the 2021S, 2022F, 2022S, 2023F and 2023S seasons, respectively. Figure 4 therefore reveals that the perennial rice production system (no tillage in consecutive seasons) can positively affect the nitrogen dry matter production efficiency of the rice plant and, in turn, significantly improve the whole year’s plant nitrogen dry matter production efficiency of the paddy rice field, with 13.37%, 11.56% and 6.17% differences for the years 2021, 2022 and 2023, respectively (p < 0.05).
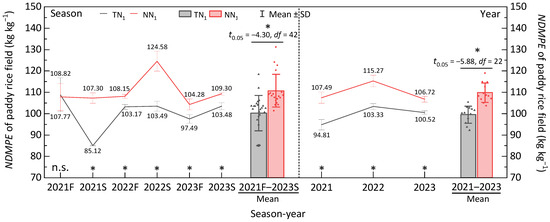
Figure 4.
Nitrogen dry matter production efficiency of perennial rice field with and without tillage. NDMPE—Nitrogen dry matter production efficiency. F—early season, S—late season, *—significant difference between TN1 and NN1 in a season/year at p < 0.05 under 2-tailed t-test, n.s.—non-significant difference between TN1 and NN1 per season. The data presented in production seasons and years are averaged.
In each of production years, nitrogen recovery efficiency of perennial rice farming was significantly higher than that of the annual system (p < 0.05). The percentage differences between the two production modes were 12.35%, 22.75% and 7.43% in the 1st (2021), 2nd (2022) and 3rd (2023) years, respectively (Table 2). Overall, no-tillage-based perennial rice cultivation in continuous cropping systems can enhance plant nitrogen recovery efficiency of perennial rice fields and improve the efficient use of nitrogen fertilizer.

Table 2.
Nitrogen recovery efficiency in perennial rice field (%).
3.4. Soil Total Nitrogen and Available Nitrogen Nutrients Change in Perennial Rice Field
In all production seasons, soil total nitrogen content under both treatment types showed a trend of decrease with an increase in soil depth (Table 3). Irrespective of plantation seasons, comparable observations between the annual and perennial farming systems were reported for the total nitrogen content of the 0–10 cm. The 10–20 cm layer of 2023F, along with the 10–20 cm and 20–30 cm soil layers of the three late rice seasons (2021S, 2022S, 2023S), showed lower soil total nitrogen content in perennial cultivation (p < 0.05).

Table 3.
Total nitrogen and available nitrogen nutrients of perennial rice field.
The three-year-based distribution pattern of ammonium nitrogen in perennial rice soil is shown in Table 3. As soil depth increased from 0 to 20 cm, the content of ammonium nitrogen at both annual and perennial conditions decreased. In the perennial rice production system, a relatively higher amount of ammonium nitrogen was available in the 0–10 cm than in the remaining two soil layers. The ammonium nitrogen content of the annual system in 20–30 cm soil layer of each season was comparable with the perennial, with a maximum difference of only 15.81% at 2023F. At the 10 to 20 cm soil layer of all of the regenerative seasons, the ammonium nitrogen content in the perennial rice production was significantly lower than that of the annual with the maximum difference of 80.66% at 2023S (p < 0.05). It is therefore revealed that no-tillage-based perennial rice cultivation tends to significantly reduce ammonium nitrogen content in the 10–20 cm soil layer but with an insignificant effect in the 20–30 cm soil layers.
Exceptionally, nitrate nitrogen content was assessed only for the 2nd and 3rd years (Table 3). When production season progressed, nitrate nitrogen content in the 0–10 cm soil layer of the perennial practice increased. Across all production seasons, nitrate nitrogen content in the 0–10 cm soil layer of the perennial paddy field was significantly higher than that in the annual (p < 0.05). In 2022S, the perennial rice production in 10–20 cm soil layer had a significantly higher nitrate nitrogen level (p < 0.05). Although nitrate nitrogen content in the 20–30 cm soil layer of perennial rice farming was significantly lower than the annual farming mode of 2022F and 2022S (p < 0.05), the deviation was normalized at both seasons of 2023.
3.5. Soil Nitrogen Storage in Perennial Rice Field
This study assessed soil nitrogen storage level at the late seasons of each production year. In the three planting years and with the exception of the annual farming of 2023S, nitrogen levels stored in both conditions declined with soil depth (Figure 5). In 0–10 cm soil layer, soil nitrogen storage of the perennial rice plantation mode was insignificantly higher than that of the annual (2021S, 2022S). In the 0–10 cm soil layer of 2023S, perennial production system possessed a significantly higher (p < 0.05) nitrogen storage than the annual production system. But in the remaining two layers of 2023S, soil nitrogen storage in the perennial mode was significantly lower (p < 0.05). Similarly, a significantly lower nitrogen storage in the perennial than annual was found in the 20–30 cm soil layer of 2022S (p < 0.05).
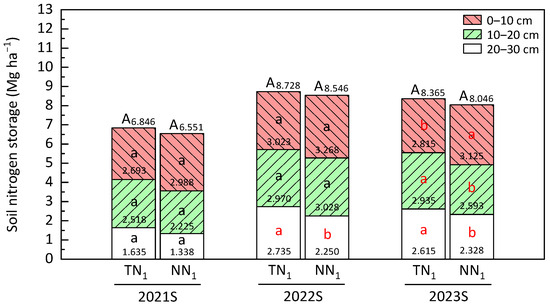
Figure 5.
Soil nitrogen storage of perennial rice fields. S—late season, different lower and uppercase letters in a season show significant differences at p < 0.05 under 2-tailed t-test. Lowercase letters designate the three soil layers, and the uppercase letter represents the entire soil layer (0–30 cm). Data presented in production seasons and soil layers are mean values (Mg ha−1). The red, light green and white bar charts represent the 0–10 cm, 10–20 cm and 20–30 cm soil layers, respectively.
In a generic term, the perennial production system did not significantly (p > 0.05) reduce soil nitrogen storage in 0–30 cm soil depth of the three years of perennial rice planting, and the differences at 2021S, 2022S and 2023S were only 4.5%, 2.13% and 3.96%, respectively.
3.6. Soil Organic Nitrogen Components in Perennial Rice Field
As soil depth increased, the content of total acid hydrolysable organic nitrogen (AHON) under both perennial and annual modes continuously decreased. In each of the regenerating seasons, AHON level at 10–20 cm and 20–30 cm soil depth of perennial farming was significantly lower than that in the annual (p < 0.05). In both seasons of the second year, AHON content at 0–10 cm soil layer of annual system was significantly higher (p < 0.05), with percentage differences of 8.46% for 2022F and 8.71% for 2022S. At the third year (both 2023F and 2023S), non-significant differences between the two farming techniques were revealed for AHON at 0–10 cm soil layer. From the 1st to the 3rd year, AHON amount at the 0–10 cm soil depth of the perennial rice farming has generally showed an increasing trend (Figure 6).
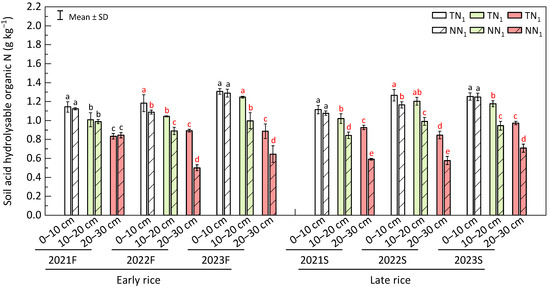
Figure 6.
Soil acid hydrolysable organic nitrogen content in paddy rice field. F—early season, S—late season, different lowercase letters in a season represent significant differences at p < 0.05 (LSD). The white, light green and red bar charts represent soil layers at 0–10 cm, 10–20 cm and 20–30 cm, respectively.
Unlike AHON, there was no significant difference in the content of soil non-hydrolysable nitrogen (NHN) at 0–10 cm soil layer of the perennial and annual cultivation methods (Table 4). Except at 2023F, where NHN level at 10–20 cm soil depth of annual production was significantly higher (p < 0.05), all the remaining seasons showed an insignificant difference. During 2022S and 2023S, the 20–30 cm soil depth at the annual system had a significantly higher (p < 0.05) NHN amount than perennial system, and the differences were 25.42% and 17.06% in 2022S and 2023S, respectively. However, at the two early seasons (2022F and 2023F), NHN level was significantly higher (p < 0.05) under the perennial rice cultivation mode by 53.61% and 69.93%, respectively. Generally, the level of NHN was reduced down in the 0–30 cm soil depth.

Table 4.
Content of soil organic nitrogen fractions in tillage and no-tillage perennial rice field (mg kg−1).
Without 2023F, where a significantly higher (p < 0.05) content of acid-hydrolyzed ammonia nitrogen (AMN) at annual cultivation mode was observed, all other seasons at 0–10 cm soil layer showed a statistically insignificant difference between the two cultivation systems (Table 4). Except for the insignificant difference reported between the two farming techniques of 2022S, no-tillage has reduced the AMN level in the 10–20 cm soil layer (p < 0.05). In all regenerating seasons, AMN was also found to be significantly lower (p < 0.05) at the perennial practice of the 20–30 cm soil depth, and the maximum difference was 83.33% at 2022F. At the 0–10 cm soil depth, amino acid nitrogen (AAN) content at 2023F and both seasons of 2022 was significantly higher in the annual system (p < 0.05). At all regenerating seasons of the 10–20 cm and 20–30 cm soil layers, the perennial rice production system had a significantly lower (p < 0.05) AAN level (Table 4). Without 2022S and 2023S, the 0–10 cm soil layer of the other regenerating seasons showed a significantly higher (p < 0.05) Amino Sugar Nitrogen (ASN) content at the annual practice. Likewise, no-tillage-based perennial farming at all regenerating seasons except 2021S of the 10–20 cm and 2023S of the 20–30 cm soil depth had a significantly lower ASN content (p < 0.05).
Except for 2022S, no-tillage-based perennial rice production has significantly increased (p < 0.05) the amount of hydrolysable unidentified nitrogen (HUN) at the 0–10 cm soil layer, with a maximum difference of 34.67% at 2023F. Perennial cultivation has also showed a significantly higher HUN content at 2022F of the 10–20 cm soil layer (p < 0.05). On the contrary, a significantly higher (p < 0.05) HUN by the annual system was reported at 2022S and 2023S of the 10–20 cm soil layer and at 2022F, 2021S and 2023S seasons of the 20–30 cm soil layer. In a general sense, the level of all components of AHON (AMN, AAN, ASN and HUN) were reduced with the increase in soil depth.
3.7. Soil Assessment of Nitrogen Mineralization in Perennial Rice Field
As shown in Figure 7, mineralization nitrogen declined with soil depth in both the annual and perennial systems. Overall, the perennial farming mode has reduced the soil’s actual nitrogen mineralization. Specifically, the 10–20 cm and 20–30 cm soil layers of the 2nd (2022) and 3rd (2023) years showed a significantly lower soil nitrogen mineralization under the perennial rice production scheme (p < 0.05). During 2022F and 2021S, the 0–10 cm soil layer’s nitrogen mineralization in perennial system was significantly lower (p < 0.05). However, as the seasons progressed (at 2023F, 2022S and 2023S), the 0–10 cm soil layer’s nitrogen mineralization difference between the two production modes became insignificant, with only 6.02% at 2023F, 10.89% at 2022S and 10.54% during 2023S.
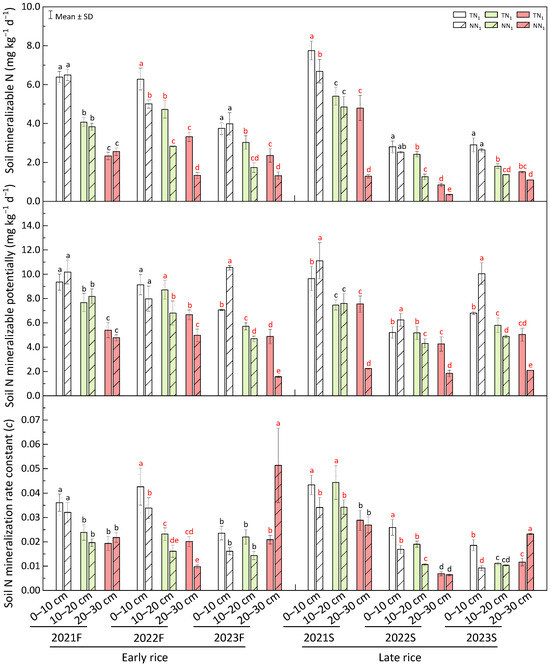
Figure 7.
Soil assessment for nitrogen mineralization in perennial rice field. F—early season, S—late season, different lowercase letters in a season represent significant differences at p < 0.05 (LSD). The white, light green and red bar charts represent soil layers at 0–10 cm, 10–20 cm and 20–30 cm, respectively.
From the three years of perennial rice plantation, spatial and temporal distribution of nitrogen mineralization potential in paddy soil is explained in Figure 7. As soil depth increased from 0 cm to 30 cm, nitrogen mineralization potential decreased in both farming methods. Excluding the non-significant difference at 0–10 cm soil layer of 2022F, nitrogen mineralization potential of this soil layer in the rest regenerating seasons was significantly higher (p < 0.05) under the perennial than that of the annual, with 49.12%, 15.10%, 20.10% and 47.86% differences for 2023F, 2021S, 2022S and 2023S, respectively. At the 2nd and 3rd years (2022, 2023), nitrogen mineralization potential of the 10–20 cm soil layer was significantly lower (p < 0.05) under perennial cultivation mode, and the maximum difference 28.18% was found at 2022F. Likewise, nitrogen mineralization potential at 20–30 cm soil layer in all regeneration seasons of the annual system was significantly higher (p < 0.05), with the maximum difference of 237.86% at 2021S. It can be seen that the perennial rice farming can increase the trend of nitrogen mineralization potential of the 0–10 cm soil layer of perennial rice field and reduce the nitrogen mineralization potential at 10–30 cm soil layer.
Excluding 2023F, all regenerating seasons at 0–10 cm soil layer had a significantly lower (p < 0.05) nitrogen mineralization rate constant (c) under perennial rice production system, with the maximum difference of 101.47% at 2023S. The 10–20 cm soil layer during 2022F, 2021S and 2022S showed significantly higher (p < 0.05) soil nitrogen mineralization rate constants under the annual system with a respective difference of 43.45%, 29.67% and 78.45%. Interestingly, nitrogen mineralization rate constant of perennial rice farming at the last two experimental seasons (2023F, 2023S) of the 20–30 cm soil layer was significantly higher (p < 0.05), and the differences were 146.70% for 2023F and 99.98% for 2023S. These results indicate that no-tillage-based perennial rice farming practice can reduce the soil nitrogen mineralization rate constant at the 0–10 cm layer with a tendency to increase the rate constant in the 20–30 cm soil layer.
3.8. Soil Ammonification Intensity in Perennial Rice Field
Ammonification intensity under both perennial and annual conditions decreased with the increase in soil depth (Figure 8). In both seasons of the 3rd year, ammonification intensity at 0–10 cm soil layer was significantly higher (p < 0.05) in the perennial mode, with the differences of 31.86% and 37.36% at 2023F and 2023S, respectively. However, in the 10–20 cm soil layer, there was no significantly different ammonification intensity between the two. In all of the regeneration seasons, ammonification intensity of the 20–30 cm soil layer of no-tillage perennial rice farming was significantly lower (p < 0.05), with 66.11%, 27.70%, 18.41%, 36.20% and 34.71% differences during 2022F, 2023F, 2021S, 2022S and 2023S, respectively. Hence, no-tillage-based perennial production can generally increase ammonification intensity in 0–10 cm soil layer and reduce in the 20–30 cm soil layer of perennial rice field.
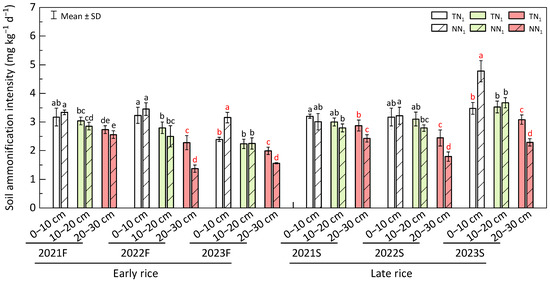
Figure 8.
Soil ammonification intensity of perennial rice field. F—early season, S—late season, different lowercase letters in a season represent significant differences at p < 0.05 (LSD). The white, light green and red bar charts represent the 0–10 cm, 10–20 cm and 20–30 cm soil layers, respectively.
3.9. Soil Urease Activity Analysis in Perennial Rice Field
In both years of 2022 and 2023 (Figure 9), urease activity in the 0–10 cm soil layer of perennial mode was found to be significantly higher (p < 0.05), depicting a respective differences of 18.49%, 62.22%, 110.85% and 10.74% at 2022F, 2023F, 2022S and 2023S. During 2022S and 2023F, urease activity at 10–20 cm soil layer of the perennial production scheme was also significantly higher (p < 0.05), and 56.68% and 31.58% differences were reported for 2022S and 2023F, respectively. At 2021S and 2022F, urease activity in the 20–30 cm soil layer of the annual farming practice was significantly higher (p < 0.05), and the differences were 30.70% at 2021S and 19.16% at 2022F. However, no-tillage-based perennial mode showed a significantly higher urease activity in the last season (2023S), and the difference was 30.24%. In conclusion, no-tillage relaying perennial farming increased urease activity in the 0–10 cm soil layer.
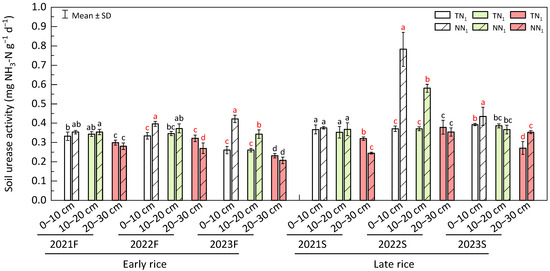
Figure 9.
Soil urease activity of perennial rice field. F—early season, S—late season, different lowercase letters in a season represent significant differences at p < 0.05 (LSD). The white, light green and red bar charts represent the respective 0–10 cm, 10–20 cm and 20–30 cm soil layers.
3.10. RDA Analysis Among Nitrogen in Perennial Rice and Soil Physicochemical Factors
The redundant analysis has shown the relationship between nitrogen utilization potential of perennial rice plants and soil physicochemical properties (Figure 10). In each soil layer (0–10 cm, 10–20 cm and 20–30 cm) of the tillage-based annual farming, the first two axes explained more than 90.8% of the correlation between the response and environmental variables. Under each soil layer (0–10 cm, 10–20 cm and 20–30 cm) of the perennial method, a relatively higher (98.4%) of the correlation was accounted by the first two axes. The total canonical eigenvalues of each soil layer of both annual and perennial production modes were greater than 0.90 (Table A6). Likewise, the cumulative interpretation of response to environmental variables was greater than 90%. As shown in Figure 10, NDMPE was negatively correlated with both the above-ground dry matter nitrogen of perennial rice (A) and dry matter nitrogen output of the paddy rice field (B). At all the three soil layers of both treatments, Soil Urease (S-UE) was positively correlated with NDMPE. In the 0–10 cm, 10–20 cm and 20–30 cm soil layers, AM of the annual mode was found to be the main positively correlated factor to NDMPE. ASN and NDMPE were positively correlated at all the three soil layers of the perennial farming method. AAN and NDMPE at 0–10 cm soil layers of the perennial production system were positively correlated. AAN at 10–20 cm soil layer of the perennial mode was also positively correlated with NDMPE. However, at the 10–20 cm soil layer, the annual system showed a negative correlation between AAN and NDMPE (Figure 10). At the 20–30 cm soil layer of both conditions, AAN was negatively correlated with NDMPE.
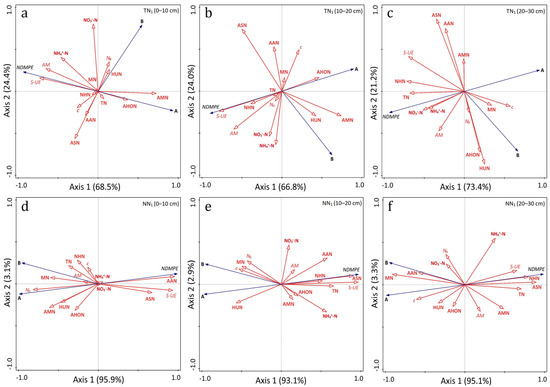
Figure 10.
Two-dimensional diagram of RDA analysis of Nitrogen Dry Matter Production Efficiency of perennial rice with stocks of soil physicochemical factors. (a) (TN1, 0–10 cm soil layer), (b) (TN1, 10–20 cm soil layer), (c) (TN1, 20–30 cm soil layer), (d) (NN1, 0–10 cm soil layer), (e) (NN1, 10–20 cm soil layer), (f) (NN1, 20–30 cm soil layer). A (above-ground dry matter nitrogen in perennial rice), B (dry matter nitrogen output of paddy rice field), MN (soil actual nitrogen mineralization), N0 (soil nitrogen mineralization potential), c (soil nitrogen mineralization rate constant), AM (ammonification intensity).
4. Discussion
4.1. Perennial Rice Farming on the Accumulation and Efficient Use of Nitrogen
Chemical composition and nutrient factors in the soil can directly determine soil fertility and affect the growth and development stages of crops. Tillage-based annual rice production can inevitably lead to a series of changes in the chemical properties of paddy soil. Significant variations in the soil’s texture and chemical properties could be derived from no-tillage farming techniques [47]. The deep roots of perennial crops can effectively absorb nutrients in soil, regulate soil chemical properties and maintain soil fertility [20,21]. Nitrogen is an important factor influencing the dry matter content of rice. Hence, nitrogen fertilizer is one of the most applicable fertilizers in rice production. But the usual nitrogen utilization rate in the rice fields of China is only 20–40% [48]. For this reason, optimizing fertilizer application and improving nitrogen use efficiency of rice have become the main research focuses.
Despite the plant above-ground dry matter nitrogen content of no-tillage-driven perennial rice farming across all regenerative seasons was significantly lower than that of the annual rice farming (p < 0.05) (Table 1), average dry matter nitrogen output per year in the perennial system was insignificantly higher (p < 0.05) (Figure 3). This observation emphasized the role of no-tillage-based perennial farming on improving nitrogen dry matter production efficiency of regenerative seasons of perennial rice field. With regard to the pooled three-year field data, the significantly (p < 0.05) higher above-ground dry matter nitrogen content of the annual than the perennial may be attributed to the naturally lower above-ground dry matter weight of perennial rice in the regenerating periods (Table 1, Figure A2). Nitrogen dry matter production efficiency of a rice field is the ratio of dry matter output per unit area of soil to dry matter nitrogen uptake. Although plant nitrogen content of the perennial system was lower than that of the annual (p < 0.05) (Table 1), a significantly higher nitrogen dry matter production efficiency was observed (Figure 4). Hence, perennial rice farming with no-tillage in successive seasons enhances nitrogen retention potential and significantly improves the plant’s nitrogen dry matter production efficiency (p < 0.05). As shown in (Table 2), the efficiency of nitrogen recovery was improved by perennial production. Hence, the significantly higher dry matter nitrogen production efficiency and nitrogen utilization rate of the perennial rice cultivation system across the whole experimental years was attributed to the positive impact of no-tillage perennial rice production on enhancing N absorption and fertilizer utilization [49].
4.2. Impact of No-Tillage-Based Perennial Rice Farming on Nitrogen Level
Insignificant difference between the two cultivation methods of the 0–10 cm soil layer indicated a negligible effect of tillage mode on the total nitrogen content (Table 3). However, perennial rice farming system was found to reduce the total nitrogen content of the 10–30 cm depth of paddy soil. Nitrogen level stored in both annual and perennial conditions was decreased from the 0–10 cm to the 20–30 cm of the paddy field (Figure 5). The level of nitrogen storage of perennial rice farming in the 0–10 cm soil layer showed an increasing trend from 2021S to 2023S. This is mainly because of the higher bulk density of the 0–10 cm soil layer under perennial than annual production methods (Table A5). During 2023S, nitrogen storage of the 0–10 cm soil layer of the perennial practice was higher (p < 0.05). It is therefore indicative that perennial rice cultivation across years is conducive for increasing nitrogen storage of the 0–10 cm soil layer of perennial rice field.
With the increase in planting years, perennial rice farming has a tendency to reduce ammonium nitrogen content at the 0–10 cm soil layer (Table 3). A reduced level of ammonium nitrogen in the topsoil of no-tillage production was also reported by Zhang et al. [50]. In a five-year continuous experiment with perennial intermediate wheatgrass, it was found that without fertilization, the nitrogen mineralization in the 0–100 cm cultivated soil layer decreased due to no-tillage practices. However, the authors did not provide a detailed report on different soil layers. According to Nyfeler et al., level of nitrate leaching after tillage is the main driving force on regulating the level of nitrate nitrogen at the surface [51]. Similarly, this study observed a reduction in nitrogen mineralization in the 10–30 cm soil layer [52]. Likewise, Obour et al. [53] indicated variability of ammonium nitrogen only among sampling depth, and the greatest concentration was reported at the topsoil depth. In this study, the perennial production method did not significantly affect the ammonium nitrogen content in the 20–30 cm soil layer. However, in all regeneration seasons, ammonium nitrogen content of perennial rice farming in the 10–20 cm soil layer was significantly lower than that of the annual system (p < 0.05). According to other related studies, inorganic nitrogen content of soil at the topsoil layer of no-tillage-based perennial farm is lower than that in the annual field. This is mainly associated with human plowing. It is further speculated that low level of residual ammonium nitrogen in 0–10 cm soil layer of perennial production mode could be linked to the release of inorganic nitrogen from urea mineralization and its excessive absorption by plants for attaining larger biomass (Table A4) and dry matter nitrogen output.
Nitrification is strongly inhibited in flooded paddy fields, so the concentration of ammonium nitrogen in soil solution is higher than that of nitrate nitrogen of a perennial rice field. Due to natural drying of rice fields, soil ventilation conditions are promoted, NH4+ gets released by nitrogen fertilizer, and soil organic nitrogen can easily be oxidized to NO3− through mineralization and finally releases more NO3−. According to Matthews et al. [54], plowing a farmland promotes NO3− leaching. Some other studies also reported that reducing the number of tillage in farmland can increase the number and activity of earthworms in soil, enlarge soil pore size and fasten leaching of nitrate nitrogen [55]. According to Huang et al. [56], no-tillage paddy field system provides higher nitrate nitrogen content at surface soil and enhances its leaching. In this study, nitrate nitrogen content in the surface soil under perennial rice production mode showed an increasing trend across production season. This assessment further proved that no-tillage of perennial rice field for several successive seasons is an efficient strategy to promote mineralization of soil organic nitrogen and enhance nitrate nitrogen content in a topsoil.
The extensive root systems of perennial crops enabled the continuous uptake of available nitrogen [57,58]. No-tillage practice of perennial rice has further contributed to the reduction in nitrate leaching. Extensive root systems of perennial crops along with the proximate microbial community influence important nutrient cycling functions. Perennial grains with deep root systems and no-tillage practice enhance soil microbial biodiversity and activity. Rhizosphere processes mediate nitrification and significantly influence nitrate nitrogen levels at soil surface. For instance, roots of a perennial wheat line OK72 can reduce nitrate leaching, and its rhizosphere bacterial community can provide nutrient availability and plant growth-promoting traits [59]. For this reason, the significantly higher nitrate nitrogen at 0–10 cm soil layer of perennial rice production mode is a function of no tillage, associated rhizosphere microbes and long root system of the perennial rice. However, a study by Zulu et al. [60], prioritized no-tillage as a main contributor for the higher level of nitrate nitrogen.
Larger root systems of perennials can form macropores and improve soil aeration for removing ammonium nitrogen and increasing nitrate nitrogen [61]. This denotes that the larger root system of the perennial rice has great potential to enhance nitrification at the surface soil in the expense of ammonium nitrogen at 10–20 cm soil layer. Along with ammonia volatilization [57], the unique mode of nutrient allocation for regeneration by perennial rice plants assimilated ammonium nitrogen [62], and thus, reduced its level at 10–20 cm soil layer.
4.3. Characterization of Soil Organic Nitrogen Components in No-Tillage Perennial Rice Filed
As one of the largest active nitrogen reservoirs, soil plays a critical role in the global nitrogen cycle [63]. Soil nitrogen is one of the necessary nutrients for regulating crops’ growth, development and yield [64,65,66,67]. Soil organic and inorganic nitrogen are the two major constituents of nitrogen pool. Soil inorganic nitrogen pools such as ammonium and nitrate can be directly utilized by plants [68]. However, over 90% of the total nitrogen exists as organic nitrogen form [69]. Soil organic nitrogen components are important raw materials for soil N mineralization and supply [70,71]. Efficient soil nitrogen mineralization from organic to inorganic nitrogen pools is therefore fundamental for optimal plant growth [72]. Soil organic nitrogen turnover further regulates soil nitrogen storage and availability [73]. Vegetation converts inorganic nitrogen into soil organic nitrogen and increases soil nitrogen inputs through root secretions, induced microbial growth and enhanced nitrogen fixation rate [74,75]. Intensive agricultural systems have caused soil erosion and infertility, destroyed vegetation, threatened ecological services and depleted soil nitrogen pools [76]. According to Huang et al. [72], no-tillage-based perennial farming is a beneficial approach for augmenting soil nitrogen supply capacity through increasing soil total nitrogen, active nitrogen, inorganic nitrogen and organic nitrogen content.
No-tillage-driven perennial cultivation practice may temporarily increase nitrogen immobilization to reduced plant-available nitrogen but later on it increases soil labile nitrogen pool for accessing available nitrogen to crops at the upper soil layers [77,78]. The level of AHON at the 0–10 cm soil depth of perennial farming was improved from the first to the third year (Figure 6). This observation could also be a response to the change in the management practice of perennial rice farming [72]. NHN is refractory to acid hydrolysis and release of available nitrogen [79]. At 0–10 cm soil layers of this study, NHN content remained the same at both annual and perennial conditions. The similar level of NHN at both production modes can be directly associated with the gradual weathering and microbial actions that can slightly decompose this complex and unavailable nitrogen component into soluble organic nitrogen types [80].
AMN and AAN are the two main sources of easily mineralized nitrogen in a soil [80,81]. Without 2023F, AMN in all other seasons of 0–10 cm soil layer showed a statistically insignificant difference between the annual and perennial rice production systems (Table 4). Here, no-tillage in the perennial farming would inevitably increase in the soil hydrolysable ammonia nitrogen [82]. However, soil disturbance arose from the annual farming practice promotes further production of ammonia nitrogen in the soil and compensated net content of hydrolysable ammonia nitrogen [83]. According to Huang et al. [72], no-tillage in the 0–5 cm and 5–10 cm soil layers increased AAN by 21.48% and 22.95%, respectively. But the perennial rice production at 0–10 cm soil depth was significantly lower during 2023F and both seasons of 2022 (Table 4). Additionally, in all seasons of the 10–20 cm and 20–30 cm soil layers, the perennial system showed a significantly lower AAN level (Table 4). This can be linked to microbial humification of the soil’s AAN [84].
Amino sugars are largely derived from the turnover of microbial residues in the soil. ASN therefore reflects the amount of dead microbes. According to Stevenson [37], implementing tillage in an annual farming technique cannot significantly affect its level. A study by Huang et al. [72] revealed a lower content of ASN in 10–30 cm soil layer in no-tillage. Likewise, most seasons of all of the three soil layers showed a significantly higher ASN at the annual production system (Table 4). HUN is a major contributor (25.92–41.19%) of the total soil nitrogen [79]. HUN in the 0–5 cm soil layer of no-tillage was significantly higher by 30.87% [72]. Except in 2022S, the perennial rice cultivation mode has significantly increased the amount of HUN in the 0–10 cm soil layer. For this reason, the accumulated HUN in the no-tillage perennial rice production system is the stock for the mineralized decomposition to utilizable nitrogen forms.
4.4. No-Tillage-Based Perennial Rice Farming on Regulating Nitrogen Mineralization
Before it can be absorbed and utilized by plants, organic nitrogen in a soil needs to be transformed into inorganic nitrogen through mineralization [85]. To some extent, mineralization of organic nitrogen defines the soil’s nitrogen supply capacity. Soil nitrogen conversion process is a biochemical enzymatic reaction facilitated by microbes [86]. The amount of nitrogen that plants can effectively absorb depends on the rate of nitrogen mineralization [87]. Tillage practices can change soil properties and affect the potential, amount and rate of soil organic nitrogen mineralization [47,50].
According to Xue et al. [88], organic nitrogen mineralization at 0–5 cm soil layer of no-tillage paddy was significantly higher than in the tilled paddy. However, soil nitrogen mineralization at deeper soil layers was lower. Like the report from EL-Haris et al. [89], a higher actual nitrogen mineralization on majority of the production seasons of this study was observed in the annual mode of 0–10 cm soil layer. In particular, actual nitrogen mineralization of the 0–10 cm soil layer at 2021S and 2022F was significantly higher in the annual than that of the perennial system.
Soil nitrogen mineralization potential is a parameter explaining nitrogen supply capacity of a soil. At 0–10 cm soil layer under 2021S, 2022S, 2023F and 2023S, nitrogen mineralization potential of the perennial system was significantly higher. Similarly, Mondal and Chakraborty [90] showed that the soil nitrogen mineralization potential in surface layer of no-tillage paddy was significantly higher than that in the tilled paddy. For both early and late rice, soil nitrogen mineralization potential of the 10–20 cm (except in 2021S) and 20–30 cm soil layers in all regenerating seasons under perennial farming was lower than that under the annual. This observation is alike with the soil nitrogen mineralization potential assessment at 10–15 cm soil depth [91].
The amount of organic nitrogen that plants can effectively absorb depends on the rate of nitrogen mineralization [87]. The nitrogen mineralization rate of soil is affected by the nitrogen mineralization potential and the nitrogen mineralization rate constant (c) of soil [40]. This three-year intensive study showed that no-tillage-based perennial rice farming method has decreased the soil nitrogen mineralization rate constant at the 0–10 cm soil layer. During the early and late seasons of 2023, nitrogen mineralization rate constant at 20–30 cm soil layer under perennial rice production mode was found to be higher than that in the annual farming system. In the course of time, perennial production model can change the 20–30 cm soil layer properties of paddy field and raise the rate of nitrogen mineralization.
4.5. Perennial Rice Cultivation as a Factor on Determining Ammonification Intensity
Size of soil organic nitrogen decomposition and transformation is reflected not only by the number of nitrogen-transforming bacteria but also in the intensity of nitrogen transformation. Intensity of ammonification is governed by organic nitrogen content and diverse environmental factors. Different studies suggested that limited or absence of tillage could improve microbial activity and raise ammonification intensity of the topsoil [90]. Thomas et al. [92] also pointed out that no tillage can significantly enhance the ammonification intensity of the topsoil in paddy field. Like nitrogen mineralization potential, no-tillage perennial rice cultivation enhances ammonification intensity at 0–10 cm soil layer with a significantly higher ammonification intensity in both early and late rice seasons of 2023 (Figure 8). It is therefore indicative that no-tillage-based perennial rice farming could enhance the decomposition and transformation ability of organic nitrogen at 0–10 cm soil layer of perennial rice field. This result is also similar to previous studies by Canisares et al. [32] and Schomberg et al. [91]. But the slight inconsistency among the reports would mainly be associated with variability in the levels of soil sampling, soil types and the no-tillage systems employed during the studies. However, the evidence is adequate enough to dictate that perennial rice production mode is vital to improve ammonification intensity of the surface soil.
4.6. Effects of No-Tillage-Driven Perennial Rice Farming on Urease Activity
Soil urease can enzymolize peptide bonds in organic molecules and directly take part in the transformation of nitrogen-containing organic matter of a soil. Hence, action of urease reflects both fertility and biological activity of a soil. Tillage affects action of urease and in turn influences soil moisture, aeration and temperature [93]. In the 2nd and 3rd years of plantation, urease activity of the 0–10 cm soil layer in the perennial rice field rice was significantly higher than that in the annual rice field (Figure 9). Wu et al. [71] had the same finding where no tillage improved urease activity in the paddy field soil. Roscoe et al. [94] also reflected a similar view where urease activity in the topsoil of no-tillage paddy field was higher than that in the tilled paddy condition. Increment of urease activity across planting years at 0–10 cm soil layer has implicated the potential of no-tillage-based perennial rice production on decomposing and transforming soil substances, enriching nitrogen nutrients of a soil and enhancing the crop’s dry matter biomass.
4.7. RDA Analysis of Nitrogen Dry Matter Production Efficiency of Perennial Rice Versus Soil Physicochemical Properties
As NDMPE was mathematically derived from the above-ground dry matter accumulation of perennial rice (A) and dry matter nitrogen output of rice field (B), the 2-dimensional RDA diagram showed negative correlation of NDMPE with both factors (Figure 10). Regardless of the rice cultivation pattern, S-UE at all the three soil layers had a positive correlation with NDMPE and a negative relationship with both A and B. This indicates that soil S-UE is an important factor affecting NDMPE. At all the three soil layers, ASN of the perennial mode was found to be a key positively correlated factor to NDMPE. AAN at both 0–10 cm and 10–20 cm soil layers of the perennial system was positively correlated with NDMPE. But at 20–30 cm soil layers, a negative correlation was observed between them (Figure 10). An increased plant dry matter observed at the perennial farming method (Figure A2) can thus be a function of ASN and AAN. In this study, N0 (soil nitrogen mineralization potential) under the perennial treatment was negatively correlated with NDMPE. Huang et al. [72] also reported that soil organic nitrogen mineralization under no-till plays an important role on influencing the dry matter nitrogen output of crops.
5. Conclusions
This study reveals the enriched nitrogen use efficiency and soil fertility potential of perennial rice farming for sustainable rice cultivation under fragile agroecosystems. On a yearly basis, no-tillage-based perennial rice farming can significantly improve both Nitrogen Dry Matter Production Efficiency and Nitrogen Recovery Efficiency. The enhanced nitrogen storage at the 0–10 cm soil layer of no-till perennial rice field can ultimately boost biomass production. Higher nitrate nitrogen accumulated at the 0–10 cm soil layer of the no-till-based perennial rice farm is also conducive to save nitrogen fertilizer through optimizing its rate of application.
Moreover, the 0–10 cm soil layer of no-till-based perennial rice cultivation possessed an increasing soil nitrogen mineralization potential, ammonification intensity and urease activity. At a soil depth of 0–20 cm, both amino acid nitrogen and amino sugar nitrogen were highly correlated with the Nitrogen Dry Matter Production Efficiency of the perennial rice. It is therefore recommended that a better Nitrogen Dry Matter Production Efficiency can be obtained by enhancing the levels of these two soil organic nitrogen components. Overall, this study systematically investigated nitrogen transformation and distribution patterns at different soil layers of perennial rice growing seasons and provided scientific basis for fertilization rating and promotion of green and sustainable perennial rice production.
6. Recommendations
As demonstrated in this study, perennial rice farming possessed promising aspects in improving the overall nitrogen level of soil. However, it was restricted to a single experimental site with only three years of testing. A multiple-location trial for prolonged period of time is therefore recommended to generate a reliable figure of perennial rice farming on ensuring sustainable productivity through maintaining soil fertility.
Author Contributions
Conceptualization, F.H. and X.Z.; methodology, X.Z. and G.H.; software, X.Z.; validation, X.Z., G.M. and Y.Z.; formal analysis, X.Z., Y.Z. and F.H.; investigation, X.Z.; re-sources, J.Z. and S.Z.; data curation, X.Z.; writing—original draft preparation, X.Z. and G.M.; writing—review and editing, Y.Z., X.Z. and G.M.; visualization, X.Z.; supervision, F.H. funding acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program Project (2023YFD2302000 to FYH), National Natural Science Foundation of China (32341025 to FYH) and New Cornerstone Fellows Program (NCI202341 to FYH) provided financial support for this research.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Time frame for the different rice cultivation phases held at perennial rice field.
Table A1.
Time frame for the different rice cultivation phases held at perennial rice field.
| Season | Activity | Planting Year | ||
|---|---|---|---|---|
| 2021 | 2022 | 2023 | ||
| Early rice | Sowing | 11 January | 9 January | 14 January |
| Transplanting | 26 February | 25 February | 22 February | |
| Harvesting | 14 July | 15 July | 20 July | |
| Late rice | Sowing | 23 June | 19 June | 2 July |
| Transplanting | 28 July | 21 July | 1 August | |
| Harvesting | 16 November | 15 November | 4 December | |

Table A2.
Soil content analysis prior to inception of the long-term experiment at perennial rice field (in 2021).
Table A2.
Soil content analysis prior to inception of the long-term experiment at perennial rice field (in 2021).
| Layer | TC | TN | TP | TK | SOC | AN | AP | AK | EC(5:1) | pHW(5:1) |
|---|---|---|---|---|---|---|---|---|---|---|
| (g·kg−1) | (g·kg−1) | (g·kg−1) | (g·kg−1) | (g·kg−1) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | (μS·cm−1) | ||
| 0–10 cm | 18.74 a | 2.03 a | 0.71 a | 15.37 c | 12.34 a | 24.21 a | 9.83 a | 201.1 a | 99.4 a | 6.05 c |
| 10–20 cm | 16.70 b | 1.64 b | 0.70 a | 16.64 c | 9.00 b | 14.81 ab | 9.51 a | 159.8 b | 84.8 b | 6.04 c |
| 20–30 cm | 12.82 c | 1.17 c | 0.56 b | 19.00 b | 5.93 c | 11.18 bc | 5.02 b | 146.5 b | 72.7 c | 6.45 b |
| 30–40 cm | 9.73 d | 0.75 d | 0.41 c | 22.85 a | 3.53 d | 6.08 c | 2.14 c | 100.7 c | 69.9 c | 6.83 a |
Different lowercase letters within a column designate significant differences at p < 0.05 (LSD). TC: total carbon, TN: total nitrogen, TP: total phosphorus, TK: total potassium, SOC: Soil organic carbon, AN: available nitrogen, AP: available phosphorus, AK: available potassium, EC(5:1) and pHW(5:1) used water to test and the ratio of water:soil = 5:1.

Table A3.
Dry matter nitrogen output of perennial rice fields (TN0) (kg·ha−1).
Table A3.
Dry matter nitrogen output of perennial rice fields (TN0) (kg·ha−1).
| Planting Duration | Treatment | Early Rice | Late Rice | Whole Year |
|---|---|---|---|---|
| 2021 | TN0 | 80.52 bc | 100.29 b | 180.81 b |
| NN0 | 80.04 c | 54.07 e | 134.11 c | |
| 2022 | TN0 | 78.42 c | 66.37 d | 78.418 d |
| NN0 | 44.88 d | 41.53 f | 44.88 e | |
| 2023 | TN0 | 87.86 b | 111.74 a | 199.61 a |
| NN0 | 96.71 a | 75.84 c | 172.55 b |
Different lowercase letters within a column represent significant differences at p < 0.05 (LSD).

Table A4.
Dry matter output in perennial rice field (Mg·ha−1).
Table A4.
Dry matter output in perennial rice field (Mg·ha−1).
| Planting Duration | Treatment | Early Rice | Late Rice | Whole Year |
|---|---|---|---|---|
| 2021 | TN1 | 14.58 c | 15.91 b | 30.49 d |
| NN1 | 15.17 c | 19.5 a | 34.31 c | |
| 2022 | TN1 | 16.42 b | 14.06 c | 30.48 d |
| NN1 | 19.52 a | 17.18 b | 36.69 ab | |
| 2023 | TN1 | 17.21 b | 18.61 a | 35.81 bc |
| NN1 | 19.16 a | 18.82 a | 37.99 a |
Different lowercase letters within a column designate significant differences at p < 0.05 (LSD).

Table A5.
Soil bulk density of perennial rice field (g cm−3).
Table A5.
Soil bulk density of perennial rice field (g cm−3).
| Layer | Treatment | Season | ||
|---|---|---|---|---|
| 2021S | 2022S | 2023S | ||
| 0–10 cm | TN1 | 1.22 c | 1.31 d | 1.25 e |
| NN1 | 1.36 b | 1.44 bc | 1.36 cd | |
| 10–20 cm | TN1 | 1.30 bc | 1.33 cd | 1.32 d |
| NN1 | 1.31 bc | 1.53 ab | 1.42 c | |
| 20–30 cm | TN1 | 1.50 a | 1.48 b | 1.51 b |
| NN1 | 1.52 a | 1.64 a | 1.71 a | |
Different lowercase letters within a column represent significant differences at p < 0.05 (LSD).

Table A6.
Eigenvalues and cumulative interpretation of RDA sequence of response-environment variable.
Table A6.
Eigenvalues and cumulative interpretation of RDA sequence of response-environment variable.
| Treatment | Layer | Axial Sequence | Eigenvalue of Response Variable | Correlation of Response and Environment Variable Factors | Cumulative Interpretation of Response Variable (%) | Cumulative Interpretation of Response-Environment Variable Factors (%) | Total Canonical Eigenvalues |
|---|---|---|---|---|---|---|---|
| TN1 | 0–10 cm | Axis 1 | 0.685 | 0.957 | 68.5 | 73.7 | 0.929 |
| Axis 2 | 0.244 | 0.986 | 92.9 | 100.0 | |||
| Axis 3 | 0.001 | 0.885 | 93.0 | 100.0 | |||
| Axis 4 | 0.067 | 0.000 | 99.7 | 100.0 | |||
| 10–20 cm | Axis 1 | 0.668 | 0.945 | 66.8 | 73.6 | 0.908 | |
| Axis 2 | 0.240 | 0.977 | 90.8 | 100.0 | |||
| Axis 3 | 0.001 | 0.764 | 90.9 | 100.0 | |||
| Axis 4 | 0.085 | 0.000 | 99.4 | 100.0 | |||
| 20–30 cm | Axis 1 | 0.734 | 0.991 | 73.4 | 77.6 | 0.946 | |
| Axis 2 | 0.212 | 0.918 | 94.6 | 100.0 | |||
| Axis 3 | 0.001 | 0.828 | 94.7 | 100.0 | |||
| Axis 4 | 0.053 | 0.000 | 100.0 | 100.0 | |||
| NN1 | 0–10 cm | Axis 1 | 0.959 | 0.998 | 95.9 | 96.9 | 0.989 |
| Axis 2 | 0.031 | 0.904 | 99.0 | 100.0 | |||
| Axis 3 | 0.000 | 0.926 | 99.0 | 100.0 | |||
| Axis 4 | 0.007 | 0.000 | 99.7 | 100.0 | |||
| 10–20 cm | Axis 1 | 0.931 | 0.984 | 93.1 | 97.0 | 0.960 | |
| Axis 2 | 0.029 | 0.873 | 96.0 | 100.0 | |||
| Axis 3 | 0.000 | 0.716 | 96.0 | 100.0 | |||
| Axis 4 | 0.033 | 0.000 | 99.3 | 100.0 | |||
| 20–30 cm | Axis 1 | 0.951 | 0.994 | 95.1 | 96.6 | 0.984 | |
| Axis 2 | 0.033 | 0.941 | 98.4 | 100.0 | |||
| Axis 3 | 0.000 | 0.955 | 98.4 | 100.0 | |||
| Axis 4 | 0.013 | 0.000 | 99.7 | 100.0 |
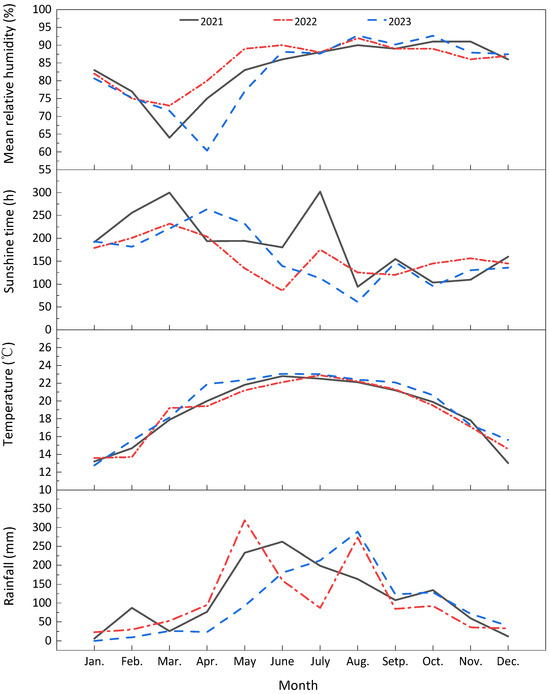
Figure A1.
Temperature, rainfall, sunshine and relative humidity of the experimental field from 2021 to 2023. Note: Meteorological data from 2021 to 2022 were obtained from the Statistical Yearbook of Menghai County, and meteorological data and for 2023 it was sourced from the meteorological station data of Menghai County Meteorological Bureau.
Figure A2.
Plant dry matter of rice fields with annual and perennial farming practices. F—early season, S—late season, *—significant difference between TN1 and NN1 per season/year at p < 0.05 under 2-tailed t-test, n.s.—non-significant difference between TN1 and NN1 in a season. Data presented in each production season or year are average values.
References
- Jain, N.; Kourampi, I.; Umar, T.P.; Almansoor, Z.R.; Anand, A.; Ur Rehman, M.E.; Jain, S.; Reinis, A. Global population surpasses eight billion: Are we ready for the next billion? AIMS Public Health 2023, 10, 849–866. [Google Scholar] [CrossRef]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.; Williams, M. Feeding 9 billion by 2050–Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Monfreda, C.; Ramankutty, N.; Foley, J.A. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Glob. Biogeochem. Cycles 2008, 22, 1022. [Google Scholar] [CrossRef]
- Gauri, S.G. Land degradation and challenges of food security. Rev. Eur. Stud. 2019, 11, 63. [Google Scholar] [CrossRef]
- Chapman, E.A.; Thomsen, H.C.; Tulloch, S.; Correia, P.M.P.; Luo, G.; Najafi, J.; DeHaan, L.R.; Crews, T.E.; Olsson, L.; Lundquist, P.-O.; et al. Perennials as future grain crops: Opportunities and challenges. Front. Plant Sci. 2022, 13, 898769. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Batáry, P.; Zou, Y.; Zhou, W.; Wang, G.; Liu, Z.; Bai, Y.; Gong, S.; Zhu, Z.; Settele, J.; et al. Agricultural diversification promotes sustainable and resilient global rice production. Nat. Food 2023, 4, 788–796. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B 2007, 363, 543–555. [Google Scholar] [CrossRef]
- Hillel, D. Out of the Earth: Civilization and the Life of the Soil; University of California Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Montgomery, D.R. Soil erosion and agricultural sustainability. Proc. Natl. Acad. Sci. USA 2007, 104, 13268–13272. [Google Scholar] [CrossRef]
- Cox, T.S.; Bender, M.; Picone, C.; Van Tassel, D.L.; Holland, J.B.; Brummer, E.C.; Zoeller, B.E.; Paterson, A.H.; Jackson, W. Breeding perennial grain crops. Crit. Rev. Plant Sci. 2002, 21, 59–91. [Google Scholar] [CrossRef]
- Cox, T.S.; Glover, J.D.; Van Tassel, D.L.; Cox, C.M.; DeHaan, L.R. Prospects for developing perennial grain crops. BioScience 2006, 56, 649–659. [Google Scholar] [CrossRef]
- Jackson, W. New Roots for Agriculture, 2nd ed.; University of Nebraska Press: Lincoln, NE, USA, 1980. [Google Scholar]
- Wagoner, P.; Schaeffer, J.R. Perennial grain development: Past efforts and potential for the future. Crit. Rev. Plant Sci. 1990, 9, 381–408. [Google Scholar] [CrossRef]
- Scheinost, P.L.; Lammer, D.L.; Cai, X.; Murray, T.D.; Jones, S.S. Perennial wheat: The development of a sustainable cropping system for the U.S. Pacific Northwest. Am. J. Altern. Agric. 2001, 16, 147–151. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased food and ecosystem security via perennial grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels—The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef]
- Glover, J.D.; Culman, S.W.; DuPont, S.T.; Broussard, W.; Young, L.; Mangan, M.E.; Mai, J.G.; Crews, T.E.; DeHaan, L.R.; Buckley, D.H.; et al. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agric. Ecosyst. Environ. 2009, 137, 3–12. [Google Scholar] [CrossRef]
- Snapp, S.; Rogé, P.; Okori, P.; Chikowo, R.; Peter, B.; Messina, J. Perennial grains for Africa: Possibility or pipedream? Exp. Agric. 2019, 55, 251–272. [Google Scholar] [CrossRef]
- Ledo, A.; Smith, P.; Zerihun, A.; Whitaker, J.; Vicente-Vicente, J.L.; Qin, Z.; McNamara, N.P.; Zinn, Y.L.; Llorente, M.; Liebig, M.; et al. Changes in soil organic carbon under perennial crops. Glob. Change Biol. 2020, 26, 4158–4168. [Google Scholar] [CrossRef]
- Peixoto, L.; Olesen, J.E.; Elsgaard, L.; Enggrob, K.L.; Banfield, C.C.; Dippold, M.A.; Nicolaisen, M.H.; Bak, F.; Zang, H.; Dresbøll, D.B.; et al. Deep-rooted perennial crops differ in capacity to stabilize C inputs in deep soil layers. Sci. Rep. 2022, 12, 5952. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Zhang, S.; Zhang, J.; Gan, S.; Cheng, M.; Hu, J.; Huang, L.; Hu, F. An innovated crop management scheme for perennial rice cropping system and its impacts on sustainable rice production. Eur. J. Agron. 2021, 122, 126186. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, S.; Huang, G.; Zhang, Y.; Lv, X.; Wan, K.; Liang, J.; Dao, J.; Wu, S.; Zhang, L.; et al. Perennial rice improves farmer livelihood and ecosystem security. Res. Sq. 2022, 22, 77. [Google Scholar] [CrossRef]
- Shanmugam, V.; Tyagi, V.C.; Gobinath, R.; Swarnaraj, A.K.; Arulanandam, M.; Kumar, V.; Peramaiyan, P.; Murugaiyan, V.; Sundaram, R.M. Perennial rice—An alternative to the ‘one-sow, one-harvest’ rice production: Benefits, challenges, and future prospects. Farming Syst. 2025, 3, 100137. [Google Scholar] [CrossRef]
- Huang, G.; Qin, S.; Zhang, S.; Cai, X.; Wu, S.; Dao, J.; Zhang, J.; Huang, L.; Harnpichitvitaya, D.; Wade, L.; et al. Performance, economics and potential impact of perennial rice PR23 relative to annual rice cultivars at multiple locations in Yunnan Province of China. Sustainability 2018, 10, 1086. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, G.; Zhang, J.; Huang, L.; Cheng, M.; Wang, Z.; Zhang, Y.; Wang, C.; Zhu, P.; Yu, X.; et al. Genotype by environment interactions for performance of perennial rice genotypes (Oryza sativa L./Oryza longistaminata) relative to annual rice genotypes over regrowth cycles and locations in southern China. Field Crops Res. 2019, 241, 107556. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, G.; Zhang, Y.; Lv, X.; Wan, K.; Liang, J.; Feng, Y.; Dao, J.; Wu, S.; Zhang, L.; et al. Sustained productivity and agronomic potential of perennial rice. Nat. Sustain. 2023, 6, 28–38. [Google Scholar] [CrossRef]
- Wang, W.; He, A.; Jiang, G.; Sun, H.; Jiang, M.; Man, J.; Ling, X.; Cui, K.; Huang, J.; Peng, S.; et al. Ratoon rice technology: A green and resource-efficient way for rice production. Adv. Agron. 2020, 159, 135–167. [Google Scholar] [CrossRef]
- Thapa, D.; Dura, R. A review on tillage system and no-till agriculture and its impact on soil health. Arch. Agric. Environ. Sci. 2024, 9, 612–617. [Google Scholar] [CrossRef]
- Pruthviraj, N.; Murali, K.; Chaitanya, A.; Harish, M.C.; Karthik, A.N. Exploring the dynamics of nitrogen from conventional manures in the soil plant atmosphere continuum: A comprehensive review. Commun. Soil Sci. Plant Anal. 2024, 55, 1690–1701. [Google Scholar] [CrossRef]
- Stevenson, F.J. Origin and Distribution of Nitrogen in the Soil. In Nitrogen in Agricultural Soils; Stevenson, F.J., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 1–42. [Google Scholar] [CrossRef]
- Canisares, L.P.; Grove, J.; Miguez, F.; Poffenbarger, H. Long-term no-till increases soil nitrogen mineralization but does not affect optimal corn nitrogen fertilization practices relative to inversion tillage. Soil Tillage Res. 2021, 213, 105080. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Zhang, S.; Zhang, J.; Hu, F.; Li, F. Density-dependent fertilization of nitrogen for optimal yield of perennial Rice. Agronomy 2022, 12, 1698. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Stevenson, F.J. Organic Forms of Soil Nitrogen. In Nitrogen in Agricultural Soils; Stevenson, F.J., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; Volume 22, pp. 67–122. [Google Scholar] [CrossRef]
- Bremner, J.M. Organic Forms of Nitrogen. In Methods of Soil Analysis, 2nd ed.; Part 2; Black, C.A., Ed.; Agronomy Monograph 9; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1238–1255. [Google Scholar]
- Waring, S.A.; Bremner, J.M. Ammonium production in soil under waterlogged conditions as an index of nitrogen availability. Nature 1964, 201, 951–952. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar] [CrossRef]
- Jezierska-Tys, S.; Rutkowska, A. Respiratory activity, intensity of processes of ammonification and nitrification in soil subjected to the effect of chemical preparates Reglone 200 SL and Elastiq 550 EC. Afr. J. Agric. Res. 2015, 10, 1565–1571. [Google Scholar] [CrossRef]
- Hoffmann, G. Eine photometrische methode zur bestimmung der phosphatase-Aktivität in Böden. J. Plant Nutr. Soil Sci. 1968, 118, 161–172. [Google Scholar] [CrossRef]
- Luo, X.; Fu, X.; Yang, Y.; Peng, C.; Peng, S.; Chen, W.; Huang, Q. Microbial communities play important roles in modulating paddy soil fertility. Sci. Rep. 2016, 6, 20326. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Li, X.; Guo, X.; Ma, J. Relationship of activities of key enzymes involved in nitrogen metabolism with nitrogen utilization in rice under water-nitrogen interaction. Acta Agron. Sin. 2009, 35, 2055–2063. [Google Scholar] [CrossRef]
- Zou, H.; Li, D.; Ren, K.; Liu, L.; Zhang, W.; Duan, Y.; Lu, C. Response of maize yield and nitrogen recovery efficiency to nitrogen fertilizer application in field with various soil fertility. Front. Plant Sci. 2024, 15, 1349180. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Wang, Y.; Abzhanov, T.; Sarsekova, D.; Zhumabekova, Z. The spatial distribution of soil nitrogen storage and the factors that influence it in central Asia’s typical arid and semiarid grasslands. Diversity 2022, 14, 459. [Google Scholar] [CrossRef]
- Sokolowski, A.C.; McCormick, B.P.; de Grazia, J.; Wolski, J.E.; Rodríguez, H.A.; Rodríguez-Frers, E.P.; Gagey, M.C.; Debelis, S.P.; Paladino, I.R.; Barrios, M.B. Tillage and no-tillage effects on physical and chemical properties of an Argiaquoll soil under long-term crop rotation in Buenos Aires, Argentina. Int. Soil Water Conserv. Res. 2020, 8, 185–194. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Zhang, X.; Xu, X.; Chen, H.; Xiong, Z. Net global warming potential and greenhouse gas intensity from the double rice system with integrated soil-crop system management: A three-year field study. Atmos. Environ. 2015, 116, 92–101. [Google Scholar] [CrossRef]
- Huang, D.; Chen, X.; Zhang, S.; Zhang, Y.; Gao, Y.; Zhang, Y.; Liang, A. No-tillage improvement of nitrogen absorption and utilization in a Chinese Mollisol using 15N-tracing method. Atmosphere 2022, 13, 530. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, D.; Ni, J.; Zeng, X. Conservation tillage practices reduce nitrogen losses in the sloping upland of the Three Gorges Reservoir area: No-till is better than mulch-till. Agric. Ecosyst. Environ. 2020, 300, 107003. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Frossard, E.; Lüscher, A. Effects of legumes and fertiliser on nitrogen balance and nitrate leaching from intact leys and after tilling for subsequent crop. Agric. Ecosyst. Environ. 2024, 360, 108776. [Google Scholar] [CrossRef]
- Crews, T.E.; Kemp, L.; Bowden, J.H.; Murrell, E.G. How the nitrogen economy of a perennial cereal-legume intercrop affects productivity: Can synchrony be achieved? Front. Sustain. Food Syst. 2022, 6, 755548. [Google Scholar] [CrossRef]
- Obour, A.K.; Holman, J.D.; Simon, L.M.; Assefa, Y. No-tillage and nitrogen fertilization on soil chemical properties under dry land wheat–sorghum–fallow rotation. Agrosyst. Geosci. Environ. 2023, 6, e20330. [Google Scholar] [CrossRef]
- Matthews, R.B.; Wassmann, R.; Arah, J. Using a crop/soil simulation model and GIS techniques to assess methane emissions from rice fields in Asia. I. Model development. Nutr. Cycl. Agroecosyst. 2000, 58, 141–159. [Google Scholar] [CrossRef]
- Tan, C.; Drury, C.F.; Soultani, M.; van Wesenbeeck, I.J.; Ng, H.Y.F.; Gaynor, J.D.; Welacky, T.W. Effect of controlled drainage and tillage on soil structure and tile drainage nitrate loss at the field scale. Water Sci. Technol. 1998, 38, 103–110. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, W.; Lindsey, L.E.; Wang, L.; Hui, D.; Tao, B.; Jacinthe, P.A.; Tian, H. No-tillage farming enhances widespread nitrate leaching in the US Midwest. Environ. Res. Lett. 2024, 19, 104062. [Google Scholar] [CrossRef]
- Shang, Y.; Olesen, J.E.; Lrke, P.E.; Manevski, K.; Chen, J. Perennial cropping systems increased topsoil carbon and nitrogen stocks over annual systems—A nine-year field study. Agric. Ecosyst. Environ. 2024, 365, 10. [Google Scholar] [CrossRef]
- Daly, E. Assessment of Perennial Cereals in Central Alberta: Environmental Performance and Productivity. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2023. [Google Scholar] [CrossRef]
- Giannelli, G.; Vecchio, L.D.; Cirlini, M.; Gozzi, M.; Gazza, L.; Galaverna, G.; Potestio, S.; Visioli, G. Exploring the rhizosphere of perennial wheat: Potential for plant growth promotion and biocontrol applications. Sci. Rep. 2024, 14, 22792. [Google Scholar] [CrossRef] [PubMed]
- Zulu, S.G.; Motsa, N.M.; Magwaza, L.S.; Ncama, K.; Sithole, N.J. Effects of different tillage practices and nitrogen fertiliser application rates on soil-available nitrogen. Agronomy 2023, 13, 785. [Google Scholar] [CrossRef]
- Jamieson, T.; Stratton, G.; Gordon, R.; Madani, A. The use of aeration to enhance ammonia nitrogen removal in constructed wetlands. Can. Biosyst. Eng. 2003, 45, 1.9–1.14. [Google Scholar]
- Li, D.; Wang, J.; Chen, R.; Chen, J.; Zong, J.; Li, L.; Hao, D.; Guo, H. Nitrogen acquisition, assimilation, and seasonal cycling in perennial grasses. Plant Sci. 2024, 342, 112054. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; An, S.; Zhao, H.; Xu, L.; Qiao, Y.; Cheng, X. Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China. Ecol. Eng. 2016, 86, 174–182. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, P.; Wu, N.; Chen, H.; Wang, G. Impacts of grazing intensity on nitrogen pools and nitrogen cycle in an alpine meadow on the eastern Tibetan plateau. Appl. Ecol. Environ. Res. 2008, 6, 69–79. [Google Scholar] [CrossRef]
- Karra, R.; Maslouhi, A.; Bamba, Y.O. Modeling of nitrogen transport in variably saturated soils. Appl. Ecol. Environ. Res. 2018, 16, 1427–1444. [Google Scholar] [CrossRef]
- Lenssen, A.W.; Waddell, J.T.; Johnson, G.D.; Carlson, G.R. Diversified cropping systems in semiarid Montana: Nitrogen use during drought. Soil Tillage Res. 2007, 94, 362–375. [Google Scholar] [CrossRef]
- Sainju, U.M.; Caesar-Tonthat, T.; Lenssen, A.W.; Evans, R.G.; Kolberg, R. Tillage and cropping sequence impacts on nitrogen cycling in dryland farming in Eastern Montana, USA. Soil Tillage Res. 2009, 103, 332–341. [Google Scholar] [CrossRef]
- Mkhabela, M.S.; Madani, A.; Gordon, R.; Burton, D.; Cudmore, D.; Elmi, A.; Hart, W. Gaseous and leaching nitrogen losses from no-tillage and conventional tillage systems following surface application of cattle manure. Soil Tillage Res. 2008, 98, 187–199. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1998, 26, 1–15. [Google Scholar] [CrossRef]
- Li, D.J.; Yang, Y.; Chen, H.; Xiao, K.; Song, T.; Wang, K. Soil gross nitrogen transformations in typical karst and non-karst forests, Southwest China. J. Geophys. Res. Biogeosci. 2017, 122, 2831–2840. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Z.; Jiang, N.; Jiang, H.; Chen, L. Effects of long-term no-tillage with different residue application rates on soil nitrogen cycling. Soil Tillage Res. 2021, 212, 105044. [Google Scholar] [CrossRef]
- Huang, D.; Chen, X.; Cao, G.; Liang, A.; Jia, S.; Liu, S. Effects of long-term conservation tillage on soil nitrogen content and organic nitrogen components in a Chinese Mollisol. Appl. Ecol. Environ. Res. 2018, 16, 5517–5528. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.; Yang, P.; Hong, Y.; Zhang, L.; Tong, C.; Lai, D.; Lin, Y.; Tan, L.; Tian, Y.; et al. Soil organic nitrogen content and composition in different wetland habitat types along the south-east coast of China. Catena 2023, 232, 104757. [Google Scholar] [CrossRef]
- Xu, L.; He, N.; Yu, G. Nitrogen storage in China’s terrestrial ecosystems. Sci. Total Environ. 2020, 709, 136201. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Hu, N.; Fu, W. Soil carbon–nitrogen coupled accumulation following the natural vegetation restoration of abandoned farmlands in a karst rocky desertification region. Ecol. Eng. 2020, 158, 106033. [Google Scholar] [CrossRef]
- Lin, T.; Shaner, P.L.; Wang, L.; Shih, Y.T.; Wang, C.; Huang, G.; Huang, J. Effects of mountain tea plantations on nutrient cycling at upstream watersheds. Hydrol. Earth Syst. Sci. 2015, 19, 4493–4504. [Google Scholar] [CrossRef]
- Sun, L.; Chang, S.; Feng, Y.; Dyck, M.; Puurveen, D. Nitrogen fertilization and tillage reversal affected water-extractable organic carbon and nitrogen differentially in a Black Chernozem and a Gray Luvisol. Soil Tillage Res. 2015, 146, 253–260. [Google Scholar] [CrossRef]
- Sainju, U.M.; Stevens, W.B.; Evans, R.G.; Iversen, W.M. Irrigation system and tillage effects on soil carbon and nitrogen fractions. Soil Sci. Soc. Am. J. 2013, 77, 1225–1234. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q.; Zhang, C.; Zhou, B.; Li, X.; Zhang, X.; Chen, J.; Liu, K. Soil organic nitrogen components and N-cycling enzyme activities following vegetation restoration of cropland in Danxia degraded region. Forests 2022, 13, 1917. [Google Scholar] [CrossRef]
- Lü, H.; He, H.; Zhao, J.; Wang, W.; Xie, H.; Hu, G.; Liu, X.; Wu, Y.; Zhang, X. Dynamics of fertilizer-derived organic nitrogen fractions in an arable soil during a growing season. Plant Soil 2013, 373, 595–607. [Google Scholar] [CrossRef]
- Amelung, W.; Zhang, X. Determination of amino acid enantiomers in soils. Soil Biol. Biochem. 2001, 33, 553–562. [Google Scholar] [CrossRef]
- Follett, R.F.; Schimel, D.S. Effect of tillage practices on microbial biomass dynamics. Soil Sci. Soc. Am. J. 1989, 53, 1091–1096. [Google Scholar] [CrossRef]
- Jiang, X. The Effect on Soil Organic Nitrogen under Different Tillage in Dryland. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2005. [Google Scholar]
- Porter, L.K.; Stewart, B.A.; Haas, H.J. Effects of long-time cropping on hydrolyzable organic nitrogen fractions in some great plains soils. Soil Sci. Soc. Am. J. 1964, 28, 368–370. [Google Scholar] [CrossRef]
- Lamb, J.A.; Fernandez, F.G.; Kaiser, D.E. Understanding Nitrogen in Soils; University of Minnesota Extension: Minneapolis, MN, USA, 2014; pp. 1–5. [Google Scholar]
- Lu, H.; Li, S.; Jin, F.; Shao, M. Contributions of organic nitrogen forms to mineralized nitrogen during incubation experiments of the soils on the loess plateau. Commun. Soil Sci. Plant Anal. 2009, 40, 3399–3419. [Google Scholar] [CrossRef]
- Sorenson, P.; Quideau, S.; Rivard, B. High resolution measurement of soil organic carbon and total nitrogen with laboratory imaging spectroscopy. Geoderma 2018, 315, 170–177. [Google Scholar] [CrossRef]
- Xue, J.; Pu, C.; Liu, S.; Chen, Z.; Chen, F.; Xiao, X.; Lal, R.; Zhang, H. Effects of tillage systems on soil organic carbon and total nitrogen in a double paddy cropping system in Southern China. Soil Tillage Res. 2015, 153, 161–168. [Google Scholar] [CrossRef]
- El-Haris, M.K.; Cochran, V.L.; Elliott, L.F.; Bezdicek, D.F. Effect of tillage, cropping, and fertilizer management on soil nitrogen mineralization potential. Soil Sci. Soc. Am. J. 1983, 47, 1157–1161. [Google Scholar] [CrossRef]
- Mondal, S.; Chakraborty, D. Soil nitrogen status can be improved through no-tillage adoption particularly in the surface soil layer: A global meta-analysis. J. Clean. Prod. 2022, 366, 132874. [Google Scholar] [CrossRef]
- Schomberg, H.H.; Wietholter, S.; Griffin, T.S.; Reeves, D.W.; Cabrera, M.L.; Fisher, D.S.; Endale, D.M.; Novak, J.M.; Balkcom, K.S.; Raper, R.L.; et al. Assessing indices for predicting potential nitrogen mineralization in soils under different management systems. Soil Sci. Soc. Am. J. 2009, 73, 1575–1586. [Google Scholar] [CrossRef]
- Thomas, G.A.; Dalal, R.C.; Standley, J. No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Tillage Res. 2007, 94, 295–304. [Google Scholar] [CrossRef]
- Moraru, P.; Teodor, R. Effect of tillage systems on soil moisture, soil temperature, soil respiration and production of wheat, maize and soybean crops. J. Food Agric. Environ. 2012, 10, 445–448. [Google Scholar]
- Roscoe, R.; Vasconcellos, C.A.; Furtini-Neto, A.E.; Guedes, G.A.A.; Fernandes, L.A. Urease activity and its relation to soil organic matter, microbial biomass nitrogen and urea-nitrogen assimilation by maize in a Brazilian Oxisol under no-tillage and tillage systems. Biol. Fertil. Soils 2000, 32, 52–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).